| Research Article | ||
Open Vet. J.. 2025; 15(5): 2218-2229 Open Veterinary Journal, (2025), Vol. 15(5): 2218-2229 Research Article Ameliorative effect of citicoline on cyclophosphamide-induced lung injuryRania Salah1,2*, Gehad El-Sayed1, El-Said El-Sherbini1, Mohamed El-Adl1,21Department of Biochemistry and Molecular Biology, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt 2Department of Basic Veterinary Sciences, Faculty of Veterinary Medicine, Delta University for Science and Technology, Mansoura, Egypt *Corresponding Author: Rania Salah. Department of Biochemistry and Molecular Biology, Faculty of Veterinary Medicine, Mansoura University, Mansoura, Egypt. Email: raniasalah285 [at] gmail.com Submitted: 26/02/2025 Revised: 12/04/2025 Accepted: 19/04/2025 Published: 31/05/2025 © 2025 Open Veterinary Journal
ABSTRACTBackground: Cyclophosphamide (CP) is known to cause pulmonary injury through free radical production and proinflammatory cytokines activation. Lung damage is associated with significant mortality, primarily due to the development of severe inflammation and pulmonary edema. On the other hand, citicoline, a key intermediate in the phosphatidylcholine biosynthesis pathway, has neurovascular protective and reparative properties. Numerous studies have highlighted the potential antioxidant and antiinflammatory effects of this agent in the context of various pathological conditions. Aim: Our study aimed to explore the potential protective effects of citicoline against oxidative stress, inflammation, and tissue damage caused by CP in an experimental model. Methods: Rats were given 200 mg/kg of CP as a single dose, either alone or in combination with citicoline (500 or 250 mg/kg), administered orally once daily for 14 days, beginning 7 days prior to CP administration. On the final day of the experiment, all animals were euthanized, and lung tissues were collected for further analysis. Results: CP administration led to a significant elevation in the lung-to-body weight ratio, inflammatory cell infiltration, and elevated levels of lactate dehydrogenase, total protein, nuclear factor kappa B, Interleukin-1β, NOD-like receptor protein 3, and caspase-1 in bronchoalveolar lavage fluid. Furthermore, CP treatment increased the concentrations of malondialdehyde and nitrate/nitrite and reduced glutathione levels in the lungs. Additionally, mRNA of Interleukin 6 and tumor necrosis factor-α levels were significantly elevated. These biochemical alterations were corroborated by histopathological findings, which revealed significant lung tissue damage. However, treatment with citicoline significantly reduced the pulmonary pathological changes induced by CP. Conclusion: These findings imply that citicoline’s antiinflammatory and antioxidant properties provide protection against CP-induced lung damage. Keywords: Citicoline, Cyclophosphamide, Lung injury, NF-kB, NLRP-3. IntroductionCyclophosphamide (CP) is a common and highly effective alkylating agent. It is used as an antitumor medication in the management of hematologic, ovarian, and breast malignancies (Hassan and Hasary, 2022). Furthermore, it has immunosuppressive properties and is used to treat organ transplant rejection and autoimmune illnesses (Moignet et al., 2014). However, CP-induced pulmonary toxicity could provide a significant barrier to the effective application of this drug (Pugh et al., 2019). CP is known to cause pulmonary injury through free radical production and proinflammatory cytokines activation (Emadi et al., 2009). Its metabolism occurs through the action of P450 enzymes, resulting in the formation of two active metabolites: acrolein and phosphoramide mustard, in the liver. While phosphoramide mustard is the main cause of the therapeutic effects of CP, acrolein is primarily associated with its adverse effects. Because acrolein interferes with the antioxidant defense system, cells produce reactive oxygen species (ROS) (MacAllister et al., 2013). CP has been linked to multiple organ toxicities, including lung injury (Şengül et al., 2017), nephrotoxicity (Seker et al., 2024), hepatotoxicity (Hao et al., 2024), and cardiotoxicity (Akram et al., 2024). Lung toxicity, in particular, is believed to result from direct damage to pneumocytes, causing the generation of ROS, the activation of pro-inflammatory pathways, and the release of pro-inflammatory cytokines (Conte et al., 2022). CP-induced lung injury is distinguished by high levels of oxidative stress markers, for example, malondialdehyde (MDA) and total nitrate/nitrite (NOx), alongside a reduction in antioxidant defenses, including total antioxidant capacity, superoxide dismutase (SOD), reduced glutathione (GSH), and nuclear factor erythroid 2-related factor 2 (Alsemeh and Abdullah, 2022; El-Kashef and Rahim, 2023). Excess ROS generation can lead to membrane lipid peroxidation and activation of intracellular signaling pathways, characterized by elevated thereby encouraging the synthesis of proinflammatory cytokines (Ahmed et al., 2015). Despite extensive research into the structural and functional changes caused by CP toxicity, no effective therapeutic intervention has been established. Citicoline (citi) is a compound utilized in numerous countries as a therapy for neurological diseases, cerebrovascular diseases, and age-related cognitive decline (Secades and