| Research Article | ||
Open Vet. J.. 2025; 15(5): 2238-2250 Open Veterinary Journal, (2025), Vol. 15(5): 2238-2250 Research Article Fertility failure of vulvar and vaginal squamous cell carcinoma in dairy cows associated with reproductive hormonal disturbance, vulvar and vaginal hyperplasia, acute phase protein production, and histopathological changesYahia A. Amin1*, Samer S. Fouad2, Rana A. Ali3, Mariam A. Fawy3, Seham A. Mobarak3, Abdellah Hassan Mahmoud4 and Amna H. M. Nour51Department of Theriogenology, Faculty of Veterinary Medicine, Aswan University, Aswan, Egypt 2Veterinary Clinical Pathology, Qena University Hospital, South Valley University, Qena, Egypt 3Department of Zoology, Faculty of Science, South Valley University, Qena, Egypt 4Department of Physiology, Faculty of Veterinary Medicine, South Valley University, Qena, Egypt 5Department of Zoology, Faculty of Science, Aswan University, Aswan, Egypt *Corresponding Author: Yahia A. Amin. Department of Theriogenology, Faculty of Veterinary Medicine, Aswan University, Aswan, Egypt. Email: yahiaamin2030 [at] gmail.com Submitted: 22/02/2025 Revised: 08/04/2025 Accepted: 13/04/2025 Published: 31/05/2025 © 2025 Open Veterinary Journal
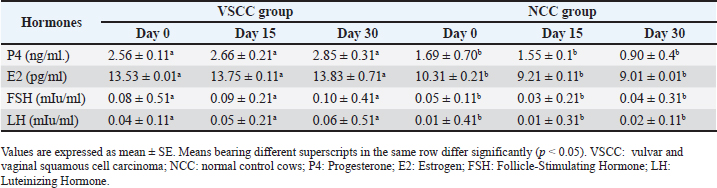
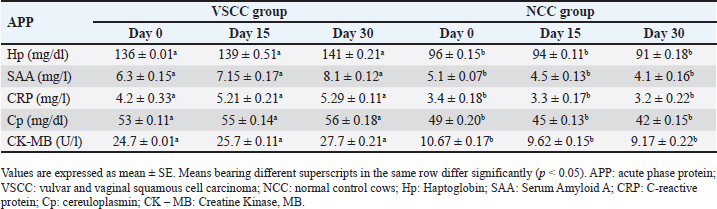
ABSTRACTBackground: The majority of neoplasms affect bovine species, and the most common are squamous cell carcinomas (SCCs) in sheep, goats, and cattle. Neoplasm of the vulva and vagina in dairy cattle undergo widespread in dairy herds. Aim: This study aimed to examine the effects of vulvar and vaginal squamous cell carcinoma (VSCC) on the fertility of dairy cows by investigating changes in reproductive hormones as well as vaginal and vulvar tissue. In addition, acute phase protein (APP) production and histopathological changes were also investigated to evaluate their relationship with tumor development and to assess their diagnostic and prognostic value. Methods: Twenty dairy cows were divided into two equal groups: the diseased group, which suffered from vulvar and vaginal squamous cell carcinoma and known as vulvar and vaginal squamous cell carcinoma group (VSCCG), and the healthy group, which served as the normal control group (NCG). Sera were used to evaluate reproductive hormones (progesterone, estrogen, follicle-stimulating hormone, and luteinizing hormone) and acute phase proteins (APPs), such as serum amyloid A (SAA), haptoglobin (Hp), ceruloplasmin (Cp), C-reactive protein (CRP), and creatine kinase (CK). Histopathological examination and immunohistochemical investigations [S100, vimentin and alpha-smooth muscle actin (α-SMA)] of the vaginal tissue were performed. Results: Results revealed that VSCCG exhibits a significant increase in the levels of reproductive hormones compared with NCG. Investigation of APPs revealed a significant increase in the levels of SAA, Hp, Cp, CRP, and CK in diseased cows compared to healthy cows. Histological investigation revealed that the vaginal mucosa from SCC cows indicated the presence of dense squamous epithelial cell invasion and variant keratin pearls. The neoplastic cells appeared as large, polyhedral cells with hyperchromatic nuclei. Additionally, koilocytic and atypical mitosis were noted. Immunohistochemical analysis of S100, vimentin, and α-SMA shows very strong positive reactions in diseased cows. Conclusion: The present study revealed that VSCC is associated with reproductive hormonal disturbance and a rise in APP production, which are responsible for fertility failure and may even lead to sudden death. Keywords: Fertility failure, Reproductive hormones, Vulva and vagina, Squamous cell carcinoma, Acute phase protein. IntroductionIn contrast to other domesticated animals, reports of tumors in cattle are quite rare. The majority of cattle do not live to an age at which they are susceptible to the formation of cancers (De Vries and Marcondes, 2020; Baumgärtner and Gruber, 2020). Ovarian, fallopian tube, uterine, cervical, vaginal, and vulvar tumors are the different subtypes of genital system cancers. Both benign and malignant vaginal tumors are possible, with the latter having a cautious prognosis because of the possibility of metastasis (Agnew and MacLachlan, 2016). Malignancy in cases of vaginal tumor was reported to be rare, while around the vulva, malignancy was illustrated to occur in the form of locally squamous cell carcinomas (SCCs) which was characterized by recurrent form. Except for solar-induced SCCs in tropical climates that affect sheep and cattle, vaginal and vulvar carcinomas, including adenocarcinomas, SCCs, and transitional cell carcinomas, are uncommon in all animals. In adult cows, ewes, and mares, SCC of the vulva has also been recorded (Foster, 2007). Hormone receptor function in several cancer types is well-established. The identification of tumor subgroups with unique etiopathological characteristics and differing therapeutic responses depends on the expression of sex hormone receptors. This is especially true for sex hormone (estrogen, androgen, and progesterone) receptors in malignancies like breast cancer (Alvarado et al., 2017; Harbeck et al., 2019) and prostate cancer (Labrecque et al., 2019). Acute-phase proteins (APPs) are blood proteins that help to preserve homeostasis and regulate the growth of microorganisms prior to the development of specific immunity in the host organism. Some factors, such as infection, trauma, and inflammation, are known to have an impact on its concentrations (Gruys et al., 1994; Andreis et al., 2010). Numerous APPs previously identified in humans and laboratory animals were identified through research on domestic animals, and variations in the acute-phase response between animal species were noted (Gruys et al., 1994; Eckersall et al., 1996). Over the past 20 years, APP reactions in animals have been closely observed for therapeutic purposes (Gruys et al., 1994; Eckersall, 2000a). Thus, numerous quantitative APP assays have been developed. Proteins in the acute phase are classified as both positive and negative molecules. Albumin and transferrin are considered negative molecules, whereas haptoglobin (Hp), C-reactive proteins (CRPs), serum amyloid-A (SAA), ceruloplasmin (Cp), fibrinogen (Fb), and a-1-acid glycoprotein are considered positive molecules. These molecules’ activity levels change in response to different stimuli (Eckersall and Bell, 2010), and their production in the liver is regulated by the release of cytokines. Inflammation, infection, or tissue injuries are examples of stimuli that cause defense-oriented cells to release cytokines, which in turn cause the production of APPs. APPs have potential use in farm animal medicine, including disease diagnosis and prognosis, despite difficulties in their detection. APPs have been shown to be highly helpful in the early identification of subclinical diseases or changes in an animal’s health condition, providing predictive information regarding the occurrence of disease (Tothova et al., 2014). This study hypothesized that vulvar and vaginal tumors contribute to disturbances in reproductive hormone levels and APP production in cows with tumors. To date, no previous investigation has studied this disease in dairy cattle. The aims of this study were to investigate the effects of vulvar and vaginal squamous cell carcinoma (VSCC) on the fertility of dairy cows by assessing the reproductive hormones and vaginal and vulvar tissue changes. In addition, the investigation involved the APP responses to evaluate their relationship with tumor production and to assess their prognostic value. Material and MethodsAnimals and sampling protocolThe animals of the current study included Holstein Friesian cows that suffered from large tumor masses protruding through the vagina and vulva (Fig. 1). The cows were assigned to two groups: cows of the first group (n=10) are that suffer from vulvar and VSCC and considered the diseased group (VSCCG), while the cows of the second group (n=10) are clinically healthy, with no history of vulvar and VSCC, and served as the normal control group (NCG). Animals from the control group underwent ultrasound to exclude pregnancy. Vulvar- and VSCC–diseased cows were selected on the basis of clinical examination and history of overgrowth of the vulva and vagina. According to the cows’ case history, they are pluriparous in their fourth lactation. They are owned by a private farm in the Qena Province. Qena province is characterized by its subtropical climate with hot and cold weather extremes (air temperatures ranging from 31°C to 41°C). The cows had an average age of 6–7 years, a weight of 250–400 kg, and body condition score (BCs) of >3.2 (scale: 1=thin, 5=fat). They appeared to be in good health. The animals were milked twice a day using a milking machine, and they produced between 4 and 7 kg of milk per head each day over the course of 200–250 days of lactation. The animals had unrestricted access to water and were fed a combination of concentrate and dry fodder materials, such as alfalfa and Egyptian clover. Barley (30%), yellow corn (21%), soybean meal (20%), wheat bran (25%), di-calcium phosphate (2%), common salt (1%), and premix (1%) comprise the concentrate mixture. Ad libitum wheat straw was available. Total digestible nutrition was 67%, and the crude protein level was 12% in these meals.
