| Research Article | ||
Open Vet. J.. 2025; 15(7): 2959-2971 Open Veterinary Journal, (2025), Vol. 15(7): 2959-2971 Research Article Contamination and antimicrobial resistance testing of Staphylococcus aureus isolates from pork in the traditional market at Central VietnamNguyen Van Chao1*, Ho Thi Dung1, Le Xuan Anh1,2, Vu Thi Thanh Tam3 and Pham Hoang Son Hung11Department of Veterinary Medicine, Faculty of Animal Sciences and Veterinary Medicine, University of Agriculture and Forestry, Hue University, Hue City, Vietnam 2Department of Veterinary Medicine, College of Veterinary Medicine, National Pingtung University of Science and Technology, Pingtung, Taiwan 3Mientrung Institute for Scientific Research, Vietnam National Museum of Nature, Vietnam Academy of Science and Technology, Hue, Vietnam *Corresponding Author: Nguyen Van Chao. Ph. D., Department of Veterinary Medicine, Faculty of Animal Sciences and Veterinary Medicine, University of Agriculture and Forestry, Hue University, Hue City, Vietnam. Email: nvchao [at] hueuni.edu.vn Submitted: 07/02/2025 Revised: 16/06/2025 Accepted: 20/06/2025 Published: 31/07/2025 © 2025 Open Veterinary Journal
ABSTRACTBackground: Staphylococcus aureus is an opportunistic pathogen widely present in humans and food-producing animals. The emergence of antimicrobial resistance (AMR) in S. aureus represents a major challenge for both animal and public health. Aim: This study investigated the presence of S. aureus in pork sold in traditional markets in Central Vietnam, analyzing its AMR profile and evaluating potential public health risks. Methods: We collected 48 retail pork samples from traditional markets in Central Vietnam. The S. aureus count (log10 CFU/g) was determined using Baird–Parker (BP) medium and was confirmed by the coagulase reaction. The S. aureus isolates were tested for AMR and AMR genes (ARGs) using the disk diffusion method and PCR, respectively. Results: All 48 retail pork samples (100%) tested positive for S. aureus, with S. aureus counts ranging from 4.17 to 4.52 log10 CFU/g. AMR was most commonly observed against penicillin (75.0%), followed by amoxicillin (64.6%), tetracycline (54.2%), ampicillin (41.7%), neomycin (39.6%), and vancomycin (39.6%). Multidrug resistance was observed in 85.4% of the S. aureus isolates, with penicillin–ampicillin–amoxicillin being the most common resistance pattern. The highest frequency of S. aureus isolates carried the tetM (30/48, 62.5%) gene, followed by blaZ (25/48, 52.1%), and femA (20/48, 41.7%). Moreover, the results revealed a positive correlation among β-lactams, specifically between ampicillin and neomycin, and between amoxicillin and erythromycin/neomycin. Conclusion: The high AMR of S. aureus isolates from retail pork samples is alarming and raises public health concerns. Food of animal origin serves as a source of AMR S. aureus, which can spread throughout the food supply chain. Therefore, controlling S. aureus requires targeting specific entry points based on findings related to risk factors and food contamination drivers. Keywords: S. aureus, Retail, Antimicrobial resistance, Vietnam, Market. IntroductionGlobal average annual meat consumption is approximately 15 kg per person for pork, 12.6 kg for poultry, and 9.6 kg for beef. In Europe, these figures are considerably higher, with per capita consumption reaching 34.2 kg for pork, 21.9 kg for poultry, and 16.1 kg for beef (World Health Organization, 2022). Pork is the most popular type of meat in Vietnam, accounting for around 73% of all meat consumption (Nguyen et al., 2006). A steady growth of per capita pork consumption was observed in the country, rising from 8.1 kg/capita/year in 1990 to 31.3 kg/capita/year in 2018 (Organisation for Economic Co-operation and Development, 2018). In 2023, pork production amounted to 3.8 million tons, an increase of 2.2% compared with 2017 (Vietnam Livestock Production, 2022). Nevertheless, food safety remains a major concern in Vietnam, which carries a high burden of foodborne illnesses, particularly those linked to pork products (Nguyen et al., 2020). Approximately 80% of pork in Vietnam is produced by smallholders, with processing and distribution occurring primarily through informal market channels, such as open-air and traditional markets (Lapar and Tiongco, 2011). Retail pork is frequently contaminated with biological hazards such as E. coli, Salmonella, Staphylococcus aureus, Streptococcus suis, and chemical hazards (Cook and Phuc, 2019). This widespread contamination contributes to the high burden of foodborne illnesses. Staphylococcus aureus is a major foodborne pathogen in fresh and processed products and is responsible for a wide range of infections worldwide (Diep et al., 2006). Staphylococcus aureus is widely distributed, but food is its most important source of infection. After Salmonella and Campylobacter, the two most common bacteria associated with food poisoning, S. aureus is the third most common foodborne pathogen, identified in approximately 241,000 cases globally per year (Wu et al., 2018a). In 2013, S. aureus caused 12.5% of all foodborne bacterial outbreaks in China (Wei-Wei et al., 2018). In Vietnam, 677 foodborne outbreaks involving 21,002 patients were recorded during the four years from 2011 to 2014 (Hung et al., 2020). The most frequent source of infection among Staphylococcal species is S. aureus, a facultatively aerobic Gram-positive bacteria. It is a component of the skin’s and the nasal mucosa’s microbiota. Nearly 20% of the general population is consistently positive for S. aureus, and this percentage can reach 80% in healthcare workers, frequent needle users, hospital patients, and people with compromised immune systems (Boucher and Corey, 2008; Foster and Geoghegan, 2023). Human and animal infections (zoonotic) caused by the bacteria S. aureus typically manifest as impetigo, pus-filled abscesses, swelling, discomfort, and pus-filled redness on the skin (Wertheim et al., 2005, 2009). Serious symptoms, such as fever, chills, and low blood pressure, might arise if the bacteria enter the bloodstream and cause sepsis. Myocarditis, pneumonia, osteomyelitis, and mastitis can all be caused by bacteria (Squier et al., 2002). Bacteria can cause serious infections when they reach the blood, leading to shock, multiple organ failure, or even death (Igbinosa et al., 2016). Food poisoning is a condition that can cause vomiting and fever in individuals who consume contaminated food containing S. aureus (Thompson and Brown, 2014). Over the past few decades, the widespread use of antimicrobials (AMs) to treat infectious diseases has contributed to the emergence of multidrug-resistant (MDR) strains, posing major challenges to public health (Ahmed et al., 2024). As a highly adaptable pathogen, S. aureus can survive in various environmental conditions and rapidly develop resistance to most AMs (Velasco et al., 2022). Currently, an increasing number of MDR S. aureus strains have been reported in food poisoning outbreaks and isolated from food products (Papadopoulos et al., 2018; Dorjgochoo et al., 2023). Furthermore, methicillin-resistant S. aureus (MRSA) has gained widespread attention. MRSA has been increasing in prevalence (Savariraj et al., 2019; Velasco et al., 2022), with recent reports indicating higher resistance rates in foodborne isolates. MRSA often exhibits MDR and was listed by the WHO in 2017 as one of the 12 bacterial families posing the greatest threat to human health (Asokan and Vanitha, 2018). In Vietnam, outbreaks of community-acquired MRSA infections have been documented by Tang et al. (2007). MRSA bloodstream infections accounted for 19% of reported cases in Vietnam, according to Song et al. (2011). The most frequently identified bacterium from the nasal mucosa was S. aureus (110/838; 13.1%), followed by 8.6% (72/838) of MRSA isolates (Thuy et al., 2017). A