| Research Article | ||
Open Vet. J.. 2025; 15(4): 1812-1822 Open Veterinary Journal, (2025), Vol. 15(4): 1812-1822 Research Article Contamination of meat and its products by Pseudomonas species and assessment of the antibacterial effect of clove (Syzygium aromaticum) essential oil on multidrug-resistant P. aeruginosaSamar E. El-Wehedy1*, Azza K. Elshafee1, Zainab F. Helal2, Yomna R. Shehab-Eldin3, Amany M. Yassin4 and Haidy T. Zaki51Nutrition Unit, Food Control Department, Zagazig University Hospitals, Zagazig University, Zagazig, Egypt 2Zagazig Scientific and Medical Research Center (ZSMRC), Pharmacology Department, Medical Faculty, Zagazig University, Zagazig, Egypt 3Pharmacology Department, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 4Laboratories Unit, Microbiology Department, Zagazig University Hospitals, Zagazig University, Zagazig, Egypt 5Nutrition Unit, Microbiology Department, Zagazig University Hospitals, Zagazig University, Zagazig, Egypt *Corresponding Author: Samar E. El-Wehedy, Nutrition Unit, Food Control Department, Zagazig University Hospitals, Zagazig University, Zagazig, Egypt. Email: drsamarelsayed89 [at] gmail.com Submitted: 22/02/2025 Accepted: 06/04/2025 Published: 30/04/2025 © 2025 Open Veterinary Journal
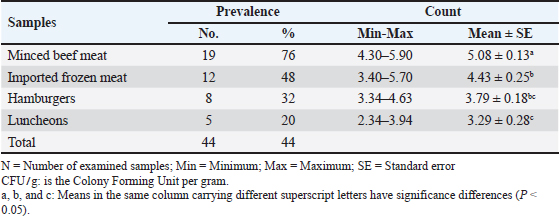
AbstractBackground: In addition to high palatability, meat and meat products are important sources of essential nutrients that are important to the growth and performance of our bodies. However, meat and its products can harbor various microorganisms. Pseudomonas species (spp.) are considered to be one of the primary causes of meat deterioration and spoilage, as well as foodborne illnesses. Aim: Different types of meat products were evaluated bacteriologically to detect the prevalence of Pseudomonas spp., in addition to the evaluation of the antibacterial effect of clove essential oil (CEO) on Pseudomonas aeruginosa. Methods: One hundred samples of minced meat, imported frozen meat, hamburgers, and luncheons were gathered randomly from various markets within the Sharkia Governorate, Egypt. Pseudomonas spp. were counted and identified; furthermore, the disk diffusion method was used to assess the antibiogram of P. aeruginosa, and some antibiotic resistance genes were detected. In addition, minimum inhibitory concentrations, minimum bactericidal concentrations, and the disc diffusion technique were used to evaluate the antibacterial properties of CEO in vitro. Furthermore, the experimental section assessed the effects of 5% and 10% CEO on the sensory characteristics and P. aeruginosa counts. Results: The results showed that 44% of samples harbored Pseudomonas spp. with a mean count of 5.08 ± 0.13, 4.43 ± 0.25, 3.79 ± 0.18, and 3.29 ± 0.28 log10 CFU/g in minced beef meat, imported frozen meat, hamburgers and luncheons, respectively. Four species were identified: P. psychrophila (40.9%), P. fluorescens (29.5%), P. fragi (18.2%), and P. aeruginosa (11.4%). Pseudomonas aeruginosa was 100% resistant to tetracycline, oxacillin, amoxicillin-clavulanic acid, lincomycin, and clindamycin; meanwhile, it was 100% sensitive to amikacin and ciprofloxacin. All tested P. aeruginosa harbored the blaSHV and Mcr1 genes; meanwhile, the blaOXA-1 gene was not detected. Furthermore, the CEO improved the sensory characteristics and reduced the number of P. aeruginosa. Conclusion: Due to growing concerns about food safety, natural antimicrobials were applied to control multidrug-resistant foodborne pathogens and spoilage bacteria. CEO had a noticeable effect on the overall acceptability of meat by sensory evaluation, and it was highly effective in meat preservation, achieving a reduction percent in P. aeruginosa count by 99.6%. Keywords: Clove, Drug resistance, Meat products, Preservation, Pseudomonas. IntroductionIn addition to being one of the most valuable sources of protein, meat and its products are also a highly rich source of vitamins, minerals, and fatty acids. All over the world, meat products constitute a principal diet component for many people because of their nutritive value, palatability, and low cost (Stadnik et al., 2024). However, based on this nutritive value, several reports about food poisoning caused by the consumption of contaminated meat and meat products by different species of microorganisms have been published (Darwish et al., 2024), which emphasizes the importance of hygiene implementation and meat inspection. Pseudomonas aeruginosa, an opportunistic Gram-negative bacterium, causes food spoilage and is responsible for many infections, including urinary, respiratory, and gastrointestinal tract infections, in addition to soft and hard tissue infections (Spagnolo et al., 2021). Food spoilage caused by P. aeruginosa is of great concern, especially in developing nations, because of the inferior quality of processing and refrigeration techniques (Rezaloo et al., 2022). It is involved in meat deterioration and spoilage, as represented by the formation of slime, pigment, malodor, and off-flavors (Abdullah et al. 2024). Many virulence factors are responsible for food infection by P. aeruginosa, such as phenazine operons (Bradbury et al., 2010), as well as exoenzymes, and drug-resistant genes (Jurado-Mart´ın et al., 2021), which are responsible for adhesions, inflammation, and host cell invasion. Drug resistance is a critical aspect related to P. aeruginosa infections; it progresses to various types of antibiotics (Rocha et al., 2019) particularly β-lactams, macrolides, tetracyclines, and quinolones (CDC, 2017), resulting in many health and economic losses. In P. aeruginosa, the blaSHV and blaOXA genes are important genes encoding extended-spectrum beta-lactamases (ESBLs) (Pang et al., 2019). Multi-drug resistance in P. aeruginosa is related to point mutations in the bacterial genome (Abdelrahman et al., 2020). To cope with the widespread of P. aeruginosa resistance to different types of antibiotics, it is necessary to search for alternative methods, such as extracts and essential oils (EOs) (Ricardo-Rodrigues et al., 2024). Clove (Syzygium aromaticum) belongs to the Myrtaceae family and is grown in different places in Indonesia. It has many uses, such as antimicrobial, antifungal, and anticancer effects (Hema et al., 2012). As one of the most important phytochemicals, it is recognized as a safe food additive (FDA, 2011). Since ancient times, clove essential oil (CEO) has been used in food; it has been reported that eugenol is the major antimicrobial component of clove (Hoquea et al., 2008); which exhibits antibacterial activities against many foodborne pathogens (Pandey et al., 2024). Due to the risk of meat contamination by Pseudomonas spp.; the recent study was to assess meat contamination by Pseudomonas spp and evaluate the antibacterial effect of CEO against P. aeruginosa. Materials and MethodsSamples collectionOne hundred samples were divided into 25 samples of