| Research Article | ||
Open Vet. J.. 2025; 15(5): 2149-2159 Open Veterinary Journal, (2025), Vol. 15(5): 2149-2159 Research Article Biochemical markers of cell death: Forensic implications for differentiating primary and secondary hypothermiaEmina Dervišević1, Aida Bešić2*, Hajrudin Spahović3, Ekrema Mujarić4, Nedim Šuta5, Muamer Dervišević6, Edina Lazović7 and Aida Selmanagić81Department of Forensic Medicine, Faculty of Medicine, University of Sarajevo, Sarajevo, Bosnia and Herzegovina 2Department of Clinical Sciences, Faculty of Veterinary Medicine, University of Sarajevo, Sarajevo, Bosnia and Herzegovina 3Urology Clinic, Clinical Center University of Sarajevo, Sarajevo, Bosnia and Herzegovina 4Cantonal Hospital Zenica, Zenica, Bosnia and Herzegovina 5Department of Biology, Faculty of Science, University of Sarajevo, Sarajevo, Bosnia and Herzegovina 6University Clinical Center-Clinic for Lung Diseases and Tuberculosis “Podhrastovi”, Sarajevo, Bosnia and Herzegovina 7Department of Pathology, Faculty of Medicine, University of Sarajevo, Sarajevo, Bosnia and Herzegovina 8Department of Tooth Morphology with Dental Anthropology and Forensics, School of Dentistry, University of Sarajevo, Sarajevo, Bosnia and Herzegovina *Corresponding Author: Department of Clinical Sciences, Faculty of Veterinary Medicine, University of Sarajevo, Sarajevo, Bosnia and Herzegovina. Email: katicaaida1 [at] gmail.com Submitted: 04/02/2025 Revised: 01/04/2025 Accepted: 08/04/2025 Published: 31/05/2025 © 2025 Open Veterinary Journal
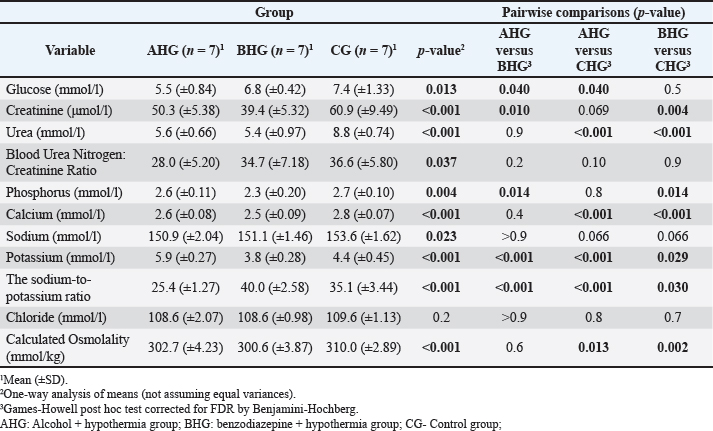
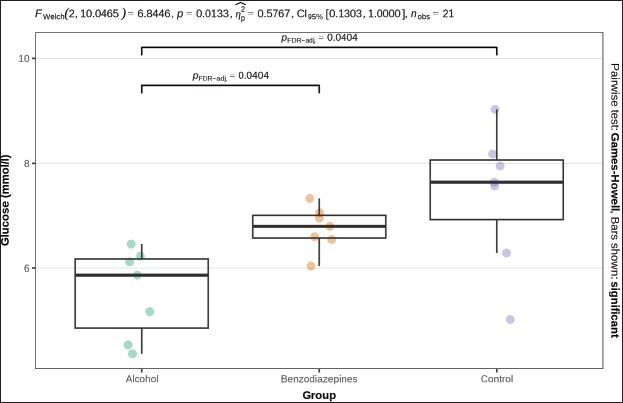
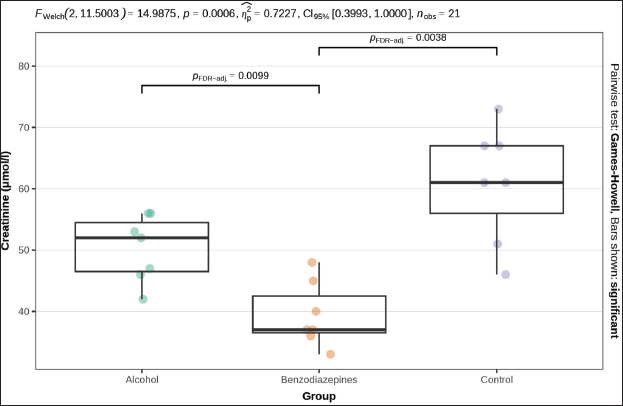
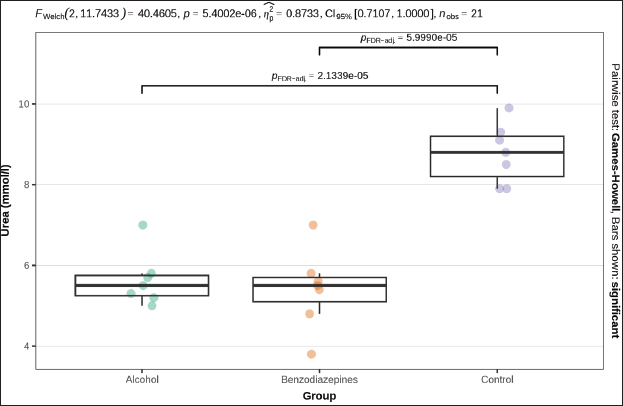
ABSTRACTBackground: Primary hypothermia occurs when the body is exposed to extremely low temperatures in an environment with no underlying health conditions. Secondary hypothermia, on the other hand, results from disruptions in thermoregulation due to diseases, trauma, surgery, drugs, alcohol, or infections. Postmortem biochemistry has become a crucial factor in forensic examinations, offering valuable apprehension into tissue of and organ dysfunction associated with the process of dying. Aim: This research aims to explore various biochemical markers and their significance in distinguishing primary from secondary hypothermia. Methods: This study involved 21 Wistar rats, which were separated into three experimental groups: CG (n=7), which were exposed only to hypothermic conditions; AHG (n=7); and BHG (n=7). We tested these parameters in each rat: glucose, urea, creatinine, blood urea nitrogen to creatinine ratio, phosphorus, calcium, sodium, potassium, sodium to potassium ratio, chloride, and calculated osmolality. Results: Distinct biochemical differences were noted between primary and secondary hypothermia. Glucose and creatinine levels exhibited significant variations (p < 0.001). Urea concentrations also manifested notable differences between the groups (p < 0.001). Phosphorus levels demonstrated significant differences (p=0.004), with post hoc analyses revealing significant contrasts between the AHG and BHG (p=0.014) and between the BHG and CG (p=0.014). Potassium levels and the sodium-to-potassium ratio differed significantly (p < 0.001). Osmolality also varied significantly across experimental groups (p < 0.001), with post hoc tests confirming significant differences between the AHG and CG (p=0.013) and between the BHG and CG (p=0.002). Conclusion: The calculated osmolality exhibited significant variation among the different groups, indicating a notable impact of the substances on the biochemical profile related to hypothermia. This study focused on the effectiveness of biochemical markers in distinguishing primary hypothermia from secondary hypothermia. Keywords: Hypothermia, Forensic, Cause of death, Biochemical analysis. IntroductionHypothermia is a state of lowered core body temperature with significant forensic implications. The condition is defined as when the core temperature falls approximately 2°C below the normal physiological range of 37.5°C–37.7°C (Paal et al., 2021). Hypothermia is classified based on the degree of temperature drop: mild (35°C–32°C), moderate (32°C–28°C), or severe (below 28°C). The forensic relevance distinguishes primary and secondary hypothermia (Duong and Patel 2024; Elmsjö et al., 2024). Primary hypothermia can lead directly to death when a person is stated to low temperatures until the person’s body thermoregulatory and metabolic processes fail. In addition, secondary hypothermia occurs when exposure to lower temperatures is compounded by other factors, such as alcohol or drug overdose, which impairs the body’s ability to obtain a stable internal temperature (Kempainen and Brunette, 2004). Exposure to cold environments can result in localized or systemic hypothermic injuries, or in some cases, fatal outcomes (Brychta and Chen, 2017; Falat, 2024; Toi et al., 2024). The duration of cold exposure and the body’s ability to compensate in its struggle to regulate temperature are critical factors. During forensic investigations, different environmental conditions may result in different forms of hypothermia, such as dry or wet hypothermia, each with distinct forensic implications. Dry hypothermia occurs in cold, dry environments where heat loss is primarily through