notable height rate of MRSA bacterial isolation was reported by Nguyen et al. (2014), with 303 of 1,016 samples (29.8%). These findings and others demonstrate a strong association between MRSA infection and increased morbidity and mortality (Shoaib et al., 2023). However, little information on S. aureus contamination in pork meat from Central Vietnam has been available to date. Given the need to better understand the dissemination of S. aureus in traditional markets, this study employed microbiological and genetic methodologies to characterize isolates and their resistance profiles. In light of these findings, the present study examined the contamination and AM resistance (AMR) prevalence of S. aureus isolates from retail pork meat sold at traditional Vietnamese markets in Hue City, Central Vietnam. Additionally, we compared these profiles with previously reported data from various regions of Vietnam to determine whether foodborne S. aureus isolates exhibit geographical variation. Materials and MethodsSample size and study areaA total of 48 retail pork samples were collected from 16 distinct meat sellers at four traditional markets in Hue City, Central Vietnam, between June and August 2023. Samples were selected based on [only sell pork (not sold with other meat), the amount of daily sales, about table form, cutting board, and knife], while samples with [the number of customers, location] were discarded. Samples were collected once a month, with 16 samples per collection. Samples were collected from the following traditional markets in Hue City: An Cuu (n=12), Ben Ngu (n=12), Dong Ba (n=12), and Tay Loc (n=12). The samples were preserved in sterile zip-lock plastic bags, placed in cold boxes at temperatures below 4°C, and transported to an accredited laboratory, where microbiological analysis was conducted within 4 hours. S. aureus counts and identificationStaphylococcus aureus counts and identification were performed according to the Viet Nam Standards and Quality Institute (2015). Briefly, a total of 25 g of each sample was cut into small pieces with sterile scissors, placed in a stomacher bag, mixed with 225 ml of sterile 0.1% Buffered Peptone water (BPW, Oxoid, Thermo Fisher, Hants, UK), and homogenized using a Stomacher® 400 Circulator (Seward, UK). For S. aureus counts, 1 ml of each homogenate was used to prepare 10-fold serial dilutions up to 104 in 0.1% (w/v) BPW. Aliquots of 0.1 ml of each dilution were spread in triplicate onto Baird–Parker (BP) agar (Becton, Dickinson, USA, 276840) containing 5% egg yolk tellurite emulsion and 6.5% NaCl. After incubation at 37°C for 24–48 hours, selected plates with 20–200 colonies displaying the typical S. aureus morphology (black center surrounded by an opaque halo) were counted. Ten to twenty colonies were randomly selected and identified them as S. aureus based on colony morphology, Gram stain appearance, and the coagulase reaction (Lin et al., 2009). Moreover, we confirmed each strain as S. aureus by polymerase chain reaction (PCR) testing for the presence of S. aureus–specific nuc gene (Brakstad et al., 1992). Staphylococcus aureus NCTC 6571 (Oxford Staphylococcus aureus NCTC 6571, Heatley Oxford, Heatley, UK) strain was used as the quality-control strain. The identified S. aureus isolates were stored at −80°C in brain heart infusion (BHI) broth (Difco, USA, 1104930500) and were supplemented with 15% glycerol until further analyses. AM susceptibility testingThe disk diffusion method was used to assess the AM susceptibility of S. aureus isolates using the Mueller–Hinton agar (MH, Oxoid, UK) plates according to the guidelines and recommendations of the Clinical and Laboratory Standards Institute (CLSI, 2020). Penicillin (PEN, 10 UI), amoxicillin (AMO, 10 µg), ampicillin (AMP, 10 µg), streptomycin (STR, 10 µg), neomycin (NEO, 30 µg), amikacin (AMK, 30 µg), gentamicin (GEN, 10 µg), enrofloxacin (ENR, 5 µg), vancomycin (VAN, 30 µg), erythromycin (ERY, 15 µg), doxycycline (DOX, 30 µg), and tetracycline (TET, 30 µg) (Oxoid™, United Kingdom) were used for AM susceptibility testing. The inoculum was prepared from cultures in BHI broth incubated for 4–6 hours before adjusting the turbidity to 0.5 according to the McFarland scale (DensiCHEKTM Plus, ALT, San Diego, US). One hundred microliters of the bacterial suspension was spread on MH agar. The appropriate AM-impregnated disks were placed on the agar surface, and the plates were incubated at 37°C for 24 hours. The zones of inhibition were measured, and the AM susceptibility of the isolates was determined using interpretative standards for Staphylococcus species (Eurl, 2018; CLSI, 2020). AMR gene amplificationFor the AM resistance genes (ARGs) of S. aureus isolates, the β-lactams (blaZ, rpoB, and femA), macrolides (ermB), and tetracycline (tetM) were detected using a single PCR method (Akanbi et al., 2017). The predicted sizes of the amplified products of the specific oligonucleotide primers used in this study are presented in Table 1. The PCR assays were performed in a 25 μl reaction volume, which included 2 μl of gDNA template (at >10 ng·μl−1), 1 µl of each primer (25 µmol·l−1), 12.5 µl of 2× Taq PCR master mix (CW Biotech, China), and 8.5 µl of ultrapure H2O. PCR products were then electrophoresed in 1.0%–1.5% agarose gels, stained with Gelred (Thermo Scientific, UK), and visualized under an ultraviolet transilluminator (Bio-Rad, USA). Statistical analysisAll data were managed in Microsoft Excel 2016 (MSO, 16.0.4266.1001) and analyzed using SPSS (IBM SPSS Statistics version 18.0, IBM, Armonk, NY, USA). The bacterial count was expressed in log10 CFU/g (colony-forming units per gram, expressed on a logarithmic scale). The S. aureus count was not normally distributed and was log10 transformed before analysis. The isolates resistant to at least two different types of AM agents were classified as MDR isolates. Two indices were used to assess the results obtained: the AMR index (ARI) and the MDR index (MARI), as described by Manjusha et al. 2005 and Krumperman (1983). An isolate carrying two or more ARGs was defined as a multiple-ARG isolate. Pairwise analysis of the association between AMR phenotypes was performed using Pearson’s correlation analysis. A positive association (>0) indicates a similarity in susceptibility or resistance between two AMs, whereas a negative association (<0) indicates an association between the susceptibility of an individual AM and the resistance to another AM. Differences were considered statistically significant when p < 0.05. Ethical approvalNot needed for this study. ResultsDetection of S. aureus contamination of samplesOverall, 100% of the retail pork samples were positive for Staphylococcus with the typical colonies growing on BP-specific agar, and there were confirmed for S. aureus by colony morphology, Gram staining, and coagulase. The colonies were determined positive by black color, shiny, rounded, and 1–1.5 mm in diameter, with a wide halo around the colony 2–5 mm (Table 2). As shown in Table 2, the contamination level of all samples with S. aureus was 4.40 log10 CFU/g. The S. aureus count in pork samples from Dong Ba market (4.52 log10 CFU/g) was highest, followed by the samples from An Cuu and Tay Loc (4.45 log10 CFU/g), while the lowest S. aureus count was observed in the samples from Ben Ngu with 4.17 log10 CFU/g. However, the S. aureus counts were not significant difference (p > 0.05) between the samples from difference markets. According to TCVN 7046:2015 (Viet Nam Standards and Quality Institute, 2015), the permissible limit of S. aureus in unprocessed meat products is 102 CFU/g sample. The results indicated that the number of S. aureus in all samples from traditional markets in Hue city was higher than the minimum allowed limit by TCVN7046:2015 (Viet Nam Standards and Quality Institute, 2015). Table 1. Primers used for ARG amplification.