Gareri, 2022). Studies have demonstrated that the administration of cytidine-5′-diphosphate-choline and choline can suppress the lipopolysaccharide-induced cyclooxygenase (COX)-2 pathway and reduce prostaglandin production, thereby improving outcomes in sepsis severity and enhancing survival rates in animal models of endotoxemia (Baris et al., 2021). The COX pathway and prostaglandin synthesis contribute to oxidative stress and tissue damage under various inflammatory conditions (Ricciotti and FitzGerald, 2011). Borovikova et al. (2000) identified a neuroimmune mechanism called the anti-inflammatory cholinergic pathway, which modulates cytokine production through vagal nerve stimulation and the subsequent release of acetylcholine (Ach). Proinflammatory cytokine expression is suppressed when the α7 nicotinic acetylcholine receptor (α7 nAChR) on immune cells is activated. This process is followed by the phosphorylation and nuclear translocation of signal transducer and activator of transcription 3 and the activation of Janus kinase 2. At the same time, α7 nAChR activation prevents inhibitory kappa B alpha from degrading, which stops nuclear factor kappa B (NF-kB) from moving to the nucleus and proinflammatory cytokines from being expressed (Mizrachi et al., 2021). Additionally, research has shown that citi treatment reduces lipid peroxidation and nitric oxide (NO) levels while increasing GSH content in the serum of tramadol-treated rats (Abdel-Salam et al., 2019). Based on these results, we speculate that citicoline might shield rats’ lungs from CP-induced damage. The goal of the current study was to investigate the possible ways that citi’s antiinflammatory and antioxidant qualities can reduce CP-induced lung toxicity. Materials and MethodsDrugs and chemicalsCP (Endoxan®) was administered as a single intraperitoneal (i.p.) dose of 200 mg/kg on day 7 to induce lung toxicity, which was supplied in a 1-g vial manufactured by Baxter Oncology GmbH, Germany. Prior to administration, 10 ml of regular saline (0.9%) was used to freshly dissolve the medication (Alsemeh and Abdullah, 2022). The study’s treatment drug, citi, was administered in sterile ampoules (500 mg/4 ml; Somazina, Ferrer International S.A., Spain). AnimalsThroughout the acclimatization and experimental phases, adult male rats were given unfettered access to tap water and regular laboratory food. All animals were handled and cared for in compliance with the National Institutes of Health’s regulations and the moral principles established by Mansoura University’s Research Ethics Committee for the Care of Laboratory Animals. Experimental designThe included rats were randomly divided into 4 groups, with 12 rats each. The treatment protocol was designed as follows:
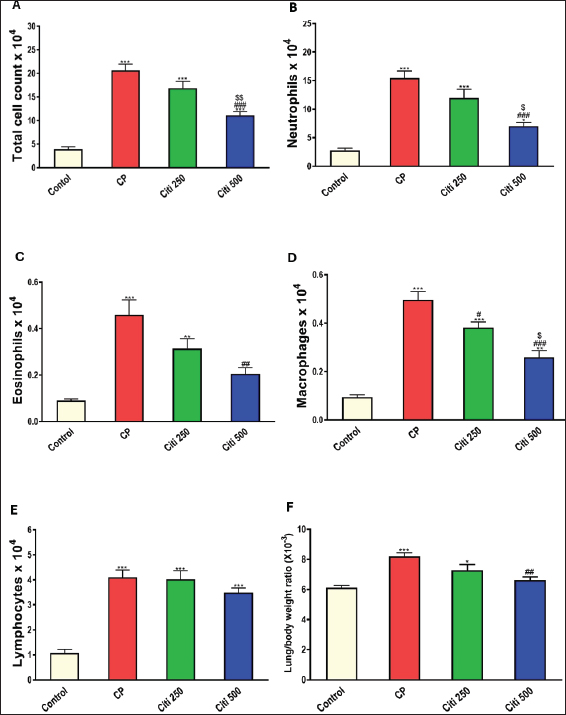
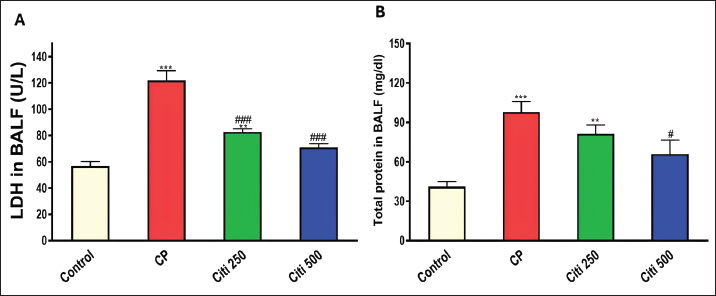
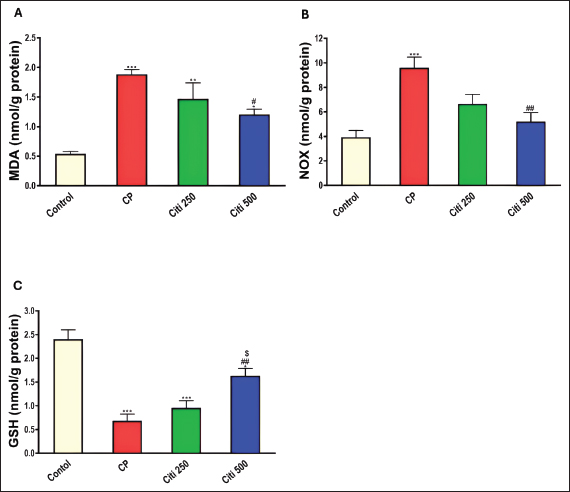
Upon completion of the experiment, the rats were anesthetized using thiopental (120 mg/kg, i.p.) (Shaaban et al., 2014). Lung tissue samples from the first six rats were homogenized (10% w/v) in a 20 mM Tris-HCl buffer containing 1 mM EDTA (pH 7.4) and subsequently centrifuged at 3,000 g for 20 minutes at 4°C. The resulting supernatants from the lung homogenates were stored at −80°C until biochemical assessments. Another portion of the lung tissue was preserved in RNA later® to facilitate quantitative polymerase chain reaction (PCR) analyses of Interleukin 6 (IL-6) and tumor necrosis factor-α (TNF-α). Additionally, the remaining samples were fixed in a 4% neutral–buffered formalin solution for histopathological examinations. Bronchoalveolar lavage was performed on the remaining six rats. The collected bronchoalveolar lavage fluid (BALF) was centrifuged using a chilled centrifuge set at 2,000 rpm for 10 minutes at (4°C). The resulting cell pellet was isolated and utilized for cell count analysis, whereas the BALF supernatant was preserved at (−80°C) for subsequent investigations. Calculating BALF’s total and differential cell countsA hemocytometer was used to measure the total number of cells. The cell-pellets were resuspended in 100 μl of 0.9% saline, centrifuged on slides, and stained with Wright-Giemsa for 8 minutes. Differential cell-counts were performed under a light-microscope at 40× magnification, analyzing a total of 200 cells per slide. The count of each cell type was determined by calculating its percentage relative to the total cell count in the BALF. Determination of the lung weight-to-body weight ratioTo assess lung edema, the index of lung-to-body weight was determined using the following formula: (lung weight divided by the total body weight of the rat). Determination of protein contentThe total protein concentration in BALF was measured to assess the pulmonary capillary permeability. This analysis was conducted using a Biomed diagnostics kit (Cat. No. TP118250, Egypt) following the manufacturer’s protocol. Determination of lactate dehydrogenase (LDH) activityThe concentration in BALF was measured using a Biomed diagnostics kit (Cat. No. LDAH 117025, Egypt) following the manufacturer’s protocol. Determination of oxidative stress and antioxidant markersThe concentrations of MDA, NOx, and GSH in the lung homogenate supernatant were measured using commercially available kits from biodiagnostics (Egypt, Cat. No. MD 2529, NO 2533, and