minced beef, imported frozen meat, hamburgers, and luncheons. Randomly, the samples were collected from various outlets and sale markets in Sharkia Province, Egypt. Samples were sent aseptically, in an ice box, as quickly as possible to the Microbiology Laboratory at Zagazig University. Samples preparationTwenty-five grams of each sample were added to 225 ml buffered peptone water (BPW) 1% (Oxoid, UK) and homogenized for 3 minutes at a rate of 2,500 rounds per minute forming 10–1 initial dilution. From the initial dilution, tenfold serial dilution was performed in a subsequent manner (American Public Health Association (APHA), 2001). Determination of the pseudodomonas countAmount of 0.1 ml from the previously made dilutions was spread on Pseudomonas Agar Base (Oxoid, UK, CM 559) containing cetrimide, cephaloridine, and fucidin supplements (SR 103; Oxoid, UK) and incubated for 24–48 hour at 37 °C (Roberts and Greenwood, 2003). Identification of Pseudomonas spp.The isolated colonies were subjected to morphological identification by Gram staining (Becerra et al. 2016) and biochemical identification according to LaBauve and Wargo (2012). API 20NE strips (BioMérieux, France) were used for the identification of P. aeruginosa (Hashem et al., 2018). AntibiogramUsing the disk diffusion technique, P. aeruginosa isolated strains were cultured on Mueller–Hinton agar (Oxoid, UK) containing various antibiotic disks (Oxoid, UK) and then incubated for 24 hour at 37°C. The antibiotic disc concentrations and interpretation of the results are presented in Table 3. The diameters of the inhibition zones were measured according to the Clinical and Laboratory Standards Institute (CLSI) (2020). The multiple antibiotic resistance index (MAR) was calculated (number of resistant antibiotics divided by the total number of antibiotics). Intermediate-resistant isolates were considered sensitive to MAR index calculation (Singh et al., 2010). Antibiotic resistance detectionThe DNA of P. aeruginosa was extracted for molecular screening of antimicrobial resistance genes based on the instructions of the QIAamp DNA mini kit. The oligonucleotide primers used were AGGATTGACTGCCTTTTTG and ATTTGCTGATTTCGCTCG (392 bp) for blaSHV; and ATATCTCTACTGTTGCATCTCC and AAACCCTTCAAACCATCC (619 bp) for BlaOXA-(Colom et al., 2003); while, CGGT CAGTCCGTTTGTTC and CTTGGTCGGTCTGTAGGG (308 bp) were used for Mcr1 (Newton-Foot et al., 2017). Gel electrophoreses were performed as described by Sambrook et al. (1989), who identified genes that are widespread in meat products in Egypt. Determination of the CEO antibacterial activityClove EO was purchased from the National Research Center in Dokki, Giza, Egypt, and analyzed by Gas Chromatography-Mass Spectrometry (GC-MS) (6890 N gas chromatograph; Agilent Technologies Inc., Palo Alto, CA, USA) using an HP-5MS capillary column. The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of CEO were determined by micro-dilution techniques using 96-well polystyrene microplates (Santos et al., 2017). CEO was diluted by adding 4.5 ml water, 0.5 ml CEO, and 0.05 ml Tween 80, forming a stock solution 10% in concentration. The used dilutions were 10%, 5%, 2.5%, 1.25%, 0.62%, 0.31%, 0.16%, and 0.08% equivalent to 104.5, 52.25, 26.12, 13.06, 6.53, 3.26, 1.63, and 0.815 mg/ml. From the bacterial suspension (overnight culture of P. aeruginosa 105 CFU/ml) adjusted to 0.5 on the McFarland scale, 10 μl were inoculated into the wells containing Mueller-Hinton (Oxoid, Basingstoke, UK), then 100 μl of different concentrations of CEO were added. After incubation at 37°C for 24 hour, the MIC was defined as the lowest concentration of CEO exhibiting no growth of P. aeurginosa. MBCs were determined according to Pozzatti et al. (2010) by plating onto the surface of Muller-Hinton Agar and incubated for 24 hours at 37°C; the lowest concentration that prevented visible growth was recorded. The agar well diffusion technique was used to evaluate CEO antibacterial activities; The standard McFarland tube 0.5 was used to standardize the bacterial suspensions. Mueller-Hinton agar plates were streaked using a cotton swab. Next, a cork borer was used to make wells with a diameter of 5 mm, and 20 μl of each extract was added. After a 24-hour incubation period at 37°C, the inhibition zones were measured (mm) (Li et al., 2015). Effects of 5% and 10% CEO exposure on the P. aeruginosa count in chilled minced beef meatFresh beef meat (1,200 g) was purchased and minced in a sterile mincer and then grouped into four equal groups, each containing 100 g of minced meat. From p. aeruginosa broth adjusted to 0.5 McFarland, 1 ml was inoculated in each group of minced meat, except the control negative group, in a sterile clean Ziploc bag and gently massaged for homogenous distribution then kept at room temperature (25°C) for 30 minutes for good attachment and absorption of the inoculated p. aeruginosa. The first group was the control group inoculated with 1 ml of sterile distilled water. The second group was treated with CEO 5% and gently massaged for homogenous distribution. The third group was treated with 10% CEO and gently massaged. The fourth group was the control group, which contained minced meat without inoculation with p. aeruginosa. The experiment was repeated in triplicate, and the groups were examined after treatment at zero time and after 1, 2, 6, 24, and 48 hours at 4 °C. Effects of CEO 5% and 10% on the sensory evaluation of chilled minced beef meatSensory characteristics (color, odor, texture, and overall acceptability) were evaluated at zero time and after 24 and 48 hours of storage at refrigeration temperature using a 10-member panel. The members were selected from the Nutrition Unit, Zagazig University Hospital, Zagazig, Egypt. The sensory scores were assessed by a nine-point hedonic scale (9=extremely accepted, 8=very accepted, 7=moderately accepted, 6=slightly accepted, and 5=the lower score of acceptability) (Aliakbarlu and Sadaghiani, 2015). Statistical analysisThe study was conducted by SPSS-21; Duncan’s multiple range test was used to compare the samples by one-way ANOVA (SAS Institute, Cary, NC). Statistical significance was considered at p < 0.05. The data are presented as means ± SE. Ethical approvalNot needed for this study. ResultsThe prevalence of Pseudomonas spp. is shown in Table 1. Forty-four of 100 (44%) samples were contaminated with Pseudomonas spp.; minced meat had the highest prevalence (76%), while luncheons harbored the lowest (20%), furthermore, imported frozen meat and hamburgers were 48% and 32% contaminated by Pseudomonas spp. The mean counts were 5.08 ± 0.13, 4.43 ± 0.25, 3.79 ± 0.18, and 3.29 ± 0.28 log10 CFU/g in minced meat, imported frozen meat, hamburgers, and luncheons respectively. Data analysis revealed significant differences between samples (P<0.05). Table 1. Prevalence and counts (log10 CFU . g) of Pseudomonas spp. in the examined samples (N=25).