Table 2. The contamination of S. aureus in pork meat samples.
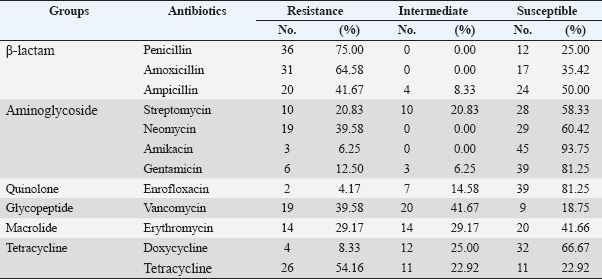
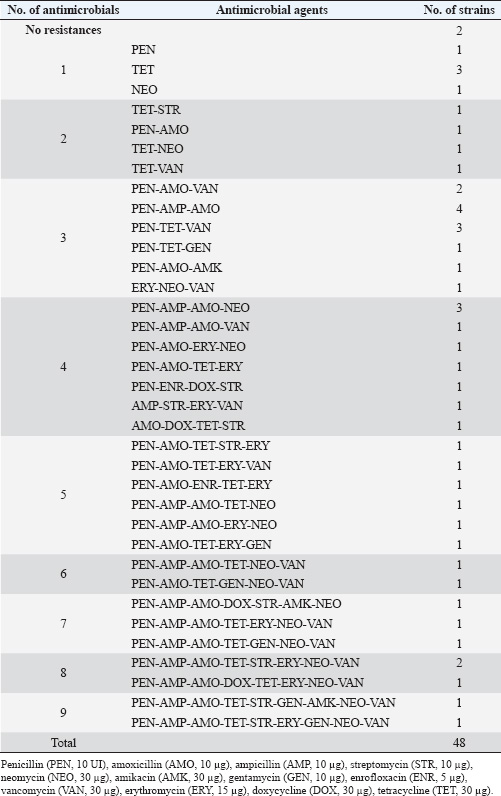
AMR profiles of S. aureus isolatesThe frequencies of resistance to 12 AM agents in six different groups were assessed. The results are summarized in Tables 3, 4, and 5. As shown in Table 3, the S. aureus isolates exhibited the highest rate of resistance to PEN (36/48, 75.00%), followed by AMO (31/48, 64.58%), and TET (26/48, 54.16%). The majority of the isolates were sensitive to AMK (45/48, 93,75%), followed by GEN and ENR (39/48, 81,25%), DOX (32/48, 66.67%), NEO (29/48, 60,42%), and AMP (24/48, 50.00%). Overall, 95.8% (46/48) of the S. aureus isolates were resistant to at least one AM (Table 5). The MARI values of the S. aureus isolates ranged from 0.17 to 0.75 (Table 5). There were 41 (85.4%) isolates resistant to two or more AMs (considered as multi-AMR), and most of the S. aureus isolates (27, 56.3%) resistant to three to five AMs (MARIs were 0.25, 0.33, and 0.42, respectively). The predominant MARI (0.17) was found in four isolates that were resistant to two out of the 12 AMs. Surprisingly, two S. aureus isolates were found to have the highest MARI of 0.75. They were resistant to 9 of the 12 AMs. A total of 32 MDR patterns were observed for the S. aureus strains for the 12 AMs tested (Table 4). Resistant to PEN-AMP-AMO was most prevalence observed in 4 (9.7%) MDR isolates. The other common patterns were PEN-TET-VAN-resistant and PEN-AMP-AMO-NEO observed in 3 (7.3%) MDR isolates. Prevalence of ARGs in the S. aureus isolatesThe ARG profiles of 48 S. aureus isolates from retail pork samples from Hue City are presented in Table 6. The most common in ARGs in the S. aureus isolates were tetM (30/48, 62.5%), blaZ (25/48, 52.1%), and femA (20/48, 41.7%). The incidences of rpoB (15/48, 31.3%) and ermB (12/48, 25.0%) were lower than those of other detected ARGs. The S. aureus isolates had multi-ARGs (35/48, 72.9%). Specifically, one isolate carried all five ARGs tested (Table 6). Correlations between the two AMR statuses of S. aureusThe pairwise correlation analysis of the AMR status of S. aureus isolates is presented in Table 7. Table 7 shows that several strong correlations among resistance to different AMs in S. aureus isolates were observed. A significant positive correlation was observed among the cell wall synthesis inhibitors, specifically between resistance to PEN and AMP, PEN and AMO, and AMP and AMO, with Pearson’s correlation coefficients of 0.39, 0.68, and 0.54, respectively (p < 0.001). Moreover, resistance to cell wall synthesis inhibitors was positively correlated with resistance to protein synthesis inhibitors, such as the resistance to AMP and NEO (0.53, p < 0.001), AMO and ERY (0.28, p < 0.05), and AMO and NEO (0.33, p < 0.05). For AMs that inhibit protein synthesis, resistance to STR, GEN, and AMK was positively correlated with resistance to DOX (0.40, p < 0.01), TET (0.35, p <0.01), and STR (0.29, p < 0.05), respectively. Table 3. Antimicrobial resistance prevalence among the S. aureus isolates.