GR 2511, respectively), as reported by Ohkawa et al. (1979), Miranda et al. (2001), and Ellman (1959), respectively. Enzyme-linked immunosorbent assay for cytokinesThe levels of Caspase-1, Interleukin-1β (IL-1β), NF-κB, and NOD-like receptor protein 3 (NLRP3) in BALF were analyzed using specific assay kits from Biovision (USA, Catalog No: E4594-100), Cloud-Clone Corp (USA, Catalog No: SEA563Ra), Elabscience (USA, Catalog No: E-EL-R0674), and Aviva Systems Biology (USA, Catalog No: OKCD04232), respectively, following the manufacturers’ recommended protocols. Real-time PCRAs directed by the manufacturer, total RNA was extracted from lung tissues using the RNeasy Mini Kit (Qiagen, Hilden, Germany, Cat no. 74104). The OD 260/280 nm absorbance ratio was used to evaluate the purity of each RNA sample; only samples with a ratio between 1.8 and 2.1 were chosen for additional examination. Following the manufacturer’s instructions, RNA was converted to complementary DNA (cDNA) using a cDNA reverse transcription kit (Qiagen, Cat no. 205113). Using the SYBR Green PCR Master Mix Kit and real-time quantitative PCR, the levels of mRNA expression for the target genes, IL-6 (Forward: 5′-AGAGACTTCCAGCCAGTTGC-3′ - Reverse : 5′-AGTCTCCTCTCCGGACTTGT-3′) and TNF-α (Forward: 5′-ACTGAACTTCGGGGTGATCG-3′ - Reverse: 5′-CCACTTGGTGGTTTGTGAGTG-3′) were measured. Melting curve analysis was used to establish the PCR amplification’s specificity. The ΔΔCT method was used to measure relative gene expression levels, with β-actin acting as the internal reference gene. Histopathological evaluation of pulmonary injuryFor 48 hours, lung tissue samples were preserved in 10% neutral–buffered formalin solution. Following fixation, a series of increasing ethyl alcohol concentrations (from 70% to absolute alcohol) were used to dry the samples. After cleaning with xylene, the tissues were embedded in paraffin. The paraffin blocks were cut into thin slices that were 5 μm thick. Hematoxylin and eosin (H&E) dyes were used to prepare and stain the slices for histological analysis. Under a light microscope, a pathologist who was blinded to the experimental setup evaluated the H&E slides to determine the extent of lung damage. Key factors, including arterial hypertrophy, congestion, inflammatory cell infiltration in the perivascular and peribronchial regions, alveolar emphysema, and alveolar wall thickening, were evaluated to assess lung damage. Statistical analysisTo compare the various groups, statistical analyses were conducted using a one-way ANOVA and the Tukey–Kramer multiple comparison test. The means ± standard error are used to express the data. p-values below 0.05 were considered statistically significant. Ethical approvalThe Mansoura University Research Ethics Committee approved this study. (No. Ph.D/90). ResultsCiti mitigated CP-induced changes in total and differential leucocytic counts in BALFTreatment with CP led to a significant increase in total leukocyte counts and the proportions of neutrophils, lymphocytes, eosinophils, and macrophages compared with the control group (Fig. 1A–E). Notably, the cellular changes induced by CP were progressively reduced in a dose-dependent manner following citi pretreatment at doses of 250 and 500 mg/kg. Citi reversed CP-induced alterations in lung/body weight ratio, LDH levels, and protein contentCP administration resulted in a marked increase in the lung-to-body weight ratio (Fig. 1F), total protein levels (Fig. 2A), and LDH activity (Fig. 2B) in the BALF compared with the control group. Interestingly, citi demonstrated a dose-dependent capacity to decrease these parameters. Citi mitigated CP-induced oxidative and nitrosative stressSubsequently, we examined the potential roles of oxidative and nitrosative stress in the protective effects of citi against CP-induced pulmonary damage. CP treatment resulted in a significant increase in MDA and NOx levels in the lungs and a reduction in GSH content (Fig. 3). These alterations induced by CP were effectively reversed with coadministration of citi, particularly at the 500 mg/kg dose.
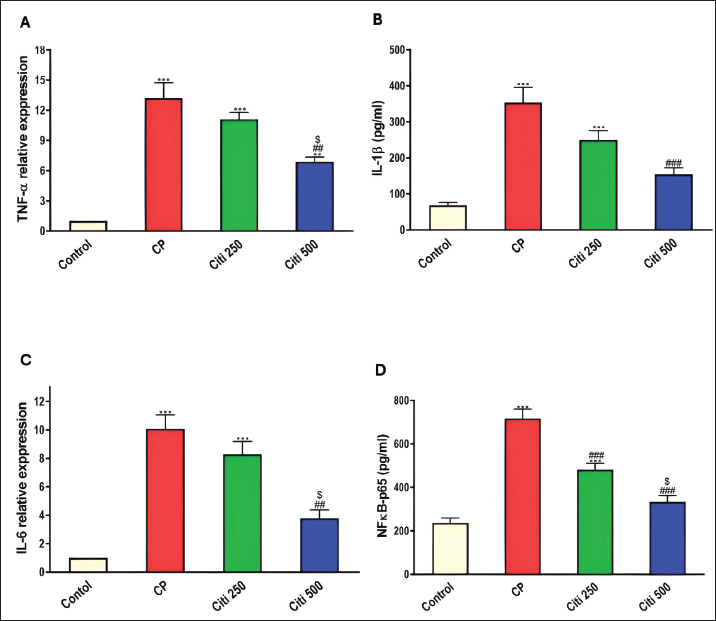
Fig. 1. Impact of citi on CP-induced alteration of total and differential leucocytic counts in the BALF and lung wt/body wt ratio. The impact of oral daily citi treatment (250, 500 mg/kg) for 14 days with a single i.p. injection of CP (200 mg/kg) on day 7 on: A. Total cell count; B. Neutrophils; C. Eosinophils; D. macrophages; E. lymphocytes; F. lung wt/body wt ratio. Values are the mean ± SEM. n=6. *** p < 0.001, ** p < 0.01, * p < 0.05 versus the control; ### p < 0.001, ## p < 0.01 versus the CP group; $$ p < 0.01, $ p < 0.05 versus the citi 250 group. CP: cyclophosphamide, citi: citicoline, BALF: bronchoalveolar lavage fluid. Citi attenuated CP-induced changes in proinflammatory cytokines and NF-kBTo investigate whether citi’s protective effect against CP-induced lung toxicity is associated with the suppression of the inflammatory cascade, we assessed the levels of the pulmonary proinflammatory cytokines TNF-α, IL-1β, IL-6, and NF-κB. The results demonstrated that CP administration significantly elevated the concentrations of these cytokines and NF-κB (Fig. 4). Pre-treatment with citi (250 and 500 mg/kg) in CP-exposed rats resulted in a dose-dependent reduction in the levels of these inflammatory markers.