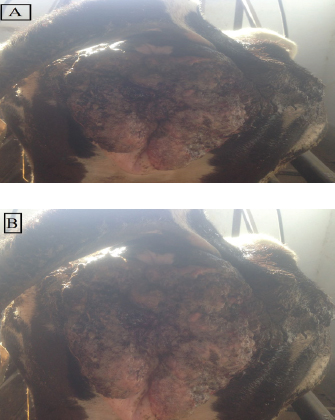
Fig. 1. (A &vagina are extensively affected by large, advanced B): Gross appearance of vulvar and vaginal squamous cell carcinoma in dairy cows. Table 1. The hormonal profile of cows with vulvar and vaginal squamous cell carcinoma compared with the normal control cows.
Table 2. Acute phase protein (APP) response in cows with vulvar and vaginal squamous cell carcinoma compared with control cows.
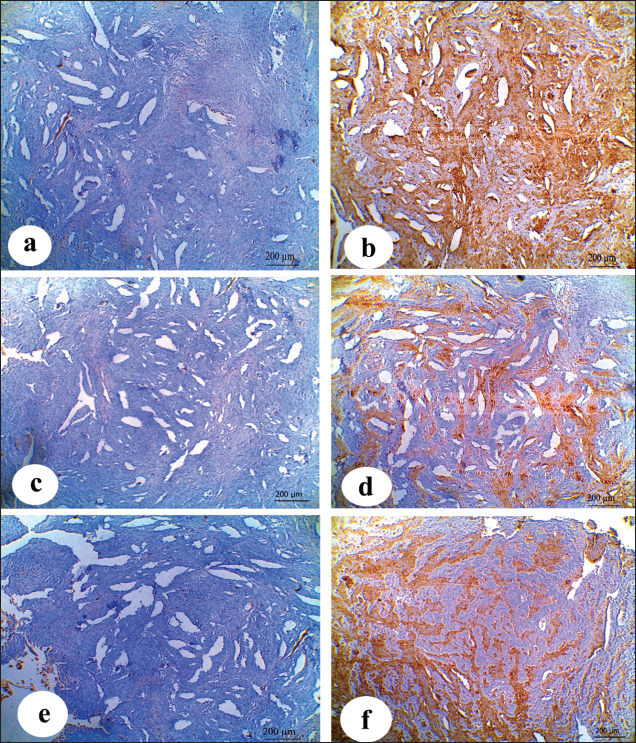
Inspection of the tumor revealed that the exposed side was friable, whereas hardness was prominent in the interior aspect. The lesion exhibited scattered ecchymotic hemorrhages on an uneven surface, resulting in a dark red color. The vulva and perineum also showed signs of enlargement and prominence. According to the animals’ case history, the tumor was initially observed as a small wound on the vulva, which later developed into a larger mass. Nevertheless, the massive tumorous mass pushing the vaginal wall during digital examination revealed limited vestibular and vaginal lumen. To remove all the trash and manure particles, the tumor mass was cleansed with potassium permanganate solution (1:10000). Upon physical inspection, the tumor mass exhibited necrotic patches and uneven, fragile surfaces. The animal was also given intramuscular injections of the long-acting antibiotic enrofloxacin 10% w/v at a dose rate of 7.5 mg/kg body weight (BW), meloxicam, an anti-inflammatory medication, and chlorpheniramine maleate, an anti-histaminic medication, at a dose rate of 0.5 mg/kg BW. Blood samplesAll of the cows in both groups underwent jugular vein blood sampling three times (on days 0, 15, and 30). Fresh plain vials were used to collect the serum. The produced serum was stored at −20°C until use. Determination of reproductive hormonesSerum progesterone (P4) levels were determined using the enzyme-linked immunosorbent assay (ELISA) kit (Catalog Number: E-OSEL-B0005) (Elabscience, USA. Serum was tested using the Estradiol (E2) ELISA Kit (Elabscience, USA (Catalog No.: E-OSEL-B0001) in accordance with the manufacturer’s instructions. ELISA kits were used to measure follicle-stimulating hormone (FSH) (Rose, 1998). ELISA kits were used to determine luteinizing hormone (LH) (Rebar et al., 1982). Determination of APPsSerum creatine kinase (CK) was measured colorimetrically using enzymatic techniques in accordance with the manufacturer’s instructions (Bio-diagnostic, Cairo, Egypt). Using a previously reported hemoglobin binding test, serum Hp was measured (Makimura and Suzuki, 1982). Using a commercially available enzyme-linked immunosorbent test kit (Phase SAA kit; Tridelta Ltd, Ireland), SAA levels were measured. Moreover, serum Cp was measured by p-phenylenediamine oxidization using a commercially available kit (catalog no. 4096–1000; Biovision Inc., CA) (Henry, 1974). The concentrations of CRPs in plasma samples were quantified using an EIA kit (CRP ELISA kit; Cloud-Clone Corp., USA). Tissue samples for histological and immunohistochemical examinationsFollowing the administration of local anesthesia, the masses were biopsyed and preserved in 10% neutral phosphate-buffered formalin (pH 7.0). For histopathological preparations, tissue sections 5 µm in thickness were stained with Harris hematoxylin and eosin (a standard stain for histological investigation), after specimens were gradually dehydrated in ethyl alcohol (50%–100%), cleared in methyl benzoate, and embedded in molten paraffin wax at 58°C–62°C. To conduct immunohistochemical examination, sections were exposed to antigen retrieval solution for 20–30 minutes, endogenous peroxidase inactivation with 3% Hydrogen Peroxide for 10 minutes, and blocking solution for 1 hour. Then, diluted mouse monoclonal vimentin (V1-10) (Cat. No. AB 20346, Abcam Co., UK), mouse monoclonal S100 beta (Cat. No. AB 218513, Abcam, UK) and Rabbit Polyclonal alpha smooth muscle actin (α-SMA) (Cat. No. AB 5694, Abcam Co., UK) (MA, 1:100) were used as primary antibodies. These reactions were allowed to proceed overnight in a humidified chamber, followed by the application of diluted Horseradish Peroxidase-conjugated anti-rabbit secondary antibody (#31460, Thermo Fisher Co., USA). Then, freshly made DAB (K3468, DAKO, Denmark) substrate was utilized for 2–5 minutes. Hematoxylin was used as a counterstain on dehydrated and mounted sections. Using a personal computer and camera software (Olympus DP74 Tokyo 163-0914 Japan) and high-power light microscopy (Olympus BX43F Tokyo 163-0914 Japan), images were analyzed. Statistical analysisThe results were statistically evaluated using SPSS® (SPSS 20, IL, USA) as the mean and standard error of the mean. A Mann-Whitney or t-Student test was used to compare outcomes between the two groups. To assess significant differences between samples at different time points (p < 0.05), a general linear ANOVA with repeated measures was employed. Ethical approvalAll procedures were performed in accordance with the ethical standards of the Ethics Committee of the Faculty of Veterinary Medicine, South Valley University, Egypt (approval number “VM/SVU/24(7)-08”). ResultsGross clinical findingsThe lesions in the affected cows appeared as raised plaques. In all affected cattle, the genital area was exposed to numerous pus-filled lesions. The vulva and vagina are extensively affected by large, advanced malignancies (Fig. 1). In addition, some parts of the surface of the lesions were hemorrhagic and ulcerative. Spontaneous regression did not occur. A case history check indicated that hyperplasia had remained for >5 months, resulting in a significant loss of BC. Cases of large, hemorrhagic, and ulcerative tumors have a poor prognosis, often leading to sudden death. Reproductive hormonal profile and APP responseTable 1 presents the findings of reproductive hormones in both diseased and control cows. Findings displayed a significant increase (p < 0.05) in the levels of reproductive hormones (Progesterone, estrogen, FSH, and LH) in the VSCC cows compared with those in the control and healthy cows. Table 2 presents the results of APP activity in both diseased and control cows. The current results (Table 2) showed that the activities of SAA, Hp, Cp, CRP, and CK were significantly (p < 0.05) higher in cows with vaginal tumor disease than in healthy control cows. HistopathologyHistological examination of vaginal sections from control cows exhibited typical vaginal tissue architecture. The vaginal mucosa reveals the diestrus stage. During this stage, the vaginal mucosa is composed of several layers, and the superficial layers change from cuboidal to flatten. Cell desquamation is also observed during this stage (Fig. 2a). In contrast, the vaginal mucosa of SCC cows showed the presence of obvious dense squamous epithelial cell invasion (Fig. 2b). The current study revealed moderately well-differentiated clear cell SCC. The tumor is characterized by large malignant cells that settle to form irregular islands with variant keratin pearls (Fig. 2c). The neoplastic cells appeared as large, polyhedral cells with hyperchromatic nuclei and eosinophilic cytoplasm. Prominent nucleoli (Fig. 2e), granular, irregular chromatin, and koilocytic (Fig. 2d) were also observed. Atypical mitosis was also noted (Fig. 2f) was noted. ImmunohistochemistryImmunohistochemical detection of vimentin, S100 and α-SMA in the VSCCG showed marked and very strong positive reactions in fine homogenous brown patches and variable degree of its localization in the cytoplasm compared with the control group (Fig. 3). DiscussionOne of the most deadly cancers, SCC is thought to be the second most prevalent type in humans. It is usually linked to papillomaviruses and has been reported in a variety of animal species (Bernard et al., 2010). Environmental factors, such as sun exposure, are the most likely carcinogenic agents for the development of this tumor.
