| Research Article | ||
Open Vet. J.. 2025; 15(5): 2171-2181 Open Veterinary Journal, (2025), Vol. 15(5): 2171-2181 Research Article Analysis of ovarian morphometry and age-related T Lymphocyte localization in adult (Phodopus roborovski) dwarf hamstersThekra Fadel Saleh, Ghada Abdulrhman Sultan and Omar Younis Altaey*Department of Anatomy, College of Veterinary Medicine, University of Mosul, Mosul, Iraq *Corresponding Author: Omar Younis Altaey. Department of Anatomy, College of Veterinary Medicine, University of Mosul, Mosul, Iraq. Email: Omar.younes [at] uomosul.edu.iq Submitted: 17/02/2025 Revised: 24/03/2025 Accepted: 06/04/2025 Published: 31/05/2025 © 2025 Open Veterinary Journal
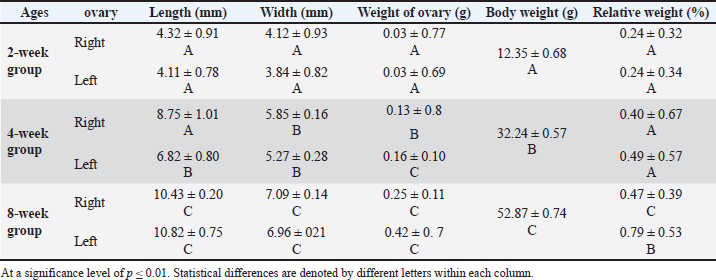
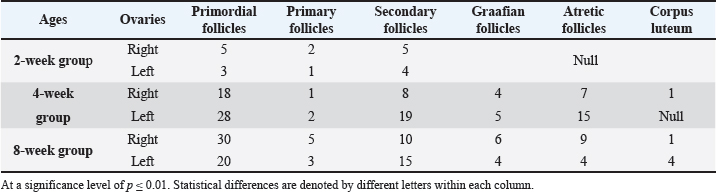
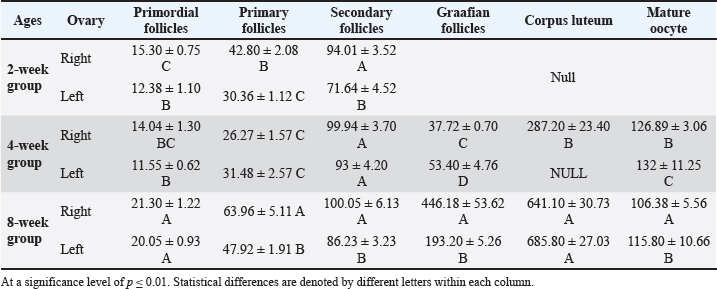
ABSTRACTBackground: Dwarf hamsters are extensively used as models in reproductive disorders studies, reproductive endocrinology, embryo transplantation, and in vivo and in vitro egg fertilization. Aim: This study aimed to explore the morphological and morphometric development of the ovary with T lymphocyte distribution at different ages using immunohistochemical cluster of differentiation 40 (CD40) expression. Methods: Fifteen dwarf hamster (Phodopus roborovski) females were used in this study and divided into three groups at three different ages (2, 4, and 8 weeks) after birth. Samples were collected, and the length, width, and weight were measured. Standard histological processing was performed, and the slides were stained with hematoxylin and eosin. Histomorphometrical analysis was performed using a 3.0 USB microscopic camera for determining the numbers and diameters of the primordial, primary, secondary, and Graafian follicles and the diameter of the mature oocyte and corpus luteum. Immunohistochemical analysis using CD40 anti-Mouse ligand antibody expression to evaluate T lymphocyte distribution within ovarian parenchyma. Results: At 2 weeks of age, the observations showed symmetric development of the ovaries, with no significant differences in dimensions and weight. Histologically, the ovaries displayed early follicle development with sparse T lymphocytes. At 4 weeks, follicular size asymmetry emerged with the presence of corpus luteum and increased T lymphocyte counts. By 8 weeks, the ovaries exhibited more developed ovarian follicles, larger corpus luteum, and a further increase in T-lymphocyte density. Conclusion: This investigation sheds light on the development of the ovary in dwarf hamsters, an understudied model with a short maturity age. These findings provide a promising understanding of ovarian morphological development and the diagnosis of various infertility developmental disorders. Keywords: Dwarf hamster, Development, Ovary, Ovarian follicles, T-lymphocytes. IntroductionRodents are among the largest and most diverse groups of mammals, with approximately 1,700 identified species (Besselsen, 2002). Hamsters, among these species, gained popularity as pets and are the fifth most commonly utilized experimental animal following mice, rats, rabbits, and guinea pigs according to a US government statistics report (County, 2019). Hamsters have been extensively studied due to their small size, ease of handling, and suitability as a model for various medical research. Examples of such research include investigations into reproductive disorders (Martorell, 2017), reproductive endocrinology (Ancel et al., 2012), embryo transplantation (Reese et al., 2008), in vivo and in vitro egg fertilization (Tateno et al., 2021), and reproductive system oncology (Tysome et al., 2012). The ovaries play a pivotal role in the differentiation and release of mature ova, facilitating successful fertilization (Taketsuru et al., 2024). The reproductive system in hamsters shows specific, unique features characterized by a short estrous cycle lasting 4 days, a short gestation period (16–17 days), poly estrous nature, and spontaneous superovulation (Hirose and Ogura 2019). The seasonal reproduction is a common strategy among hamsters, enabling them to breed at specific times of the year when it is most advantageous for offspring to survive and grow (Weems et al., 2015). T lymphocytes play an important