| Research Article | ||
Open Vet. J.. 2025; 15(5): 2127-2137 Open Veterinary Journal, (2025), Vol. 15(5): 2127-2137 Research Article Molecular characterization of fowl and pigeon pox viruses: A recent outbreak in LibyaMarwa H.A. Elshwihdi1, Abdulwahab M. Kammon1,2 and Abdulatif A. Asheg1*1Department of Poultry and Fish Diseases, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya 2National Research Center for Tropical and Transboundary Diseases, Alzintan, Libya *Corresponding Author: Abdulatif A. Asheg. Department of Poultry and Fish Diseases, Faculty of Veterinary Medicine, University of Tripoli, Tripoli, Libya. Email: a.asheg [at] uot.edu.ly Submitted: 21/01/2025 Revised: 28/03/2025 Accepted: 06/04/2025 Published: 31/05/2025 © 2025 Open Veterinary Journal
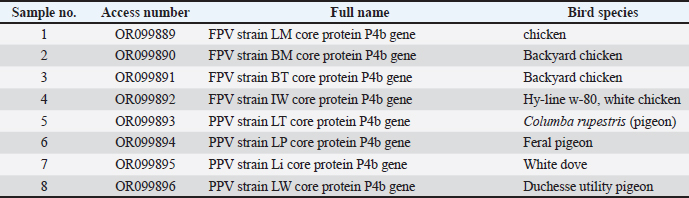
ABSTRACTBackground: Poxvirus infections in poultry, particularly fowl poxviruses (FPV), pose significant challenges to the global poultry industry. A notable outbreak of cutaneous fowl (FP) and pigeon pox in Libya has primarily affected backyard chickens, pigeons, and some commercial layers, marking the region’s first official record of these viruses. Aim: This study aimed to isolate, identify, and characterize FP and pigeon pox viruses (PPVs) associated with the outbreak. Methods: Histopathological examinations were conducted alongside clinical observations of lesions in affected chickens and pigeons. Virus isolation was performed using embryonated chicken eggs, and molecular diagnosis was achieved via polymerase chain reaction targeting the P4b core protein gene. Sequencing and phylogenetic analyses were also performed. Results: Characteristic lesions, such as wart-like growths and scabs, were observed in backyard chickens and pigeons. Histopathological analyses confirmed the presence of eosinophilic intracytoplasmic inclusion bodies. Molecular analysis revealed high genetic similarity among the four FPV isolates, which were 100% identical to selected isolates from Iraq, Iran, and Brazil. The PPV isolates also showed significant genetic homogeneity, with 100% identity to strains from Egypt and India and high similarities to other isolates. Conclusion: Our findings underscore the need for further investigation into the epidemiology and transmission dynamics of fowlpox and PPVs. Future research should focus on the genetic diversity of PPVs and their implications for pathogenicity and host specificity. Ongoing global surveillance and genetic analysis of avian viruses are crucial for understanding their impact on poultry populations and developing effective disease management strategies. Keywords: Fowl pox, Pigeon pox, Backyard chicken, Pigeon, Libya. IntroductionThe genus Avipox encompasses several DNA viruses, including Fowlpox virus (FPV), Pigeon Pox virus (PPV), Turkey Pox virus, Canary Pox virus, and Penguin Pox virus. Fowl pox (FP), a virus of this genus within the Poxviridae family, is a globally distributed and slowly spreading viral disease affecting chickens. All bird species are vulnerable to avian pox, as evidenced by reported cases of natural infections in various wild and domestic birds (Fauquet et al., 2005). In contrast to DNA viruses, poxviruses replicate and develop in the cytoplasm of infected cells. The transmission of pox infections is significantly facilitated by wild birds and insects. The FPV has a DNA structure of approximately 288 to 300 kilobase pairs in length (Asif et al., 2021). Characteristically, poxviruses possess a distinctive brick-like shape and spread slowly, leading to the development of proliferative lesions and scabs (dry form) on the skin, along with diphtheritic lesions (wet form) in the upper digestive and respiratory systems (Williams et al., 2021). FP has considerable economic implications because it can decrease egg production and hinder growth in younger birds. The risk of mortality increases when both dry and wet forms are present (Singh et al., 2000). Typical clinical symptoms include weight loss, feather loss, and scaly skin on the head, neck, and back. Secondary bacterial infections are common in both forms and can result in pneumonia or further infections at blister locations (Tripathy and Reed, 2013). Prompt recognition of these clinical signs, timely intervention, and appropriate management practices are crucial for mitigating the impact of poxvirus outbreaks on poultry health and welfare. Veterinarian consultation and diagnostic testing are essential for confirming poxvirus infections and implementing targeted control measures. Preventing poxvirus outbreaks in poultry requires a multifaceted approach that incorporates robust biosecurity measures, vaccination programs, hygiene protocols, and effective farm management practices. By integrating these practices into daily operations and continuously monitoring strategies to meet the evolving needs of the flock, poultry farmers can significantly reduce the risk of poxvirus outbreaks and enhance the health and well-being of their poultry populations. Collaborations with veterinary authorities, industry experts, and peers can further bolster disease prevention efforts and strengthen the overall biosecurity of poultry production systems (Giotis and Skinner, 2019). In Libya, there is significant interest in backyard chickens and pigeons, yet no official reports exist on poxvirus infections, and data on clinical cases from