DiscussionThe main objective of this study was to assess the contamination of retail pork sold in traditional markets with S. aureus. This is the first study on the prevalence of AMR S. aureus from traditional markets in Central Vietnam. This study found a high frequency of S. aureus in retail pork samples from all markets (100%). The frequencies of S. aureus contamination in retail pork samples ranged from low (5.0%) (Yakubu et al., 2020) to high (72.5%) (Owuna et al., 2015). A study by Ton et al. (2021) in Hue City reported the absence of S. aureus in processed meat. However, Fahrion et al. (2013) reported that 41% of pork samples in the pork supply chain in Hanoi were positive for S. aureus. In other countries such as China, India, and Thailan, the frequencies of S. aureus in retail pork samples were 35.0% (Wu et al., 2018b), 76,67% (Savariraj et al., 2019) and 28.18% (Kanungpean et al., 2021), respectively. Numerous factors contribute to the microbial contamination of meat, including slaughtering and transportation processes, as well as the sanitary conditions of the meat counter (Akagha et al., 2015; Bodunde et al., 2019). Throughout this process, meat is inadequately stored, facilitating the development of microorganisms over time. Moreover, cross-contamination may occur during its sale at the market. The majority of meat found at markets originates from slaughterhouses that employ manual slaughtering processes, which often fail to guarantee safe and hygienic conditions. Consequently, during the slaughtering process, meat can become contaminated with microorganisms present in water, on floors, utensils, slaughter tools, and through contact with workers (Klaharn et al., 2022). Furthermore, the sample collection time for this research at the market is from 7 to 9 am, a period of high customer traffic. During this time, meat undergoes frequent handling by both sellers and buyers, increasing the likelihood of cross-contamination. Additionally, the cutting boards and knives used during the trading process are not adequately maintained, further elevating the risk of microbial contamination in meat (Sekoai et al., 2020). The previous study conducted by Ly and Nguyen (2016) revealed that pork samples sold at the market tend to accumulate higher levels of S. aureus over time postslaughter. Within 6 hours after slaughter, 70.63% of the samples obtained from local markets contained more than 102 CFU/g of S. aureus, whereas only 16.67% of samples from supermarkets exhibited similar levels. These findings underscore the significant impact of storage temperature and exposure conditions on product quality. Overall, a comprehensive assessment of food safety levels for fresh meat involves a multidimensional approach that addresses microbial, chemical, and physical hazards throughout the production and distribution process. Regular monitoring, compliance with regulations, and continuous improvement efforts are essential to ensure the quality and safety of fresh pork and processed meat for consumers. There was no significant difference in S. aureus counts between the markets (p > 0.05), which may reflect the uniformity of trading conditions across sites. These conditions include pork sourced from slaughterhouses with similar hygiene standards and transportation methods, comparable customer volumes, and consistent sampling times. According to Lapar and Tiongco (2011), approximately 80% of pork in the region is produced by smallholders, with processing and retail activities primarily conducted within the informal market value chain, such as open-air and traditional markets. AM-resistant S. aureus isolates from food are common and are a major cause of economic losses in the food supply chain (Richter et al., 2012). In the present study, 95.8% of S. aureus isolates from retail pork samples were resistant to at least one type of AM. A significantly higher resistance to PEN, AMO, and TET was observed. These results are consistent with previous studies in Vietnam (Hoang et al., 2024) and other nations (Ge et al., 2017; Kraushaar et al., 2017; Wang et al., 2017; Wu et al., 2018b). A reason for the high level of AMR in S. aureus is the widespread use of AMs in animal production (Chu et al., 2013; Jibril et al., 2021). Vancomycin is a key drug for treating MRSA and other Gram-positive infections (Dasgupta, 2012). In 1997, Hiramatsu et al. 1997 first reported vancomycin-resistant S. aureus (VRSA). Recently, other studies reported VRSA isolates from pork meat (Kanungpean et al., 2021; Sineke et al., 2021; Velasco et al., 2022). The high frequency of VRSA (39.58%) in food may reduce the effectiveness of VAN for treating important human infectious diseases. In June 2002, the first VRSA strains were identified, carrying the vanA operon within the Tn1546 transposon (Centers for Disease Control Prevention, 1997). Tn1546, which confers VAN resistance, was first described in the Enterococcus genus in 1988 (Uttley et al., 1988). Subsequent research has shown that Tn1546 is expressed in most staphylococcal strains (Wang et al., 2022; Vo et al., 2024). Glycopeptide resistance encoded by the vanA operon is more frequently expressed in S. aureus strains harboring mutations in their restriction-modification systems and/or carrying pSK41-like conjugative plasmids, which increase the likelihood of vanA operon transfer (LeBard et al., 2008; Rossi et al., 2014). The detection of vanA in both VRSA and vancomycin-resistant Enterococcus (VRE) suggests horizontal gene transfer from Enterococcus to Staphylococcus. The clinical significance of VRE lies in its ability to transmit vancomycin resistance genes to MRSA. Table 4. Antimicrobial resistance patterns of the S. aureus isolate.
Table 5. Number of AMRs and ARGs in the S. aureus isolates.
Table 6. Antimicrobial resistance genes prevalence among the S. aureus isolates (n=48).
Moreover, 41 out of 48 (85.4%) S. aureus isolates were MDR, and 37 (77.08%) had MARI > 0.25. The results of this study are similar to those of a study in China, in which 94.6% of S. aureus isolates were MDR (Wu et al., 2018b). Additionally, according to Waters et al. (2011), up to 52% of S. aureus isolates from pork samples in the US were MDR. In this study, the MARI of S. aureus isolates ranged from 0.17 to 0.75. Compared with a previous study in Malaysia, the MARI of S. aureus isolates from cutting boards ranged from 0.18 to 0.55 (Mahyudin et al., 2019). Moreover, the results in the present study showed that the MARI of S. aureus isolates was >0.2 MAR. This indicates that these isolates originated from a high-risk source of contamination with a high frequency of AM use (Davis and Brown, 2016; Onaolapo et al., 2016). In 2006, class I integrons previously identified in Gram-negative bacteria were first detected in staphylococci (Shi et al., 2006). These were initially found in coagulase-negative staphylococci and later in S. aureus. These integrons contained gene cassettes associated with resistance to STR (aadA2, aadA5), CHL (cmlA1), and TXT (dfrA12, dfr17) (Xu et