role in the regression of the corpus luteum. Through direct cytotoxic effects on the luteal cells. Furthermore, their attraction and activation of macrophages within the ovarian parenchyma trigger phagocytosis of the damaged luteal cells (Vinatier et al., 1995). The dynamic interplay between the reproductive and immune systems is important to optimize the survival of embryos. While studies have focused on describing the developmental morphology of the female reproductive system in various rodent species, such as rabbits (Al-Saffer and Amayah, 2018), rats (Gaytan et al., 2014), mice (Lui et al., 2010), and guinea pigs (Fernández et al., 2022). There is a notable gap in understanding ovarian structural development in dwarf hamsters (Phodopus roborovski) across different postnatal ages. Specifically, no study has comprehensively examined the distribution of T lymphocytes in the ovaries of these species or correlated these findings with age-related changes in ovarian morphometry. This study aimed to address this gap by providing insights into the anatomical, histological, and histomorphometric aspects of ovarian development in dwarf hamsters, with a special emphasis on understanding the distribution of T lymphocytes in the ovaries using immunohistochemical cluster of differentiation 40 (CD40) expression at different postnatal ages. Materials and MethodsSamples preparationFifteen clinically healthy dwarf hamsters (Phodopus roborovski) females were used in this study, divided into three equal groups at three different ages (2, 4, and 8 weeks) after birth. Animals were offered food and water ad libitum during the experiment, which was performed in the Anatomy Department Laboratories of Veterinary Medicine College - University of Mosul-Iraq during June to December 2023. Most adult animals (>4 weeks of age) were confirmed to be in the diestrous phase of the estrous cycle during the short photoperiod, which was determined by vaginal cytology. Vaginal smears revealed a predominance of leukocytes with minimal epithelial cells. Animals were euthanized with decapitation using laboratory sterilized tools following sedition with intraperitoneal injection of 120 mg/kg Pentobarbital sodium (Nembutal®), consistent with American Veterinary Medical Association (AVMA) guidelines) Underwood and Anthony, 2020). An abdominal incision was made across the pelvic symphysis to expose the ovaries in situ. The organs were then extracted, and the right and left ovaries were separated, washed with normal saline, and kept in plastic containers containing 10% buffered formalin for fixation. The macroscopic measurements were performed using a digital Vernier caliper (zhart-CT-ZT, India) and weighed using a sensitive scale (Aliston-Atom, China ). The measurements included: the length, width, and weight of the right and left ovaries and the relative weight=(ovary Weight (g) / body Weight (g)×100) in each age group. Histological analysisSubsequent to fixation for 72 hours, the samples were dehydrated by replacing them with increasing concentrations of ethyl alcohol, starting with 70% for 24 hours, then 90% (two changes) for 3 hours each, then 100% (two changes) for 3 hours each for dehydration; afterward, samples were cleared using xylene (two changes) for 15 minutes each samples later. Followed by impregnation of the sample using melted paraffin at 58°C for 3 hours, cast into blocks, and cut using a rotary microtome to obtain 5 μm-thick sections loaded on positively charged histological slides (Suvarna et al., 2019). The slides were stained with Delafield’s Hematoxylin and Eosin stain to determine the general histological structure of the ovary and as a prelude to take microscopic measurements (Carson and Cappellano, 2015). Morphometrical analysisA morphometrical study was conducted using a 3.0 USB microscopic mounted camera (AmScope 1.3MP-A3513, China). The images were taken at different magnification powers (4X,10X,40X). Then, measurements were performed using the Toupview software (Omax, China) in 10 different microscopic fields and in 5 different slides from each age group. The measurement parameters included the numbers and diameters of the primordial, primary, secondary, and Graafian follicles) (the diameter of the