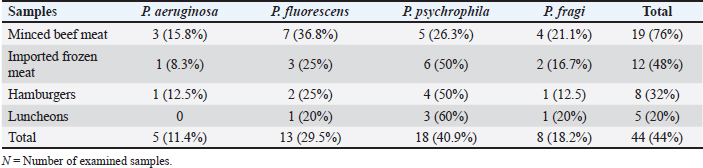
Table 2. Identified Pseudomonas spp. in the examined samples (N=25, of each).
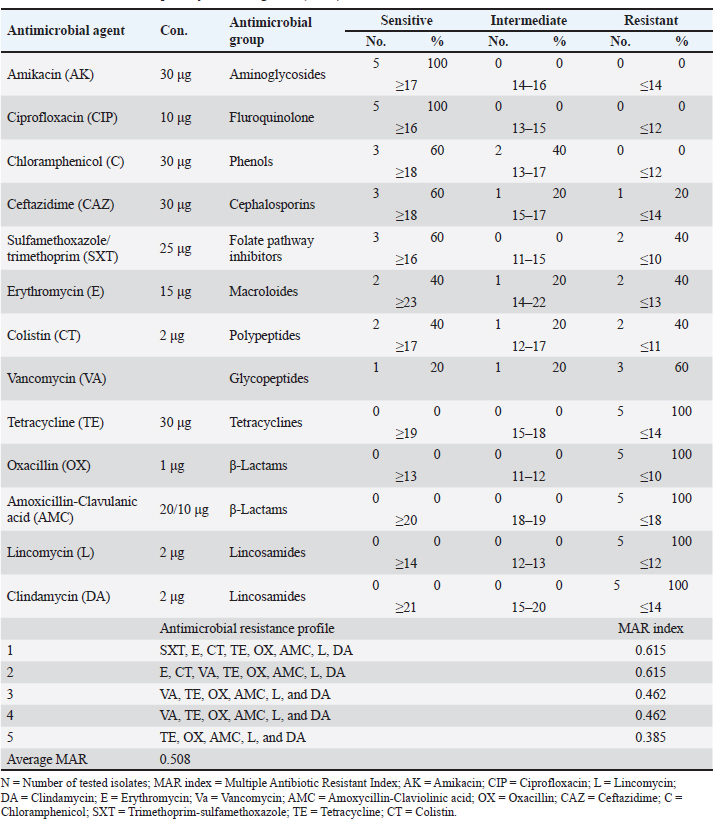
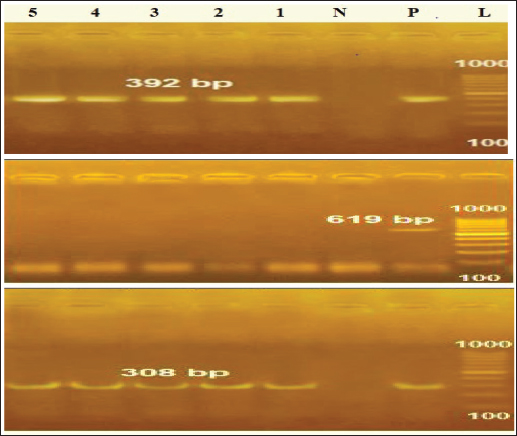
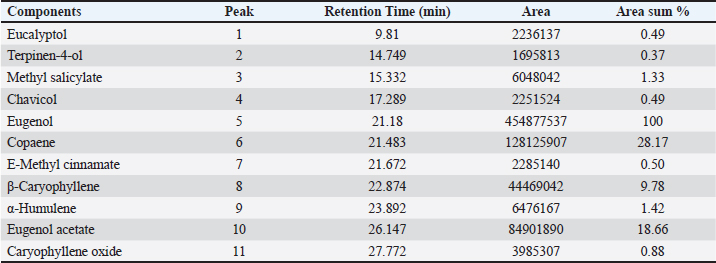
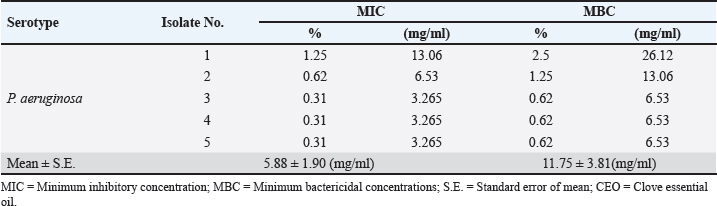
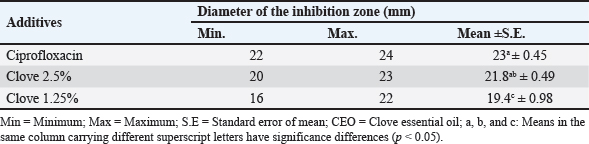
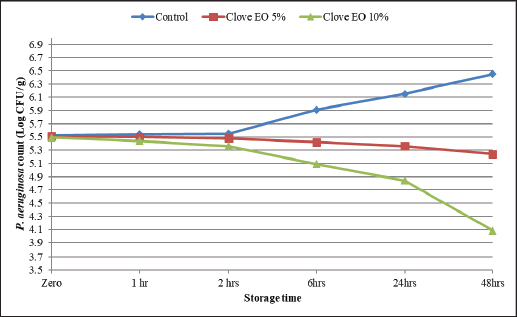
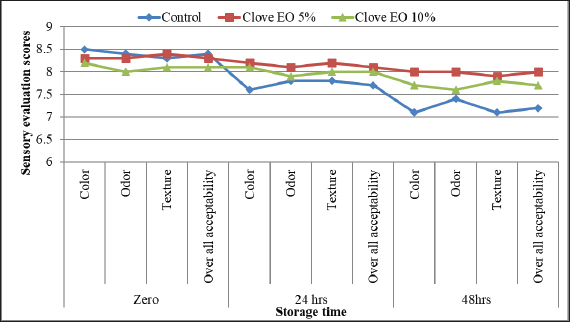
Identification of different species of Pseudomonas isolated from the examined meat and meat product samples is illustrated in Table 2. Four species were identified; P. psychrophila (40.9%), P. fluorescens (29.5%), P. fragi (18.2%), and P. aeruginosa (11.4%). The prevalence of Pseudomonas spp. in minced meat samples was 15.8%, 36.8%, 26.3%, and 21.1%; imported frozen meat was 8.3%, 25%, 50%, and 16.7%; Hamburgers was 12.5%, 25%, 50%, and 12.5%; and luncheons was 0, 20%, 60%, and 20% for P. aeruginosa, P. fluorescens, P. psychrophila, and P. fragi, respectively. The antibiotic resistance profile of P. aeruginosa is illustrated in Table 3. It was highly resistant to tetracycline, oxacillin, amoxicillin-clavulanic acid, lincomycin, and clindamycin (100%), whereas resistance toward vancomycin, colistin, erythromycin, sulfamethoxazole-trimethoprim, and ceftazidime were 60%, 40%, 40%, 40%, and 20%, respectively. The tested strains were sensitive to amikacin, ciprofloxacin, chloramphenicol, and ceftazidime (100%, 100%, 60%, and 60%, respectively). The MAR index of the examined strains varied from 0.615 to 0.385 with 0.508 as a mean value. Figure 1 shows some resistance genes to antibiotics. Gel electrophoresis of the PCR products illustrated that all of the tested P. aeruginosa strains harbored the blaSHV and Mcr1 genes; meanwhile, the blaOXA-1gene failed to be detected in any of the tested strains. The chemical composition of CEO in Table 4 revealed that many active components include eucalyptol, terpinen-4-ol, methyl salicylate, chavicol, eugenol, copaene, e-methyl cinnamate, β-caryophyllene, α-humulene, eugenol acetate, and caryophyllene oxide with an area sum of 0.49%, 0.37%, 1.33%, 0.49%, 100%, 28.17%, 0.50%, 9.78%, 1.42%, 18.66%, and 0.88%, respectively. The values of MIC and MBC for the CEO against P. aeruginosa are illustrated in Table 5; the MIC ranged from 0.31% to 1.25%; while the MBC ranged from 0.62% to 2.5%. The inhibition zone diameter of CEO 2.5% ranged from 20 to 23 mm with a mean value of 21.8 ± 0.49 mm; while it was 19.4c ± 0.98 mm for CEO 1.25%. Statistical analysis of data revealed significant differences (p<0.05), with individual variation between the inhibition zone diameters of ciprofloxacin and CEO 1.25%; while there is no variation between ciprofloxacin and CEO 2.5% as recorded in Table 6. The evaluation of the antibacterial effect of CEO 5% and CEO 10% on minced meat inoculated with P. aeruginosa is illustrated in Figure 2. At the beginning of the experiment, the P. aeruginosa count was 5.52 ± 0.14, 5.51 ± 0.13, and 5.49 ± 0.13 log10 CFU/g in control, treated samples by CEO 5% and CEO 10%, respectively; meanwhile, after one hour, the count decreased from 5.54 ± 0.14 in control samples to 5.50 ± 0.13 and 5.44 ± 0.13 log10 CFU/g after treatment by CEO 5% and CEO 10%, respectively. The percent reduction increased after