al., 2008). The emergence and spread of such integrons in staphylococci and other Gram-positive bacteria raise significant concerns regarding the potential for rapid horizontal gene transfer across species and genera. This includes the risk of Gram-positive bacteria acquiring resistance genes typically associated with Gram-negative organisms, thereby accelerating the dissemination of AMR. A high frequency of pork samples were contaminated with S. aureus (in the present study), E. coli, and Salmonella (in the same samples of our previous study) (Le et al., 2023). The cooccurrence of these bacteria may increase the likelihood of horizontal transfer of antibiotic resistance genes within pork samples. These findings highlight the potential public health risk associated with AMR S. aureus isolates from freath foods sold in traditional markets. These findings highlight the potential public health risk associated with AMR S. aureus isolates from freath foods sold in traditional markets. Table 7. Pairwise correlation between two antimicrobial resistance statuses.
The high frequency of ARGs in S. aureus isolates is concerning because it highlights the potential for transmission of resistance traits from the food supply chain to the clinical setting. This study showed that 62.5% of the S. aureus isolates carried the tetM gene, which encodes for TET resistance. A high prevalence (82.8%) of S. aureus isolates from hospitals in Vietnam carrying the tetM/K gene was reported by Son et al. (2019). The high frequency of the tetM gene may indicate the overuse of TETs in livestock production in recent years (Nguyen et al., 2016; Nguyen Van Chao et al., 2022). The tetM gene binds to the EF-G site on the ribosome, preventing TETs from binding and exerting their effects (Grossman, 2016). Tetracycline resistance may be caused by mutations in chromosomal genes (such as Tet38) that lead to increased expression of TETs efflux pumps on the cell membrane (Truong-Bolduc et al., 2022). In agreement with the present study, Akanbi et al. (2017) confirmed that tetM, blaZ, rpoB, and ermB were the most prevalent ARGs in S. aureus isolates from food samples. The high resistance to PEN (75.0%) and AMO (64.58%) raises concerns because these AMs are still widely used in veterinary medicine. The presence of the blaZ gene in 52.1% of isolates suggests that β-lactamase production is a key driver of resistance. The more common AMR S. aureus isolates from food (as reported in the present study and others) significantly reduced the effectiveness of AMs in treating infectious diseases. Recently, the use of combination therapy instead of monotherapy has been found to be considerably more effective for treating MDR infections and preventing the emergence of new resistant strains (Dai et al., 2017). Hu et al. (2015) reported that the combination of a beta-lactam and GEN exerted a strong synergistic effect against S. aureus infection. However, in this study, the positive correlations among b-lactams (PEN and AMP), b-lactams (AMO), macrolides (ERY), and aminoglycosides (NEO) were investigated. Moreover, high-level resistance to b-lactams (PEN, AMO) and TET in the S. aureus isolates were also observed. Yu et al. (2020) demonstrated a marked synergistic activity of the combination regimens, including VAN/rifampicin, VAN/oxacillin, levofloxacin/oxacillin, and GEN/oxacillin, in treating S. aureus infection. In this study, no significant correlation was observed between VAN and the other AMs (except VAN and TET) was not observed. Therefore, combination therapy with VAN plus NEO or GEN may be an option for the treatment of S. aureus infections. Further studies are needed to evaluate the efficacy of AM combinations for treating S. aureus infections in humans and animals. ConclusionTo the best of our knowledge, this is the first study on the prevalence and AMR of S. aureus isolates from retail pork in Hue City, Vietnam. This study found that 100% of the retail pork samples collected from traditional markets in Hue City was contaminated with S. aureus. The S. aureus counts in all samples were higher than the minimum allowed limit by TCVN7046:2015. The S. aureus isolates exhibited high resistance to penicillin, amoxicillin, and tetracycline but remained susceptible to amikacin, gentamicin, enrofloxacin, and doxycycline. The mutiple AMR index ranged from 0.17 to 0.75 (resistance to two to nine different AMs). A high frequency of S. aureus isolates was resistant to three different AMs (ARI=0.25). Pairwise correlation analysis suggested that combining penicillin, ampicillin, and amoxicillin with neomycin may not be an effective option for treating S. aureus infections. Furthermore, a high rate of MDR S. aureus isolates from retail pork samples from traditional markets underscores the role of foodborne pathogens in the spread of AMR. The findings of this study underscore the need for strengthened surveillance throughout the food supply chain. Additionally, expanding the research scale and conducting studies over extended periods would yield comprehensive insights. Integrating advanced methodologies, such as large-scale genomic epidemiology, metagenomic analysis, and machine learning models for AMR predictions will greatly enhance our understanding of the evolution and dissemination of resistance within the food supply chain. AcknowledgmentsNone. Conflict of interestThe authors declare that they have no conflicts of interest regarding any financial, personal, or other relationships with individuals or organizations related to the material discussed in this manuscript. FundingThis work was supported by the University of Agriculture and Forestry, Hue University under Strategic Research Group Program Number NCM.ĐHNL.2022.02. Authors’ contributionAll authors contributed to the study conception and design. Nguyen Van Chao, Ho Thi Dung and Pham Hoang Son Hung developed the original hypotheses and designed the experiments; Ho Thi Dung and Pham Hoang Son Hung, Le Xuan Anh and Vu Thi Thanh Tam collected the data for this study and conducted the statistical analyses; Nguyen Van Chao, Ho Thi Dung, and Pham Hoang Son Hung collaborated in interpreting the results and finalized the manuscript. All authors have read and approved the finalized manuscript. Data availabilityData supporting the findings of this study are available from the corresponding author upon reasonable request under the Project funding. ReferencesAhmed, S.K., Hussein, S., Qurbani, K., Ibrahim, R.H., Fareeq, A., Mahmood, K.A. and Mohamed, M.G. 2024. Antimicrobial resistance: impacts, challenges, and future prospects. J. Med. Surg. Public Health 2(1), 100081. Akagha, T., Gugu, T., Enemor, E., Ejikeugwu, P., Ugwu, B. and Ugwu, M. 2015. Prevalence and antibiogram of Salmonella species and Staphylococcus aureus in retail meats sold in Awka metropolis, southeast Nigeria. Int. J. Biol. Pharm. Res. 6(12), 924–929. Akanbi, O.E., Njom, H.A., Fri, J., Otigbu, A.C. and Clarke, A.M. 2017. Antimicrobial susceptibility of Staphylococcus aureus isolated from recreational waters and beach sand in Eastern Cape Province of South Africa. Int. J. Environ. Res. Public Health 14(9), 1001. Asokan, G.V. and Vanitha, A. 2018. WHO global priority pathogens list on antibiotic resistance: an urgent need for action to integrate One Health data. Perspect. Public Health 138(2), 87–88. Bodunde, R., Ogidi, C. and Akinyele, B. 2019. Load and antibiotic susceptibility pattern of microorganisms in muscle foods sold in Akure, Southwest Nigeria. J. Food Qual. Hazards Control 6(1), 30–36. Boucher, H.W. and Corey, G.R. 2008. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 46(5), S344–S349. Brakstad, O.G., Aasbakk, K. and Maeland, J.A. 1992. Detection of Staphylococcus aureus by polymerase chain reaction amplification of the nuc gene. J. Clin. Microbiol. 30(7), 1654–1660. Centers for Disease Control Prevention. 1997. Update: Staphylococcus aureus with reduced susceptibility to vancomycin--United States, 1997. MMWR Morb. Mortal Wkly. Rep. 46(35), 813–815. Chu, H., Zhao, L., Zhang, Z., Gui, T., Han, L. and Ni, Y. 2013. Antibiotic resistance and molecular epidemiology of methicillin-resistant Staphylococcus aureus from lower respiratory tract: multi-resistance and high prevalence of SCC mec III type. Cell Biochem. Biophys. 124(4), 795–801. CLSI. 2020. Performance standards for antimicrobial susceptibility testing. 30th ed. CLSI supplement M100S. Wayne, PA: CLSI. Cook, M.A. and Phuc, P.D. 2019. Review of biological and chemical health risks associated with pork consumption in Vietnam: major pathogens and hazards identified in Southeast Asia. J. Food Qual. 2019(1), 1048092. Dai, C., Zhao, T., Yang, X., Xiao, X., Velkov, T. and Tang, S. 2017. Pharmacokinetics and relative bioavailability of an oral amoxicillin-apramycin combination in pigs. PLoS One 12(4), e0176149. Dasgupta, A. 2012. Chapter 3 - Advances in antibiotic measurement. In Advances in clinical chemistry. Ed. Makowski, G.S., Basel, Switzerland: Elsevier. No. 56, pp: 75–104. Davis, R. and Brown, P.D. 2016. Multiple antibiotic resistance index, fitness and virulence potential in respiratory Pseudomonas aeruginosa from Jamaica. J. Med. Microbiol. 65(4), 261–271. Diep, B.A., Gill, S.R., Chang, R.F., Phan, T.H., Chen, J.H., Davidson, M.G., Lin, F., Lin, J., Carleton, H.A., Mongodin, E.F., Sensabaugh, G.F. and Perdreau-Remington, F. 2006. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 367(9512), 731–739. Dorjgochoo, A., Batbayar, A., Tsend-Ayush, A., Erdenebayar, O., Byambadorj, B., Jav, S. and Yandag, M. 2023. Detection of virulence genes of Staphylococcus aureus isolated from raw beef for retail sale in the markets of Ulaanbaatar city, Mongolia. BMC Microbiol. 23(1), 372. Eurl, A. 2018. Isolation of methicillin-resistant Staphylococcus aureus (MRSA) from food producing animals and farm environment. DTU National Food Institute 1(1), 1–8. Fahrion, A.S., Lapar M. L., Toan, N.N., Thuy, D.N. and Grace, D. 2013. Food-borne hazards in a transforming pork value chain in Hanoi: basis for future risk assessments. Vietnam J. Pre. Med. XXIII(4), 18–25. Foster, T.J. and Geoghegan, J.A. 2023. Staphylococcus aureus infection. In Molecular medical microbiology, 3rd ed. Eds. Tang, Y.W., Hindiyeh, M., Liu, D., Sails, A., Spearman, P., Zhang, J.R. London: Elsevier, pp: 655–679. Ge, B., Mukherjee, S., Hsu, C.-H., Davis, J.A., Tran, T.T.T., Yang, Q., Abbott, J.W., Ayers, S.L., Young, S.R. and Crarey, E.T. 2017. MRSA and multidrug-resistant Staphylococcus aureus in US retail meats, 2010–2011. Food Microbiol. 62(1), 289–297. Grossman, T.H. 2016. Tetracycline antibiotics and resistance. Cold Spring Harb. Perspect. Med., 6(4), a025387. Hiramatsu, K., Hanaki, H., Ino, T., Yabuta, K., Oguri, T. and Tenover, F.C. 1997. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 40(1), 135–136. Hoang, M.D., Tran, T.K.H. and Hoang, M.S. 2024. Occurrence, antibiotic resistance profile and molecular characterization of Staphylococcus aureus isolated from pork and chicken meat sold in Gia Lam District, Hanoi City. Vietnam J. Food Control 7(2), 78–88. Hu, Y., Liu, A., Vaudrey, J., Vaiciunaite, B., Moigboi, C., McTavish, S.M., Kearns, A. and Coates, A. 2015. Combinations of β-lactam or aminoglycoside antibiotics with plectasin are synergistic against methicillin-sensitive and methicillin-resistant Staphylococcus aureus. PLoS One 10(2), e0117664. Hung, L., Nguyen, H., Yen, T., Le, V.-H., Ba, T., Quyen, P., Huong, D., Trung, N. and Hao, L. 2020. Isolation and characterization of Staphylococcus aureus from two large-scale food poisoning outbreaks in Vietnam. Health Risk Anal. 3(3), 139–147. Igbinosa, E.O., Beshiru, A., Akporehe, L.U., Oviasogie, F.E. and Igbinosa, O.O. 2016. Prevalence of methicillin-resistant Staphylococcus aureus and other Staphylococcus species in raw meat samples intended for human consumption in Benin City, Nigeria: implications for Public Health. Int. J. Env. Res. Public Health, 13(10), 949. Jibril, A.H., Okeke, I.N., Dalsgaard, A. and Olsen, J.E. 2021. Association between antimicrobial usage and resistance in Salmonella from poultry farms in Nigeria. BMC Vet. Res. 17(1), 234. Kanungpean, D., Takai, S. and Kakuda, T. 2021. Contamination and antimicrobial susceptibility testing of Staphylococcus aureus isolated from pork in fresh markets, Nongchok District, Thailand. Vet. Med. Int. 2021(1), 1–3. Klaharn, K., Pichpol, D., Meeyam, T., Harintharanon, T., Lohaanukul, P. and Punyapornwithaya, V. 2022. Bacterial contamination of chicken meat in slaughterhouses and the associated risk factors: a nationwide study in Thailand. PLoS One, 17(6), e0269416. Kraushaar, B., Ballhausen, B., Leeser, D., Tenhagen, B.-A., Käsbohrer, A. and Fetsch, A. 2017. Antimicrobial resistances and virulence markers in methicillin-resistant Staphylococcus aureus from broiler and turkey: a molecular view from farm to fork. Vet. Microbiol. 200(1), 25–32. Krumperman, P.H. 1983. Multiple antibiotic resistance indexing of Escherichia coli to identify high-risk sources of fecal contamination of foods. Appl. Environ. Microbiol. 46(1), 165–170. Lapar, M.L. and Tiongco, M.M. 2011. Private standards in pork value chains: role, impact and potential for local innovation to improve food safety and enhance smallholder competitiveness. Farm Policy J. 8(3): 39–53. Le, M.D., Ho, T.D., Pham, H.S.H., Nguyen, T.T., Phan, T.H., Le, T.H. and Nguyen, V.C. 2023. Assessment of microbial contamination in retail pork at some markets in Hue city (in Vietnamese). E-J. Agric. Sci. Technol. 7(3), 3814–3821. LeBard, R.J., Jensen, S.O., Arnaiz, I.A., Skurray, R.A. and Firth, N. 2008. A multimer resolution system contributes to segregational stability of the prototypical staphylococcal conjugative multiresistance plasmid pSK41. FEMS Microbiol. Lett. 284(1), 58–67. Lin, J., Yeh, K.-S., Liu, H.-T. and Lin, J.