mature oocyte and corpus luteum). All microstructural and morphometric analysis were performed using (Amscope-B120 series, China) light microscope (Altaey et al., 2025). Immunohistochemical analysisFollowing the removal of paraffin and rehydration of the sections, antigen retrieval was conducted by heating the (ethylenediaminetetraacetic acid) EDTA buffer. The CD40 anti-Mouse ligand clone antibody (12-1541-82, eBioscience™, USA) was used and incubated overnight, followed by incubation with a secondary antibody that was combined with horseradish peroxidase. The reaction was observed using DABi (diaminobenzidine) substrate and later counterstained with Gill’s hematoxylin. The slides were then examined under a light microscope (Lin et al., 2022: Saleh and Altaey, 2023). Statistical analysisData of macroscopic and microscopic examinations were analyzed for descriptive statistics using (IBM,Spss v27,UK). Data were confirmed to be normally distributed using Shapiro–Wilk test. Differences among age groups were demonstrated using one-way analysis of variance and Duncun post hoc test. All tests were performed at a significance level of p ≤ 0.01 (Petrie and Watson, 2013). Ethical approvalThe study was approved by the Institutional Animal Care and Use Committee of the College with Ref No. of (UM.VET.2023.035) dated 17/5/2023. ResultsTwo-week age groupMacroscopic observations showed that the dwarf hamster female had left and right ovaries located inside the upper abdominal cavity between the medial surface abdominal wall and the kidney. The ovaries were very small in size and had a compressed, round shape and smooth surface. The oviduct extended along the medial border of the ovary (Fig. 1). The right ovary was wider and longer than the left ovary. However, the weight was similar between the right and left ovaries, and the macroscopic measurements showed no significant differences between the right and left ovaries among animals of this group (Table 1). Histologically: The ovaries were covered with simple cuboidal epithelium, and the ovarian cells overlain fibrous connective tissue, tunica albuginea. The parenchyma was composed of two zones, the outer cortex and inner medulla, with the shallow notch representing the primary ovarian hilum. Within the cortex, there were clusters of oocytes (Fig. 2) and numerous primordial, primary, secondary, and degenerative follicles (Fig. 3); the ovaries at this age are devoid (absant) of mature follicles (Graafian follicle) and the corpus luteum. The ovarian medulla was clear and easily recognized at this age, and it comprised connective tissue with prominent blood vessels (Fig. 4). The microscopic measurements showed that the numbers of primordial, primary, and secondary follicles were higher in the right ovary than in the left ovary, and the diameters of these follicles were significantly higher in the right ovary than in the left, at a significant level of p ≤ 0.01 (Tables 2 and 3). The immunohistochemical observations showed that T lymphocytes were scarce and distributed in the ovarian parenchyma and around the follicles (Fig. 5). Four-week age groupThe length, width, and weight gradually and significantly increased with age. The surface remained smooth grossly, and the shape turned to bean-like. Within the same age group, the measurements also showed variations. The right ovary was longer than the left ovary, while there were no noticeable differences seen in the width and weight of both ovaries (Figs. 1 and 6) (Table 1).
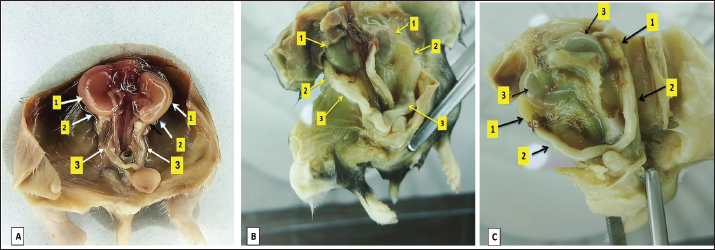
Fig. 1. Illustrates the position of the ovary in the abdominal cavity at different developmental stages: at 2 weeks of age (A), at 4 weeks of age (B), and within the 8-week age group (C). The noticeable changes include variations in the size and positioning of the ovary (2) and oviduct (3) in relation to the kidney (1). Table 1. Dimensional measurements of the left and right ovaries among age groups.
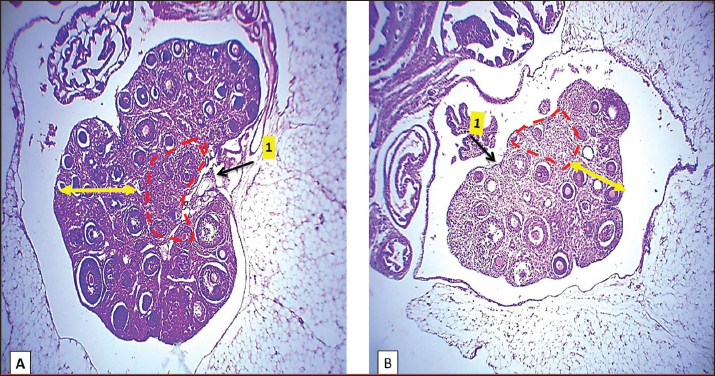
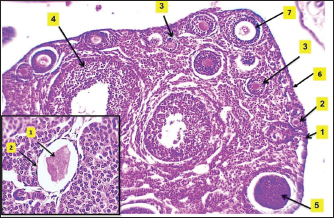
Fig. 2. Microphotograph showing the microscopic structure of the ovary in 2 weeks age group, the left ovary (A), the right ovary (B), the medulla (dashed circle), the cortex (arrows), and the ovarian hilum (1). H&E 40X.