Fig. 2. (a): Photomicrographs of normal vaginal mucosa stained with H&E. (b) & (f) photomicrographs of vaginal SCC stained with H&E. Vaginal mucosa (VM), squamous cell invasion The present macroscopic analysis of the diseased cows indicated that the tumor had spread and caused significant infiltration in the vulva and vagina, which was linked to surface ulceration and purulent infection. Similar findings were reported in a previous study involving sheep, which showed that SCC was observed at the vulvar mucocutaneous interface and that the tumor progressed with widespread infiltration in the vulva and vagina. Additionally, the tumor was associated with purulent infection and surface ulceration (Del Fava et al., 2001). Usually starting on the vulva’s hairless, lighter-colored skin, SCC in the genital area resembles SCC in appearance and behavior when it develops in other places (Foster, 2007). Purulent infections are common in the reproductive tract, particularly after parturition and during the postpartum period. It has been reported that metritis and placental retention are predisposing factors for the incidence of purulent infection in dairy cattle (Amin et al., 2021a). It can be noted in the current study that tumor development in the vagina is associated with purulent exudate, suggesting that tumor affection, particularly in dairy animals that undergo pregnancy and parturition regularly, is a predisposing factor that facilitates purulent infection.
Fig. 3. (a–f) Photomicrographs of cow’s vaginal sections immunostained with vimentin (a, b), S100 (c, d), and α-SMA (e, f); control group (a, c & e) showed negative reaction while SCC-infected cow’s (b, d & f) showed strong positive reaction. (IHC, scale bar=200 μm). It was announced that steroid hormones play a role in the development of some types of cancer. It has been stated that experimental evidence indicates the presence of a link between steroid hormones, human Papillomavirus (HPV), and cervical cancer. Previous studies in humans have revealed that lesions linked to HPV have altered patterns of steroid hormone receptor expression (Gao et al., 1983; Monsonego et al., 1991). Estrogen is one of the most important steroid hormones that play significant roles in female reproductive life. In the current trial, its examination indicated its high concentration in the VSCCG compared with the NCG. Similarly, it was stated that estrogen plays a role in the development of HPV16 in SiHa cervical carcinoma cells as it stimulates the transcription of this type of tumor (Mitrani-Rosenbaum et al., 1989). In an alternative HPV transgenic mouse model, the endogenous viral promoter maintained control of the high-risk HPV18 E6 and E7 oncogenes. Estrogen stimulated the expression of E6 and E7, which are associated with a greater degree of cervical dysplasia (Park et al., 2003). These findings support the theory that estrogen plays a crucial role in genital cancer. Similarly, in the current study, the development of vaginal tumors may depend on an increase in the secretion of steroid hormones such as estrogen and progesterone. Progesterone is one of the most important hormones, and its evaluation indicates the fertility status of animals. The increase referred to physiological conditions such as pregnancy and pathological conditions such as abortion (Amin et al., 2023) and pyometra (Amin et al., 2021b), while the decrease referred to cases of failure of reproduction such as inactive ovaries (Amin and Said, 2021). Progesterone detection in the current study revealed its high concentration in VSCCG compared with that in NCG. These findings are similar to those previously reported in cases of cows with luteoma in which P4 secretion was high due to the presence of tumors that caused the cows to exhibit anestrosis (Grunert, 1999). It has been reported that P4 plays a role in the development of tumors resulting from papillomavirus. Many studies have indicated that P4 receptors regulate the expression of the HPV by triggering the viral promoter found in the body and assisting high-risk HPV in undergoing cellular transformations (Gloss et al., 1987; Pater et al., 1988; Pater et al., 1994). The pituitary gland produces an important glycoprotein hormone known as FSH. FSH production and/or its signaling pathways are disrupted in several reproductive diseases. In the current study, FSH and LH concentrations were significantly increased in diseased cows compared with normal healthy cows. Several theories have been proposed to explain the causes of cancer that may develop in the reproductive system. The gonadotropin hypothesis is an important one. Numerous transgenic or knockout animal models that display hypergonadotropism with high levels of circulating FSH and LH comparable to the postmenopausal state in women have been found to develop ovarian cancers (Risma et al., 1995; Keri et al., 2000). In addition, increasing evidence indicates that the hormonal environment is responsible for the development and progression of ovarian surface epithelium and ovarian epithelial cancer cells. Granulosa cell tumors (GCTs) have been shown to grow in transgenic mice with persistently increased LH, an analog of which is frequently utilized in fertility regimens (Keri et al., 2000). Therefore, a later study indicated that gonadotropin may be involved in the pathogenesis of GCT. In our study, the significant increase in FSH and LH levels in the affected patients could be attributed similarly to this gonadotropin hypothesis. In the context of clinical investigations, elevated levels of APPs in patients with various malignancies could function as prognostic or diagnostic markers. It is interesting to note that a variety of cancer types have the ability to express APPs, which may have an impact on metastasis, treatment resistance, and cancer progression (Orr et al., 2011). Prior research documented the emergence of the APP response