-H. 2009. Staphylococcus aureus isolated from pork and chicken carcasses in Taiwan: prevalence and antimicrobial susceptibility. J. Food Prot. 72(3), 608–611. Ly, T.L.K. and Nguyen, T.T. 2016. Study on the varation of quality of pork at markets and supermarkets. Can Tho Uni. J. Sci. 2(1), 61–68. Mahyudin, N.A., Sahil, S.M., Radu, S., Mahmud, N.K. and Rashid, A. 2019. Multiple drug resistance among Staphylococcus aureus strains isolated from cutting boards of commercial food premises: a threat to food and public health safety. J. Biochem. Microbiol. Biotechnol. 7(1), 48–51. Manjusha, S., Sarita, G.B., Elyas, K.K. and Chandrasekaran, M. 2005. Multiple antibiotic resistances of vibrio isolates from coastal and brackish water areas. Am. J. Biochem. Biotechnol. 1(4), 193–198. Nguyen Thi Thuy, M., Dorny, P., Lebailly, P., Le Thi Minh, C., Nguyen Thi Thu, H. and Dermauw, V. 2020. Mapping the pork value chain in Vietnam: a systematic review. Trop. Anim. Health Prod. 52(6), 2799–2808. Nguyen Thuy Minh, N.T.M., Yutaka, T., Fukuda, S. and Kai, S. 2006. The pork consumption and distribution in urban areas of Vietnam before WTO accession. J. Fac. Agric. Kyushu Univ. 51(2), 459–466. Nguyen, V.C., Nguyen, T.Q.A., Tran, T.N., Le Minh, D. and Bui, N.B. 2022. Antimicrobial usage in pig farms and the antimicrobial resistance of Staphylococcus aureus isolates from pigs and farmers in Huong Tra town, Thua Thien Hue province. HUAF J. Agri. Sci. Technol. 6(1), 2816–2825. Nguyen, V.C., Nguyen, T.N., Nguyen, H.N., Nguyen, T.M.H., Nguyen, V.T., Thwaites, G. and Carrique-Mas, J. 2016. Antimicrobial consumption in medicated feeds in Vietnamese pig and poultry production. EcoHealth, 13(3), 490–498. Nguyen, V.K., Zhang, T., Vu, T.B.N., Dao, T.T., Tran, K.T., Nguyen, T.D.N., Tran, T.H.K., Nguyen, T.C.K., Fox, A. and Horby, P. 2014. Staphylococcus aureus nasopharyngeal carriage in rural and urban northern Vietnam. Trans. R. Soc. Trop. Med. Hyg. 108(12), 783–790. Onaolapo, J., Olayinka, B., Adeshina, G., Igwe, J. and Obajuluwa, A. 2016. Antimicrobial susceptibility pattern of Staphylococcus aureus isolates from orthopaedic patients in Abuth Zaria. J. Food Indust. Microbiol. 2(1), 1–6. Organisation for Economic Co-operation and Development. 2018. Meat consumption 2018, Available via https://data.oecd.org/agroutput/meat-consumption.htm Owuna, G., Abimiku, R., Nkene, I., Joseph, G. and Ijalana, O. 2015. Isolation and antibiotic susceptibility of Staphylococcus aureus from fresh poultry meat sold in Keffi metropolis, Nigeria. Int. J. Res. Stud. Biosci. 3(11), 1–5. Papadopoulos, P., Papadopoulos, T., Angelidis, A.S., Boukouvala, E., Zdragas, A., Papa, A., Hadjichristodoulou, C. and Sergelidis, D. 2018. Prevalence of Staphylococcus aureus and of methicillin-resistant S. aureus (MRSA) along the production chain of dairy products in north-western Greece. Food Microbiol. 69(1), 43–50. Richter, A., Sting, R., Popp, C., Rau, J., Tenhagen, B.A., Guerra, B., Hafez, H. and Fetsch, A. 2012. Prevalence of types of methicillin-resistant Staphylococcus aureus in turkey flocks and personnel attending the animals. Epidemiol. Infect. 140(12), 2223–2232. Rossi, F., Diaz, L., Wollam, A., Panesso, D., Zhou, Y., Rincon, S., Narechania, A., Xing, G., Gioia, T.S.R.D., Doi, A., Tran, T.T., Reyes, J., Munita, J.M., Carvajal, L.P., Hernandez-Roldan, A., Brandão, D., Heijden, I.M.v.d., Murray, B.E., Planet, P.J., Weinstock, G.M. and Arias, C.A. 2014. Transferable vancomycin resistance in a community-associated MRSA lineage. New Engl. J. Med. 370(16), 1524–1531. Savariraj, W.R., Ravindran, N.B., Kannan, P., Paramasivam, R., Senthilkumar, T., Kumarasamy, P. and Rao, V.A. 2019. Prevalence, antimicrobial susceptibility and virulence genes of Staphylococcus aureus isolated from pork meat in retail outlets in India. J. Food Saf. 39(1), e12589. Sekoai, P.T., Feng, S., Zhou, W., Ngan, W.Y., Pu, Y., Yao, Y., Pan, J. and Habimana, O. 2020. Insights into the microbiological safety of wooden cutting boards used for meat processing in Hong Kong’s wet markets: a focus on food-contact surfaces, cross-contamination and the efficacy of traditional hygiene practices. Microorg. 8(4), 579. Shi, L., Zheng, M., Xiao, Z., Asakura, M., Su, J., Li, L. and Yamasaki, S. 2006. Unnoticed spread of class 1 integrons in gram – positive clinical strains isolated in Guangzhou, China. Microbiol. Immunol. 50(6), 463–467. Shoaib, M., Aqib, A.I., Muzammil, I., Majeed, N., Bhutta, Z.A., Kulyar, M.F.-e.-A., Fatima, M., Zaheer, C.-N.F., Muneer, A. and Murtaza, M. 2023. MRSA compendium of epidemiology, transmission, pathophysiology, treatment, and prevention within one health framework. Front. Microbiol. 13(1), 1067284. Sineke, N., Asante, J., Amoako, D.G., Abia, A.L.K., Perrett, K., Bester, L.A. and Essack, S.Y. 2021. Staphylococcus aureus in intensive pig production in South Africa: antibiotic resistance, virulence determinants, and clonality. Pathogens 10(3), 317. Son, N.T., Huong, V.T.T., Lien, V.T.K., Nga, D.T.Q., Au, T.T.H., Nga, T.T., Hoa, L.N.M. and Binh, T.Q. 2019. First report on multidrug-resistant methicillin-resistant Staphylococcus aureus isolates in children admitted to tertiary hospitals in Vietnam. J. Microbiol. Biotechnol. 29(9), 1460–1469. Song, J.H., Hsueh, P.R., Chung, D.R., Ko, K.S., Kang, C.I., Peck, K.R., Yeom, J.S., Kim, S.W., Chang, H.H., Kim, Y.S., Jung, S.I., Son, J.S., So, T.M., Lalitha, M.K., Yang, Y., Huang, S.G., Wang, H., Lu, Q., Carlos, C.C., Perera, J.A., Chiu, C.H., Liu, J.W., Chongthaleong, A., Thamlikitkul, V. and Van, P.H. 2011. Spread of methicillin-resistant Staphylococcus aureus between the community and the hospitals in Asian countries: an ANSORP study. J. Antimicrob. Chemother. 66(5), 1061–1069. Squier, C., Rihs, J.D., Risa, K.J., Sagnimeni, A., Wagener, M.M., Stout, J., Muder, R.R. and Singh, N. 2002. Staphylococcus aureus rectal carriage and its association with infections in patients in a surgical intensive care unit and a liver transplant unit. Infect. Control Hosp. Epidemiol. 23(9), 495–501. Tang, C.T., Nguyen, D.T., Ngo, T.H., Nguyen, T.M., Le, V.T., To, S.D., Lindsay, J., Nguyen, T.D., Bach, V.C., Le, Q.T., Le, T.H., Le, D.L., Campbell, J., Nguyen, T.K., Nguyen, V.V., Cockfield, J., Le, T.G., Phan, V.N., Le, H.S., Huynh, T.S., Le, V.P., Counahan, M., Bentsi-Enchill, A., Brown, R., Simmerman, J., Nguyen, T.C., Tran, T.H., Farrar, J. and Schultsz, C. 2007. An outbreak of severe infections with community-acquired MRSA carrying the Panton-Valentine leukocidin following vaccination. PLoS One 2(9), e822. Thompson, T. and Brown, P.D. 2014. Comparison of antibiotic resistance, virulence gene profiles, and pathogenicity of methicillin-resistant and methicillin-susceptible Staphylococcus aureus using a Caenorhabditis elegans infection model. Pathog. Glob. Health, 108(6), 283–291. Thuy, D.B., Campbell, J., Hoang, N.V.M., Trinh, T.T.T., Duong, H.T.H., Hieu, N.C., Duy, N.H.A., Hao, N.V., Baker, S. and Thwaites, G.E. 2017. A one-year prospective study of colonization with antimicrobial-resistant organisms on admission to a Vietnamese intensive care unit. PLoS One 12(9), e0184847. Ton, T.N.T., Ngo, T.T.M. and Pham, T.N.L. 2021. Assessment of microbiological contamination levels in processed meat products from markets in southern Hue city. Vietnam J. Food Contr. 