radiation and convection, whereas wet hypothermia occurs in cold, wet conditions where heat loss is accelerated due to water’s high thermal conductivity. These variations can affect the body’s cooling rate and the preservation of specific postmortem signs, such as rigor mortis or livor mortis, making it important for forensic experts to consider the environmental context when determining the time of death or other relevant factors (Bohl et al., 2018; Savioli et al., 2023). These distinctions are important for understanding the circumstances surrounding deaths related to exposure to cold environments. Overall, hypothermia in forensic research underscores the complex interplay of environmental factors, physiological responses, and secondary influences like drugs or alcohol, all of which contribute to the forensic assessment of such cases. Postmortem biochemistry has proven to be a crucial factor in forensic examinations, providing important insights into tissue impairment and organ dysfunction linked to the process of death (Wang et al., 2014). This highlights the need for further research in this area to better understand how these biochemical markers can aid in forensic death investigations, particularly in delineating specific patterns of tissue damage and systemic dysfunction associated with various death processes (Wang et al., 2014; Jeican, 2014). Because of the absence of visible macroscopic evidence, hypothermia has long been one of the most challenging issues in forensic pathology. While forensic autopsy signs, such as red livers, purple spots on the limbs, and reddish blood, provide important clues, they must be understood within the context of the limitations of macroscopic evidence. These visible signs can indicate certain conditions, but they often lack the specificity needed to determine the exact cause of death on their own. The reliance on these surface-level observations without further microscopic or contextual investigation can lead to misinterpretations, highlighting the need for a more comprehensive approach in forensic analysis (Zhang et al., 2023). As a result, macroscopic changes are generally nonspecific and do not provide conclusive evidence for diagnosing death due to hypothermia (Cappaert et al., 2008; Polderman et al., 2009; Petrone et al., 2014; Cheshire, 2016; Yang et al., 2020). Exposure to cold triggers a complex array of physiological responses to maintain core body temperature. This process involves the release of catecholamines, such as adrenaline and noradrenaline, and counterregulatory hormones, which are crucial for thermoregulation. In the context of hypothermia, the body’s response includes metabolic changes driven by the secretion of insulin antagonist hormones. These hormonal shifts lead to increased fat catabolism and elevated production of ketone bodies, such as acetoacetate and β-hydroxybutyrate (Hirvonen, 1976; Nixdorf-Miller et al., 2006; Lim et al., 2008; Palmiere et al., 2014; Režić-Mužinić et al., 2018). These metabolic by-products can provide valuable insights into the physiological state of the body during hypothermia. The type of hypothermia is critical in forensic pathology because it can significantly impact the interpretation of death investigations. Primary hypothermia typically occurs from environmental exposure to cold, leading to direct physiological responses and metabolic changes. In contrast, secondary hypothermia is often induced by underlying medical conditions or external substances, such as alcohol and benzodiazepines, which may alter the body’s metabolic and hormonal responses differently. Despite advancements in forensic science, a gap remains in the understanding of how specific biochemical markers can reliably distinguish between hypothermia types. Traditional forensic methods may not always capture the biochemical differences necessary to distinguish primary from secondary hypothermia. Thus, a more nuanced approach is required to improve diagnostic accuracy. This study addresses this gap by systematically evaluating a range of biochemical markers to differentiate between primary and secondary hypothermia, respectively. By analyzing key parameters such as glucose, creatinine, urea, phosphorus, potassium, sodium-to-potassium ratio, and osmolality, this research aims to identify distinctive biochemical profiles associated with each type of hypothermia. The use of a controlled animal model, including the administration of substances like alcohol and benzodiazepines, allows for a rigorous examination of how these agents influence biochemical responses under hypothermic conditions. The novelty of this study lies in its comprehensive approach to integrating biochemical analysis with forensic investigation, enhancing the ability to accurately differentiate between primary and secondary hypothermia. This research not only contributes to a deeper understanding of hypothermia’s metabolic and hormonal effects but also offers methodological advancements that could improve forensic assessments and interpretations of hypothermia-related deaths. This research aims to explore various biochemical markers and their significance in distinguishing primary from secondary hypothermia. Materials and MethodsThe study was designed as an experimental and prospective investigation using an albino rat model of hypothermia. This study was conducted at the Veterinary Faculty of the University of Sarajevo, in accordance with the Principles for the Care of Laboratory Animals (Režić-Mužinić et al., 2018; Dervišević et al., 2022). 