veterinarians are scarce despite observed cases in the field. The Libyan Animal Health Authority recommends vaccinating against poxvirus at 4–7 weeks of age for protection against poxvirus. However, the lack of recorded data on FP and pigeon pox, along with limited characterization of the viruses, leaves significant gaps in our understanding of these diseases. This study is the first official documentation of fowl and PPVs in Libya, utilizing isolation, identification, and genetic and phylogenetic analyses. Materials and MethodsSamplingBetween January 2022 and December 2022, cases submitted to the Faculty of Veterinary Medicine Hospital revealed pox lesions in commercial chickens, backyard chickens, and pigeons. These cases were observed and humanely euthanized, and skin samples were collected from the lesions. The samples were divided into two portions: one was preserved in 10% buffered formalin, and the other was stored in a deep freezer for egg inoculation. Additionally, older frozen samples were obtained from private veterinary clinics that had stored them in a deep freezer since 2014. These included samples from feral pigeons collected in the Tripoli area, as well as samples from backyard chickens gathered in 2016 and 2017 from the Souq Aljuma and Janzour areas of Tripoli, respectively. Another sample of the pigeon species, Columba rupestris, was collected in 2019 from the Tajoura area in Tripoli. All frozen samples were thawed, and the material from the pox lesions was used for egg inoculation. Table 1 summarizes the samples received during this period. HistopathologySections of skin with pox lesions were cut into pieces, including all layers of the skin, from samples from the current study. The skin samples were preserved in neutral formalin until processing. After being washed overnight in running water and dehydrated in increasing concentrations of alcohol, the tissues were embedded in paraffin. Sections 5 μm thick were then cut and stained with hematoxylin and eosin. Methods of egg inoculationThe methods used for chorioallantoic membrane (CAM) inoculation were described in (Baddeley and Tripathy, 1998) with minor modifications. Fertile eggs were obtained from flocks under restricted control and observation, in which previously does not receive any type of vaccination during the entire period of their lives. polymerase chain reaction (PCR)The QIAamp DNA Mini Kit. Oligonucleotide primers used in conventional polymerase chain reaction (Midland Certified Reagent Company_ oilgos, USA). Oligonucleotide primer sequences were used according to (Prukner-Radovčić et al., 2006) with amplified product 578 bp of gene 4b core primer Sequence (5′-3′). The extraction of viral DNA was conducted according to QIAamp DNA mini kit instructions. Briefly, each cycle of the reaction consisted of primary denaturation at 94°C for 2 minutes, secondary denaturation at 94°C for 1 minute, annealing at 60°C for 1 minute, and extension at 72°C for 1 minute. The cycle was repeated 35 times with a final extension at 72°C for 2 minutes. The gel was then photographed by a gel documentation system. A purified PCR product was sequenced in the forward and/or reverse directions on an Applied Biosystems 3130 automated DNA Sequencer (ABI, 3130, USA) using a cycle sequencing kit (Perkin-Elmer/Applied Biosystems, Foster City, CA). Table 1. Samples collected during the studies indicating the type and breed of birds and location.
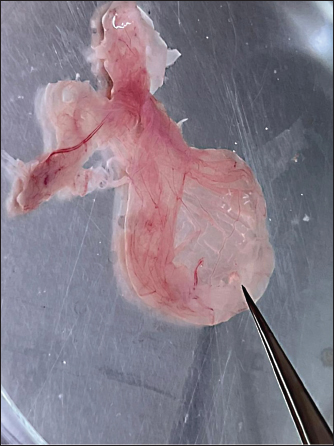
Fig. 1. Commercial Novo gen white-laying chickens aged 18 weeks with pox lesions on their combs and wattles. The Basic Local Alignment Search Tool (A BLAST® analysis) was initially used to establish sequence identity for GenBank accessions. The sequence reaction was performed according to the instructions of the manufacturer. Phylogenetic analysisA comparative sequence analysis was conducted using the CLUSTAL W multiple sequence alignment program, version 12.1 of the MegAlign module from LaserGene DNAStar software (Madison, Wisconsin, USA), developed by (Tamura et al., 2013). Phylogenetic analyses were performed using maximum likelihood neighbor joining (MEGA11 software). Ethical approvalThis research was approved by the Scientific Research and Ethics Committee of the University of Tripoli (No.: SREC/010/33) dated on 31/07/2023. ResultsClinical signs and lesionsSamples included in the current study exhibited Pox lesions consisting of a cutaneous form (dry pox) characterized by the growth of proliferative lesions, which varied from small nodules to spherical wart-like masses on the skin of the comb, wattle, and other featherless areas. The samples belonged to Novogen chickens (Fig. 1), commercial chickens (Fig. 2), and backyard chickens (Fig. 3). In addition, four samples from a private clinic were frozen from feral pigeons since 2014. Additionally, a sample of a C. rupestris pigeon was frozen in 2019. Two samples from backyard chickens were also frozen, one in 2016 and the other in 2017. Histopathology of skin samplesOnly fresh chicken and pigeon samples were sent for histopathological analysis. Histologic sections of the skin from areas over the eye, combs, wattles, and around the mouth exhibited significant epithelial hyperplasia, resulting in deep folds. Some differences were noted in the extent of severity of histopathological lesions among the samples included in the current study.