Fig. 2. Influence of citi on CP-induced changes in LDH levels and protein content in BALF: The impact of oral daily citi treatment (250, 500 mg/kg) for 14 days with a single i.p. injection of CP (200 mg/kg) on day 7 on: A. LDH level; B. protein content. The mean ± SEM is used to express data. n=6. ***p < 0.001, ** p < 0.01 versus the control; ### p < 0.001, # p < 0.01 versus the CP group. CP: cyclophosphamide, citi: citicoline, LDH: lactate dehydrogenase, BALF: bronchoalveolar lavage fluid.
Fig. 3. Impact of citi on oxidative injury and antioxidant suppression caused by CP in lung tissue homogenates: The impact of oral daily citi treatment (250, 500 mg/kg) for 14 days with a single i.p. injection of CP (200 mg/kg) on day 7 on: A. MDA; B. NOx; C. GSH. The mean ± SEM is used to express data. n=6. ***p < 0.001, ** p < 0.01, * p < 0.05 versus the control; ## p < 0.01, # p < 0.05 versus the CP group; $ p < 0.05 versus the citi 250 group. CP: cyclophosphamide, citi: citicoline, MDA: malondialdehyde, NOx: total nitrate/nitrite, GSH: reduced glutathione.
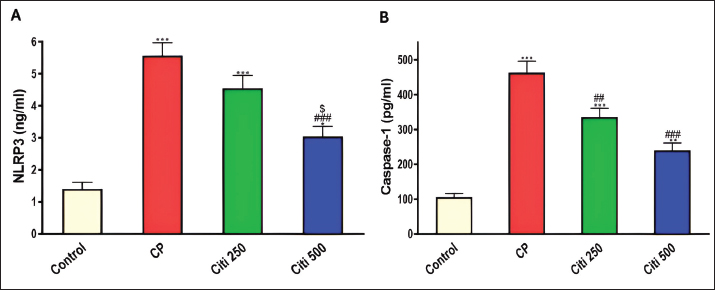
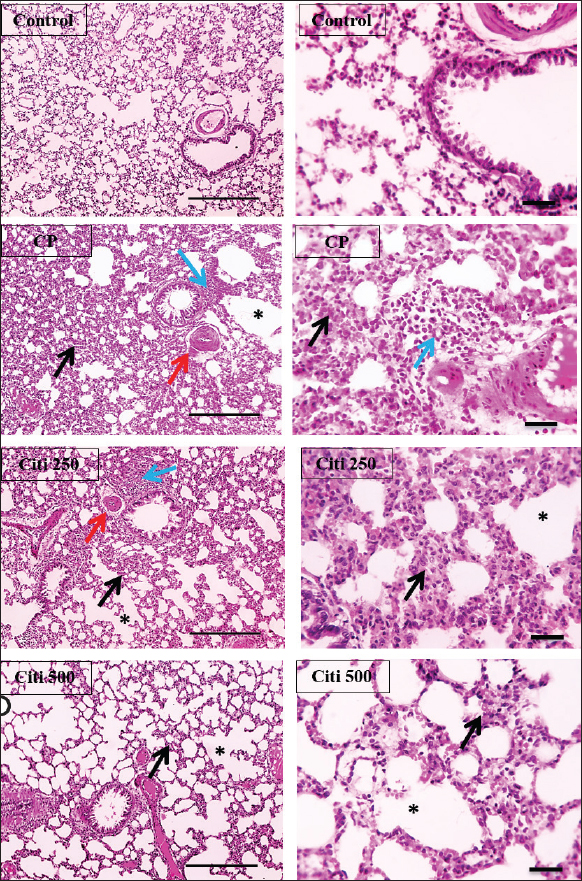
Fig. 4. Impact of citi on CP-induced alteration of proinflammatory cytokines and NF-kB: The impact of oral daily citi treatment (250, 500 mg/kg) for 14 days with a single i.p. injection of CP (200 mg/kg) on day 7 on: A. Relative mRNA expression of TNF-α in lung tissue; B. Relative mRNA expression of IL-6 in lung tissue; C. IL-1B level in BALF; D. NF-kB level in BALF. The mean ± SEM is used to express data. n=6. ***p < 0.001, ** p < 0.01 versus the control; ### p < 0.001, ## p < 0.01 versus the CP group; $ p < 0.05 versus the citi 250 group. CP: cyclophosphamide, citi: citicoline, TNF-α: tumor necrosis factor-α, IL-6: Interleukin-6, IL-1B: Interleukin-1B, NF-kB: nuclear factor kappa B, BALF: bronchoalveolar lavage fluid. Citi ameliorated CP-induced elevation of NLRP3 and caspase-1Furthermore, rats in the CP group exhibited a notable increase in caspase-1 activity and NLRP3 levels (Fig. 5) compared with healthy rats in the control group. However, citi treatment significantly reduced the activity of caspase-1 and the content of NLRP3, with the higher dose group showing the most pronounced effect. Citi improved CP-induced lung injuryAs indicated in Figure 6, histopathological analysis of the rat lungs revealed that CP administration caused thickening of the alveolar walls, along with significant infiltration of inflammatory cells, hemorrhage, and congestion. Additionally, alveolar emphysema, arterial hypertrophy, and inflammatory cells infiltration around the blood vessels and bronchi were observed. However, citi treatment dose-dependently reduced the harmful effects of CP. DiscussionCP is a highly effective cancer chemotherapeutic agent; however, it is also one of the most toxic agents, leading to lung toxicity and other pathological complications (Bhattacharjee et al., 2015). The primary mechanism of CP toxicity is the toxic metabolite acrolein, which induces cellular apoptosis, promotes oxidative stress, and triggers inflammation, resulting in widespread organ damage. This study aimed to explore whether citicoline can alleviate lung injury caused by CP and, if so, how it exerts its protective effects. For the first time, we have presented compelling evidence supporting the therapeutic potential of citi in mitigating pulmonary damage. In our study, inflammatory infiltration of cells and emphysema, which are associated with macrophage activation, were most prevalent in the CP-treated group. Inflammatory cells move more quickly from the blood vessels into the alveolar cavities as a result of this activation. Protein-rich pulmonary edema results from cell activation, which damages the alveolar-capillary membrane and increases the accessibility of the lung vasculature (Kim et al., 2014; Ali et al., 2023). This was demonstrated by the increased lung weight-to-body weight ratio and high protein levels in BALF (El-Kashef and Rahim, 2023). Furthermore, the activity of LDH in the BALF was markedly higher in the CP group, indicating cell damage and death.