21 Wistar rats, each weighing between 200 and 250 g, were allocated into three experimental groups: CG (n=7): Control group exposed only to hypothermic conditions defined as primary hypothermia; AHG (n=7)- Alcohol + hypothermia group defined as (SH) secondary hypothermia; and BHG (n=7)- Benzodiazepines + hypothermia group defined as SH. Anesthesia was administered to the rats via intramuscular injection of ketamine into the quadriceps muscle. The dosage, based on body weight, was calculated as 1.2 ml/kg, with a ±10% variation. A dose of 100 mg/ml of Ketaminol 10, provided by Animal Health, was used for the procedure (Dervišević et al., 2022). Prior to placement in the fluid, an esophageal probe was inserted into the anesthetized rat esophagus (5 cm) to measure the internal temperature (Jeican, 2014). Temperature monitoring was performed using a thermometer, TH-8-Thermalert, with readings recorded at various intervals. Both temperature probes were produced by Physitemp Instruments (Clifton, USA). Hypothermia modelThe water bath temperature was monitored using a submerged probe and thermometer. The anesthetized rat’s head was positioned to elevate the water level, fastened to a wooden board, and subsequently immersed in pre-cooled ice water, maintained at a target temperature of 7°C. Hypothermia was defined as a 2°C drop in internal temperature, in accordance with previous definitions (Kempainen and Brunette, 2004; Paal et al., 2021). Blood samplesBefore blood collection, animals were euthanized using an approved humane method, such as euthanasia by overdose of anesthetic (e.g., isoflurane or euthasol), ensuring compliance with ethical guidelines and animal welfare standards. This method was chosen to minimize stress and pain to the animals, in accordance with institutional animal care protocols, before proceeding with any further procedures like blood collection (Kempainen and Brunette, 2004). Blood samples were obtained via thoracic aortic puncture in vacuum tubes with anticoagulant. We tested the following parameters in each rat: glucose (mmol/l), Urea (mmol/l), Creatinine (μmol/l), blood urea nitrogen to creatinine ratio, phosphorus (mmol/l), Calcium (mmol/l), Sodium (mmol/l), Potassium (mmol/l), Sodium to Potassium Ratio, Chloride (mmol/l), and calculated osmolality (mmol/kg) using an automated cell counter (IDEXX, Netherland). Statistical analysisStatistical analysis was performed using R 4.4.0 (R Foundation for Statistical Computing, Vienna, Austria). A significance level of α=0.05 was set, with p-values below this threshold considered statistically significant. The normality of quantitative variables was assessed using the Shapiro–Wilk test, with visual inspection through QQ plots and histograms. The homogeneity of variances was evaluated using Levene’s test prior to conducting bivariate tests to compare group means. Welch’s one-way ANOVA was used to assess differences in means when variances were unequal. Post hoc tests, adjusted for false discovery rate (FDR) to control for type I error, were performed following significant results from Welch’s ANOVA. The Benjamini–Hochberg procedure was applied to control for FDR, and pairwise differences between groups were investigated using the Games–Howell test after a significant Welch one-way ANOVA. Ethical approvalThe experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals by the National Institutes of Health. The use of animals and study protocol were approved on September 2, 2024, by the Veterinary faculty of the University of Sarajevo Institutional Animal Care and Use Committee under protocol number 07-03-161-2/23. ResultsBiochemical parameters were measured in samples from all 3 groups, and the results are shown in Table 1. The blood urea nitrogen-to-creatinine ratio was initially significant (p=0.037), but post hoc tests did not confirm this significance for distinction between groups (Table 1). No statistically significant distinctions were observed in chloride and sodium levels among the analyzed groups. Welch’s one-way ANOVA revealed significant differences in glucose levels between groups (p=0.013). Post-hoc tests, adjusted for FDR, weakly confirmed significant distinctions between alcohol and benzodiazepine groups (p=0.040) and alcohol and control groups (p=0.040), but not between the benzodiazepine and control groups (Fig. 1). FWelch-Welch ANOVA; p-probability; CI-confidence interval; np2 -η² or eta-squared; nobs-number of observations Significant distinctions in creatinine levels were observed (p < 0.001). Post-hoc tests, corrected for FDR, confirmed significant differences between the alcohol and benzodiazepine groups (p < 0.010) and the benzodiazepine and control groups (p=0.004), whereas the difference between the alcohol and control groups was not significant (Fig. 2). Urea levels also presented significant distinctions in groups (p < 0.001), with post-hoc tests confirming differences between alcohol and control groups (p < 0.001), benzodiazepine and control groups (p < 0.001), but not between alcohol and benzodiazepine groups (Fig. 3). Table 1. Biochemical analysis of the groups.