in a variety of tumor types, including human breast cancer and canine mammary cancers (Szczubiał et al., 2004; Machado et al., 2015). In certain oncologic diseases, APPs have also been shown to be clinically useful biomarkers (Selting et al., 2000; Tamamoto et al., 2013). To the best of our knowledge, this is the first study to thoroughly assess the APP responses of VSCC. It is interesting to note that the findings of this study, regarding the significantly elevated Hp in VSCC-affected cows compared to controls, may be the first to address Hp as a biomarker for the condition. Hp is an important factor in the pathophysiology of cancer (Naryzhny and Legina, 2021) and serves as a prognostic biomarker for non-small-cell lung cancer (Arredouani et al., 2003; Tai et al., 2017). The main role of Hp is to bind free hemoglobin that has been liberated from erythrocytes, thereby preventing its oxidative activity (Yang et al., 2003). Moreover, the importance of Hp as a clinically valuable metric for assessing the frequency and intensity of inflammatory reactions in cattle with diverse illnesses has been previously demonstrated (Eckersall, 2000a). A previous study on acute puerperal metritis reported that Hp concentrations were significantly higher in puerperal metritis cows than in healthy cows (Chan et al., 2010). Furthermore, the data obtained in recent years suggest that APPs may be used as early predictors or risk factors for some diseases, such as metritis. Cows with Hp concentrations >1 g/l on day 3 postpartum were 6.7 times more likely to develop mild or severe metritis (Huzzey et al., 2009). In the current study, SAA levels were significantly increased in VSCC-diseased cows compared with normal control cows. SAA is an apolipoprotein that functions as a chemoattractant, interferes with the transport of high-density lipoproteins and cholesterol, aids in the detoxification of endotoxins, and prevents the growth of lymphocytes and endothelial cells (Kisilevsky and Manley, 2012). SAA has a high diagnostic value in ruminants and can be used to identify subclinical diseases as well as to evaluate the herd’s overall health (Eckersall, 2000b). This was confirmed by previous studies reporting that one of the important predictive biomarkers of solid tumors is SAA. It was discovered to be raised in melanoma, renal cancer, gastric cancer, colorectal cancer, and neuroblastoma (Cho et al., 2010; Urieli-Shoval et al., 2010). The increase in the level of SAA in the VSCC cows in the current study indicates the poor prognosis of this disease and indicates that these cows may suffer from inflammation along with tumor presence. Several studies have demonstrated an intimate connection between oxidative stress, carcinogenesis, and chronic inflammation (Cruz-Gregorio et al., 2018). One potential promoter of carcinogenesis could be a component that can initiate and maintain an inflammatory process that involves activation of several cell types and an increase in the formation of reactive oxygen species (Reuter et al., 2010). Thus, persistent inflammation can aid in the development of cancer, even as a type of host defense against a pathogen (Ben-Baruch, 2006). Furthermore, according to recent data, the ability of cancer cells to create SAA increases their resistance to T-cell immunity by inducing immunosuppressive granulocytes (De Santo et al., 2010; Greten et al., 2011). These findings indicate that SAA levels can be used as a diagnostic marker for VSCC. CRP has a vital function in the pathophysiology of malignant tumors, such as colorectal and rectal cancer. In addition, variations in CRP concentration are influenced by some factors, such as the tumor’s size and the stage of the neoplastic process (Heikkilä et al., 2007). CRP concentration was found to be significantly increased in VSCCG compared with normal control in the present trials. It has been demonstrated that CRP is a solid tumor prognostic marker. Increased tumor levels were discovered in melanoma, neuroblastoma, colorectal, esophagus, ovarian, stomach, pancreatic, and renal cancers (Shrotriya et al., 2018; Sproston and Ashworth, 2018; Yang et al., 2019). This indicates that CRP can be used as a prognostic marker for VSCC. The increase in CRP levels in the VSCC cows in the current study indicates a poor prognosis of this disease. It has been shown that estrogens have anti-inflammatory properties, as evidenced by a decrease in the expression of cytokines and a reduction in the adhesion of molecules (Straub, 2007). It has been reported that endogenous E2 release during the follicular phase lowers CRP levels in women (Gaskins et al., 2012). However, in the current study, VSCCG was found to have high levels of both E2 and CRP, indicating that cancer cells are responsible for disturbing steroid hormone secretion and the anti-inflammatory effect of E2. Among the APPs that can scavenge toxins and free oxygen radicals generated during inflammation and protect the host from tissue injury, Cp (Cousins and Swerdel, 1985). As a measure of the wellbeing and health of animals, Cp was tested. Numerous studies conducted on cattle have suggested that the swine grazer can be used as a diagnostic tool for a wide range of medical disorders (Sheldon et al., 2002; Szczubiał et al., 2008). In a study on mastitis, the authors illustrated that a reliable indicator of early mastitis cases was Cp (Chassagne et al., 1998). In our study, evaluation of Cp revealed a significant increase in its levels in the group of cows with VSCC compared with the control group. An increase in Cp levels may occur as a method of protection against cellular changes that occur during tumor. However, the increase in its use in the present study indicates that it can be used as a biomarker of VSCC. Ceruloplasmin has been considered a potential cancer biomarker because it is increased in hepatocellular carcinoma, breast cancer, cervical cancer, and bile duct cancer (Ferrin et al., 2015; Han et al., 2017). CK is considered a muscle-specific enzyme. Therefore, muscle disorders can be diagnosed by the biochemical assessment of CK (Abd-Elhamid et al., 2025) and aspartate transaminase activity. There are relatively few studies on CK in cows. Mounting data suggest that blood CK levels could be a useful marker for predicting the onset and course of several malignancies, such as breast cancer (Pan et al., 2013). In the current study, the level of CK was significantly increased in VSCCG compared with NCG. Both spontaneous and chemically produced animal cancers have been observed to contain high levels of CK (Shatton et al., 1979). Furthermore, patients with illnesses such as brain injuries, severe shock syndrome, hypothermia, and cancer have occasionally had their CK-MB activity significantly exaggerated (Li et al., 2023). The majority of CK-MB is found in cardiac muscle, where it accounts for 25%–46% of the total CK activity in the