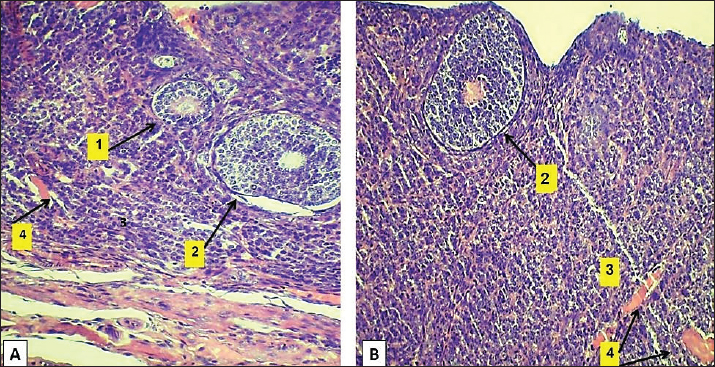
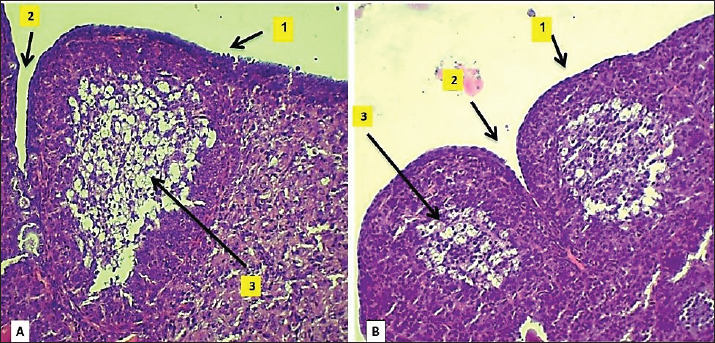
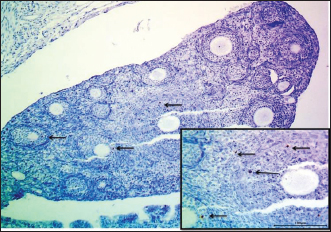
Fig. 3. Microphotograph showing the cortex of the ovary in 2-week-old animals, at the left ovary (A) and right ovary (B). The figure illustrates the primary follicles (1), secondary follicles (2), cortex (3), ovarian blood vessels (4), and H&E 100X. Histologically, the microscopic structure of the ovaries was similar to that of the younger animal group. Single line of simple cuboidal epithelium covering the ovarian surface and the parenchyma filled with progressively developing primordial, primary, secondary, Graafian, and atretic follicles. The number of left ovarian follicles was greater than that of right ovarian follicles (Fig. 7) (Table 2).
Fig. 4. Microphotograph showing the ovarian cortex in the 2-week age group. The left ovary (A) and right ovary (B). The figure illustrates the ovarian epithelium (1), ovarian notch (2), the oocytes clusters (3), H&E 400X. Table 2. Numbers of ovarian follicles and corpus luteum in left and right ovaries among age groups.
Table 3. Diameters of ovarian follicles and related structures in the left and right ovaries among age groups.
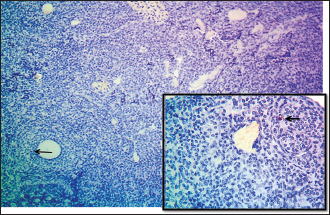
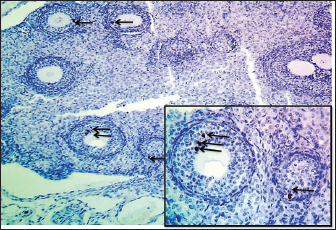
Fig. 5. Microphotograph showing immunohistochemical positive expression of CD40 in T-lymphocytes (arrows) within the ovarian parenchyma of the 2-week age group, IHC 100x (magnified figure 400X).
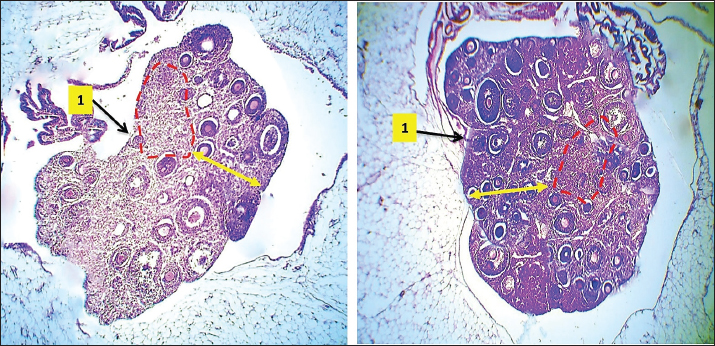
Fig. 6. Microphotograph showing the microscopic structure of the ovary in 4-week age group, The left ovary (A), right ovary (B), medulla (dashed circle), cortex (arrows), ovarian hilum (1), H&E 40X.
Fig. 7. Microphotograph showing the ovarian follicles at 4 weeks of age, primordial follicles (1), primary follicles (2), secondary follicles (3), Graafian follicles (4), atretic follicles (5), H&E 100X. The microscopic measurements revealed that the diameters of the primordial and secondary follicles were longer in the right ovary compared to the left ovary. The primary and Graafian follicles were significantly longer in the left ovary than in the right ovary, and the mature ovary was larger. In addition, multiple corpus luteum were developed in the right ovaries. The immunohistochemical observations showed a slightly increased number of greater T-lymphocytes population compared with the younger (2 weeks of age) group distributed around the follicles (Fig. 8) (Table 3). Eight-week age groupThe ovaries are impacted among the medial wall of the abdominal cavity, kidney, small intestine, and the visceral fat. The ovaries extended beyond the dorsal surface of the kidneys, and the ovarian hilum became deeper and more prominent in the ovarian medial border (Fig. 1). The ovaries turned more longer and wider compared with the second and fourth week age groups, and were slightly heavier (Table 1). The shape of the right ovary was bean-like, and the left ovary was heart-like. Within similar age animals, the length, width, and weight were nearly equal between the right and left ovaries. Furthermore, there were no statistically significant differences observed among these measurements.
Fig. 8. Microphotograph showing immunohistochemical positive expression of CD40 in T-lymphocytes (arrows) distributed within the ovarian parenchyma of 4-week-old animals, IHC 100x (magnified figure 400X).