Fig . 1. Serum glucose concentrations. FWelch-Welch ANOVA; p-probability; CI-confidence interval; np2 -η² or eta-squared; nobs-number of observations.
Fig. 2. Serum creatinine levels. FWelch-Welch ANOVA; p-probability; CI-confidence interval; np2 -η² or eta-squared; nobs-number of observations
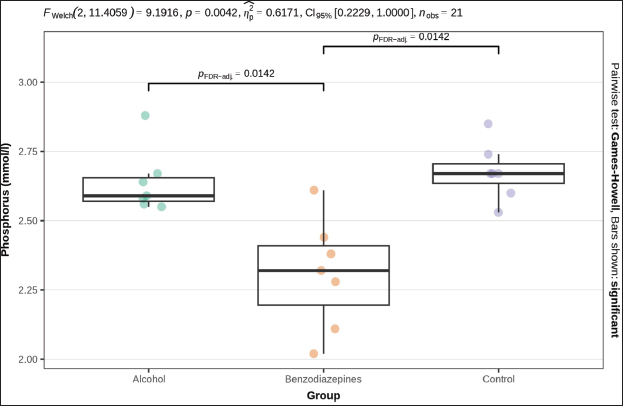
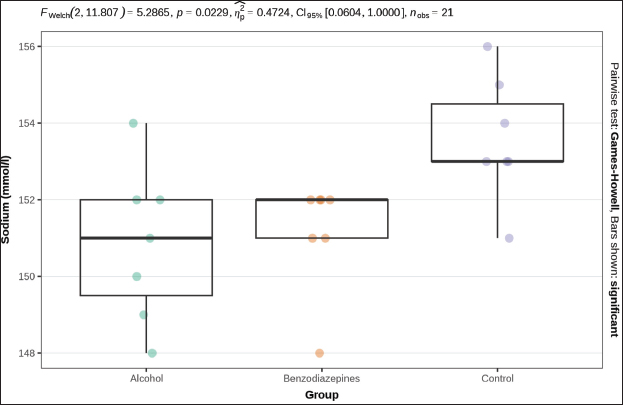
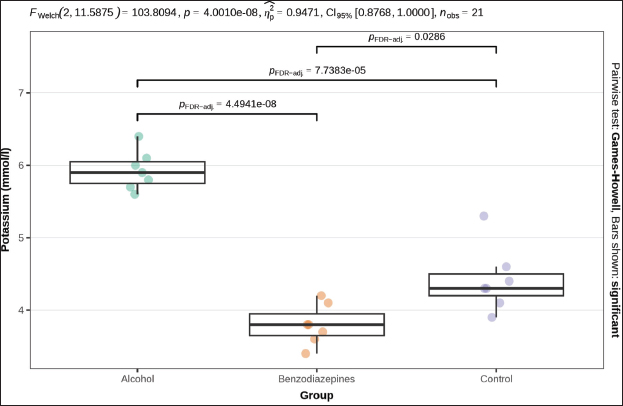
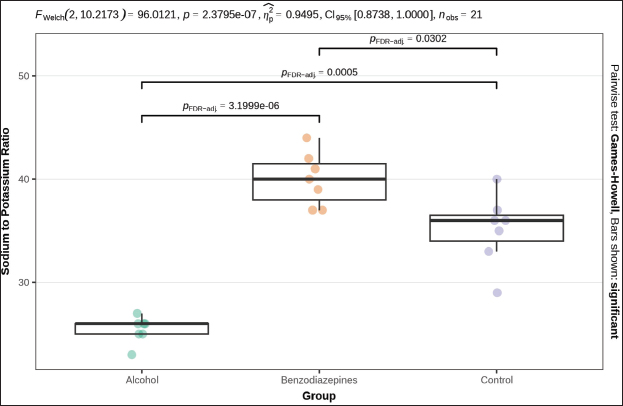
Fig. 3. Serum urea concentration between groups. Phosphorus levels showed a significant distinction (p=0.004), with post-hoc tests confirming significant distinctions between the alcohol and benzodiazepine groups (p=0.014), benzodiazepine and control groups (p=0.014), but not between the alcohol and control groups (Fig. 4). Sodium levels manifested remarkable distinctions (p=0.023), although post hoc comparisons did not confirm significant differences between the groups (Fig. 5). The potassium levels and sodium-to-potassium ratio were significantly distinguished (p < 0.001) (Fig. 6). Additionally, calculated osmolality varied significantly in the groups (p < 0.001), with post-hoc analysis confirming significant difference for alcohol versus control (p=0.013) and benzodiazepine versus control (p=0.002) comparisons (Fig. 7). DiscussionThe findings of this study highlight the significant impact of environmental temperature on global mortality, contributing to approximately 8% of deaths worldwide in the past 10 years. Interestingly, most of this mortality burden (7.29%) is attributed to cooler temperatures than warmer temperatures (0.42%) (Gasparrini et al., 2015). In Bosnia and Herzegovina, autopsy reports indicate a higher incidence of deaths from hypothermia than from heatstroke. This reflects a broader trend observed globally, where deaths from long-term exposure to moderately freezing and hot temperatures are more prevalent than those from acute exposure to extreme temperature peaks. Studies that comprehensively examine the interaction between environmental conditions and human health are crucial. These data provide vital insights that help shape public health policies aimed at reducing the harmful effects of extreme temperatures on global populations. Cold exposure can significantly contribute to fatalities (Gasparrini et al., 2015). However, the diagnosis of fatal hypothermia is typically based on clinical observations rather than specific biochemical markers. Common clinical signs include frost-erythema (reddening of the skin due to frostbite) and Wischnewski’s spots (small, red pinpoint hemorrhages often found in the lungs), which are present in approximately 2/3 of hypothermia-related deaths (Takasu et al., 2024). Searching for the difference in biochemical parameters that will make the difference between primary and secondary hypothermia, we have come to the conclusion that there is, unfortunately, no such information. Based on this experience, we purposefully conducted this experimental study to determine biochemical indicators of hypothermia-related death. Analyses of biochemical parameters were performed on samples from the 3 groups, with results showing a significant difference in glucose levels between groups (p=0.013). Post-hoc tests, adjusted for FDR, weakly confirmed significant differences between the alcohol and benzodiazepine groups (p=0.040) and the alcohol and control groups (p=0.040), but not between the benzodiazepine and control groups. In the study (Bodman et al., 2024) and (Mack et al., 2024), alcohol consumption and high glucose trajectories were positively and significantly correlated. Significant differences in creatinine levels were observed (p < 0.001). Post-hoc tests, corrected for FDR, confirmed significant differences ranging between the alcohol and benzodiazepine groups (p < 0.010) and benzodiazepine and control groups (p=0.004), whereas the distinguishing between the alcohol and control group was not significant. Urea levels also presented significant differences between groups (p < 0.001), with post-hoc tests which confirmed differences between alcohol and control groups (p < 0.001) and benzodiazepine and control groups (p < 0.001), but not between alcohol and benzodiazepine groups. Phosphorus levels showed a significant difference (p=0.004), with post-hoc tests confirming important variations between the alcohol and benzodiazepine groups (p=0.014) and the benzodiazepine and control groups (p=0.014), but not between the alcohol and control groups (Danzl et al., 1987). In their study found the different levels in laboratory parameters, such as phosphorus level, in hypothermia-related death.