Fig. 5. Impact of citi on CP-induced activation of the NLRP3 inflammasome pathway: The impact of oral daily citi treatment (250, 500 mg/kg) for 14 days with a single i.p. injection of CP (200 mg/kg) on day 7 on: A. NLRP3 level in BALF; B. Caspase-1 level in BALF. The mean ± SEM is used to express data. n=6. ***p < 0.001, ** p < 0.01, * p < 0.05 versus the control; ### p < 0.001, ## p < 0.01 versus the CP group; $ p < 0.05 versus the citi 250 group. CP: cyclophosphamide, citi: citicoline, NLRP3: NOD-like receptor protein 3, BALF: bronchoalveolar lavage fluid. Lung tissue from rats exposed to CP exhibited thickened alveolar walls, extensive inflammatory cell infiltration, hemorrhage, and congestion. These observations are consistent with a previous study reporting alveolar cell damage, increased alveolar septal thickness, and infiltration of edema, epithelial cells, polymorphonuclear cells, and erythrocyte cells in the alveolar lumen following CP administration (Alsemeh and Abdullah, 2022). In contrast, rats treated with citi showed significant improvement in lung damage, characterized by reduced alveolar wall thickness and milder alveolar emphysema in a dose-dependent manner. This protective effect is likely attributable to citi’s ability to mitigate inflammatory responses (Ek et al., 2014). Citi is known to inhibit NF-κB, thereby reducing the production and release of proinflammatory cytokines (Cavalu et al., 2024). ROS directly causes tissue damage and triggers inflammatory responses by activating various proinflammatory cytokines. Oxidative stress elevation and subsequent lipid peroxidation contribute to tissue inflammation and necrosis (Zhang and An, 2007). Acrolein, a toxic metabolite of CP, causes cell damage through free radical generation, which binds to GSH, depleting its levels in the cell (Araghi et al., 2018). Lipid peroxidation is characterized by the presence of MDA, which functions as both a marker and signal of lipid peroxidation (Ibrahim et al., 2020). CP administration is known to induce oxidative stress, significantly raising MDA levels in the lungs while decreasing GSH, SOD, catalase, and GH-Px levels in comparison to the control group (Ghosh et al., 2015). Another study reported reduced GSH levels and SOD activity, alongside increased MDA levels, following CP exposure (Şengül et al., 2017). However, treatment with citicoline was found to enhance the antioxidant defense system, restoring GSH levels and decreasing lipid peroxidation and MDA concentrations. Multiple studies have demonstrated that citicoline effectively inhibits ROS production (Abdel-Salam et al., 2019; El-Baz et al., 2023). NO plays a significant role in CP-induced toxicity (Elsayed et al., 2022). In this study, CP administration caused a notable elevation of NOx levels, consistent with previous findings suggesting that the elevation in NOx is linked to the overexpression of inducible nitric oxide synthase (iNOS) (Sun et al., 2021). NO can react with superoxide anions to produce peroxynitrite (Radi, 2018), which may cause NF-κB activation, thus an elevation in proinflammatory cytokine production and inflammatory response (Yee et al., 2021). Conversely, citi treatment reduced the rise in NOx levels, as observed in prior studies investigating its effects in animal models of tissue injury, such as head irradiation-induced brain damage and sodium arsenite-induced nephrotoxicity (Abdel-Aziz et al., 2021; Khodayar et al., 2024). Furthermore, citi has been shown to inhibit iNOS activity in studies examining its impact on γ-radiation-induced splenic inflammation in rats (Abdel-Aziz and Saif-Elnasr, 2024).
Fig. 6. Effect of citi on CP-induced histopathological lung damage: CP induced thickening of the alveolar wall (black arrow) with numerous inflammatory cell infiltrations (blue arrows), together with hemorrhage and congestion. This condition is associated with arterial hypertrophy (red arrow) and emphysema (*). Meanwhile, the deleterious manifestations of CP were dose-dependently mitigated by citi treatment. Lung sections were stained with H&E. Low magnification ×:100 bar 100 and high magnification ×:400 bar 50. CP: cyclophosphamide, citi: citicoline, H&E: Hematoxylin and eosin. This study demonstrated that CP induced inflammation by increasing the release of proinflammatory cytokines, including TNF-α, IL-6, and IL-1β. This phenomenon may be ascribed to the activation of neutrophils and macrophages, which discharge free radicals and proteolytic enzymes, finally resulting in damage to connective tissues (El-Kashef, 2018). Consistent with other studies (Badawi, 2022; Balaha et al., 2023). Treatment with citi effectively reduced elevated cytokine levels. This beneficial effect may be mediated by its ability to inhibit phospholipase A2, an enzyme implicated in neuroinflammation, which consequently reduces ROS production and the release of proinflammatory cytokines (Adibhatla and Hatcher, 2003). The NF-κB signaling system is crucially activated by oxidative stress and is critical to cell survival, inflammation, and immunological responses. This transcription factor governs the synthesis of several inflammatory cytokines. In standard settings, the NF-κB p65 subunit is associated with its inhibitor, IκBα, and additional IκB proteins, creating an inactive complex in the cytoplasm. Upon activation, IκBα is phosphorylated, resulting in the activation and subsequent translocation of NF-κB p65 into the nucleus, where it promotes the production of inflammatory genes (El-Agamy et al., 2018). Numerous studies have emphasized the critical role of NF-κB activation in mediating the inflammatory response associated with CP-induced