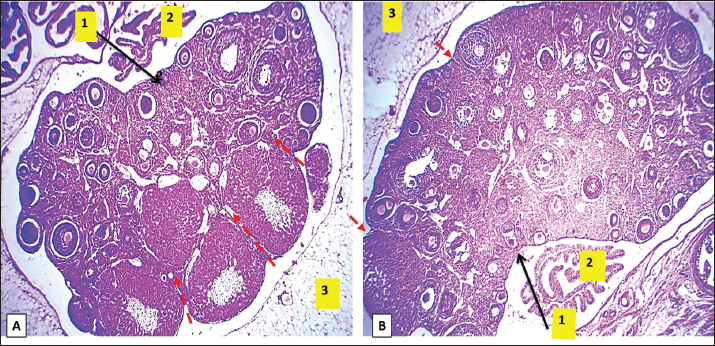
Fig. 9. Microphotograph showing the microscopic structure of the ovary in 8 weeks age group, the left ovary (A), the right ovary (B), the ovarian hilum (1), oviduct, and the surface of left ovary shows deep lobulation compared with right one (dashed lines) (2), mesovarium (3), H&E 40X.
Fig. 10. Microphotograph showing the ovarian follicles at 8 weeks age animals, the primordial follicles (1), primary follicles (2), secondary follicles (3), Graafian follicles (4), atretic follicles (5), ovarian epithelium (6), zona pellucida (7), (Magnified figure show the corona radiata (1), interstitial glands (2), H&E 100X. Histologically, the ovarian surface was lobulated and lobules were deeper in the left ovary compared to the right ovary, and the number of Graafian follicles were higher compared to the younger age groups with complete development of zona pellucida (Figs. 9 and 10), and in the right ovary were more than in the left ovary, the corpus luteum were larger and more distinct compared to the younger group, and in the left ovary more than in the right one, the mature corpus luteum composed of peripheral small luteal and central large luteal cells penetrated with septal connective tissues (Figs. 10 and 11). Multiple Graafian follicles had two or three mature ova in this age group ovaries. The number of atretic follicles was increased. Additionally, there was a cluster of interstitial glands accumulate around the mature corpus luteum, after ovulation, the internal granulosa cells covering the degenerated corona radiate, (Figs. 10 and 11), the microscopic measurements revealed that follicular dimeters of the primary, secondary, and Graafian follicles were significantly longer in the right ovary compared with left ovary. However, the proportions of primordial follicles were nearly equal in both ovaries. Furthermore, the mature oocyte was larger in the left ovary than in the right ovary (Table 3). Immunohistochemical observations showed a further increase in T-lymphocyte density in this group of animals, reflecting the relationship between corpus luteum development and T-lymphocyte population and their distribution within the ovarian parenchyma (Fig. 12).
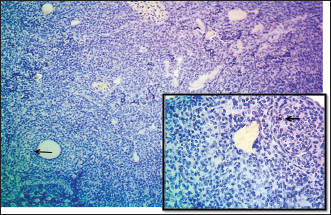
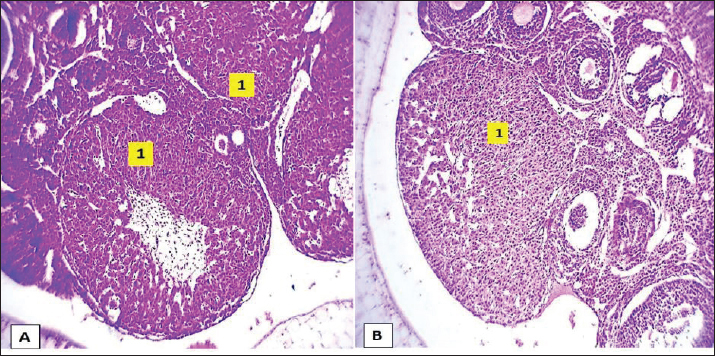
Fig. 11. Microphotograph showing the ovarian cortex in 8-week age group, pointing to the corpus luteum (1), in the left ovary (A), and right ovary (B), H&E 100X.
