| Research Article | ||
Open Vet. J.. 2025; 15(5): 2049-2058 Open Veterinary Journal, (2025), Vol. 15(5): 2049-2058 Research Article Determination of risk factors for foot and mouth disease emergence in East Java, IndonesiaSaifur Rehman1, Shakeeb Ullah1, Mutasem Abuzahra2, Mustofa Helmi Effendi2*, Budiastuti Budiastuti3, Kholik Kholik4, Muhammad Munawarah5, Ali Zaman6, Atta Ur Rahman6, Muhammad Inamullah Malik6, Sana Ullah6 and Saqib Ali Rustam61Faculty of Veterinary and Bio-Sciences, University of Veterinary and Animal Sciences Swat KPK, Swat, Pakistan 2Division of Veterinary Public Health, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 3Study Program of Pharmacy Science, Faculty of Health Science, Universitas Muhammadiyah Surabaya, East Java, Indonesia 4Department of Veterinary Public Health, Faculty of Veterinary Medicine, Universitas Pendidikan Mandalika, Mataram, Indonesia 5Department of Basic Medical Veterinary Medicine, Faculty of Veterinary Medicine, Universitas Pendidikan Mandalika, Mataram City, Indonesia 6Faculty of Veterinary and Animal Sciences, Gomal University, Dera Ismail Khan, Pakistan *Corresponding Author: Mustofa Helmi Effendi. Division of Veterinary Public Health, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: mhelmieffendi [at] gmail.com Submitted: 16/01/2025 Revised: 27/03/2025 Accepted: 07/04/2025 Published: 31/05/2025 © 2025 Open Veterinary Journal
ABSTRACTBackground: Foot and mouth disease (FMD) is a highly infectious disease, affecting animals with divided hooves. FMD has seen significant outbreaks in East Java, Indonesia, highlighting the urgent need to understand the risk factors contributing to its emergence in this region. FMD spreads easily across borders and leads to significant economic losses due to illness, death, and trade limitations. Aim: The current study aimed to identify risk variables connected to the FMD outbreak in East Java. Methods: We conducted risk factor investigations for FMD in seven districts of East Java province, in collaboration with the Faculty of Veterinary Medicine at Airlangga University and the Department of Livestock, East Java. A case–control study of 76 (case=53, control=23) livestock farms with a case−control ratio of approximately 2:1 was conducted in East Java, Indonesia. Data on multiple possibly correlated parameters were collected using a standardized questionnaire. Results: Univariate analysis of the current study showed that five risk factors (the presence of animals other than cattle, visits of the veterinarian, calf sections, mixing young calves with other animal species, and regular disinfection) were significantly associated (p < 0.05) with the occurrence of FMD. Multiple logistic regression showed that visiting veterinary doctors (odds ratio [OR]=0.08, 95% CI=0.01–0.52, p=0.008), regular disinfection (OR=3.98, 95% CI=1.06–14.93, p=0.04), and sharing equipment between healthy and infected farms (OR=3.39, 95% CI=0.95–12.1, p=0.04) were significantly associated with the FMD outbreak in the vicinity in 2022. Conclusion: Strengthening biosecurity protocols, limiting admissions of animals lacking documented vaccination records, and ensuring routine vaccination against FMD are all effective countermeasures to significantly reduce and alleviate the prevalence of FMD on Indonesian livestock farms. Keywords: Biosecurity, East Java, Foot and mouth disease, Risk factors, Vaccination. IntroductionFoot and mouth disease (FMD) is a highly contagious viral disease that affects livestock and has significant economic implications. The disease affects various species of cloven-hoofed ruminants, including cattle, swine, sheep, goats, and other similar animals. The disease occurs in outbreak form and is caused by a virus, often known as Foot and Mouth Disease Virus, an RNA virus classified within the Aphthovirus genus and the Picornaviridae family (Domingo et al., 2002; Belsham, 2005; Can-Sahna et al., 2024). The bovine respiratory disease complex, which causes bronchopneumonia, arises from a complex array of factors, including infectious agents, host-related variables, environmental stresses, and the interactions among these elements (Khan et al., 2024; Ozbek et al., 2024; Tursunov et al., 2024). It is believed that the earliest descriptions of FMD were made in cattle in Italy during the 16th century (Grubman and Baxt, 2004). FMD is endemic in Asia, South America, Africa, and the Middle East; however, the World Organization of Animal Health (WOAH) has designated 69 countries as FMD-free (WOAH, 2002). There are seven immunologically distinct serotypes, designated as A, O, C, SAT1, SAT2, SAT3, and Asia1 (Spickler and Roth, 2015). The most prevalent serotypes in South America, Africa, and Asia are O and A (Di Nardo et al., 2011; Lloyd-Jones et al., 2017; WHO, 2022; WHOA, 2022). The SAT and Asia 1 serotypes are typically limited to Africa and Asia, respectively; however, there have been instances where they have spread to other areas (Wubshet et al., 2019). Serotype C, which was last identified during outbreaks in Brazil and Kenya in 2004, seems to be nonexistent in animals and may even be extinct, while there are worries about iatrogenic re-introduction with vaccinations due to laboratory escapes or poor deactivation (Sangula et al., 2011; Paton et al., 2021; Chen et al., 2022). FMDV serotype O, namely the O/ME-SA/Ind-2001e sub-lineage, is responsible for the outbreak that is currently occurring in Indonesia (Chen et al., 