Fig. 4. Serum phosphorous concentrations. FWelch-Welch ANOVA; p-probability; CI-confidence interval; np2 -η² or eta-squared; nobs-number of observations
Fig. 5. Serum sodium concentrations. FWelch-Welch ANOVA; p-probability; CI-confidence interval; np2 -η² or eta-squared; nobs-number of observations
Fig. 6. Serum potassium concentrations. FWelch-Welch ANOVA; p-probability; CI-confidence interval; np2 -η² or eta-squared; nobs-number of observations
Fig. 7. Serum sodium/potassium ratio. FWelch-Welch ANOVA; p-probability; CI-confidence interval; np2 -η² or eta-squared; nobs-number of observations Sodium levels showed significant variations (p=0.023), although post hoc comparisons did not confirm significant differences between the groups. The potassium levels and the sodium-to-potassium ratio were significantly different (p < 0.001). Additionally, calculated osmolality was notably diverse among the experimental groups, with post-hoc analysis confirming important variations for the AHG versus CG (p=0.013) and BHG versus CG (p=0.002) comparisons on PH and SH. In a study by the plasma levels of sodium, chlorine, potassium, and calcium in the hypothermic group were much higher in comparison with the normotermic groups. In the hypothermic group, the levels of plasma osmolality at instant, 2 hours, and 5 hours after immersion were 111%, 109%, and 108% of those in the normothermic group, respectively, all p < 0.01 (Wang et al., 2023). Biochemical markers such as urea, creatinine, blood urea nitrogen/creatinine ratio (BUN/creatinine), glucose, phosphorus, calcium, potassium, and sodium have long been used in clinical practice and research to assess kidney function, metabolic disturbances, and other physiological conditions. In the context of cause of death, these markers have shown statistically significant differences among different groups, suggesting potential relevance in understanding the underlying causes. However, it is important to note that these biochemical markers lack specificity, and their alteration can occur due to various factors, including metabolic disorders, organ failure, and systemic diseases. They should not be considered definitive indicators of the cause of death. While certain biochemical markers can provide valuable insights into physiological disturbances, they do not directly correlate with a single cause of death (Zhu and Diao, 2001; Ishikawa et al., 2004). Apart from the natural decline in cold tolerance with age, various medical conditions can contribute to hypothermia. Conditions such as strokes, infections, tumors, bleeding, brain trauma, neurological diseases as Parkinson’s disease or multiple sclerosis, can impair central thermoregulation (Hearse et al., 1984; Rankin et al., 1984). Autonomic dysfunction affecting the control of peripheral blood vessels is also a factor in diabetes, infections, and heart failure. In some endocrine disorders, such as hypoadrenalism, hypopituitarism, and hypothyroidism, it comes to reduced heat production (Maclean et al., 1968; Hirvonen et al., 1976; Chernow et al., 1983). Hypoglycemia, especially when combined with alcohol consumption, can increase the risk of hypothermia. Pancreatitis and diabetic ketoacidosis are also potential causes to be considered, even if not immediately obvious clinically. Diabetes itself, particularly in malnourished individuals, increases the risk of accidental hypothermia (Danzl et al., 1994). In older individuals, sepsis is a common precipitating factor, and it has been observed in most older patients with hypothermia in several studies. In approximately 9%–10% of cases where infection leads to hypothermia rather than fever, the prognosis is significantly worse, with mortality rates approximately increased, possibly due to a higher incidence of shock (Maclean et al., 1968; Hirvonen et al., 1976; Chernow et al., 1983). Sepsis can predispose patients to hypothermia by impairing vasoconstriction or affecting hypothalamic responses. In hypothermic patients, even though the exact mechanism is not clear, there is evidence of an increased cytokine response and increased levels of TNF-α,interleukin-6, thromboxane B2 metabolites, and prostacyclin (Mallet et al., 2002). Biochemical analysis using postmortem blood (or serum if available) and vitreous humor has become essential for investigating various causes of death, including those related to alcohol, diabetes, anaphylactic