Fig. 12. Microphotograph showing immunohistochemical positive expression of CD40 in T-lymphocytes distribution (arrows) within the ovarian parenchyma of the 8-week age group, IHC 100x (magnified figure 400X). DiscussionThe findings of our study revealed notable morphological changes in the ovaries of dwarf hamsters with age progression. The length, width, and weight of the ovaries increase with age. The right ovary exhibited a bean-like shape, and the left ovary appeared heart-shaped. The ovarian surface remained smooth and later became more lobular (segmented) at 8 weeks of age. These morphological developmental changes are consistent with those of previous studies. For instance, Karimi et al. (2014) reported similar findings in dimensional measurements of golden hamster ovaries, which were consistent with our anatomical data on dwarf hamsters. Moreover, the ovaries of adult hamsters were deeply lobulated and densely occupied with different follicles. Al-Saffar and Al-Ebbadi, (2019), documented rounded ovaries in guinea pigs and found that preovulatory ovary dimensions and weights were comparable to our observations in 2-week-old hamsters. Furthermore, Ozdemir et al. (2005) described porcupine ovaries as smaller bean-like structures with a rough lobulated surface, corresponding to our findings. The differences in ovarian morphology among laboratory animals can be attributed to the inherent biological diversity present across species and reflect the developmental process governing maturity and adulthood in these animals. Furthermore, the current study revealed significant differences in the size and follicular activity between the left and right ovaries within each age group. The right ovaries exhibited a notably larger size compared with their left ovary, with slight variations in weight also observed. Such asymmetry in ovarian characteristics is widely recognized in animals. For instance, McKey et al. (2022) observed higher weight and functional activity in the right ovary of mice compared to the left. Similarly, Karamishabankareh et al. (2015) recorded greater length, width, and weight in the right ovaries of adult Bengal goats. Additionally, Reddy et al. (2005) reported increased follicular activity and size in the right ovary of cows compared with the left. The authors agree that changes in follicular size and growth are closely associated with functionality and development. Histologically, the dwarf hamster’s ovary is surrounded by a single layer of cuboidal cells and overlies fibrous connective tissue called tunica albuginea. The ovarian parenchyma comprises a cortex containing different developing follicles and a highly vascularized medulla. This microstructure is similar to that observed in the ovaries of different rodents, such as the European hamster (Karimi et al., 2014), rabbits (Al-Saffar and Almayahi, 2018), and mice (McKey et al., 2022). The identification of the medulla was clear in 2-week-old animals in this study, while it was not obvious in older animals. Al-Saffar and Almayahi, (2018), also mentioned the same notes in rabbits’ ovaries and attributed this to the small follicles in younger animals compared to the large ones in adult animals. The number of primordial follicles was scant in this age group compared with the other types of follicles, which increased gradually with age, and the primary oocytes were organized in clusters surrounded by stratified epithelium. This observation was confirmed by Reddy et al. (2005) in rats and mice. The number of primary, secondary, and Graafian follicles increased gradually with age, with obvious corpus luteum seen in 4–8 week-old animals. Furthermore, the diameter of the follicles also increased with age. Lo et al. (2019) ascribed this progression to the development and release of mature eggs during ovulation, which plays a crucial role in the reproductive cycle in animals. The critical age of ovarian development in dwarf hamsters extends from 4 to 8 weeks of age, when ovulation is initiated. Takagi et al. (2007) found that the development of the corpus luteum is a characteristic feature after 8 weeks of age in rats, and it is more prominent in the right ovary than the left ovary. The microscopic observations in this study also revealed complete development of the zona pellucida within mature follicles, an increased number of atretic follicles, and an increased depth of the ovarian hilum in 8-week-old hamsters. Similar observations were reported by Picut et al., 2015 in rats at 32 postnatal days (4 weeks old). They ascribed that to the increased sensitivity and responsiveness of the ovaries to the pituitary gland gonadotropins, corresponding to FSH elevation Reddy et al., (2005) also mentioned similar developmental events in the ovary of mice and correlated the understanding of follicular development with the treatment of infertility and oocyte transplantation. The density of T-lymphocytes increased with age and was noticeable within the follicular tissue of 8-week-old animals. Vinatier et al., (1995) suggested that the function of T-lymphocytes increases with age as a result of corpus luteum development and regression, due to the cytotoxic effect on luteal cells and the protective role of T-lymphocytes after ovulation. Bukulmez and Arici, (2000), also mentioned that T-lymphocytes contribute to the regulation of gonadal activity and the modulation of mature follicular