2022). After 36 years of ofnoo FMD cases in animals in Indonesia, the disease reemerged in livestock species in different foci of the country in 2022 and created great concern among livestock owners. Despite the lack of clear evidence, it is believed that the source of this problem is the illegal import of animals (Thomson et al., 2003) and may be from neighboring countries (Cambodia, Lao PDR, Malaysia, Myanmar, Thailand, and Vietnam), in which Serotype O is endemic (Chen et al., 2022). On April 12, 2022, in the province of East Java, the disease was identified in cattle for the first time. In light of the 3,496 instances certified by the WOAH in the provinces of East Java and Aceh on May 6, 2022, Indonesia’s FMD-free designation was revoked as of April 12, 2022 (Indonesia, 2022; Zainuddin et al., 2023). As of November 3, 2022, out of a total population of 54,767,135 livestock, there have been 570,137 cases of the disease (which corresponds to a morbidity rate of 1.04%), 9,785 deaths (which corresponds to a mortality rate of 0.02%), 12,650 livestock have been culled, and 5,199,595 have been vaccinated. The majority of reported cases were found in cattle (94.27%), followed by buffalo (4.56%), goats (0.80%), sheep (0.36%), and swine (0.02%) (Ministry of Agriculture, Republic of Indonesia, 2022). The present outbreak of FMD is posing considerable economic and biosecurity problems for Indonesia, with damage estimated to range from $1.37 to $6.60 billion US dollars (Indonesia, 2022; Karyza, 2022) because farmers had not been routinely vaccinated their animals against FMD in the recent past and Indonesian naïve population was entirely open to infection (Belsham, 2020; Zhang et al., 2024). Even if the Indonesian authorities have identified the strain of the current outbreak, that is, harming animals (Belsham, 2020), it will take some time to either import sufficient doses of an effective vaccine or produce enough of it locally. The Mojokerto, Gresik, Sidoarjo, and Lamongan Regencies of East Java were designated as areas of FMD outbreaks by the government through the Decree of the Minister of Agriculture Number 403 of 2022. The movement of cattle, sheep, pigs, and goats concentrated in close quarters between Java and Bali played a part in the spread of the FMD epidemic to Bali (Sarsana and Merdana, 2022). In June 2022, it was reported that cows in the Gerokgak district of Buleleng Regency were suspected of having FMD (Sarsana and Merdana, 2022). Various factors (herd size, presence of animals other than bovines, equipment shared with infected farms, higher beef cattle density, mixing young calves with other animals species, proximity to a cattle market or slaughterhouse, distribution of feeding stations, and higher pig density, etc.) have been highlighted by previous studies as elevating the probability of an FMD outbreak from Palestine (Ghaith and Ghazaleh, 2021), Pakistan (Ali et al., 2022), Indonesia (Sarsana and Merdana, 2022; Yustendi et al., 2022), Libya (Abosrer et al., 2022), Afghanistan (Wajid et al., 2020), Iran (Ilbeigi et al., 2018), Sri Lanka (Gunasekera et al., 2017), Thailand (Sansamur et al., 2020), and Japan (Muroga et al., 2013). Previous studies have linked FMD outbreaks to a variety of factors, including proximity to a cattle market or slaughterhouse (Hernandez, 2007), transportation network (roads), herd size (Ali et al., 2022), movement of humans (Allepuz et al., 2015), purchase of new animals and distribution of feeding stations (Cleland et al., 1996), presence or absence of a history of FMD outbreaks, grazing practices, and farmer knowledge (Wajid et al., 2020). This study represents the first investigation conducted in Indonesia to examine risk factors associated with the occurrence of FMD in the province of East Java. An epidemic of FMD approximately affected all districts of the province was detected in 2022, which could be related to a variety of factors, including inadequate biosecurity measures, poor husbandry techniques, extensive cross-border trade, and unavailability of vaccines for the re-emerging strain. Therefore, it is necessary to identify the relevant risk variables that may be possibly linked to the spread of FMDV and to develop effective FMD prevention and control measures at the national level, in particular, and at the global level. Materials and MethodsStudy areaThe study was carried out in a livestock-rich belt of seven different adjacent districts, including Gresik, Jombang, Sidoarjo, Sooko, Pasuruan, Wringinanom, and Kesamben Kulon, located in East Java province, Indonesia (Fig. 1). The Department of Livestock of East Java (DLD) was responsible for providing veterinary services, particularly symptomatic and supportive therapies. There is a disease diagnostic laboratory in each district, three of which are specifically dedicated to FMDV detection and serotyping. The local DLD staff carried out FMD control measures such as (1) placing suspected FMD animals in quarantine at the individual farm level; (2) sending a sample of tissue lesions to a laboratory; (3) restricting the movement of animals and animal products in the outbreak region (infected farm) for a period of 30 days; (4) vaccinating animals in a ring (vaccination of all FMD suspected animal in certain area); (5) cleaning and spraying vehicles at control points (entrance of the farm); (6) disposing of contaminated materials and carcasses; and (7) raising public awareness regarding the risk factors.