shock, drowning, and hypothermia. Vitreous humor is particularly advantageous in postmortem investigations because it undergoes fewer changes after death compared with blood, and it can be analyzed using standard clinical chemistry methods originally designed for plasma or serum (Belsey and Flanagan, 2016). However, despite these advancements, significant challenges remain. For instance, determining the role of external substances like potassium or insulin in causing death is complex. To gain widespread acceptance in forensic practice, new tests must undergo extensive research and rigorous validation. They must demonstrate accuracy and reliability in detecting specific markers or conditions associated with different causes of death. Furthermore, these testing methodologies must be practical and accessible for use in both clinical and forensic laboratories. This underscores several complexities in forensic and clinical biochemistry, particularly in the interpretation of postmortem laboratory results. Challenges include differences in sample types, limited availability of reference materials, issues with method validation, and uncertainties regarding the equilibration of analyses between plasma and vitreous humor during life. These factors contribute to the complexity of accurately interpreting postmortem biochemistry data. ConclusionThe calculated osmolality demonstrated significant variation among the different groups, indicating a notable impact of substances on the biochemical profile related to hypothermia. Post-hoc analyses revealed that osmolality was significantly different between the AHG and CG, as well as between the BHG and CG. These findings suggest that both alcohol and benzodiazepines notably influence osmolality in the context of primary and secondary hypothermia, distinguishing them from primary hypothermia. In summary, while biochemical analyses have advanced the understanding and investigation of deaths related to primary and secondary hypothermia, ongoing research and methodological developments are crucial to address current challenges and enhance the reliability of forensic biochemical testing. The introduction of novelty into research methodologies and analytical techniques may offer new perspectives and innovative solutions to these enduring problems. AcknowledgmentsNone. Conflict of interestThe authors declare no conflict of interest. FundingThis research was supported by the Federal Ministry of Science, Higher Education of Bosnia and Herzegovina 2024 No: 05.35.5504-7/24. Authors’ contributionsAll authors contributed equally to this research. Data availabilityAll data were provided in the manuscript. ReferencesBelsey, S.L. and Flanagan, R.J. 2016. Postmortem biochemistry: current applications. J Forensic Leg. Med. 41, 49–57; doi: 10.1016/j.jflm.2016.04.011 Bodman, M.A., Dreyer, M.A. and Varacallo, M. 2024. Diabetic peripheral neuropathy. In: StatPearls [Internet]. Treasure Island, FL: StatPearls Publishing. Bohl, M.A., Martirosyan, N.L., Killeen, Z.W., Belykh, E., Zabramski, J.M., Spetzler, R.F. and Preul, M.C. 2018. History of therapeutic hypothermia and its use in neurosurgery. J. Neurosurg. 130(3), 1006–1020; doi: 10.3171/2017.10.JNS171282 Brychta, R.J. and Chen, K.Y. 2017. Cold-induced thermogenesis in humans. Eur. J. Clin. Nutr. 71(3), 345–352. Cappaert, T.A., Stone, J.A., Castellani, J.W., Krause, B.A., Smith, D. and Stephens, B.A. 2008. National Athletic Trainers’ Association position statement: environmental cold injuries. J. Athl. Train. 43, 640–658; doi: 10.4085/1062-6050-43.6.640 Chernow, B., Lake, C.R., Zaritsky, A., Finton, C.K., Casey, L, Rainey, T.G. and Fletcher, J.R. 1983. Sympathetic nervous system ‘switch off’ with severe hypothermia. Crit. Care Med. 11, 677–680. Cheshire, W.P. 2016. Thermoregulatory disorders and illnesses related to heat and cold stress. Auton. Neurosci. 