myocardium. Smaller levels of skeletal muscle are observed (Serdar et al., 2005). When either cardiogenic or noncardiogenic tissue is damaged, a significant number of muscle enzymes are released into the bloodstream, which causes the serum CK-MB level to rise rapidly. Therefore, a significant increase in CK-MB levels in serum or plasma is an essential diagnostic tool for coronary syndromes, particularly acute myocardial infarction (Kodatsch et al., 2001). The increased level of CK in the present trial indicates that tumor-infected cows are predisposed to coronary syndrome, which exposes cows to sudden death. SCC of the vagina is the most common type of vaginal cancer and can spread to other organs (Mitchell et al., 2007). The present study revealed well-differentiated clear cell SCC, which is characterized by the differentiation of keratinocytes. Depending on the tumor’s development, neoplastic cells create varying amounts of keratin, the keratinocytes that appear as a result of hydropic swelling. The neoplastic cells appeared to invade epithelial cells in the underlying tissue beyond the basement membrane. Keliocytosis and abnormal mitotic figures with irregular or abnormal divisions are often observed. These results are in line with previous research that mentioned similar outcomes (Freedberg et al., 2003; Khodakaram-Tafti et al., 2013). A number of characteristics, including breed, extended exposure to UV light, absence of pigment in the epidermis at tumor locations, and extremely sparse or no hair at the afflicted areas, are linked to the formation of the SCC (Foster, 2007). Positive immunohistochemical reaction of some molecules is used as an indicator for tumor malignancy, such as vimentin, S100, and α-SMA. In the current study strong positive reaction was observed for vimentin, S100, and α-SMA in the VSCCG compared to NCG. In agreement with our study, increased vimentin expression was reported in various cancers, such as prostate cancer, breast cancer, gastrointestinal tumors, lung cancer, malignant melanoma, cervical cancer, and other types of cancer (Satelli and Li, 2011). It has been shown that one useful indication for the incidence of metastases in cancers generated from epithelial cells is vimentin, which is the standard marker of epithelial–mesenchymal transition (EMT) (Richardson et al., 2018). This indicates that evaluating the vimentin expression pattern in normal and cancer tissues is important for cancer diagnosis and prognosis. Recent research has demonstrated an association between S100 proteins and various types of cancer, including roles in carcinogenesis, cancer development, and soft tissue cancers (Kuberappa et al., 2016). Therefore, S100 protein is a useful marker in the evaluation of soft tissue tumors (Hu et al., 2023). α-SMA is an EMT marker. The isoform of actin is positive in myofibroblasts (El-Kammar et al., 2019). Elevation of α-SMA expression could indicate neoplasm propensity to progress. Such this in the case of ovarian cancer (Parikh et al., 2014). Vimentin, together with S100 and α-SMA, are the markers for the EMT process. One process thought to be involved in the transformation of normal cells into malignant cells is the EMT (Kalluri and Weinberg, 2009; Jin et al., 2010), which has been shown to indicate that the VSCC is malignant and should undergo metastasis. The prognosis of VSCC is very bad in the current study. This bad prognosis originated from sudden death that occurred in the affected cases, positive expression of vimetin, S100, and actin smooth muscle which evidenced the malignancy of the tumor. Although the tumor is located in the genital organs, it can cause animal death and subsequently financial loss. A similar result was mentioned previously in a study of hemangiosarcoma in calves (Badylak, 1983). Although the hemangiosarcoma type was congenital, it was capable of causing death in the infected calves. Older age, squamous hyperplasia, lichen sclerosus, HPV infection, and immunocompromised status are recognized risk factors (Dohopolski et al., 2019; Weinberg and Gomez-Martinez, 2019). The overall VSCC recurrence rate after the primary treatment was 30% (Ragupathy et al., 2016). Although there is variability in recurrence statistics, research has suggested that tumor size, margin status, lymph node involvement, precursor lesions, and tumor depth of invasion are all related to the disease (Ragupathy et al., 2016; Te Grootenhuis et al., 2019). ConclusionThe present study revealed that cases of vulvar and VSCC are associated with reproductive hormone disturbance and APP production. Disturbance in the secretion of steroid hormones (E2 and P4) and gonadotropins (FSH and LH) may play a role in the fertility failure of such cases and may predispose to its development. Increased APPs (SAA, Hp, and Cp) production was shown to be a useful biomarker for the prognosis and/or diagnosis of VSCC in dairy cows. Furthermore, histopathology and immunohistochemical analysis revealed a positive expression of vimentin, S100 and α-SMA in the tissues of diseased cows, which suggested tumor metastization. Understanding the physiopathological role of these biomarkers (reproductive hormonal profile and APP) in this devastating disease is an important and understudied area that could help inform clinical screening processes and improve drug therapies, thus improving the survival rate for such cases of dangerous cancer. AcknowledgmentsNone. Conflict of interestThere are no conflicts of interest to declare. FundingThis manuscript has not received any funding. Authors’ contributionConceptualization: YAA; Methodology: YAA, SSF, SAM, AHMN; Validation: YAA, RAA, MAF, AHM; Formal Analysis: YAA, RAA, MAF, AHM; Investigation: YAA, SSF, SAM, AHMN; Data Curation: YAA, SAM, AHMN; Supervision: YAA; Project Administration: YAA; Funding Acquisition: YAA, SSF, RAA, MAF, SAM, AHM, AHMN; Writing-Original Draft Preparation: YAA, SAM, AHMN; Writing-Review and Editing: YAA; All authors have read and agreed to the publication of the manuscript. Data availabilityThis article includes all the data generated or evaluated during the research. ReferencesAbd-Elhamid, T.H., Ismail, N.S., Amin, Y.A., Meligy, F.Y., Galal, A.T., Abdel-Ziz, H.A.M. and Ahmed, M.A.E.B. 2025. Treatment of corticosteroid-induced myopathy through Filgrastim induced endogenous stem cells mobilization in male albino rats. Cells Tissues Organs 11, 1–36. Agnew, D.W. and MacLachlan, N.J. 2016. Tumors of the genital systems. In Tumors in domestic animals. Ed., Meuten, D.J. Hoboken, NJ: Wiley, p: 689–722. Alvarado, A., Gil da Costa, R.M., Faustino-Rocha, A.I., Ferreira, R., Lopes, C., Oliveira, P.A. and Colaço, B. 2017. Effects of exercise training on breast cancer metastasis in a rat model. Int. J. Exp. Pathol. 98, 40–46. Amin, Y.A., Ali, R.A., Fouad, S.S. and Ibrahim, R.M. 2021b. The deleterious effect of postpartum pyometra on the reproductive indices, the metabolic profile, and oxidant/antioxidant parameters of dairy cows. Vet. World. 