rupture by secreting numerous regulatory soluble factors. The challenges in our study of dwarf hamsters stem from their early maturity and seasonal effects, which distinctly conceal these changes. On the other hand, the findings of these studies provide great potential benefits for the use of this model to understand ovarian development in other animals and humans. ConclusionThe current study explained the macroscopic and microscopic morphological development, symmetry between left and right ovaries, and distribution of T-lymphocytes within dwarf hamster ovaries. This investigation sheds light on the critical developmental age in the ovary of dwarf hamsters, a relatively understudied model characterized by a notably short maturity age compared with other laboratory animals. Such insights hold considerable promise for advancing our understanding of ovarian development in humans and diagnosing various infertility developmental disorders. AcknowledgmentsThe author thanks the staff of the Anatomy Department, College of Veterinary Medicine, University of Mosul, Iraq, for their assistance. Conflicts of interestThere are no conflicts of interest. FundingThe study was self-funded. Authors’ contributionsThekra F. conceptualized the project, wrote the initial draft, and conducted data analysis. Ghada A. performed the histological sectioning. Omar Y. conducted the microscopic evaluation and collected and analyzed the data. All authors contributed equally to the analysis, reviewing, and presentation of the final work. Data availabilityThe data used in this study are available in the manuscript. ReferencesAl-Saffar, F.J. and Al-Ebbadi, H.N.H. 2019. Histomorphological and histochemical study of the ovary and the uterine tubes of the adult guinea pigs (Cavia porcellus). Egypt. Acad. J. Biol. 11(2), 1–22; doi: 10.21608/eajbsd.2019.56024 Al-Saffar, F.J. and Almayahi, M.S. 2018. Histomorphological postnatal developmental study of the ovaries of the local rabbits (Oryctolagus cuniculus). Basra J. Vet. Res. 17(2), 124–146. Altaey, O.Y., Hasan, A.A. and Alhaaik, A.G. 2025. Early-life development of spleen in white rabbit (Oryctolagus cuniculus): a morphometric and histochemical analysis. Vet. Integr. Sci. 23(1), 1–13; doi: 10.12982/VIS.2025.012 Ancel, C., Bentsen, A.H., Sébert, M.E., Tena-Sempere, M., Mikkelsen, J.D. and Simonneaux, V. 2012. Stimulatory effect of RFRP-3 on the gonadotrophic axis in the male Syrian hamster: the exception proves the rule. Endocrinol. 153(3), 1352–1363; doi: 10.1210/en.2011-1622 Besselsen, D.G. 2002. Biology of laboratory Rodents. Res. Anim. Methods 443–453. Bukulmez, O. and Arici, A. 2000. Leukocytes in ovarian function. Hum. Reprod. Update 6(1), 1–15; doi: 10.1093/humupd/6.1.1 Carson, F.L. and Cappellano, C.H. 2015. Connective and muscle tissue. In: Histotechnology: a self-instructional text. Chicago, IL: American Society for Clinical Pathology, pp: 160–188. County, D . 2019. Annual report fiscal year 2017. Athens-Clarke County, GA. Avaialble via https://speakingofresearch.files.wordpress.com/2021/08/united-states-2019.pdf Fernández, E.A., Rosales, C.A., Garzón, J.P., Argudo, D.E., Ayala, L.E., Guevara, G.E. and Perea, F.P. 2022. Morphological and histological characteristics of ovaries from two genetic groups of guinea pigs (Cavia porcellus) from South America. Rev. Investig. Vet. Perú 33(4), e23349. Gaytán, F., Morales, C., Manfredi-Lozano, M. and Tena-Sempere, M. 2014. Generation of multi-oocyte follicles in the peripubertal rat ovary: link to the invasive capacity of granulosa cells?. Fertil. Steril. 101(5), 1467–1476; doi: 10.1016/j.fertnstert.2014.01.037 Hirose, M. and Ogura, A. 2019. The golden (Syrian) hamster as a model for the study of reproductive biology: past, present, and future. Reprod. Med. Biol. 18(1), 34–39; doi: 10.1002/rmb2.12241 Karamishabankareh, H., Hajarian, H., Shahsavari, M. and Moradinejad, R. 2015. In vivo and in vitro study of the function of the left and right bovine ovaries. Theriogenol 84(5), 724–731; doi: 10.1016/j.theriogenology.2015.04.033 Karimi, H., Abbasi, M., Balazadehkocheh, F. and Hasanzadeh, B. 2014. Histomorphometry of golden hamster ovaries. Anat. Sci. J. 11(1), 15–24. Available via http://anatomyjournal.ir/article-1-61-en.html Lin , F., Prichard, J.W., Liu, H. and Wilkerson, M.L. 2022. Handbook of practical immunohistochemistry: frequently asked questions. Springer Nature. Lo, B.K., Sheikh, S. and Williams, S.A. 2019. In vitro and in vivo mouse follicle development in ovaries and reaggregated ovaries. Reprod. 157(2), 135–148; doi: 10.1530/REP-18-0115 Martorell, J. 2017. Reproductive disorders in pet rodents. Vet. Clin. Exot. Anim. Pract. 20(2), 589–608; doi: 10.1016/j.cvex.2016.11.015 McKey, J., Anbarci, D.N., Bunce, C., Ontiveros, A.E., Behringer, R.R. and Capel, B. 2022. Integration of mouse ovary morphogenesis with developmental dynamics of the oviduct, ovarian ligaments, and rete ovarii. Elife 11, e81088; doi: 10.7554/elife.81088 Özdemir, D., Yılmaz, S., Dinç, G., Aydın, A. and Atalar, Ö. 2005. Observations on the morphological of the ovaries of the porcupine (Hystrix cristata). Vet. Arh. 75(2). 129–135. Petrie, A. and Watson, P. 2013. Hypothesis tests: the F-test. 3rd ed. In: Statistics for veterinary and animal science . Hoboken, NJ: Wiley-Blackwell, pp: 105–111. Picut, C.A., Dixon, D., Simons, M.L., Stump, D.G., Parker, G.A. and Remick, A.K. 2015. Postnatal ovary development in the rat: morphologic study and correlation of morphology to neuroendocrine parameters. Toxicol. Pathol. 