one hour from 4.4% and 7.4% to 10.9% and 19.7% of CEOs 5% and CEO 10%, respectively. After 2 hours of treatment, the P. aeruginosa count decreased from 5.55 ± 0.13 log10 CFU/g in control untreated minced meat samples to 5.48 ± 0.13 and 5.36 ± 0.16 log10 CFU/g after treatment by CEO at 5% and 10%. The percentage reductions were 13.7% and 29%. After six hours, the count decreased from 5.91 ± 0.04 to 5.42 ± 0.12 log10 CFU/g after treatment by CEO 5% with 62.6% reduction, whereas 10% of the CEOs decreased the count to 5.09 ± 0.10 log10 CFU/g, with a 83.1% reduction. A significant difference between the control and treated samples according to the CEO was recorded at p < 0.05. After 24 hours, the percent reduction was 87% and 96.4% with a mean count of 5.36 ± 0.09 log10 CFU/g and 4.84 ± 0.06 log10 CFU/g after treatment by CEO 5% and 10% compared with control samples (6.15 ± 0.16 log10 CFU/g), with significant differences (p < 0.05) between the samples. At the end of the experiment, the P. aeruginosa count decreased from 6.45 ± 0.17 log10 CFU/g in control samples to 5.48 ± 0.13 and 5.36 ± 0.16 log10 CFU/g after treatment by CEO at 5% and 10%, respectively, achieving a reduction of 95% and 99.6%. Significant differences were detected between control and treated samples (p < 0.05). A sensory evaluation of the effects of CEO 5% and CEO 10% on minced meat is presented in Figure 3. At the beginning of the experiment, the sensory evaluation scores were 8.4 ± 0.10, 8.3 ± 0.99, and 8.1 ± 0.09; while after 24 hours, it was 7.7 ± 0.08, 8.1 ± 0.10, and 8 ± 0.09 and it was 7.2 ± 0.11, 8 ± 0.10, and 7.7 ± 0.09 for control, treated samples by CEO 5% and CEO 10%, respectively. Table 3. Antimicrobial susceptibility of P. aeruginosa (N=5).
DiscussionPseudomonas spp. are emerging food bacteria extensively found in the environment, resulting in the spoilage of different foods, particularly meat and meat products (Abdullah et al., 2024). Thus, it is important to determine its prevalence and identify highly pathogenic stains that belong to this family. In our study, minced meat and imported frozen meat were highly contaminated by these strains due to the psychrophilic nature of these bacteria and the importation of inferior quality beef that has been improperly stored for a long time (Marcelli et al., 2024). Inadequate heat treatment and post-cooking contamination of meat products are possible causes of contamination. The examined minced meat samples had the highest prevalence of P. aeruginosa, followed by imported frozen meat and burgers due to improper handling, low quality of raw materials, and unsanitary conditions either during production or storage, while it failed to be detected in the examined luncheon samples. The results of this study disagree with those of Sheir et al. (2020), who found P. aeruginosa in 4% of burger samples. A higher prevalence of P. aeruginosa (53.04%) was reported by Benie et al. (2017), whereas a lower prevalence (6.7%) was detected in frozen imported meat by Ibrahim et al. (2016). Contamination of meat and meat products by P. aeruginosa could be attributed to its environmental adaptability, low water activity, which ranged from 72% to 97%, as well as a wide range of temperatures, which ranged from 4°C to 42°C (Gu et al., 2016).