Fig. 2. Commercial laying chicken (Hy-line W- 80 white) aged 16 weeks with a wart-like lesion on the tops of their peaks.
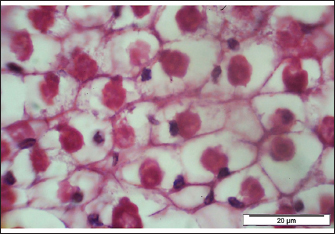
Fig. 3. Backyard chicken aged 40 days suffered from severe pox lesions on the comb, wattles, and around the eyes. The skin of the backyard chicken showed multifocal hyperplasia of the epidermal epithelium with mild ballooning degeneration (swollen) of keratocyte and numerous eosinophilic viral inclusion bodies (Bollinger bodies) within the cytoplasm of the affected cells. A section is characterized by the presence of a moderate amount of superficial amorphous eosinophilic necrotic material, admixed with cellular debris. Whereas in sample skin of backyard chicken and sample skin of pigeon, the epidermal and follicular epithelium is diffusely hyperplastic with marked thickness of the stratum spinosum that shows extensive swollen keratinocytes and the presence of eosinophilic viral inclusion bodies within the cytoplasm of the affected cells displacing the nuclei to the periphery (Figs. 4 and 5).
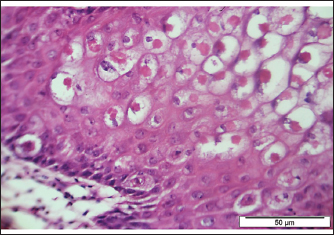
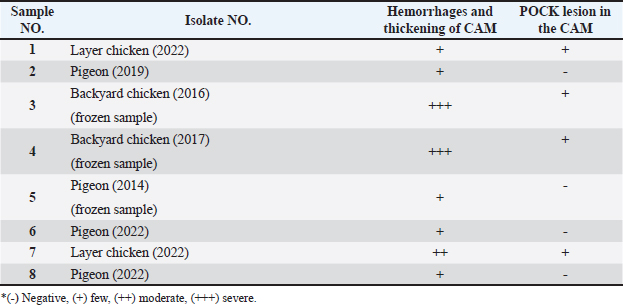
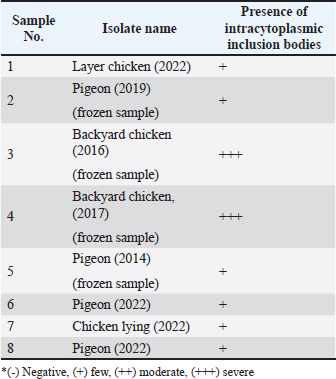
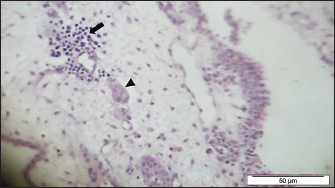
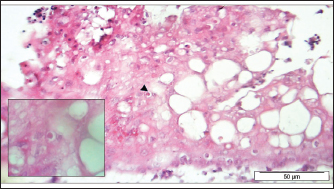
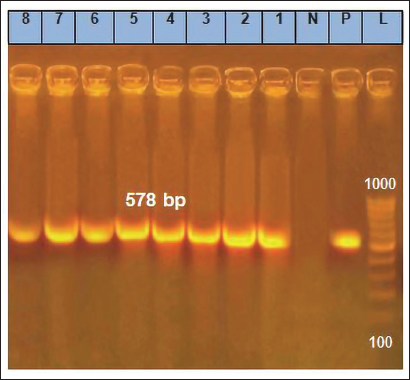
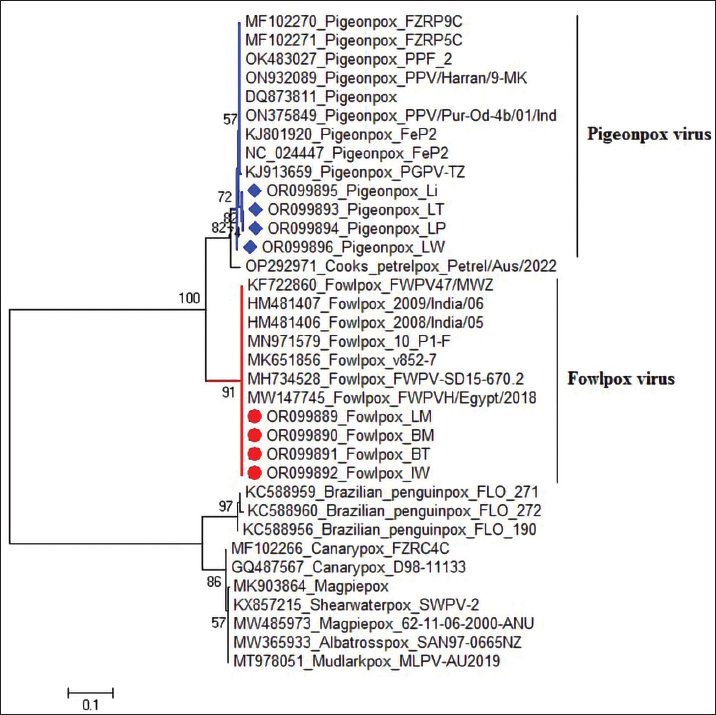
Fig. 4. Section of sample showing extensive lesions (ballooning degeneration and inclusion bodies) caused by PPV in the skin of C. rupestris pigeons (HE, Bar=50 μm). Virus isolationSamples of live birds and frozen samples were used for egg inoculation to propagate the virus. Three passages were conducted on the eggs. All isolates grew well on the CAM. Pock lesions were clear on CAMs inoculated with samples from backyard chickens and commercial chickens (Fig. 6). However, isolates collected from pigeons did not exhibit clear pock lesions on the CAM, whereas all samples showed thickening of the CAM accompanied by varying degrees of hemorrhage. The results of egg