43(3), 343–353; doi: 10.1177/0192623314544380 Reddy, P., Shen, L., Ren, C., Boman, K., Lundin, E., Ottander, U., Lindgren, P., Liu, Y.X., Sun, Q.Y. and Liu, K. 2005. Activation of Akt (PKB) and suppression of FKHRL1 in mouse and rat oocytes by stem cell factor during follicular activation and development. Dev. Biol. 281(2), 160–170; doi: 10.1016/j.ydbio.2005.02.013 Reese, J., Wang, H., Ding, T. and Paria, B.C. 2008. The hamster as a model for embryo implantation: insights into a multifaceted process. Semin. Cell. Dev. Biol. 19(2), 194–203; doi: 10.1016/j.semcdb.2007.11.001 Saleh, T.F. and Altaey, O.Y. 2023. Histomorphometrical and histochemical study of caecum in adult Muscovy ducks (Cairina moschata). Adv. Anim. Vet. Sci, 11(6), 1021–1029; doi: 10.17582/journal.aavs/2023/11.6.1021.1029 Suvarna, S.K., Layton, C. and Bancroft, J.D. 2019. Tissue processing. In Bancroft’s theory and practice of histological techniques. Elsevier, pp: 193–201. Takagi, K., Yamada, T., Miki, Y., Umegaki, T., Nishimura, M. and Sasaki, J. 2007. Histological observation of the development of follicles and follicular atresia in immature rat ovaries. Acta Med. Okayama 61(5), 283–298; doi: 10.18926/AMO/32892 Taketsuru, H., Hirayama, R., Nakatsukasa, E., Natsume, R., Takao, K., Abe, M. and Sakimura, K. 2024. Generation of rat offspring from ovarian oocytes by xenotransplantation. Sci. Rep. 14(1), 20109; doi: 10.1038/s41598-024-71030-0 Tateno, H., Tamura-Nakano, M., Kusakabe, H., Hirohashi, N., Kawano, N. and Yanagimachi, R. 2021. Sperm acrosome status before and during fertilization in the Chinese hamster (Cricetulus griseus), and observation of oviductal vesicles and globules. Mol. Reprod. Dev. 88(12), 793–804; doi: 10.1002/mrd.23547 Tysome, J.R., Li, X., Wang, S., Wang, P., Gao, D., Du, P., Chen, D., Gangeswaran, R., Chard, L.S., Yuan, M. and Alusi, G. 2012. A novel therapeutic regimen to eradicate established solid tumors with an effective induction of tumor-specific immunity. Clin. Cancer. Res. 18(24), 6679–6689; doi: 10.1158/1078-0432.CCR-12-0979 AVMA Guidelines for the Euthanasia of Animals AVMA Guidelines for the Euthanasia of Underwood, W. and Anthony, R. 2020. AVMA guidelines for the euthanasia of animals: 2020 edition. American Veterinary Medical Association. Vinatier, D., Dufour, P., Tordjeman-Rizzi, N., Prolongeau, J.F., Depret-Moser, S. and Monnier, J.C. 1995. Immunological aspects of ovarian function: role of the cytokines. Eur. J. Obst. Gynecol. Reprod. Biol. 63(2), 155–168; doi: 10.1016/0301-2115(95)02227-9 Weems, P.W., Goodman, R.L. and Lehman, M.N. 2015. Neural mechanisms controlling seasonal reproduction: principles derived from the sheep model and its comparison with hamsters. Front. Neuroendocrinol. 37, 43–51; doi: 10.1016/j.yfrne.2014.12.002 | ||
| How to Cite this Article |
| Pubmed Style Saleh TF, Sultan GA, Altaey OY. Analysis of ovarian morphometry and age-related T Lymphocyte localization in adult (Phodopus roborovski) dwarf hamsters. Open Vet. J.. 2025; 15(5): 2171-2181. doi:10.5455/OVJ.2025.v15.i5.35 Web Style Saleh TF, Sultan GA, Altaey OY. Analysis of ovarian morphometry and age-related T Lymphocyte localization in adult (Phodopus roborovski) dwarf hamsters. https://www.openveterinaryjournal.com/?mno=243296 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i5.35 AMA (American Medical Association) Style Saleh TF, Sultan GA, Altaey OY. Analysis of ovarian morphometry and age-related T Lymphocyte localization in adult (Phodopus roborovski) dwarf hamsters. Open Vet. J.. 2025; 15(5): 2171-2181. doi:10.5455/OVJ.2025.v15.i5.35 Vancouver/ICMJE Style Saleh TF, Sultan GA, Altaey OY. Analysis of ovarian morphometry and age-related T Lymphocyte localization in adult (Phodopus roborovski) dwarf hamsters. Open Vet. J.. (2025), [cited January 25, 2026]; 15(5): 2171-2181. doi:10.5455/OVJ.2025.v15.i5.35 Harvard Style Saleh, T. F., Sultan, . G. A. & Altaey, . O. Y. (2025) Analysis of ovarian morphometry and age-related T Lymphocyte localization in adult (Phodopus roborovski) dwarf hamsters. Open Vet. J., 15 (5), 2171-2181. doi:10.5455/OVJ.2025.v15.i5.35 Turabian Style Saleh, Thekra Fadel, Ghada Abdulrhman Sultan, and Omar Younis Altaey. 2025. Analysis of ovarian morphometry and age-related T Lymphocyte localization in adult (Phodopus roborovski) dwarf hamsters. Open Veterinary Journal, 15 (5), 2171-2181. doi:10.5455/OVJ.2025.v15.i5.35 Chicago Style Saleh, Thekra Fadel, Ghada Abdulrhman Sultan, and Omar Younis Altaey. "Analysis of ovarian morphometry and age-related T Lymphocyte localization in adult (Phodopus roborovski) dwarf hamsters." Open Veterinary Journal 15 (2025), 2171-2181. doi:10.5455/OVJ.2025.v15.i5.35 MLA (The Modern Language Association) Style Saleh, Thekra Fadel, Ghada Abdulrhman Sultan, and Omar Younis Altaey. "Analysis of ovarian morphometry and age-related T Lymphocyte localization in adult (Phodopus roborovski) dwarf hamsters." Open Veterinary Journal 15.5 (2025), 2171-2181. Print. doi:10.5455/OVJ.2025.v15.i5.35 APA (American Psychological Association) Style Saleh, T. F., Sultan, . G. A. & Altaey, . O. Y. (2025) Analysis of ovarian morphometry and age-related T Lymphocyte localization in adult (Phodopus roborovski) dwarf hamsters. Open Veterinary Journal, 15 (5), 2171-2181. doi:10.5455/OVJ.2025.v15.i5.35 |