Figure 1. Agarose gel electrophoresis of cPCR of P. aeruginosa blaSHV gene (392bp), Mcr1gene (308bp) and blaOXA-1gene (619bp); L=Ladder (100bp); P=Positive control; N=Negative control. Table 4. Chemical composition of CEO by Gas chromatographic-mass spectrometric analysis (GC/MS).
Table 5. Minimum inhibitory concentration (MIC) and minimum bactericidal concentrations (MBC) of CEO against P. aeruginosa.
Table 6. Antimicrobial activity of CEO against P. aeruginosa using the disc diffusion technique.
Figure 2. Antibacterial effects of CEO 5% and CEO 10% on minced meat inoculated with P. aeruginosa. The percent reduction increased after one hour from 4.4% and 7.4% to 10.9% and 19.7% of CEOs 5% and CEO 10%, respectively; meanwhile, at the end of the experiment, the P.aeruginosa count decreased from 6.45 ± 0.17 log10 CFU/g in control samples to 5.48 ± 0.13 and 5.36 ± 0.16 log10 CFU/g after treatment by CEOs 5% and 10%, achieving a reduction percent of 95% and 99.6%.
Figure 3. Sensory evaluation of the effect of CEO 5% and CEO 10% on minced meat. At the beginning of the experiment, the sensory evaluation scores were 8.4 ± 0.10, 8.3 ± 0.99, and 8.1 ± 0.09; while after 24 hours, it was 7.7 ± 0.08, 8.1 ± 0.10, 8a ± 0.09 and it was 7.2 ± 0.11, 8 ± 0.10, and 7.7 ± 0.09 for control, treated samples by CEO 5% and CEO 10%, respectively. Pseudomonas aeruginosa was resistant to most antibiotics used in this study. Antibiotic resistance among the examined strains was also evaluated by the presence of some antibiotic resistance genes (blaSHV and Mcr1). Similar to our results, a higher prevalence of P. aeruginosa resistance has been reported in different countries, including China (Meng et al., 2020), Australia (Khan et al., 2020), and Germany (Yayan et al., 2015). In contrast, Benie et al. (2017) reported that patients were 100%, 97.60%, and 35.40% resistant to kanamycin, aztreonam, and ciprofloxacin, respectively. Resistance to P. aeruginosa is attributed to the importation of inferior-quality meat; also, cross-contamination from other foodstuffs during storage (Rezaloo et al., 2022), and improper and irregular use of antibiotics. Our results revealed that the gene encoding resistance to -lactams (blaSHV) and Mcr1 were detected among the isolated bacteria; while blaOXA-1 be detected. Bahrami et al. (2018) blaSHV and blaOXA genes in 23.08% and 12.5% of the examined P. aeruginosa. Elhariri et al., (2017) found blaSHV in 33.30% of camel meat samples. In the United States, multidrug-resistant strains of P. aeruginosa resulted in 32,500 infections and 2,700 deaths (Center of Diseases Control and Prevention (CDC), 2017). One of the newest strategies is using nonantibiotic compounds to control antibiotic-resistant foodborne pathogens and spoilage bacteria (Ricardo-Rodrigues et al., 2024). Many natural products, including EOs, have antibacterial activities. Medicinal plants are very important sources of natural products rich in various secondary metabolites (alkaloids, tannin, terpenoids, and flavonoid) which have antimicrobial properties, as well as phenolic acids, steroidal saponins, quinones (Bouzada et al., 2009). EOs are mixtures of many natural volatile secondary metabolites that are extracted from plants by steam or hydro-distillation and are responsible for biological properties (Assadpour et al., 2024). Concerning sensory evaluation, CEO, particularly CEO 5%, had a noticeable effect on minced meat color, odor, texture, and overall acceptability scores after 24 and 48 hours of treatment, with a remarkable significance difference (p < 0.05) compared with control untreated samples. Nearly similar findings were reported by Abdel-Aziz and Morsy (2014) and Aliakbarlu and Sadaghiani (2015). In this experiment, P. aeruginosa isolates were evaluated for their susceptibility to CEO; similar results were reported in other studies (Hu et al., 2018; Islamieh et al., 2019; Awad et al., 2024). Results of the agar well diffusion method and disk diffusion test revealed that CEO evoked variable degrees of inhibition, which agreed with Burt (2004) and Hoquea et al. (2008); attributed to its high content of eugenol, which has wide-spectrum antimicrobial effects against many bacteria (Burt, 2004). Since ancient times, the CEO has antioxidant and antibacterial effects that make it a valuable natural food preservative through its interaction with bacterial cells, bacterial enzymes, and polysaccharides (Cortés-Rojas et al., 2014). In our experiment, 5% and 10% of CEO (four times more than MIC and MBC values) were used because high protein and fat content in meat acts as a barrier that protects it from the effect of CEO; therefore, higher concentrations of CEOs are required to control the bacteria effectively (Hoquea et al., 2008). During 48 hours at chilling temperature, the CEO at 10% was more effective than the CEO at 5% in meat preservation; this agreed with Hoquea et al. (2008), who reported that clove has the ability to inhibit Gram-positive and Gram-negative meat-borne pathogen growth. In addition, Freires et al. (2015) reported that CEO has bactericidal effects on P. aeruginosa due to its effective interaction against P. aeruginosa outer membrane lipopolysaccharide. The observed reduction in P. aureginosa was attributed to the effect of CEO on bacterial virulence factors (Husain et al., 2013). Therefore, the CEO can act as an alternative natural control for P. aeruginosa. ConclusionThe meat and meat products under examination were contaminated by different species of