inoculation for each isolate are presented in Table 2. Histopathology of the CAM samplesThe samples showed differences in the severity of the reaction, especially in the presence of intracytoplasmic inclusion bodies, as shown in Table 3. The histopathological lesions observed on CAM inoculated from samples from backyard chickens were thickening of the CAM tissue with infiltration of lymphocytes, presence of syncytial cells and hemorrhages (Fig. 7), presence of intracytoplasmic eosinophilic inclusion bodies, and ballooning of some other cells (Fig. 8). PCRThe PCR results are shown in Figure 9. All eight samples included in the current study were positive for the pox virus using conventional PCR. The 578 bp of 4 b gene fragments (fpv167 locus) were amplified according to Lüschow et al. (2004). Sequencing and phylogenetic analysesSequencing and phylogenetic analysis of the isolates from chickens and pigeons were performed. The sequences were published in GenBank using the access numbers and names listed in Table 4. Partial sequences of the 4b core protein gene were amplified from four FP and four pigeon virus isolates. The obtained sequences (FASTA files) were subjected to NCBI BLASTN analysis to determine their identity with other isolates in the NCBI data archive. Phylogenetic analyses were performed using maximum likelihood, neighbor-joining, and maximum parsimony in MEGA11 software, as shown in Figure 10. The four FPV isolates of the current study were very similar to each other, and the four pigeon virus isolates were also very similar to each other.
Fig. 5. Section of sample showing Bollinger bodies displacing the nuclei of keratocyte to the periphery (HE, 20 μm).
Fig. 6. Section of CAMs harvested from embryonated eggs inoculated with samples from backyard chickens. CAMs showing a membrane, hemorrhage, and pock lesions. Table 2. Samples collected from the third passage on CAM after 5 days.
Table 3. the presence and intensity of cytoplasmic inclusion bodies in CAM inoculated with different isolates.