lung injury (Balaha et al., 2023; Salama et al., 2023). Interestingly, citi has been shown to significantly inhibit NF-κB activation, resulting in a marked reduction in downstream inflammatory cytokines, such as NOx, TNF-α, and IL-6. Research has suggested that citi can modulate NF-κB signaling in various inflammatory conditions, including diabetes-induced retinal neurodegeneration and γ-radiation-induced splenic inflammation (Bogdanov et al., 2018; Abdel-Aziz and Saif-Elnasr, 2024). Additionally, citicoline may enhance ACh activity by increasing the expression of choline acetyltransferase and α7 nAChR. Furthermore, phosphatidylcholine is considered an agonist of α7nAChR (Conant and Schauss, 2004). Activation of α7nAChR reduces IκBα degradation, preventing nuclear translocation of NF-κB and subsequent expression of proinflammatory cytokines (Mizrachi et al., 2021). The NLRP3 inflammasome consists of proteins containing nucleotide binding domains and leucine-rich repeat sequences, the adaptor protein apoptosis-associated speck-like protein containing a CARD (ASC), and procaspase-1 (Schroder et al., 2010). The phosphorylation-induced nuclear translocation of NF-κB p65 activates the NLRP3 inflammasome, resulting in elevated levels of IL-1β and IL-18. Various endogenous and exogenous stimuli trigger a secondary signal that promotes the assembly of NLRP3, ASC, and procaspase-1 into a complex (Yang et al., 2019). This activation results in the maturation of caspase-1, which then cleaves the proforms of IL-1β and IL-18 into their active forms (Vanaja et al., 2015). Previous studies have shown that CP triggers the activation of NLRP3 (Lin et al., 2020; Zhang et al., 2021). In this investigation, we noted that CP dramatically enhanced the expression of NLRP3 and caspase-1 in the NLRP3 inflammasome pathway in the lung tissues of rats treated with CP. Nonetheless, these effects were significantly mitigated by the administration of citi. Our research suggests that the antiinflammatory properties of citi in alleviating lung toxicity may be partly due to the suppression of the NLRP3 inflammasome pathway. These findings correspond with earlier studies indicating that citi may mitigate inflammatory responses by suppressing the NLRP3 inflammasome or other elements of this pathway (Silva-Palacios et al., 2022). ConclusionIn summary, citi exerted a protective effect against CP-induced lung injury, primarily through its antioxidative and anti-inflammatory properties. Therefore, citi is a potential option for lung damage management associated with CP treatment. AcknowledgmentsNone. Conflict of interestThe authors declare that they have no conflicting interests. FundingThis study was not funded. Authors’ contributionsGehad El-Sayed, El-Said El-Sherbini, and Mohamed El-Adl: study design and supervision. Rania Salah: experimental work, data collection, statistical analysis, and writing of the original draft. Gehad El-Sayed, El-Said El-Sherbini, and Mohamed El-Adl: reviewing and editing the article. Data availabilityThe manuscript contains all the data that support the study’s conclusions. ReferencesAbdel-Aziz, N., Moustafa, E.M. and Saada, H.N. 2021. The impact of citicoline on brain injury in rats subjected to head irradiation. Environ. Sci. Pollut. R. 28(8), 9742–9752. Abdel-Aziz, N. and Saif-Elnasr, M. 2024. Citicoline modulates inflammatory signaling pathways in the spleen of rats exposed to gamma-radiation. Immunopharmacol. Immunotoxicol. 46(4), 564–571. Abdel-Salam, O., Youness, E., Mohamed, N., El-Moneim, O. and Shaffie N. 2019. Citicoline protects against tramadol-induced oxidative stress and organ damage. React. Oxyg. Species. 7(20), 106–120. Adibhatla, R.M. and Hatcher, J.F. 2003. Citicoline decreases phospholipase A2 stimulation and hydroxyl radical generation in transient cerebral ischemia. J. Neurosci. Res. 73(3), 308–315. Ahmed, L.A., El-Maraghy, S.A. and Rizk, S.M. 2015. Role of the KATP channel in the protective effect of nicorandil on cyclophosphamide-induced lung and testicular toxicity in rats. Sci. Rep. 5(1), 14043. Akram, W., Najmi, A.K., Alam, M.M. and Haque, S.E. 2024. Levocabastine ameliorates cyclophosphamide-induced cardiotoxicity in Swiss albino mice: targeting TLR4/NF-κB/NLRP3 signaling pathway. Toxicol. Appl. Pharmacol. 483, 116838. Ali, Y.M., Lynch, N.J., Shaaban, A.A., Rizk, D.E., Abdel-Rahman, S.H., Khatri, P., Yabuki, M., Yaseen, S., Dudler, T., Demopulos, G. and Schwaeble W.J. 2023. Inhibition of the lectin pathway of complement activation reduces LPS-induced acute respiratory distress syndrome in mice. Front. Immunol. 14, 1192767. Alsemeh, A.E. and Abdullah, D.M. 2022. Protective effect of alogliptin against cyclophosphamide-induced lung toxicity in rats: impact on PI3K/Akt/FoxO1 pathway and downstream inflammatory cascades. Cell Tissue Res. 388(2), 417–438. Araghi, A., Golshahi, H., Baghban, F. and Abouhosseini Tabari, M. 2018. Ameliorative action of farnesol on cyclophosphamide induced toxicity in mice. J. Herbmed. Pharmacol. 7(1), 37–43. Badawi, M. 2022. The protective effect of β-cryptoxanthin against cyclophosphamide-induced lung injury in adult male albino rats. Bull. Natl. Res. Cent. 46, 106. Balaha, M.F., Alamer, A.A., Aldossari, R.M., Aodah, A.H., Helal, A.I. and Kabel, A.M. 2023. Amentoflavone mitigates cyclophosphamide-induced pulmonary toxicity: involvement of-SIRT-1/Nrf2/Keap1 Axis, JAK-2/STAT-3 signaling, and apoptosis. Medicina (Kaunas) 59(12), 2119. Baris, E., Simsek, O., Efe, H., Oncu, S., Gelal, A., Hamurtekin, E., Tosun, M., Ozbal, S., Yuce, Z. and Arici, M.A. 2021. Effects of CDP-choline and choline on COX pathway in LPS-induced inflammatory response in rats. Int. J. Pharmacol. 