4(2), 34–42. Truong-Bolduc, Q., Wang, Y. and Hooper, D. 2022. Role of Staphylococcus aureus Tet38 in transport of tetracycline and its regulation in a salt stress environment. J. Bacteriol. 204(7), e00142–00122. Uttley, A.C., Collins, C.H., Naidoo, J. and George, R.C. 1988. Vancomycin-resistant Enterococci. Lancet 331(8575), 57–58. Velasco, V., Mallea, A., Bonilla, A.M., Campos, J. and Rojas-García, P. 2022. Antibiotic-resistance profile of Staphylococcus aureus strains in the pork supply chain. Chil. J. Agric. Anim. Sci. 38(1), 234–240. Viet Nam Standards and Quality Institute, 2015. Food additive - Microbiological analyses. Part 6: detection and enumeration of Staphylococcus aureus by colony count technique., TCVN 11039-6:2015, Hanoi, Viet Nam Standards and Quality Institute. Vietnam Livestock Production. 2022. Thống kê chăn nuôi Việt Nam năm 2022 về số lượng đầu con và sản phẩm gia súc, gia cầm, vật nuôi khác (in Vietnamese), Available via https://nhachannuoi.vn/thong-ke-chan-nuoi-viet-nam-nam-2022-ve-so-luong-dau-con-va-san-pham-gia-suc-gia-cam-vat-nuoi-khac/ Vo, T., Pontarotti, P., Rolain, J.M. and Merhej, V. 2024. Mechanisms of acquisition of the vanA operon among vancomycin-resistant Staphylococcus aureus genomes: the tip of the iceberg? Int. J. Antimicrob. Agents. 63(6), 107154. Wang, H., Wu, D., Di, L., Zhu, F., Wang, Z., Sun, L., Chen, Y., Jiang, S., Zhuang, H., Chen, M., Ji, S. and Chen, Y. 2022. Genetic characteristics of multiple copies of Tn1546-like elements in ermB-positive methicillin-resistant Staphylococcus aureus from Mainland China. Front. Microbiol. 13, 814062. Wang, W., Baloch, Z., Jiang, T., Zhang, C., Peng, Z., Li, F., Fanning, S., Ma, A. and Xu, J. 2017. Enterotoxigenicity and antimicrobial resistance of Staphylococcus aureus isolated from retail food in China. Front. Microbiol. 8(1), 2256. Waters, A.E., Contente-Cuomo, T., Buchhagen, J., Liu, C.M., Watson, L., Pearce, K., Foster, J.T., Bowers, J., Driebe, E.M. and Engelthaler, D.M. 2011. Multidrug-resistant Staphylococcus aureus in US meat and poultry. Clin. Infect. Dis. 52(10), 1227–1230. Wei-Wei, L.I., Jiang-hui, Z.H.U., Shi-qi, Z., Xiao-cheng, L., Yu-yan, J., Ning, L.I., Jiao, X.U., Xiu-mei, L.I.U. and Yun-chang, G.U.O. 2018. Analysis of foodborne disease outbreaks in China mainland in 2011. Chin. J. Food Hyg. 30(3), 283–288. Wertheim, H.F., Melles, D.C., Vos, M.C., Van Leeuwen, W., Van Belkum, A., Verbrugh, H.A. and Nouwen, J.L. 2005. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 5(12), 751–762. Wertheim, H.F.L., Nguyen, H.N., Taylor, W., Lien, T.T.M., Ngo, H.T., Nguyen, T.Q., Nguyen, B.N.T., Nguyen, H.H., Nguyen, H.M., Nguyen, C.T., Dao, T.T., Nguyen, T.V., Fox, A., Farrar, J., Schultsz, C., Nguyen, H.D., Nguyen, K.V. and Horby, P. 2009. Streptococcus suis, an important cause of adult bacterial meningitis in Northern Vietnam. PLoS One 4(6), e5973. World Health Organization. 2022. World Food Safety Day 2022 in pictures and numbers. World Health Organization, Viale delle Terme di Caracalla 00153 Rome, Italy. Wu, S., Huang, J., Wu, Q., Zhang, F., Zhang, J., Lei, T., Chen, M., Ding, Y. and Xue, L. 2018a. Prevalence and characterization of Staphylococcus aureus isolated from retail vegetables in China. Front. Microbiol. 9(14), 1263. Wu, S., Huang, J., Wu, Q., Zhang, J., Zhang, F., Yang, X., Wu, H., Zeng, H., Chen, M., Ding, Y., Wang, J., Lei, T., Zhang, S. and Xue, L. 2018b. Staphylococcus aureus isolated from retail meat and meat products in China: incidence, antibiotic resistance and genetic diversity. Front. Microbiol. 9(14), 2767. Xu, Z., Li, L., Alam, M., Zhang, L., Yamasaki, S. and Shi, L. 2008. First confirmation of integron-bearing methicillin-resistant Staphylococcus aureus. Curr. Microbiol. 57, 264–268. Yakubu, A., Abdullahi, I., Whong, C. and Olayinka, B. 2020. Prevalence and antibiotic susceptibility profile of Staphylococcus aureus from milk and milk products in Nasarawa State, Nigeria. Sokoto J. Vet. Sci. 18(1), 1–12. Yu, Y., Huang, H.-L., Ye, X.-Q., Cai, D.-T., Fang, J.-T., Sun, J., Liao, X.-P. and Liu, Y.-H. 2020. Synergistic potential of antimicrobial combinations against methicillin-resistant Staphylococcus aureus. Front. Microbiol. 11(7), 1919. | ||
| How to Cite this Article |
| Pubmed Style Chao NV, Dung HT, Anh LX, Tam VTT, Hung PHS. Contamination and antimicrobial resistance testing of Staphylococcus aureus isolates from pork in the traditional market at Central Vietnam. Open Vet. J.. 2025; 15(7): 2959-2971. doi:10.5455/OVJ.2025.v15.i7.7 Web Style Chao NV, Dung HT, Anh LX, Tam VTT, Hung PHS. Contamination and antimicrobial resistance testing of Staphylococcus aureus isolates from pork in the traditional market at Central Vietnam. https://www.openveterinaryjournal.com/?mno=241577 [Access: January 12, 2026]. doi:10.5455/OVJ.2025.v15.i7.7 AMA (American Medical Association) Style Chao NV, Dung HT, Anh LX, Tam VTT, Hung PHS. Contamination and antimicrobial resistance testing of Staphylococcus aureus isolates from pork in the traditional market at Central Vietnam. Open Vet. J.. 2025; 15(7): 2959-2971. doi:10.5455/OVJ.2025.v15.i7.7 Vancouver/ICMJE Style Chao NV, Dung HT, Anh LX, Tam VTT, Hung PHS. Contamination and antimicrobial resistance testing of Staphylococcus aureus isolates from pork in the traditional market at Central Vietnam. Open Vet. J.. (2025), [cited January 12, 2026]; 15(7): 2959-2971. doi:10.5455/OVJ.2025.v15.i7.7 Harvard Style Chao, N. V., Dung, . H. T., Anh, . L. X., Tam, . V. T. T. & Hung, . P. H. S. (2025) Contamination and antimicrobial resistance testing of Staphylococcus aureus isolates from pork in the traditional market at Central Vietnam. Open Vet. J., 15 (7), 2959-2971. doi:10.5455/OVJ.2025.v15.i7.7 Turabian Style Chao, Nguyen Van, Ho Thi Dung, Le Xuan Anh, Vu Thi Thanh Tam, and Pham Hoang Son Hung. 2025. Contamination and antimicrobial resistance testing of Staphylococcus aureus isolates from pork in the traditional market at Central Vietnam. Open Veterinary Journal, 15 (7), 2959-2971. doi:10.5455/OVJ.2025.v15.i7.7 Chicago Style Chao, Nguyen Van, Ho Thi Dung, Le Xuan Anh, Vu Thi Thanh Tam, and Pham Hoang Son Hung. "Contamination and antimicrobial resistance testing of Staphylococcus aureus isolates from pork in the traditional market at Central Vietnam." Open Veterinary Journal 15 (2025), 2959-2971. doi:10.5455/OVJ.2025.v15.i7.7 MLA (The Modern Language Association) Style Chao, Nguyen Van, Ho Thi Dung, Le Xuan Anh, Vu Thi Thanh Tam, and Pham Hoang Son Hung. "Contamination and antimicrobial resistance testing of Staphylococcus aureus isolates from pork in the traditional market at Central Vietnam." Open Veterinary Journal 15.7 (2025), 2959-2971. Print. doi:10.5455/OVJ.2025.v15.i7.7 APA (American Psychological Association) Style Chao, N. V., Dung, . H. T., Anh, . L. X., Tam, . V. T. T. & Hung, . P. H. S. (2025) Contamination and antimicrobial resistance testing of Staphylococcus aureus isolates from pork in the traditional market at Central Vietnam. Open Veterinary Journal, 15 (7), 2959-2971. doi:10.5455/OVJ.2025.v15.i7.7 |