Pseudomonas, particularly the multi-drug-resistant species of P. aeruginosa, resulting in many health problems for consumers. Therefore, the use of natural antibacterial agents, particularly clove EO, is an absolute necessity to control microorganisms in meat and improve its sensory characteristics. AcknowledgmentsThe authors are grateful for their departments and institutions. Author contributionsSEE and AKE collected the samples and prepared them for examination. AMY and HTZ performed the bacteriological and molecular techniques. ZFH and YRS evaluated the effect of the essential oil used. SEE, AKE, and ZFH supervised the work. SEE and AMY drafted the manuscript. Conflicts of interestThere are no conflicts of interest. Funding statementThis study was self-funded. Data availabilityData will be available upon reasonable request. ReferencesAbdel-Aziz, M.E. and N.F. 2015. Keeping quality of frozen beef patties by marjoram and clove essential oils. J. Food Process. Preserv. 39(6), 956–965. https://doi.org/10.1111/jfpp.12309. Abdelrahman, D.N., Taha, A.A., Dafaallah, M.M., Mohammed, A.A., El Hussein, A.R., Hashim, A.I., Hamedelni, Y.F. and Altayb, H.N. 2020. β-lactamases (bla TEM, bla SHV, bla CTXM-1, bla VEB, bla OXA-1 and class C β-lactamases gene frequency in Pseudomonas aeruginosa isolated from various clinical specimens in Khartoum State, Sudan: a cross sectional study. F1oo Res. 9, 774. https://doi.org/10.12688/f1000research.24818.3. Abdullah, R.M., Ali, S., Aslam, B. and Arshad, M.I. 2024. Molecular characterization and drug resistance pattern of Pseudomonas aeruginosa isolated from poultry meat and meat products. Pakistan Vet. J. 20(10), 30. http://dx.doi.org/10.29261/pakvetj/2024.204. Aliakbarlu, J. and Sadaghiani, S.K. 2015. Effect of avishane Shirazi (Zataria multiflora) and clove (Syzygium aromaticum) essential oils on microbiological, chemical and sensory properties of ground sheep meat during refrigerated storage. J. Food Quality, 38(4), 240–247; doi:10.1111/jfq.12147. American Public Health Association (APHA) 2001. Compendium of methods for the microbiological examination of foods fourth edition. Washington, DC: F.P. Downes and K. Ito (editors). Assadpour, E., Karaãa, A.C., Fasamanesh, M., Mahdavi, S.A., Shariat-Alavi, M., Feng, J., Kharazmi, M.S., Rehman, A. and Jafari, S.M. 2024. Application of essential oils as natural biopesticides; recent advances. Crit. Rev. Food Sci. Nutr. 64, 19; doi:10.1080/10408398.2023.2170317. Awad, F.A., Mohammed, R., Karama, T. and Al-Taee, A. 2024. The effect of sub-inhibitory concentration of clove essential oil on the expression of Pseudomonas aeruginosa virulence genes. Trop. J. Nat. Prod. Res. 8(3), 6498–6502; doi:10.26538/tjnpr/v8i3. Bahrami, M., Mmohammadi-Sichani, M. and Karbasizadeh, V. 2018. Prevalence of SHV, TEM, CTX-M and OXA-48β-lactamase genes in clinical isolates of Pseudomonas aeruginosa in bandar-abbas, Iran, Avicenna. J. Clin. Microbiol. Infect. 5(4), 86–90; doi:10.34172/ajcmi.2018.18. Becerra, S.C., Roy, D.C. Sanchez, C.J., Christy, R.J. and Burmeiste, D.M. 2016. An optimized staining technique for the detection of Gram-positive and Gram-negative bacteria within the tissue. BMC Res. Notes. 9, 216. https://doi.org/10.1186/s13104-016-1902-0. Benie, C., Nathalie, G. and Adjehi, D. 2017. Prevalence and antibiotic resistance of Pseudomonas aeruginosa isolated from bovine meat, fresh fish and smoked fish. Arch. Clin. Microbiol. 8(3), 1–9; doi:10.4172/1989-8436.100040. Bergspica, I., Kaprou, G., Alexa, E.A., Prieto, M. and Alvarez-Ordoãez, A. 2020. Extended spectrum β-lactamase (ESBL) producing Escherichia coli in pigs and pork meat in the European Union. Antibiotics. 9(10), 678. https://doi.org/10.3390/antibiotics9100678. Bouzada, M.L.M., Fabri, R., Nogueira, M., Konno, T.U., Garcia, G.D. and Scio, E. 2009. Antibacterial, cytotoxic and phytochemical screening of some traditional medicinal plants in Brazil. Pharmaceut. Biol. 47(1), 44–52; doi: 10.1080/13880200802411771 Bradbury, R.S., Roddam, L.F., Merritt, A., Reid, D.W. and Champion, A.C. 2010. Virulence gene distribution in clinical, nosocomial and environmental isolates of Pseudomonas aeruginosa. J. Med. Microbiol. 59(8), 881–890. https://doi.org/10.1099/jmm.0.018283-0. Burt, S. 2004. Essential oils: their antibacterial properties and potential applications in foods”a review. Int. J. Food Microbiol. 94, 223”253; doi:10.1016/j.ijfoodmicro.2004.03.022. Castanheira, M., Simner, P.J. and Bradford, P.A. 2021. Extended spectrum β-lactamases: an update on their characteristics, epidemiology and detection. JAC-Antimicrob. Resist. 3(3), 92; doi:10.1093/jacamr/dlab092. Center of Diseases Control and Prevention (CDC) (2017). Multi drug-resistant Pseudomonas aeruginosa, CDC report. Available via https://www.cdc.gov/hai/organisms/pseudomonas.html. Clinical and Laboratory Standards Institute (CLSI) (2020). Performance standards for antimicrobial susceptibility testing; thirtieth informational supplement. M100-S30. Wayne, PA: Clinical and Laboratory Standards Institute. Colom, K., Perez, J., Alonso, R., Fernãndez-Aranguiz, A., Larião, A. and Cisterna, R. 2003. Simple and reliable multiplex PCR assay for detection of blaTEM, blaSHV and blaOXA-1 genes in Enterobacteriaceae. FEMS Microbiol. Lett. 223, 147–151; doi:10.1016/S0378-1097(03)00306-9. Cortés-Rojas, D.F., de Souza, C.R. and Oliveira, W.P. 2014. Clove (Syzygium aromaticum): a precious spice. Asian Pacific J. Trop. Biomed. 