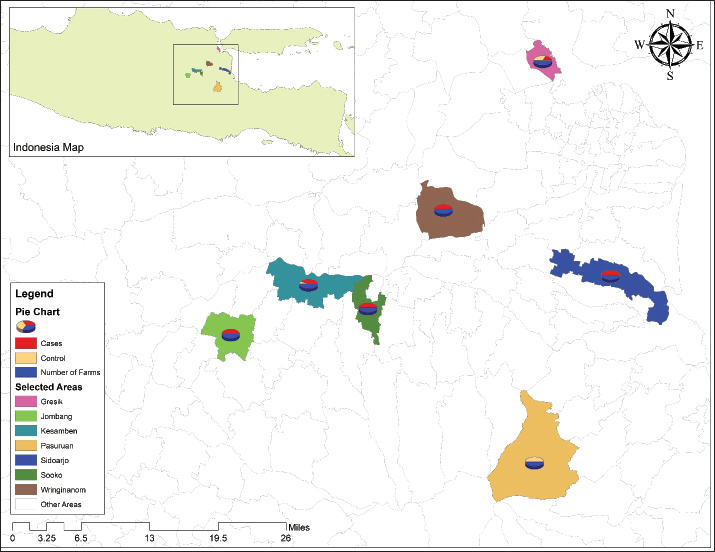
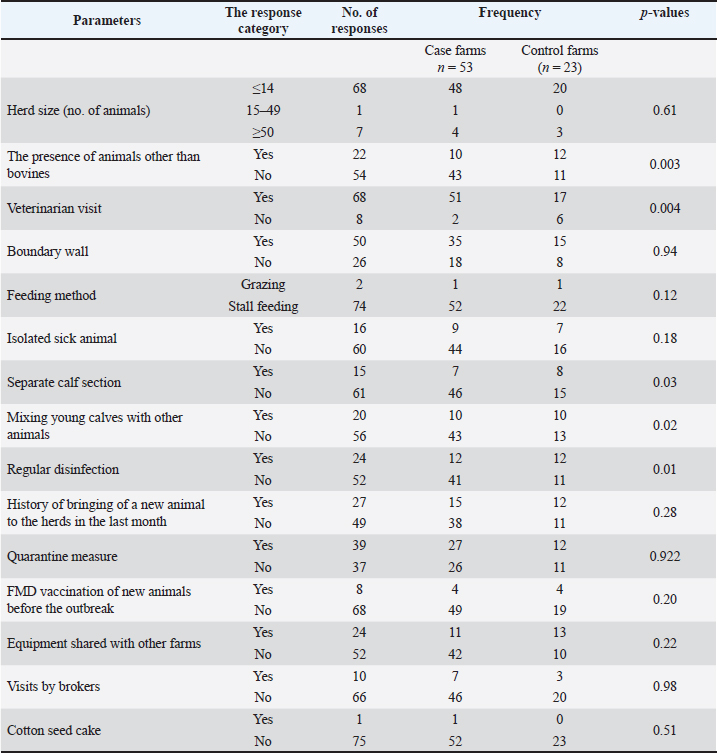
Fig. 1. Map of FMD outbreaks in East Java province study districts. Data collectionFMD outbreak data and case definition Data were collected with the collaboration of the provincial livestock office of East Java and Faculty of Veterinary Medicine Airlangga University regarding FMD outbreak risk factors, as district livestock officers identified FMD outbreaks at different farms. Those farms were considered case farms where a Swab or tissue sample from an animal exhibiting classic clinical signs of FMD was confirmed to be FMDV positive using PCR or enzyme-linked immunosorbent assay techniques in the dedicated FMD laboratories of the relevant districts. Evaluation of risk factors using case–control study The probability of FMD outbreaks occurring on farms in 2022 was investigated using a case−control study. The goal of this research was to determine the risk factors most closely associated with FMD outbreaks. In the current study, a farm is defined as any piece of land that is used to raise cows or buffaloes for the purpose of producing milk or meat, independent of the number of animals that are kept on the land. If a farm had at least one animal that tested positive for FMD, it was classified as a case farm. In addition, any farm that tests positive for FMD from this point onward will be referred to as an epidemic of FMD. During this period, farmers from the control farms who had not seen any clinical symptoms suggestive of FMD were randomly chosen from the same location as the FMD-infected farm. Veterinary professionals inspected the farms to confirm their FMD-free status. Sample size for the case–control model EpiTools was used to calculate the sample size for a case–control study (Sergeant, 2018). This was done under the assumption of an odds ratio (OR) of 2.8 for large herd sizes (with >50 animals), because a previous study conducted by Ali et al. (2022) identified herd size as a risk factor related to FMD outbreaks. A power of 80% is required to detect an OR of 2 with a 95% confidence interval (due to technical issues, the search service is temporarily unavailable). The study initially aimed for a 1:1 case-control ratio, requiring 56 case farms and 56 control farms, as determined by an online sample size calculator. However, due to the high incidence of FMD in Indonesia, only 53 case farms and 23 control farms (totaling 76 farms) were available for analysis. Geographical matching was not performed due to the widespread nature of FMD. The final case−control ratio was approximately 2:1. Field data collection and variablesA standardized questionnaire was used as the research instrument. The questionnaire was modified from published research by Ali et al. (2022) and Cao et al. (2024) to identify risk factors. The questionnaire focused on information about the farm, such as herd size based on the number of animals that were categorized into three groups, including small herd (less than 14 animals), medium herd (between 15 and 49 animals), and large herd (more than 50 animals) (Mulisa et al., 2011), dedicated space for calves, cleaning and disinfection, feeding methods, isolation of sick animals from healthy, sharing of equipment with infected farms, visiting of brokers, acquisition of new livestock, and the implementation