196, 91–104; doi: 10.1016/j.autneu.2016.01.001 Danzl, D.F. and Pozos, R.S. 1994. Accidental hypothermia. N. Engl. J. Med. 331, 1756–1760. Danzl, D.F., Pozos, R.S., Auerbach, P.S., Glazer, S., Goetz, W., Johnson, E., Jui, J., Lilja, P., Marx, J.A. and Miller, J. 1987. Multicenter hypothermia survey. Ann. Emerg. Med. 16(9), 1042–1055; doi: 10.1016/s0196-0644(87)80757-6 Dervišević, E., Hasić, S., Katica, M., Salihbegović, A., Ajanović, Z. and Sarajlić, N. 2022. Forensic significance of serum cTnI levels for the detection of terminal myocardial damage in rats (Rattus norvegicus) caused by hyperthermia. J. King Saud Univ. Sci. 34(2), 1018–3647; doi: 10.1016/j. jksus.2021.101753 Duong, H. and Patel, G. 2024. Hypothermia. In: StatPearls [internet]. Treasure Island, FL: StatPearls Publishing. Elmsjö, A., Ward, L.J., Horioka, K., Watanabe, S., Kugelberg, F.C., Druid, H. and Green, H. 2024. Biomarker patterns and mechanistic insights into hypothermia from a postmortem metabolomics investigation. Sci. Rep. 14(1), 18972; doi: 10.1038/s41598-024-68973-9 Falat, C. 2024. Environmental hypothermia. Emerg. Med. Clin. North Am. 42(3), 493–511; doi: 10.1016/j.emc.2024.02.011 Gasparrini, A., Guo, Y., Hashizume, M., Lavigne, E., Zanobetti, A., Schwartz, J., Tobias, A., Tong, S., Rocklöv, J., Forsberg, B., Leone, M., De Sario, M., Bell, M.L., Guo, Y.L., Wu, C.F., Kan, H., Yi, S.M., de Sousa, Z.S.C.M., Saldiva, P.H., Honda, Y., Kim, H. and Armstrong, B. 2015. Mortality risk attributable to high and low ambient temperatures: a multicountry observational study. Lancet 386(9991), 369–375; doi: 10.1016/S0140-6736(14)62114-0 Hearse, D.J., Yamamoto, F. and Shattaock, M.J. 1984. Calcium antagonists and hypothermia: the temperature dependency of the negative inotropic and anti-ischemic properties of verapamil in the isolated rat heart. Circulation 70, I54–I64. Hirvonen, J. 1976. Necropsy findings in fatal hypothermia cases. Forensic Sci. 8, 155–164. Available via 10.1016/0300-9432(76)90059-5Search in Google Scholar Ishikawa T., Miyaishi South, Tachibana T., Ishizu H. Zhu B.L. and Maeda H. 2004. Fatal hypothermia related vacuolation of hormone-producing cells in the anterior pituitary. Legal Med. 6(3), 157–163; doi: 10.1016/j.legalmed.2004.05.004 Jeican, I.I. 2014. Pathophysiological mechanisms of the onset of death through accidental hypothermia and the presentation of “The little match girl” case. Clujul Med. 87(1), 54–60; doi: 10.15386/cjm.2014.8872.871.iij1 Kempainen, R.R. and Brunette, D.D. 2004. Evaluation and management of accidental hypothermia. Respir. Care. 49(2), 192–205. Lim, C. and Duflou, J. 2008. Hypothermia fatalities in a temperate climate: sydney, Australia. Pathology 40, 46–51; doi: 10.1080/0031302070171646610.1080/00313020701716466 Mack, T., Parai, J.L. and Milroy, C.M. 2024. Establish vitreous glucose and beta-hydroxybutyrate thresholds to assist in the diagnosis of hypothermia. Forensic Sci. Int. 356, 111963; doi: 10.1016/j.forsciint.2024.111963 Maclean, D., Griffiths, P.D. and Emslie-Smith, D. 1968. Serum-enzymes in relation to electrocardiographic changes in accidental hypothermia. Lancet 2, 1266–1270. Mallet, M.L. 2002. Pathophysiology of accidental hypothermia. QJM 95(12), 775–785; doi: 10.1093/qjmed/95.12.775 Nixdorf-Miller, A., Hunsaker, D.M. and Hunsaker, J.C. 2006. Hypothermia and hyperthermia as a medicolegal investigation of morbidity and mortality from exposure to environmental temperature extremes. Arch. Pathol. Lab. Med. 130, 1297–1304. Available via 10.5858/2006-130-1297-HAHMIOSearch in Google ScholarPubMed Paal, P., Pasquier, M., Darocha, T., Lechner, R., Kosinski, S., Wallner, B., Zafren, K. and Brugger, H. 2021. Accidental hypothermia. Int. J. Environ. Res. Public Health. 19(1), 501; doi: 10.3390/ijerph19010501 Palmiere, C., Teresiński, G. and Hejna, P. 2014. Postmortem diagnosis of hypothermia. Int. J. Legal. Med. 128, 607–614; doi: 10.1007/s00414-014-0977 Petrone, P., Asensio, J.A. and Marini, C.P. 2014. Management of accidental hypothermia and cold injury. Curr. Probl. Surg. 51, 417–431; doi: 10.1067/j.cpsurg.2014.07.004 Polderman, K.H. 2009. Mechanisms of action, physiological effects, and complications of hypothermia. Crit. Care Med. 37(7), S186–S202; doi: 10.1097/CCM.0b013e3181aa5241 Rankin, A.C. and Rae, A.P. 1984. Cardiac arrhythmias during rewarming in patients with accidental hypothermia. Br. Med. J. 289, 874–877. Režić-Mužinić, N., Mastelić, A., Benzon, B., Markotić, A., Mudnić, I. and Grković, I. 2018. Expression of adhesion molecules on granulocytes and monocytes following myocardial infarction in rats drinking white wine. PLoS One 13(5), 196; doi: 10.1371/journal.pone.0196842 Savioli, G., Ceresa, I.F., Bavestrello Piccini, G., Gri, N., Nardone, A., La Russa, R., Saviano, A., Piccioni, A., Ricevuti, G. and Esposito, C. 2023. Hypothermia: beyond the narrative review-the point of view of emergency physicians and medico-legal considerations. J. Pers. Med. 13(12), 1690; doi: 10.3390/jpm13121690 Takasu, S., Matsumoto, S. and Iwadate, K. 2024. Prominent black esophagus, Wischnewsky spots, and black duodenum in a patient with fatal hypothermia and underlying diabetic