4(2), 90–96; doi:10.1016/S2221-1691(14)60215-X. Darwish, W.S., El Bayoumi, R.M., Mohamed, N.H. and Hussein, M.A. 2024. Microbial contamination of meat at a low temperature storage: A review. J. Adv. Vet. Res. 14(2), 322–325. Elhariri, M., Hamza, D., Elhelw, R. and Dorgham, S. 2017. Extended-spectrum beta-lactamase-producing Pseudomonas aeruginosa in camel in Egypt: potential human hazard. Ann. Clin. Microbiol. Antimicrob. 16(1), 21–26. https://doi.org/https://doi.org/10.1186/s12941-017-0197-x">10.1186/s12941-017-0197-x. Freires, I.A., Denny, C., Benso, B., Matias Alencar, S. and Rosalen, P. 2015. Antibacterial activity of essential oils and their isolated constituents against cariogenic bacteria: a systematic review. Molecules. 20(4), 7329–7358; doi:10.3390/molecules20047329. Gu, X., Sun, Y., Tu, K., Dong, Q. and Pan, L. 2016. Predicting the growth situation of Pseudomonas aeruginosa on agar plates and meat stuffs using gas sensors. Sci. Rep. 6(1), 38721–38722. https://doi.org/https://doi.org/10.1038%2Fsrep38721">10.1038/srep38721. Hashem, H., Hanorab, A., Abdallac, S., Shawkyd, A. and Saad, A. 2018. Pseudomonas aeruginosa identification and MIC assessment from clinical isolates. Pharmacol. Biomed. Sci. 1(2) 21–24; doi:10.21608/rpbs.2018.5925. Hema, R., Kumaravel, S. and Sivasubramanian, C. 2012. GC-MS study on the potentials of Syzygium aromaticum. Res. J. 12, 1–4. http://www.sciencepub.net. Hoquea, M., Barib, M.L. Vijay, K. and Kawamoto, S. 2008. Antimicrobial activity of cloves and cinnamon extracts against food borne pathogens and spoilage bacteria, and inactivation of Listeria monocytogenes in ground chicken meat with their essential oils. Nat. Food Res. Inist. 72, 9”21. Hu, Q., Zhou, M. and Wei, S. 2018. Progress on the antimicrobial activity research of clove oil and eugenol in the food antisepsis field. J. Food Sci. 83(6), 1476–1483; doi:10.1111/1750-3841.14180. Husain, F.M., Ahmad, I., Asif, M. and Tahseen, Q. 2013. Influence of clove oil on certain quorum-sensing-regulated functions and biofilm of Pseudomonas aeruginosa and Aeromonas hydrophila. J. Biosci. 38(5), 835–844. https://doi.org/10.1007/s12038-013-9385-9. Ibrahim, H.M., Bou El-Roos, N.A. and Abd Elsalam, M. 2016. Prevalence and molecular characterization of Pseudomonas species in frozen imported meat. Benha Vet. Med. J. 31(2), 220–224. https://doi.org/10.21608/bvmj.206.31301. Islamieh, D.I., Afshar, D. and Esmaeili, D. 2019. Effect of Satureja khuzistanica essential oil (SKEO) extract on expression of lasA and lasB genes in Pseudomonas aeruginosa. Iran J. Microbiol. 11(1), 55–59. Jurado-Mart´ın, I., Sainz-Mej´ıas, M. and McClean, S. 2021. Pseudomonas aeruginosa: an audacious pathogen with an adaptable arsenal of virulence factors. Int. J. Molec. Sci. 22(6), 3128; doi:10.3390/ijms22063128. Khan, M., Stapleton, F., Summers, S., Rice, S. and Willcox, M. 2020. Antibiotic resistance characteristics of Pseudomonas aeruginosa isolated from keratitis in Australia and India. Antibiotics. 9(9), 600; doi:10.3390/antibiotics9090600. LaBauve, A.E. and Wargo, M.J. 2012. Growth and laboratory maintenance of Pseudomonas aeruginosa. Curr. Protoc. Microbiol. 6E, 1; doi:10.1002/9780471729259.mc06e01s25. Li, G., Ma, X., Deng, L., Zhao, X., Wei, Y., Gao, Z., Jia, J., Xu, J. and Sun, C. 2015. Fresh garlic extract enhances the antimicrobial activities of antibiotics on resistant strains in vitro. Jundishapur J. Microbiol. 8, 5. https://doi.org/https://doi.org/10.5812%2Fjjm.14814">10.5812/jjm.14814. Marcelli, V., Osimani, A. and Aquilanti, L. 2024. Research progress in the use of lactic acid bacteria as natural bio-preservatives against Pseudomonas spp. in meat and meat products: a review. Food Res. Int. 196, 115129. https://doi.org/10.1016/j.foodres.2024.115129. Meng, L., Liu, H. and Lanetal, T. 2020. Antibiotic resistance patterns of Pseudomonas spp. isolated from raw milk revealed by whole genome sequencing. Front. Microbiol. 11, 1005. https://doi.org/https://doi.org/10.3389%2Ffmicb.2020.01005">10.3389/fmicb.2020.01005. Newton-Foot, M., Snyman, Y., Maloba, M. and Whitelaw, A. 2017. Plasmid-mediated mcr-1 colistin resistance in Escherichia coli and Klebsiella spp. Clinical isolates from the Western Cape region of South Africa. Antimicrob. Resist. Infect. Cont. 6, 78; doi:10.1186/s13756-017-0234-8. Pandey, V. K., Srivastava, S., Dash, K. K., Singh, R., Dar, A., Singh, T., Farooqui, A., Shaikh, A.M. and Kovacs, B. 2024. Bioactive properties of clove (Syzygium aromaticum) essential oil nanoemulsion: a comprehensive review. Cell Biomaterials. 10, 1; doi:10.1016/j.heliyon. 2023.e22437. Pang, Z., Raudonis, R., Glick, B., Lin, T. and Cheng, Z. 2019. Antibiotic resistance in Pseudomonas aeruginosa: mechanisms and alternative therapeutic strategies. Biotech. Adv. 37(1), 177–192; doi:10.1016/j.biotechadv.2018.11.013. Pozzatti, P., Loreto, E. S., Lopes, P. G., Athayde, M., Santurio, J. and Alve, S. 2010. Comparison of the susceptibilities of clinical isolates of Candida albicans and Candida dubliniensis to essential oils. Mycoses. 53 (1), 12–15; doi:10.1111/j.1439-0507.2008. 01643.x. Rezaloo, M., Motalebi, A., Mashak, Z., Mashak, Z. and Anvar, A. 2022. Prevalence, antimicrobial resistance, and molecular description of Pseudomonas aeruginosa isolated from meat and meat products. J. Food Qual. 11, 9899338. https://doi.org/10.1155/2022/9899338. Ricardo-Rodrigues, S., Rouxinol, M. I., Agulheiro-Santos, A.C., Potes, M. A., Laranio, M. and Elias, M. 2024. The antioxidant and antibacterial potential of thyme and clove essential oils for meat preservation”an overview. App. Biosci. 