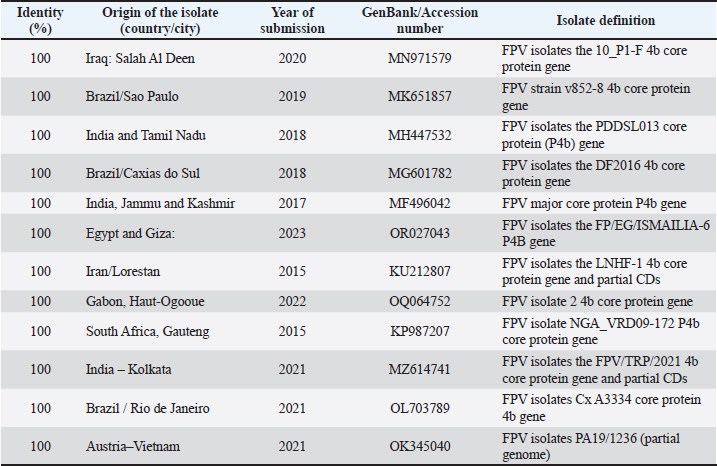
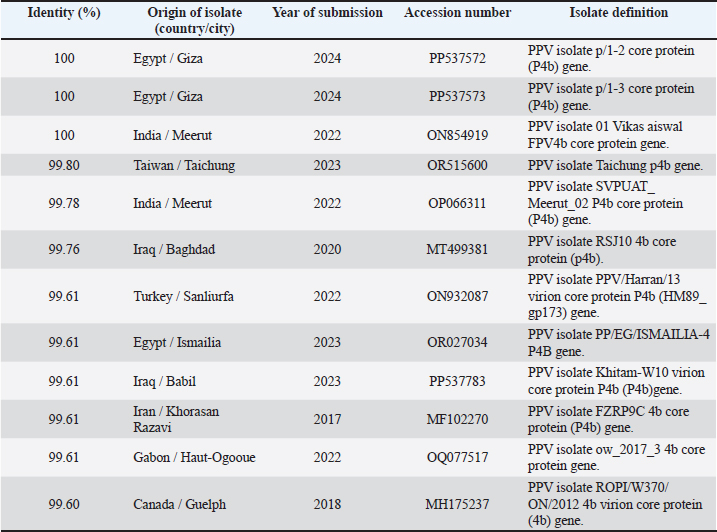
The FPV isolated in the current study were 100% identical to some selected isolates from Iraq, Iran, India, Brazil, South Africa, Gabon, and Egypt (Table 5). The similarities of the PPVs isolated in this study are shown in Table 6, in which 100% similarities were found to isolates from Egypt and India. Identities of 99.80%, 99.78%, and 99.76% were isolated from Taiwan, India, and Iraq, respectively. Moreover, our isolates were 99.61% identical to other isolates from Egypt, Iraq, Iran, and Gabon, whereas our isolates were 99.60% identical to one isolate from Canada.
Fig. 7. Section of the CAM showing thickening of the tissue with infiltration of lymphocytes (arrow), presence of syncytial cells (arrow head) and hemorrhages (HE, Bar=50 μm).
Fig. 8. Section of the CAM showing some infected cells (Arrowhead) with intracytoplasmic eosinophilic inclusion bodies and ballooning of other cells (HE, Bar=50 μm, inlet 100×).
Fig. 9. Result of PCR showing the positive eight isolates from CAM. Bands of successful amplification of viral DNA (578 bp) compared with the positive and negative controls. A 1,000 bp DNA marker (Ladder) was used to determine the molecular weight of the bands. Table 4. Sequencing data of poxviruses isolated from chickens and pigeons published in GenBank.
DiscussionPoxvirus infections in poultry have been documented for many years (Minhas et al., 2024). Despite being a recognized disease in poultry, FPV continue to challenge the global poultry industry. Clinical manifestations of poxvirus infections in chickens and pigeons can be diagnosed by observing characteristic gross and microscopic lesions (Tripathy and Reed, 2013). The results of this study indicate that poxvirus infections in backyard chickens result in the formation of scabs on the comb, wattles, eyelids, and feet, consistent with the lesions described by Minhas et al. (2024). However, the lesions observed in commercial laying hens in the current study were atypical; they presented as wart-like lesions solely on the beak near the nasal orifice, with no lesions on the feathered areas. Similar atypical lesions have been previously reported in vaccinated commercial laying hen flocks in Brazil (Chacón et al., 2020). In the current study, backyard chickens and pigeons had more severe lesions, including small, bleeding, wart-like growths on areas such as the face, head, neck, beak, and toes. These findings are similar to those described by Hibl et al. (2019). The differences in the severity of lesions among backyard chickens, pigeons, and commercial laying hens may be due to the lack of vaccination in the backyard chicken and pigeon populations.