14, 329. Amin, Y.A., Omran, G.A., Fouad, S.S., Fawy, M.A., Ibrahim, R.M., Khalifa, F.A. and Ali, R.A. 2023. Abortion associated with postpartum opportunistic bacterial invasion reduces fertility and induces disturbances of reproductive hormones, hematological profile, and oxidant/antioxidant profiles in dairy cows. J. Adv. Vet. Anim. Res. 10, 654. Amin, Y. and Said, A. 2021. The addition of chitosan to GnRH analog induces ovarian resumption and improves conception rates in buffaloes. Trop. Anim. Sci. J. 44, 1–9. Amin, Y., Zakaria, A. and Hasan, A. 2021a. The efficacy of treatment of retained placenta with chlortetracycline and oxytetracycline through local intrauterine route in dairy cows. J. Anim. Health Prod. 9, 100–106. Andreis, I., Batinić, D., Čulo, F., Grčević, D., Lukinivić-Škudar, V., Marušić, M., Taradi, M. and Višnjić, D. 2010. Imunologija, 7th izdanje. Zagreb, Croatia: Medicinska naklada, pp: 167–168. Arredouani, M., Matthijs, P., Van Hoeyveld, E., Kasran, A., Baumann, H., Ceuppens, J. and Stevens, E. 2003. Haptoglobin directly affects T cells and suppresses T helper cell type 2 cytokine release. Immunology 108, 144–151. Badylak, S. 1983. Congenital multifocal hemangiosarcoma in a stillborn calf. Los Angeles, CA: SAGE Publications Sage CA, pp: 245–247. Ben-Baruch, A. 2006. Inflammation-associated immune suppression in cancer: the roles played by cytokines, chemokines and additional mediators. Semin. Cancer Biol. 16(1), 38–52. Bernard, H.-U., Burk, R.D., Chen, Z., Van Doorslaer, K., Zur Hausen, H. and De Villiers, E.-M. 2010. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology 401, 70–79. Baumgärtner, W. and Gruber, A. 2020. Tumorpathologie [Tumor pathology]. In Allgemeine Pathologie für die Tiermedizin [General pathology for veterinary medicine], 3rd ed. Stuttgart, Germany: Thieme, pp: 222–269. Chan, J.P.W., Chang, C.C., Hsu, W.L., Liu, W.B. and Chen, T.H. 2010. Association of increased serum acute-phase protein concentrations with reproductive performance in dairy cows with postpartum metritis. Vet. Clin. Pathol. 39, 72–78. Chassagne, M., Barnouin, J. and Chacornac, J.-P. 1998. Biological predictors for early clinical mastitis occurrence in Holstein cows under field conditions in France. Prev. Vet. Med. 35, 29–38. Cho, W.C.S., Yip, T., Cheng, W. and Au, J.S. 2010. Serum amyloid A is elevated in the serum of lung cancer patients with poor prognosis. Br. J. Cancer 102, 1731–1735. Cousins, R.J. and Swerdel, M.R. 1985. Ceruloplasmin and metallothionein induction by zinc and 13-cis-retinoic acid in rats with adjuvant inflammation. Proc. Soc. Exp. Biol. Med. 179, 168–172. Cruz-Gregorio, A., Manzo-Merino, J. and Lizano, M. 2018. Cellular redox, cancer and human papillomavirus. Virus Res. 246, 35–45. Del Fava, C., Verissimo, C., Rodrigues, C., Cunha, E., Ueda, M., Maiorka, P. and Angelino, D. 2001. Occurrence of squamous cell carcinoma in sheep from a farm in Sao Paulo state, Brazil. Arq. Inst. Biol. Sao Paulo 68, 35–40. De Santo, C., Arscott, R., Booth, S., Karydis, I., Jones, M., Asher, R., Salio, M., Middleton, M. and Cerundolo, V. 2010. Invariant NKT cells modulate the suppressive activity of IL-10-secreting neutrophils differentiated with serum amyloid A. Nat. Immunol. 11, 1039–1046. De Vries, A. and Marcondes, M.I. 2020. Overview of factors affecting productive lifespan of dairy cows. Animal 14, s155–s164. Dohopolski, M.J., Horne, Z.D., Pradhan, D., Bhargava, R., Edwards, R.P., Kelley, J.L., Comerci, J.T., Olawaiye, A.B., Courtney-Brooks, M. and Berger, J.L. 2019. The prognostic significance of p16 status in patients with vulvar cancer treated with vulvectomy and adjuvant radiation. Int. J. Radiat. Oncol. Biol. Phys. 103, 152–160. Eckersall, P. 2000a. Acute phase proteins as markers of infection and inflammation: monitoring animal health, animal welfare and food safety. Ir. Vet. J. 53(6), 307–311. Eckersall, P. 2000b. Recent advances and future prospects for the use of acute phase proteins as markers of disease in animals. Rev. Med. Vet. 151, 577–584. Eckersall, P. and Bell, R. 2010. Acute phase proteins: biomarkers of infection and inflammation in veterinary medicine. Vet. J. 185, 23–27. Eckersall, P., Saini, P. and McComb, C. 1996. The acute phase response of acid soluble glycoprotein, α1-acid glycoprotein, ceruloplasmin, haptoglobin and C-reactive protein, in the pig. Vet. Immunol. Immunopathol. 51, 377–385. El-Kammar, H., Afifi, N.S. and AbdulKhalik, D. 2019. Role of alpha smooth muscle actin in oral squamous cell carcinoma progression. Egypt. Dent. J. 65, 2387–2396. Ferrin, G., Rodriguez-Peralvarez, M., Aguilar-Melero, P., Ranchal, I., Llamoza, C., Linares, C.I., Gonzalez-Rubio, S., Muntane, J., Briceno, J. and Lopez-Cillero, P. 2015. Plasma protein biomarkers of hepatocellular carcinoma in HCV-infected alcoholic patients with cirrhosis. PLoS One 10, e0118527. Foster, A.R. 2007. Female reproductive system. In Pathologic basis of veterinary disease, 4th ed. Eds., McGavin, D. and James Zachary, J.F. St. Louis, MO: Mosby Elsevier, pp: 1306–1307. Freedberg, I.-M., Eisen, A.Z., Austen, K.F., Goldsmith, L.A. and Katz, S.I. 2003. Fitzpatrick’s dermatology in general medicine, 6th ed. New York, NY: McGraw-Hill, p: 743. Gao, Y.L., Twiggs, L.B., Leung, B.S., Winston, C., Potish, R.A., Okagaki, T., Adcock, L.L. and Prem, K.A. 1983. Cytoplasmic estrogen and progesterone receptors in primary cervical carcinoma: clinical and histopathologic correlates. Am. J. Obstet. Gynecol. 146, 299–306. Gaskins, A.J., Wilchesky, M., Mumford, S.L., Whitcomb, B.W., Browne, R.W., Wactawski-Wende, J., Perkins, N.J. and Schisterman, E.F. 2012. Endogenous reproductive hormones and C-reactive protein across the menstrual cycle: the BioCycle Study. Am. J. Epidemiol. 175, 423–431. Gloss, B., Bernard, H., Seedorf, K. and Klock, G. 1987. The upstream regulatory region of the human papilloma virus-16 contains an E2 protein-independent enhancer which is specific for cervical carcinoma cells and regulated by glucocorticoid hormones. EMBO J. 6, 3735–3743. Greten, T.F., Manns, M.P. and Korangy, F. 2011. Myeloid derived suppressor cells in human diseases. Int. Immunopharmacol. 11, 802–807. Grunert, E. 1999. Ovarielle dysfunktionen, fertility disorders in female cattle. Fertilitätsstörungen beim weiblichen Rind 3, 111–142. Gruys, E., Obwolo, M. and Toussaint, M. 1994. Diagnostic significance of the major acute phase proteins in veterinary clinical chemistry: a review. Vet. Bull. 64(11), 1009–1018. Han, I.W., Jang, J.-Y., Kwon, W., Park, T., Kim, Y., Lee, K.B. and Kim, S.-W. 2017. Ceruloplasmin as a prognostic marker in patients with bile duct cancer. Oncotarget 8, 29028. Harbeck, N., Penault-Llorca, F., Cortes, J., Gnant, M., Houssami, N., Poortmans, P., Ruddy, K., Tsang, J. and Cardoso, F. 2019. Breast cancer. Nat. Rev. Dis. Primers. 