3(1), 87–101. https://doi.org/10.3390/applbiosci3010006. Roberts, D. and Greenwood, M. 2003. Practical food microbiology. 3rd edition. London, UK: Blackwell Publishing Ltd. pp: 273–274. https://doi.org/10.1002/9780470757512. Rocha, J., Barsottini, M., Rocha, R., Laurindo, M., Laurindo de Moraes, F. and Lília da Rocha, S. 2019. Pseudomonas aeruginosa: virulence factors and antibiotic resistance genes. Brazilian Arch. Biol. & Tech. 62, 180503; doi:10.1590/1678-4324-2019180503. Sambrook, J., Fritscgh, E. and Mentiates, F. 1989. Molecular cloning. A laboratory manual. , New York, NY: Cold Spring Harbor Laboratotry Press. Santos, C.H., Piccoli, R.H. and Tebaldi, V.M. 2017. Antimicrobial activity of essential oils and isolated composts with pathogenic agents of clinical and food origin. Revesta Do Instituto Adolfo Lutz. 76, 1–8. https://periodicoshomolog.saude.sp.gov.br/index.php/RIAL/article/view/33539. Sheir, S.H., Ibrahim, H.M. and Hassan, M.A. 2020. Incidence of Psychotropic bacteria in frozen chicken meat products with special reference to Pseudomonas species. Benha Vet. Med. J. 39(1), 165–168; doi:10.21608/bvmj.2020.37744.1238. Spagnolo, M., Sartini, M. and Cristina, M.L. 2021. Pseudomonas aeruginosa in the healthcare facility setting. Rev. Med. Microbiol. 32(3), 169–175. https://doi.org/10.1097/MRM.0000000000000271. Stadnik, J. 2024. Nutritional value of meat and meat products and their role in human health. Nutrients. 16(10), 1446. https://doi.org/10.3390/nu16101446. Wei, M.C., Xiao, J. and Yang, Y.C. (2016). Extraction of α-humuleneenriched oil from clove using ultrasound-assisted supercritical carbon dioxide extraction and studies of its fictitious solubility. Food Chem. 210, 172–181; doi:10.1016/j.foodchem.2016.04.076. Yayan, J., Ghebremedhin, B. and Rasche, K. 2015. Antibiotic resistance of Pseudomonas aeruginosa in pneumonia at a single university hospital center in Germany over a 10-year period. PLoS One, 10, 10; doi:10.1371/journal.pone.0139836. | ||
| How to Cite this Article |
| Pubmed Style El-wehedy SE, Elshafee AK, Helal ZF, Shehab-eldin YR, Yassin AM, Zaki HT. Contamination of meat and its products by Pseudomonas species and assessment of the antibacterial effect of clove (Syzygium aromaticum) essential oil on multidrug-resistant P. aeruginosa. Open Vet. J.. 2025; 15(4): 1812-1822. doi:10.5455/OVJ.2025.v15.i4.34 Web Style El-wehedy SE, Elshafee AK, Helal ZF, Shehab-eldin YR, Yassin AM, Zaki HT. Contamination of meat and its products by Pseudomonas species and assessment of the antibacterial effect of clove (Syzygium aromaticum) essential oil on multidrug-resistant P. aeruginosa. https://www.openveterinaryjournal.com/?mno=248815 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i4.34 AMA (American Medical Association) Style El-wehedy SE, Elshafee AK, Helal ZF, Shehab-eldin YR, Yassin AM, Zaki HT. Contamination of meat and its products by Pseudomonas species and assessment of the antibacterial effect of clove (Syzygium aromaticum) essential oil on multidrug-resistant P. aeruginosa. Open Vet. J.. 2025; 15(4): 1812-1822. doi:10.5455/OVJ.2025.v15.i4.34 Vancouver/ICMJE Style El-wehedy SE, Elshafee AK, Helal ZF, Shehab-eldin YR, Yassin AM, Zaki HT. Contamination of meat and its products by Pseudomonas species and assessment of the antibacterial effect of clove (Syzygium aromaticum) essential oil on multidrug-resistant P. aeruginosa. Open Vet. J.. (2025), [cited January 25, 2026]; 15(4): 1812-1822. doi:10.5455/OVJ.2025.v15.i4.34 Harvard Style El-wehedy, S. E., Elshafee, . A. K., Helal, . Z. F., Shehab-eldin, . Y. R., Yassin, . A. M. & Zaki, . H. T. (2025) Contamination of meat and its products by Pseudomonas species and assessment of the antibacterial effect of clove (Syzygium aromaticum) essential oil on multidrug-resistant P. aeruginosa. Open Vet. J., 15 (4), 1812-1822. doi:10.5455/OVJ.2025.v15.i4.34 Turabian Style El-wehedy, Samar E., Azza K. Elshafee, Zainab F. Helal, Yomna R. Shehab-eldin, Amany M. Yassin, and Haidy T. Zaki. 2025. Contamination of meat and its products by Pseudomonas species and assessment of the antibacterial effect of clove (Syzygium aromaticum) essential oil on multidrug-resistant P. aeruginosa. Open Veterinary Journal, 15 (4), 1812-1822. doi:10.5455/OVJ.2025.v15.i4.34 Chicago Style El-wehedy, Samar E., Azza K. Elshafee, Zainab F. Helal, Yomna R. Shehab-eldin, Amany M. Yassin, and Haidy T. Zaki. "Contamination of meat and its products by Pseudomonas species and assessment of the antibacterial effect of clove (Syzygium aromaticum) essential oil on multidrug-resistant P. aeruginosa." Open Veterinary Journal 15 (2025), 1812-1822. doi:10.5455/OVJ.2025.v15.i4.34 MLA (The Modern Language Association) Style El-wehedy, Samar E., Azza K. Elshafee, Zainab F. Helal, Yomna R. Shehab-eldin, Amany M. Yassin, and Haidy T. Zaki. "Contamination of meat and its products by Pseudomonas species and assessment of the antibacterial effect of clove (Syzygium aromaticum) essential oil on multidrug-resistant P. aeruginosa." Open Veterinary Journal 15.4 (2025), 1812-1822. Print. doi:10.5455/OVJ.2025.v15.i4.34 APA (American Psychological Association) Style El-wehedy, S. E., Elshafee, . A. K., Helal, . Z. F., Shehab-eldin, . Y. R., Yassin, . A. M. & Zaki, . H. T. (2025) Contamination of meat and its products by Pseudomonas species and assessment of the antibacterial effect of clove (Syzygium aromaticum) essential oil on multidrug-resistant P. aeruginosa. Open Veterinary Journal, 15 (4), 1812-1822. doi:10.5455/OVJ.2025.v15.i4.34 |