17(2), 84–96. Bhattacharjee, A., Basu, A., Biswas, J. and Bhattacharya, S. 2015. Nano-Se attenuates cyclophosphamide-induced pulmonary injury through modulation of oxidative stress and DNA damage in Swiss albino mice. Mol. Cell. Biochem. 405(1-2), 243–256. Bogdanov, P., Sampedro, J., Solà-Adell, C., Simó-Servat, O., Russo, C., Varela-Sende, L., Simó, R. and Hernández, C. 2018. Effects of liposomal formulation of citicoline in experimental diabetes-induced retinal neurodegeneration. Int. J. Mol. Sci. 19(8), 2458. Borovikova, L.V., Ivanova, S., Zhang, M., Yang, H., Botchkina, G.I., Watkins, L.R., Wang, H., Abumrad, N., Eaton, J.W. and Tracey, K.J. 2000. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature 405(6785), 458–462. Cavalu, S., Saber, S., Ramadan, A., Elmorsy, E.A., Hamad, R.S., Abdel-Reheim, M.A. and Youssef, M.E. 2024. Unveiling citicoline’s mechanisms and clinical relevance in the treatment of neuroinflammatory disorders.FASEB J. 38(17), e70030. Conant, R. and Schauss, A.G. 2004. Therapeutic applications of citicoline for stroke and cognitive dysfunction in the elderly: a review of the literature. Altern. Med. Rev. 9(1), 17–31. Conte, P., Ascierto, P.A., Patelli, G., Danesi, R., Vanzulli, A., Sandomenico, F., Tarsia, P., Cattelan, A., Comes, A., De Laurentiis, M., Falcone, A., Regge, D., Richeldi, L. and Siena, S. 2022. Drug-induced interstitial lung disease during cancer therapies: expert opinion on diagnosis and treatment. ESMO Open 7(2), 100404. Ek, R.O., Serter, M., Ergin, K., Cecen, S., Unsal, C., Yildiz, Y. and Bilgin, M.D. 2014. Protective effects of citicoline on TNBS-induced experimental colitis in rats. Int. J. Clin. Exp. Med. 7(4), 989–997. El-Agamy, D.S., El-Harbi, K.M., Khoshhal, S., Ahmed, N., Elkablawy, M.A., Shaaban, A.A. and Abo-Haded, H.M. 2018. Pristimerin protects against doxorubicin-induced cardiotoxicity and fibrosis through modulation of Nrf2 and MAPK/NF-kB signaling pathways. Cancer Manag. Res. 11, 47–61. El-Baz, A.M., El-Ganiny, A.M., Hellal, D., Anwer, H.M., El-Aziz, H.A.A., Tharwat, I.E., El-Adawy, M.A., Helal, S.E.M., Mohamed, M.T.A., Azb, T.M., Elshafaey, H.M., Shalata, A.A., Elmeligi, S.M., Abdelbary, N.H., El-Kott, A.F., Al-Saeed, F.A., Salem, E.T., El-Sokkary, M.M.A., Shata, A. and Shabaan, A.A. 2023. Valuable effects of lactobacillus and citicoline on steatohepatitis: role of Nrf2/HO-1 and gut microbiota. AMB Express 13(1), 57. El-Kashef, D.H. 2018. Role of venlafaxine in prevention of cyclophosphamide-induced lung toxicity and airway hyperactivity in rats. Environ. Toxicol. Pharmacol. 58, 70–76. El-Kashef, D.H. and Rahim, M.A. 2023. Levocetrizine attenuates cyclophosphamide-induced lung injury through inhibition of TNF-α, IL-1β, TGF-β and MMP-9. BMC Pharmacol. Toxicol. 24(1), 76. Ellman, G.L. 1959. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 82(1), 70–77. Elsayed, F.F., Elshenawy, W.M., Khalifa, E.M., Rizq, M.R. and Abdelaziz, R.R. 2022. Ameliorative effect of flavocoxid on cyclophosphamide-induced cardio and neurotoxicity via targeting the GM-CSF/NF-κB signaling pathway. Environ. Sci. Pollut. Res. Int. 29(46), 69635–69651. Emadi, A., Jones, R.J. and Brodsky, R.A. 2009. Cyclophosphamide and cancer: golden anniversary. Nat. Rev. Clin. Oncol. 6(11), 638–647. Ghosh, P., Bhattacharjee, A., Basu, A., Singha Roy, S. and Bhattacharya, S. 2015. Attenuation of cyclophosphamide-induced pulmonary toxicity in Swiss albino mice by naphthalimide-based organoselenium compound 2-(5-selenocyanatopentyl)-benzo[de]isoquinoline 1,3-dione. Pharm. Biol. 53(4), 524–532. Hao, H., Xu, Y., Chen, R., Qi, S., Liu, X., Lin, B., Chen, X., Zhang, X., Yue, L. and Chen, C. 2024. Protective effects of chlorogenic acid against cyclophosphamide induced liver injury in mice. Biotech. Histochem. 99(1), 33–43. Hassan, D.S. and Hasary, H.J.M. 2022. Clinical implication of cyclophosphamide in oncology, hematology and bone marrow transplantation (BMT). IJISRT 7(5), 1073–1079. Ibrahim, H., Mohammed Geba, K., Tawfic, A. and El-Magd, M. 2020. Camel milk exosomes modulate cyclophosphamide-induced oxidative stress and immuno-toxicity in rats. Food Funct. 10(11), 7523–7532. Khodayar, M.J., Shirani, M., Nikravesh, M., Mohammadi, E., Khorsandi, L.S. and Shariati, S. 2024. Protective effect of citicoline on sodium arsenite-induced nephrotoxicity in mice. Jundishapur J. Nat. Pharm. Prod. 19(2), e144745. Kim, D.H., Chung, J.H., Son, B.S., Kim, Y.J. and Lee, S.G. 2014. Effect of a neutrophil elastase inhibitor on ventilator-induced lung injury in rats. J. Thorac. Dis. 6(12), 1681–1689. Lin, X., Yang, F., Huang, J., Jiang, S., Tang, Y. and Li, J. 2020. Ameliorate effect of pyrroloquinoline quinone against cyclophosphamide-induced nephrotoxicity by activating the Nrf2 pathway and inhibiting the NLRP3 pathway. Life Sci. 256, 117901. MacAllister, S.L., Martin-Brisac, N., Lau, V., Yang, K. and O’Brien, P.J. 2013. Acrolein and chloroacetaldehyde: an examination of the cell and cell-free biomarkers of toxicity. Chem. Biol. Interact. 202(1-3), 259–266. Miranda, K.M., Espey, M.G. and Wink, D.A. 2001. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide 5(1), 62–71. Mizrachi, T., Vaknin-Dembinsky, A., Brenner, T. and Treinin, M. 2021. Neuroinflammation modulation via α7 nicotinic acetylcholine receptor and its chaperone, RIC-3. Molecules 26(20), 6139. Moignet, A., Hasanali, Z., Zambello, R., Pavan, L., Bareau, B., Tournilhac, O., Roussel, M., Fest, T., Awwad, A., Baab, K., Semenzato, G., Houot, R., Loughran Jr, T.P. and Lamy, T. 2014. Cyclophosphamide as a first-line therapy in LGL leukemia. Leukemia 28(5), 1134–1136. Ohkawa, H., Ohishi, N. and Yagi, K. 1979. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 95(2), 351–358. Pugh, D., Farrah, T.E., Gallacher, P.J., Kluth, D.C. and Dhaun, N. 2019. Cyclophosphamide-induced lung injury. Kidney Int. Rep. 4(3), 484–486. Radi, R. 2018. Oxygen radicals, nitric oxide, and peroxynitrite: redox pathways in molecular medicine. Proc. Natl. Acad. Sci. U. S. A. 115(23), 5839–5848. Ricciotti, E. and FitzGerald, G.A. 2011. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 31(5), 986–1000. Salama, A., Elgohary, R., Amin, M.M. and Elwahab, S.A. 2023. Impact of protocatechuic acid on alleviation of pulmonary damage induced by cyclophosphamide targeting peroxisome proliferator activator receptor, silent information regulator type-1, and fork head box protein in rats. Inflammopharmacology 31(3), 1361–1372. Schroder, K., Zhou, R. and Tschopp, J. 2010. The NLRP3 inflammasome: a sensor for metabolic danger? Science 327(5963), 296–300. Secades, J.J. and Gareri, P. 2022. Citicoline: pharmacological and clinical review, 2022 update. Rev. Neurol. 75(s05), S1–S89. Seker, U., Kavak, D.E., Dokumaci, F.Z., Kizildag, S. and Irtegun-Kandemir, S. 2024. The nephroprotective effect of quercetin in cyclophosphamide-induced renal toxicity might be associated with MAPK/ERK and NF-κB signal modulation activity. Drug Chem.Toxicol. 47(6), 1165–1174. Şengül, E., Gelen, V., Gedikli, S., Özkanlar, S., Gür, C., Çelebi, F. and Çınar, A. 2017. The protective effect of quercetin on cyclophosphamide-Induced lung toxicity in rats. Biomed. Pharmacother. 92, 303–307. Shaaban, A.A., Shaker, M.E., Zalata, K.R., El-kashef, H.A. and Ibrahim, T.M. 2014. Modulation of carbon tetrachloride-induced hepatic oxidative stress, injury and fibrosis by olmesartan and omega-3. Chem. Biol. Interact. 207, 81–91. Silva-Palacios, A., Arroyo-Campuzano, M., Flores-García, M., Patlán, M., Hernández-Díazcouder, A., Alcántara, D., Ramírez-Camacho, I., Arana-Hidalgo, D., Soria-Castro, E., Sánchez, F., González-Pacheco, H. and Zazueta, C. 2022. Citicoline modifies the expression of specific miRNAs related to cardioprotection in patients with ST-segment elevation myocardial infarction subjected to coronary angioplasty. Pharmaceuticals (Basel) 15(8), 925. Sun, D., Sun, C., Qiu, G., Yao, L., Yu, J., Al Sberi, H., Fouda, M.S., Othman, M.S., Lokman, M.S., Kassab, R.B. and Abdel Moneim, A.E. 2021. Allicin mitigates hepatic injury following cyclophosphamide administration via activation of Nrf2/ARE pathways and through inhibition of inflammatory and apoptotic machinery. Environ. Sci. Pollut. Res. Int. 28(29), 39625–39636. Vanaja, S.K., Rathinam, V.A. and Fitzgerald, K.A. 2015. Mechanisms of inflammasome activation: recent advances and novel insights. Trends cell Biol. 25(5), 308–315. Yang, Y., Wang, H., Kouadir, M., Song, H. and Shi, F. 2019. Recent advances in the mechanisms of NLRP3 inflammasome activation and its inhibitors. Cell Death Dis. 10(2), 128. Yee, Y.H., Chong, S.J.F., Kong, L.R., Goh, B.C. and Pervaiz, S. 2021. Sustained IKKβ phosphorylation and NF-κB activation by superoxide-induced peroxynitrite-mediated nitrotyrosine modification of B56γ3 and PP2A inactivation. Redox Biol. 41, 101834. Zhang, H., Zhao, J., Lu, Q., Sun, B., Liu, X., Yang, C., Li, S., Li, L., Yi, S., Yang, Z. and Xu, J. 2021. Luteolin improves cyclophosphamide-induced cystitis through TXNIP/NLRP3 and NF-κB pathways. Evid. Based Complement Alternat. Med. 2021(1), 1718709. Zhang, J.M. and An, J. 2007. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 45(2), 27–37. | ||
| How to Cite this Article |
| Pubmed Style Salah R, El-sayed G, El-sherbini E, El-adl M. Ameliorative effect of citicoline on cyclophosphamide-induced lung injury. Open Vet. J.. 2025; 15(5): 2218-2229. doi:10.5455/OVJ.2025.v15.i5.39 Web Style Salah R, El-sayed G, El-sherbini E, El-adl M. Ameliorative effect of citicoline on cyclophosphamide-induced lung injury. https://www.openveterinaryjournal.com/?mno=244881 [Access: January 23, 2026]. doi:10.5455/OVJ.2025.v15.i5.39 AMA (American Medical Association) Style Salah R, El-sayed G, El-sherbini E, El-adl M. Ameliorative effect of citicoline on cyclophosphamide-induced lung injury. Open Vet. J.. 2025; 15(5): 2218-2229. doi:10.5455/OVJ.2025.v15.i5.39 Vancouver/ICMJE Style Salah R, El-sayed G, El-sherbini E, El-adl M. Ameliorative effect of citicoline on cyclophosphamide-induced lung injury. Open Vet. J.. (2025), [cited January 23, 2026]; 15(5): 2218-2229. doi:10.5455/OVJ.2025.v15.i5.39 Harvard Style Salah, R., El-sayed, . G., El-sherbini, . E. & El-adl, . M. (2025) Ameliorative effect of citicoline on cyclophosphamide-induced lung injury. Open Vet. J., 15 (5), 2218-2229. doi:10.5455/OVJ.2025.v15.i5.39 Turabian Style Salah, Rania, Gehad El-sayed, El-said El-sherbini, and Mohamed El-adl. 2025. Ameliorative effect of citicoline on cyclophosphamide-induced lung injury. Open Veterinary Journal, 15 (5), 2218-2229. doi:10.5455/OVJ.2025.v15.i5.39 Chicago Style Salah, Rania, Gehad El-sayed, El-said El-sherbini, and Mohamed El-adl. "Ameliorative effect of citicoline on cyclophosphamide-induced lung injury." Open Veterinary Journal 15 (2025), 2218-2229. doi:10.5455/OVJ.2025.v15.i5.39 MLA (The Modern Language Association) Style Salah, Rania, Gehad El-sayed, El-said El-sherbini, and Mohamed El-adl. "Ameliorative effect of citicoline on cyclophosphamide-induced lung injury." Open Veterinary Journal 15.5 (2025), 2218-2229. Print. doi:10.5455/OVJ.2025.v15.i5.39 APA (American Psychological Association) Style Salah, R., El-sayed, . G., El-sherbini, . E. & El-adl, . M. (2025) Ameliorative effect of citicoline on cyclophosphamide-induced lung injury. Open Veterinary Journal, 15 (5), 2218-2229. doi:10.5455/OVJ.2025.v15.i5.39 |