5, 66. Heikkilä, K., Ebrahim, S. and Lawlor, D.A. 2007. A systematic review of the association between circulating concentrations of C reactive protein and cancer. J. Epidemiol. Community Health. 61, 824–833. Henry, R.J. 1974. Clinical chemistry: principles and technics, 2nd ed. Medical Dept., Harper & Row, p: 1629. Hu, Y., Han, Y., He, M., Zhang, Y. and Zou, X. 2023. S100 proteins in head and neck squamous cell carcinoma. Oncol. Lett. 26, 1–12. Huzzey, J., Duffield, T., LeBlanc, S., Veira, D., Weary, D. and Von Keyserlingk, M. 2009. Haptoglobin as an early indicator of metritis. J. Dairy Sci. 92, 621–625. Jin, H., Morohashi, S., Sato, F., Kudo, Y., Akasaka, H., Tsutsumi, S., Ogasawara, H., Miyamoto, K., Wajima, N. and Kawasaki, H. 2010. Vimentin expression of esophageal squamous cell carcinoma and its aggressive potential for lymph node metastasis. Biomed. Res. 31, 105–112. Kalluri, R. and Weinberg, R.A. 2009. The basics of epithelial-mesenchymal transition. J. Clin. Invest. 119, 1420–1428. Keri, R.A., Lozada, K.L., Abdul-Karim, F.W., Nadeau, J.H. and Nilson, J.H. 2000. Luteinizing hormone induction of ovarian tumors: oligogenic differences between mouse strains dictates tumor disposition. Proc. Natl. Acad. Sci. U. S. A. 97, 383–387. Khodakaram-Tafti, A., Motaghypisheh, M. and Shirian, S. 2013. Pathological study of naturally occurring vulvar and vaginal squamous cell carcinoma (SCC) in cattle. Comp. Clin. Pathol. 22, 713–716. Kisilevsky, R. and Manley, P.N. 2012. Acute-phase serum amyloid A: perspectives on its physiological and pathological roles. Amyloid 19, 5–14. Kodatsch, I., Finsterer, J. and Stöllberger, C. 2001. Serum creatine kinase elevation in a medical department. Acta. Med. Austriaca. 28, 11–15. Kuberappa, P.H., Bagalad, B.S., Ananthaneni, A., Kiresur, M.A. and Srinivas, G.V. 2016. Certainty of S100 from physiology to pathology. J. Clin. Diagn. Res. JCDR. 10, ZE10. Labrecque, M.P., Coleman, I.M., Brown, L.G., True, L.D., Kollath, L., Lakely, B., Nguyen, H.M., Yang, Y.C., da Costa, R.M.G. and Kaipainen, A. 2019. Molecular profiling stratifies diverse phenotypes of treatment-refractory metastatic castration-resistant prostate cancer. J. Clin. Invest. 129, 4492–4505. Li, Y., Chen, Y., Shao, B., Liu, J., Hu, R., Zhao, F., Cui, X., Zhao, X. and Wang, Y. 2023. Evaluation of creatine kinase (CK)-MB to total CK ratio as a diagnostic biomarker for primary tumors and metastasis screening. Pract. Lab. Med. 37, e00336. Machado, V.S., Crivellenti, L.Z., Bottari, N.B., Tonin, A.A., Pelinson, L.P., Borin-Crivellenti, S., Santana, A.E., Torbitz, V.D., Moresco, R.N. and Duarte, T. 2015. Oxidative stress and inflammatory response biomarkers in dogs with mammary carcinoma. Pathol. Res. Pract. 211, 677–681. Makimura, S. and Suzuki, N. 1982. Quantitative determination of bovine serum Haptoglobin and its elevation in some inflammatory diseases. Japanese J. Vet. Sci. 44, 15–21. Mitchell, R.S., Kumar, V., Abbas, A.K. and Fausto, N. 2007. Robbins basic pathology. Philadelphia, PA: Saunders, vol. 8, pp: 72–79. Mitrani-Rosenbaum, S., Tsvieli, R. and Tur-Kaspa, R., 1989. Oestrogen stimulates differential transcription of human papillomavirus type 16 in SiHa cervical carcinoma cells. J. Gen. Virol. 70, 2227–2232. Monsonego, J., Magdelenat, H., Catalan, F., Coscas, Y., Zerat, L. and Sastre, X. 1991. Estrogen and progesterone receptors in cervical human papillomavirus related lesions. Int. J. Cancer. 48, 533–539. Naryzhny, S. and Legina, O. 2021. Haptoglobin as a biomarker. Biomed. Khim. 67, 105–118. Orr, W.S., Malkas, L.H., Hickey, R.J., Sandoval, J.A. and Veas, F. 2011. Acute phase proteins as cancer biomarkers. In Acute phase proteins as early non-specific biomarkers of human and veterinary diseases. Ed., Veas, F, pp: 408. Pan, H., Xia, K., Zhou, W., Xue, J., Liang, X., Cheng, L., Wu, N., Liang, M., Wu, D. and Ling, L. 2013. Low serum creatine kinase levels in breast cancer patients: a case-control study. PLoS One 8, e62112. Parikh, J.G., Kulkarni, A. and Johns, C. 2014. αsmooth muscle actinpositive fibroblasts correlate with poor survival in hepatocellular carcinoma. Oncol. Lett. 7, 573–575. Park, J.S., Rhyu, J.W., Kim, C.J., Kim, H.S., Lee, S.Y., Kwon, Y.I., Namkoong, S.E., Sin, H.S. and Um, S.J. 2003. Neoplastic change of squamo-columnar junction in uterine cervix and vaginal epithelium by exogenous estrogen in hpv-18 URR E6/E7 transgenic mice*.Gynecol. Oncol. 89, 360–368. Pater, M.M., Hughes, G.A., Hyslop, D.E., Nakshatri, H. and Pater, A. 1988. Glucocorticoid-dependent oncogenic transformation by type 16 but not type 11 human papilloma virus DNA. Nature 335, 832–835. Pater, M.M., Mittal, R. and Pater, A. 1994. Role of steroid hormones in potentiating transformation of cervical cells by human papillomaviruses. Trends Microbiol. 2, 229–235. Ragupathy, K., Grandidge, L., Strelley, K., Wang, H. and Tidy, J. 2016. Early and late vulval cancer recurrences: are they different? J. Obstet. Gynaecol. 36, 518–521. Rebar, R.W., Erickson, G.F. and Yen, S.S. 1982. Idiopathic premature ovarian failure: clinical and endocrine characteristics. Fertil Steril. 37, 35–41. Reuter, S., Gupta, S.C., Chaturvedi, M.M. and Aggarwal, B.B. 2010. Oxidative stress, inflammation, and cancer: how are they linked? Free Radic. Biol. Med. 49, 1603–1616. Richardson, A.M., Havel, L.S., Koyen, A.E., Konen, J.M., Shupe, J., Wiles IV, W., Martin, W.D., Grossniklaus, H.E., Sica, G. and Gilbert-Ross, M. 2018. Vimentin is required for lung adenocarcinoma metastasis via heterotypic tumor cell–cancer-associated fibroblast interactions during collective invasion. Clin. Cancer Res. 24, 420–432. Risma, K.A., Clay, C.M., Nett, T.M., Wagner, T., Yun, J. and Nilson, J.H. 1995. Targeted overexpression of luteinizing hormone in transgenic mice leads to infertility, polycystic ovaries, and ovarian tumors. Proc. Natl. Acad. Sci. U. S. A. 92, 1322–1326. Rose, M.P. 1998. Follicle stimulating hormone: international standards and reference preparations for the calibration of immunoassays and bioassays. Clin. Chim. Acta. 273, 103–117. Satelli, A. and Li, S. 2011. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell. Mol. Life Sci. 68, 3033–3046. Selting, K., Ogilvie, G., Lana, S., Fettman, M., Mitchener, K., Hansen, R., Richardson, K., Walton, J. and Scherk, M. 2000. Serum alpha 1-acid glycoprotein concentrations in healthy and tumor-bearing cats. J. Vet. Intern. Med. 14, 503–506. Serdar, M.A., Tokgoz, S., Metinyurt, G., Tapan, S., Erinç, K., Haşimi, A., Kenar, L., Bilgİ, C. and Kutluay, T. 2005. Effect of macro-creatine kinase and increased creatine kinase BB on the rapid diagnosis of patients with suspected acute myocardial infarction in the emergency department. Mil. Med. 170, 648–652. Shatton, J.B., Morris, H.P. and Weinhouse, S. 1979. Creatine kinase activity and isozyme composition in normal tissues and neoplasms of rats and mice. Cancer Res. 39, 492–501. Sheldon, I., Noakes, D., Rycroft, A. and Dobson, H. 2002. Effect of postpartum manual examination of the vagina on uterine bacterial contamination in cows. Vet. Rec. 151, 531–534. Shrotriya, S., Walsh, D., Nowacki, A.S., Lorton, C., Aktas, A., Hullihen, B., Benanni-Baiti, N., Hauser, K., Ayvaz, S. and Estfan, B. 2018. Serum C-reactive protein is an important and powerful prognostic biomarker in most adult solid tumors. PLoS One 13, e0202555. Sproston, N.R. and Ashworth, J.J. 2018. Role of C-reactive protein at sites of inflammation and infection. Front. Immunol. 