Fig. 10. Phylogenetic analysis of the partial 4b core protein gene of avian pox virus isolates. Isolate names and GenBank accession numbers. Libyan FP isolates from this study are highlighted in red circles, whereas Libyan pigeon isolates are highlighted in blue squares. The phylogenetic tree was constructed in MEGA 11 using the neighbor-joining method with 1,000 bootstrap replicates. Histopathological examination of fresh samples from backyard chickens, commercial layers, and pigeons demonstrates the presence of multiple eosinophilic intracytoplasmic inclusion bodies (Bollinger bodies), which are a diagnostic feature of avian poxvirus infection (Tripathy and Reed, 2013). The epidermis showed marked hyperplasia (acanthosis) due to the swelling and increased number of cells in the stratum spinosum. These cells exhibited ballooning degeneration and contained various sizes of pale eosinophilic inclusion bodies, which are characteristic of avian pox (Tripathy et al., 2000). Pustular lesions showed superficial crusts of hemorrhages and necrotic cells, with lymphocyte and histiocyte infiltration in the dermis. Occasionally, fibroblast proliferation and fibrosis were observed in the affected areas. Table 5. Comparison of Libyan FP isolates with those from other populations (BLASTN).
In this study, the initial virus isolation was successful using 9-11-day-old embryonated chicken eggs (ECE) for inoculation of the isolate from chickens or pigeons, according to Tripathy and Reed (2013). Moreover, frozen old samples from 2014 to 2019 from pigeons and backyard chickens were readily grown and propagated in ECE, showing the typical histopathological changes of chicken embryos. These findings were similar to those of the studies by Offerman et al. (2013), which explained the hypertrophy and the appearance of many intracytoplasmic inclusion bodies in the ectoderm. Three serial passages were continued for the propagation of FPV and PPV, as previously described (Sultana et al., 2019). The CAM thickened upon inoculation with FPV and PPV samples, consistent with the findings of Kabir et al. (2015), Masola et al. (2015), and Roy et al. (2013). However, pock lesions on the CAM were absent in the pigeon samples. These findings are similar to the results reported (Audarya et al., 2018). The results here agreed with earlier studies, except for the low number of pock lesions in most of the samples tested. The P4b core protein gene is a unique genetic marker of poxviruses (Sarker et al., 2021). The highly conserved characteristics of this gene enable the molecular diagnosis and differentiation of pox viruses across different host species, as well as among various strains of the same species (Abdallah and Hassanin, 2013; Gyuranecz et al., 2013). PCR analysis targeting the P4b gene was performed on four FPV and four PPV field samples, and all samples tested positive (100%). The results obtained were similar to those reported by Sultana et al. (2019). The present study demonstrated partial sequencing of the 4b core protein gene of four FP and four pigeon virus isolates. The resulting sequences, formatted as FASTA files, were then analyzed using NCBI BLASTN to assess their identity with existing isolates in the NCBI database. To further explore the relationships among these viruses, phylogenetic analyses were performed using maximum likelihood neighbor-joining with MEGA11 software. The analysis revealed that the four FPV isolates in this study exhibited a high degree of similarity to one another, indicating a close genetic relationship, although some samples were frozen many years ago. Similarly, the four-pigeon virus isolates also showed significant homogeneity within their groups, and no molecular characterization study of the FPV in Libya. The FPV isolated in this study were 100% identical to selected isolates from various countries, including Iraq, Iran, India, Brazil, South Africa, Gabon, and Egypt. The Iraqi isolate was described by Hasan et al. (2021), who showed that nucleotide sequence alignments revealed three Iranian and one Egyptian virus isolates from chicken hosts that were identical to the Iraqi FPV isolate. Table 6. Comparison of Libyan pigeon pox isolates with those from other populations (BLASTN).