ketoacidosis. Forensic Sci. Med. Pathol; doi: 10.1007/s12024-024-00837-0 Toi, T., Tsuneya, S., Inokuchi, G., Chiba, F., Hoshioka, Y., Nagasawa, S., Yoshida, M., Yamaguchi, R., Torimitsu, S., Inoue, H., Motomura, A., Yajima, D., Makino, Y. and Iwase, H. 2024. Characteristics of indoor and outdoor fatal hypothermia cases in Chiba, Japan. Leg. Med. 71, 102494; doi: 10.1016/j.legalmed.2024.102494 Wang, J.G., Fan, Z., Zhang, B., Gao, H.Y. and Wang. 2014. The global burden of liver disease: the major impact of China. Hepatology 60, 2099–2108. Wang, L.L., Tian, Y.M., Hu, S., Zhang, H.P., Meng, X.X., Zhang, H.P., Zhong, Y.X., Du, M.H. and Ding, Y. 2023. An animal model of seawater immersion injury following hemorrhagic shock. J. Surg. Res. 287, 24–32; doi: 10.1016/j.jss.2023.01.012 Yang, C., Sugimoto, K., Murata, Y., Hirata, Y., Kamakura, Y., Koyama, Y., Miyashita, Y., Nakama, K., Higashisaka, K., Harada, K., Katada, R. and Matsumoto, H. 2020. Molecular mechanisms of Wischnewski spot development in the gastric mucosa in fatal hypothermia: an experimental study in rats. Sci. Rep. 10(1), 1877; doi: 10.1038/s41598-020-58894-8 Zhang, C., Puligheddu, M., Zhang, L, Car, R. and Galli, G. 2023. Thermal conductivity of water at extreme conditions. J. Phys. Chem. B; 127(31), 7011–7017; doi: 10.1021/acs.jpcb.3c02972 Zhu, L. and Diao, C. 2001. Theoretical simulation of brain temperature distribution during mild hypothermia treatment for brain injury. Med. Biol. Eng. Comput. 39(6), 681–687; doi: 10.1007/BF02345442 | ||
| How to Cite this Article |
| Pubmed Style Dervišević E, Bešić A, Spahović H, Mujarić E, Šuta N, Dervišević M, Lazović E, Selmanagić A. Biochemical markers of cell death: Forensic implications for differentiating primary and secondary hypothermia. Open Vet. J.. 2025; 15(5): 2149-2159. doi:10.5455/OVJ.2025.v15.i5.33 Web Style Dervišević E, Bešić A, Spahović H, Mujarić E, Šuta N, Dervišević M, Lazović E, Selmanagić A. Biochemical markers of cell death: Forensic implications for differentiating primary and secondary hypothermia. https://www.openveterinaryjournal.com/?mno=241053 [Access: January 24, 2026]. doi:10.5455/OVJ.2025.v15.i5.33 AMA (American Medical Association) Style Dervišević E, Bešić A, Spahović H, Mujarić E, Šuta N, Dervišević M, Lazović E, Selmanagić A. Biochemical markers of cell death: Forensic implications for differentiating primary and secondary hypothermia. Open Vet. J.. 2025; 15(5): 2149-2159. doi:10.5455/OVJ.2025.v15.i5.33 Vancouver/ICMJE Style Dervišević E, Bešić A, Spahović H, Mujarić E, Šuta N, Dervišević M, Lazović E, Selmanagić A. Biochemical markers of cell death: Forensic implications for differentiating primary and secondary hypothermia. Open Vet. J.. (2025), [cited January 24, 2026]; 15(5): 2149-2159. doi:10.5455/OVJ.2025.v15.i5.33 Harvard Style Dervišević, E., Bešić, . A., Spahović, . H., Mujarić, . E., Šuta, . N., Dervišević, . M., Lazović, . E. & Selmanagić, . A. (2025) Biochemical markers of cell death: Forensic implications for differentiating primary and secondary hypothermia. Open Vet. J., 15 (5), 2149-2159. doi:10.5455/OVJ.2025.v15.i5.33 Turabian Style Dervišević, Emina, Aida Bešić, Hajrudin Spahović, Ekrema Mujarić, Nedim Šuta, Muamer Dervišević, Edina Lazović, and Aida Selmanagić. 2025. Biochemical markers of cell death: Forensic implications for differentiating primary and secondary hypothermia. Open Veterinary Journal, 15 (5), 2149-2159. doi:10.5455/OVJ.2025.v15.i5.33 Chicago Style Dervišević, Emina, Aida Bešić, Hajrudin Spahović, Ekrema Mujarić, Nedim Šuta, Muamer Dervišević, Edina Lazović, and Aida Selmanagić. "Biochemical markers of cell death: Forensic implications for differentiating primary and secondary hypothermia." Open Veterinary Journal 15 (2025), 2149-2159. doi:10.5455/OVJ.2025.v15.i5.33 MLA (The Modern Language Association) Style Dervišević, Emina, Aida Bešić, Hajrudin Spahović, Ekrema Mujarić, Nedim Šuta, Muamer Dervišević, Edina Lazović, and Aida Selmanagić. "Biochemical markers of cell death: Forensic implications for differentiating primary and secondary hypothermia." Open Veterinary Journal 15.5 (2025), 2149-2159. Print. doi:10.5455/OVJ.2025.v15.i5.33 APA (American Psychological Association) Style Dervišević, E., Bešić, . A., Spahović, . H., Mujarić, . E., Šuta, . N., Dervišević, . M., Lazović, . E. & Selmanagić, . A. (2025) Biochemical markers of cell death: Forensic implications for differentiating primary and secondary hypothermia. Open Veterinary Journal, 15 (5), 2149-2159. doi:10.5455/OVJ.2025.v15.i5.33 |