9, 754. Straub, R.H. 2007. The complex role of estrogens in inflammation. Endocr. Rev. 28, 521–574. Szczubiał, M., Dąbrowski, R., Kankofer, M., Bochniarz, M. and Albera, E. 2008. Concentration of serum amyloid A and activity of ceruloplasmin in milk from cows with clinical and subclinical mastitis. Bull. Vet. Inst. Pulawy. 52, 391–395. Szczubiał, M., Kankofer, M., Łopuszyński, W., Dąbrowski, R. and Lipko, J. 2004. Oxidative stress parameters in bitches with mammary gland tumours. J. Vet. Med. Ser. A. 51, 336–340. Tai, C.-S., Lin, Y.-R., Teng, T.-H., Lin, P.-Y., Tu, S.-J., Chou, C.-H., Huang, Y.-R., Huang, W.-C., Weng, S.-L. and Huang, H.-D. 2017. Haptoglobin expression correlates with tumor differentiation and five-year overall survival rate in hepatocellular carcinoma. PLoS One 12, e0171269. Tamamoto, T., Ohno, K., Takahashi, M., Nakashima, K., Fujino, Y. and Tsujimoto, H. 2013. Serum amyloid A as a prognostic marker in cats with various diseases. J. Vet. Diagn. Invest. 25, 428–432. Te Grootenhuis, N.C., Pouwer, A., de Bock, G.H., Hollema, H., Bulten, J., van der Zee, A.G., de Hullu, J.A. and Oonk, M.H. 2019. Margin status revisited in vulvar squamous cell carcinoma. Gynecol. Oncol. 154, 266–275. Tothova, C., Nagy, O. and Kovac, G. 2014. Acute phase proteins and their use in the diagnosis of diseases in ruminants: a review. Vet. Med. 59, 163–180. Urieli-Shoval, S., Finci-Yeheskel, Z., Dishon, S., Galinsky, D., Linke, R.P., Ariel, I., Levin, M., Ben-Shachar, I. and Prus, D. 2010. Expression of serum amyloid a in human ovarian epithelial tumors: implication for a role in ovarian tumorigenesis. J. Histochem. Cytochem. 58, 1015–1023. Weinberg, D. and Gomez-Martinez, R.A. 2019. Vulvar cancer. Amsterdam, The Netherlands: Elsevier Inc vol. 46, pp: 125–135. Yang, F., Haile, D.J., Berger, F.G., Herbert, D.C., Van Beveren, E. and Ghio, A.J. 2003. Haptoglobin reduces lung injury associated with exposure to blood. Am. J. Physiol. Lung Cell Mol. Physiol. 284, L402–L409. Yang, J.-R., Xu, J.-Y., Chen, G.-C., Yu, N., Yang, J., Zeng, D.-X., Gu, M.-J., Li, D.-P., Zhang, Y.-S. and Qin, L.-Q. 2019. Post-diagnostic C-reactive protein and albumin predict survival in Chinese patients with non-small cell lung cancer: a prospective cohort study. Sci. Rep. 9, 8143. | ||
| How to Cite this Article |
| Pubmed Style Amin YA, Fouad SS, Ali RA, Fawy MA, Mobarak SA, Mahmoud AH, Nour AHM. Fertility failure of vulvar and vaginal squamous cell carcinoma in dairy cows associated with reproductive hormonal disturbance, vulvar and vaginal hyperplasia, acute phase protein production, and histopathological changes. Open Vet. J.. 2025; 15(5): 2238-2250. doi:10.5455/OVJ.2025.v15.i5.41 Web Style Amin YA, Fouad SS, Ali RA, Fawy MA, Mobarak SA, Mahmoud AH, Nour AHM. Fertility failure of vulvar and vaginal squamous cell carcinoma in dairy cows associated with reproductive hormonal disturbance, vulvar and vaginal hyperplasia, acute phase protein production, and histopathological changes. https://www.openveterinaryjournal.com/?mno=248812 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i5.41 AMA (American Medical Association) Style Amin YA, Fouad SS, Ali RA, Fawy MA, Mobarak SA, Mahmoud AH, Nour AHM. Fertility failure of vulvar and vaginal squamous cell carcinoma in dairy cows associated with reproductive hormonal disturbance, vulvar and vaginal hyperplasia, acute phase protein production, and histopathological changes. Open Vet. J.. 2025; 15(5): 2238-2250. doi:10.5455/OVJ.2025.v15.i5.41 Vancouver/ICMJE Style Amin YA, Fouad SS, Ali RA, Fawy MA, Mobarak SA, Mahmoud AH, Nour AHM. Fertility failure of vulvar and vaginal squamous cell carcinoma in dairy cows associated with reproductive hormonal disturbance, vulvar and vaginal hyperplasia, acute phase protein production, and histopathological changes. Open Vet. J.. (2025), [cited January 25, 2026]; 15(5): 2238-2250. doi:10.5455/OVJ.2025.v15.i5.41 Harvard Style Amin, Y. A., Fouad, . S. S., Ali, . R. A., Fawy, . M. A., Mobarak, . S. A., Mahmoud, . A. H. & Nour, . A. H. M. (2025) Fertility failure of vulvar and vaginal squamous cell carcinoma in dairy cows associated with reproductive hormonal disturbance, vulvar and vaginal hyperplasia, acute phase protein production, and histopathological changes. Open Vet. J., 15 (5), 2238-2250. doi:10.5455/OVJ.2025.v15.i5.41 Turabian Style Amin, Yahia A., Samer S. Fouad, Rana A. Ali, Mariam A. Fawy, Seham A. Mobarak, Abdellah Hassan Mahmoud, and Amna H. M. Nour. 2025. Fertility failure of vulvar and vaginal squamous cell carcinoma in dairy cows associated with reproductive hormonal disturbance, vulvar and vaginal hyperplasia, acute phase protein production, and histopathological changes. Open Veterinary Journal, 15 (5), 2238-2250. doi:10.5455/OVJ.2025.v15.i5.41 Chicago Style Amin, Yahia A., Samer S. Fouad, Rana A. Ali, Mariam A. Fawy, Seham A. Mobarak, Abdellah Hassan Mahmoud, and Amna H. M. Nour. "Fertility failure of vulvar and vaginal squamous cell carcinoma in dairy cows associated with reproductive hormonal disturbance, vulvar and vaginal hyperplasia, acute phase protein production, and histopathological changes." Open Veterinary Journal 15 (2025), 2238-2250. doi:10.5455/OVJ.2025.v15.i5.41 MLA (The Modern Language Association) Style Amin, Yahia A., Samer S. Fouad, Rana A. Ali, Mariam A. Fawy, Seham A. Mobarak, Abdellah Hassan Mahmoud, and Amna H. M. Nour. "Fertility failure of vulvar and vaginal squamous cell carcinoma in dairy cows associated with reproductive hormonal disturbance, vulvar and vaginal hyperplasia, acute phase protein production, and histopathological changes." Open Veterinary Journal 15.5 (2025), 2238-2250. Print. doi:10.5455/OVJ.2025.v15.i5.41 APA (American Psychological Association) Style Amin, Y. A., Fouad, . S. S., Ali, . R. A., Fawy, . M. A., Mobarak, . S. A., Mahmoud, . A. H. & Nour, . A. H. M. (2025) Fertility failure of vulvar and vaginal squamous cell carcinoma in dairy cows associated with reproductive hormonal disturbance, vulvar and vaginal hyperplasia, acute phase protein production, and histopathological changes. Open Veterinary Journal, 15 (5), 2238-2250. doi:10.5455/OVJ.2025.v15.i5.41 |





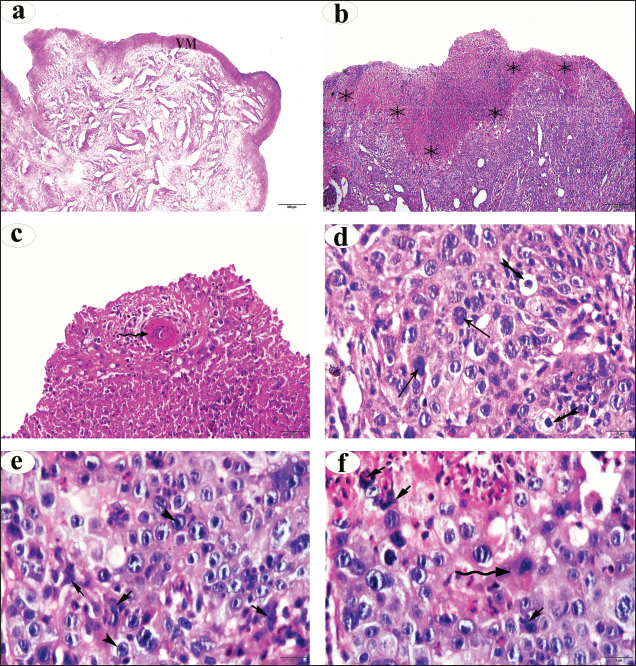
 , keratin pearls
, keratin pearls  , koilocytosis
, koilocytosis  , hyperchromic nuclei (arrow), prominent nucleoli
, hyperchromic nuclei (arrow), prominent nucleoli  , atypical mitosis
, atypical mitosis  .
.