The similarities among the PPVs isolated in this study were 100% identical to those isolated from Egypt and India. Furthermore, our analysis revealed identities of 99.80%, 99.78%, and 99.76% with isolates from Taiwan, India, and Iraq, respectively. These high levels of genetic similarity suggest a close evolutionary relationship among these viruses, which may indicate shared transmission pathways or common ancestral origins. Additionally, our isolates demonstrated 99.61% identity with other isolates from Egypt, Iraq, Iran, and Gabon, highlighting the interconnectedness of PPV strains across these regions. Notably, our isolates also showed 99.60% identity with one isolate from Canada, suggesting that the genetic diversity of PPVs may extend beyond traditional geographic boundaries. This level of genetic congruence suggests potential avenues for understanding the epidemiology and transmission dynamics of the FPV across different geographic regions. The similarities observed among the PPVs warrant further investigation. These findings underscore the importance of continued surveillance and genetic analysis of avian viruses globally, as they can provide crucial insights into their epidemiology and potential impacts on avian populations. AcknowledgmentsThe author would like to thank Dr. Muhammed Qurish, Dr. Mohamed Subah, and Dr. Mohamed Ishtawi for providing the samples that exhibited symptoms of pox. Additionally, the author extends sincere gratitude to Dr. Ahmed Erfan, Head of Biotechnology at the Animal Health Research Institute in Dokki, Giza, Egypt, for his invaluable contributions in performing the PCR and sequencing of the isolate. Conflict of interestAbdulatif Asheg hereby declares that he has no financial, personal, or professional conflicts of interest related to the subject matter of this publication. If any potential conflicts arise in the future, he commits to disclosing them promptly to the relevant parties to ensure transparency and maintain the integrity of the work. Abdulatif Asheg [Date] 29-3-2025. FundingThis work was not funded. Authors’ contributionsDr. Abdulatif Asheg served as the primary supervisor for this work. Dr. Marwa H.A. Elshwihdi was responsible for sample collection and conducted the laboratory work. Dr. Abdulwahab M. Kammon acted as a co-advisor and was responsible for histopathological readings. Data availabilityThe data that support the findings of this study are available upon reasonable request. ReferencesAbdallah, F.M. and Hassanin, O. 2013. Detection and molecular characterization of avipoxviruses isolated from different avian species in Egypt. Vi. Genes. 46, 63–70. Asif, K., O’Rourke, D., Legione, A.R., Shil, P., Marenda, M.S. and Noormohammadi, A.H. 2021. Whole-genome based strain identification of fowlpox virus directly from cutaneous tissue and propagated virus. PLoS One 16(12), e0261122. Audarya, S., Riyesh, T., Kumar, N., Chhabra, D., Sikrodia, R., Sharda, R., Barua, S. and Garg, U. 2018. Molecular diagnosis of a cutaneous form of pox in pigeons at Mhow in Madhya Pradesh, India. Int. J. Curr. Microbiol. App. Sci. 37(9), 1318–1323. Baddeley, R. and Tripathy, S.P. 1998. Insights into motion perception by observer modeling. JOSA A 15(2), 289–296. Chacón, R.D., Astolfi-Ferreira, C.S., De la Torre, D.I., de Sá, L.R. and Piantino Ferreira, A.J. 2020. An atypical clinicopathological manifestation of fowlpox virus associated with reticuloendotheliosis virus in commercial laying hen flocks in Brazil. Transbound. Emerg. Dis. 67(6), 2923–2935. Fauquet, C.M., Mayo, M.A., Maniloff, J., Desselberger, U. and Ball, L.A. 2005. Virus taxonomy: VIIIth report of the ICTV. Cambridge, MA: Academic Press. Giotis, E.S. and Skinner, M.A. 2019. Spotlight on avian pathology: fowlpox virus. Avian Pathol. 48(2), 87–90. Gyuranecz, M., Foster, J.T., Dán, Á., Ip, H.S., Egstad, K.F., Parker, P.G., Higashiguchi, J.M., Skinner, M.A., Höfle, U. and Kreizinger, Z. 2013. Worldwide phylogenetic relationship of avian poxviruses. J. Virol. 87(9), 4938–4951. Hasan, I.I., Rasheed, S.T., Shakor, M.K. and Science, A. 2021. Pathological, molecular and phylogenic study of fowlpox virus in domesticated chickens of Tikrit City, Iraq. Brazil. J. Vet. Res. Anim. Sci. 58, e176255. Hibl, B.M., Blackwood, R.S., Simons, B.W. and Collins, D.E. 2019. Poxvirus infection in a colony of laboratory pigeons (Columba livia). Comp. Med. 69(3), 179–183. Kabir, M.L., Haque, M.E., Borty, S.C., Mustafa, K., Kamal, M.M., Khasruzzaman, A.K.M., Khan, M.S.R. and Islam, M.A. 2015. Isolation and molecular detection of fowl pox and pigeon pox viruses from recent outbreak in Bangladesh.Indian J. Life Sci. 5(1), 1. Lüschow, D., Hoffmann, T.H.M. and Hafez, H.M. 2004. Differentiation of avian poxvirus strains on the basis of nucleotide sequences of 4b gene fragment. Avian Dis. 48(3), 453–462. Masola, S., Mzula, A., Mwega, E., Kasanga, C. and Wambura, P. 2015. Detection and genetic characterization of an avipox virus isolate from domestic pigeon (Columba livia domestica) in Morogoro region, Eastern Tanzania. Adv. Res. 3(5), 460–469. Minhas, S.K., Kumar, P., Panghal, R., Mehtani, R., Yadav, R., Kalonia, S. and Gowthaman, V. 2024. Fowl pox virus: a minireview. World Poult. Sci. J. 80(2), 329–347. Offerman, K., Carulei, O., Gous, T.A., Douglass, N. and Williamson, A.-L. 2013. Phylogenetic and histological variation in avipoxviruses isolated in South Africa. J. Gen. Virol. 94(10), 2338–2351. Prukner-Radovčić, E., Lüschow, D., Grozdanić, I.C., Tišljar, M., Mazija, H., Vranešić, Ł. and Hafez, H. 2006. Isolation and molecular biological investigations of avian poxviruses from chickens, a turkey, and a pigeon in Croatia. Avian Dis. 50(3), 440–444. Roy, B., Joardar, S.N., Samanta, I., Das, P.K., Halder, A. and Nandi, S. 2013. Molecular characterization of fowl pox virus isolates from backyard poultry. Adv. Anim. Vet. Sci. 1(4S), 54–58. Sarker, S., Hannon, C., Athukorala, A. and Bielefeldt-Ohmann, H. 2021. Emergence of a novel pathogenic poxvirus infection in the endangered green sea turtle (Chelonia mydas) highlights a key threatening process. Viruses 13(2), 219. Singh, P., Kim, T.-J. and Tripathy, D. 2000. Re-emerging fowlpox: evaluation of isolates from vaccinated flocks. Avian Pathol. 29(5), 449–455. Sultana, R., Nazir, K.N.H., Rahman, M.T., Nipa, S.A., Rahman, M.M., Soma, S.S. and Rahman, M.B. 2019. Isolation and molecular detection of Fowl pox and Pigeon pox viruses for the development of live attenuated vaccine seeds from the local isolates: isolation and molecular detection of Fowl pox and Pigeon pox viruses. J. Bangladesh Agr. Univ. 17(2), 211–219. Tamura, K., Stecher, G., Peterson, D., Filipski, A. and Kumar, S. 2013. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 30(12), 2725–2729. Tripathy, D.N. and Reed, W.M. 2020. Laboratory manual for the isolation, identification, and characterization of avian pathogens, 5th ed. Athens, GA: AAAP, pp: 116–119. Tripathy, D.N., Schnitzlein, W.M., Morris, P.J., Janssen, D.L., Zuba, J.K., Massey, G. and Atkinson, C.T. 2000. Characterization of poxviruses from forest birds in Hawaii. J. Wildl. Dis. 36(2), 225–230. Williams, R.A., Truchado, D.A. and Benitez, L. 2021. A review on the prevalence of poxvirus disease in free-living and captive wild birds. Microbiol. Res. 12(2), 403–418. | ||
| How to Cite this Article |
| Pubmed Style Elshwihdi MH, Kammon AM, Asheg AA. Molecular characterization of fowl and pigeon pox viruses: A recent outbreak in Libya. Open Vet. J.. 2025; 15(5): 2127-2137. doi:10.5455/OVJ.2025.v15.i5.31 Web Style Elshwihdi MH, Kammon AM, Asheg AA. Molecular characterization of fowl and pigeon pox viruses: A recent outbreak in Libya. https://www.openveterinaryjournal.com/?mno=239055 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i5.31 AMA (American Medical Association) Style Elshwihdi MH, Kammon AM, Asheg AA. Molecular characterization of fowl and pigeon pox viruses: A recent outbreak in Libya. Open Vet. J.. 2025; 15(5): 2127-2137. doi:10.5455/OVJ.2025.v15.i5.31 Vancouver/ICMJE Style Elshwihdi MH, Kammon AM, Asheg AA. Molecular characterization of fowl and pigeon pox viruses: A recent outbreak in Libya. Open Vet. J.. (2025), [cited January 25, 2026]; 15(5): 2127-2137. doi:10.5455/OVJ.2025.v15.i5.31 Harvard Style Elshwihdi, M. H., Kammon, . A. M. & Asheg, . A. A. (2025) Molecular characterization of fowl and pigeon pox viruses: A recent outbreak in Libya. Open Vet. J., 15 (5), 2127-2137. doi:10.5455/OVJ.2025.v15.i5.31 Turabian Style Elshwihdi, Marwa H., Abdulwahab M. Kammon, and Abdulatif A. Asheg. 2025. Molecular characterization of fowl and pigeon pox viruses: A recent outbreak in Libya. Open Veterinary Journal, 15 (5), 2127-2137. doi:10.5455/OVJ.2025.v15.i5.31 Chicago Style Elshwihdi, Marwa H., Abdulwahab M. Kammon, and Abdulatif A. Asheg. "Molecular characterization of fowl and pigeon pox viruses: A recent outbreak in Libya." Open Veterinary Journal 15 (2025), 2127-2137. doi:10.5455/OVJ.2025.v15.i5.31 MLA (The Modern Language Association) Style Elshwihdi, Marwa H., Abdulwahab M. Kammon, and Abdulatif A. Asheg. "Molecular characterization of fowl and pigeon pox viruses: A recent outbreak in Libya." Open Veterinary Journal 15.5 (2025), 2127-2137. Print. doi:10.5455/OVJ.2025.v15.i5.31 APA (American Psychological Association) Style Elshwihdi, M. H., Kammon, . A. M. & Asheg, . A. A. (2025) Molecular characterization of fowl and pigeon pox viruses: A recent outbreak in Libya. Open Veterinary Journal, 15 (5), 2127-2137. doi:10.5455/OVJ.2025.v15.i5.31 |