of quarantine procedures before they entered the farm. The vaccination topic was covered in the questionnaire, and farmers were asked whether or not they had vaccinated their animals against FMD and when they had vaccinated their animals. The data were collected from respondents via face-to-face interviews (conducted at 55 farms) and the distribution of Google forms (conducted at 21 farms) via WhatsApp and emails with the assistance of the regional veterinarian. Statistical analysisUnivariate analysis was used to examine all categorical and binary explanatory variables. A logistic regression model was used for the multiple logistic regression. Variables with univariate p < 0.05 were chosen for further consideration in the multivariate analysis. The phi coefficient was used to analyze the degree of correlation between potential variables in multiple logistic regression. For the multiple logistic regression analysis, researchers chose two highly connected variables that were less irrational (phi coefficient > 0.4). Because the herd size was considered a confounding factor, it was included in all logistic regression models. Interactions between variables in the final model were evaluated after being selected in a stepwise backward elimination fashion, with a p < 0.05 indicating retention. To obtain the best possible fit, Hosmer and Lemeshow statistics were used to evaluate the final model. IBM SPSS Statistics v26 (SPSS Inc., Chicago, IL) was used for all statistical analyses. Ethical approvalThe Animal Care and Use Committee, Faculty of Veterinary Medicine, University of Airlangga, Surabaya, authorized this study, as evidenced by approval letter number 2.KE.096.07.2022. ResultsDescriptive statisticsA total of 76 farms were included in this study at the following distribution values: 39.5% Gresik (n=30), 31.60% Kesamben Kulon (n=24), 11.80% Sooko (n=9), 9.20% Sidoarjo (n=7), 5.30% Wringinanom (n=4), and 1.30% from Jombang (n=1) and Pasuruan (n=1) districts of East Java province (Fig. 1). According to Table 1, the majority (n=68, or 89.50%) of the farms that participated in the study had small-sized herds (i.e., ≤14 animals), followed by farms with large herds (n=7, 9.20%) and farms with medium herds (n=1, 1.30%). Most of the (n=68, 89.47%) respondents depicted that veterinarians visited their farms during the FMD outbreak, whereas only 10.53% reported that no veterinarians visited their farms throughout the outbreak. Most farmers (n=74, 97.37%) employed stall feeding to feed their cattle, whereas only 2.63% grazed; 78.94% of farmers reported that they never separate sick animals from healthy animals, indicating a lack of farm biosecurity. Most farmers (n=61, 80.26%) stated that they do not have separate calf areas for newborn calves. Out of 76 farms, 24 (31.57%) frequently performed disinfection and had a disinfection dip at the entrance of their facility. The farmers at 89.5% of the 76 farms that were surveyed stated that prior to the outbreak of FMD, they had never vaccinated their new or old animals against FMD. The farmers stated that this was because the previous outbreak of FMD occurred 36 years ago, and at that time, the country was declared to be free from FMD. Most farmers (n=66, 86.84%) forbade brokers from visiting their farms during the outbreak. Variables used in the univariate logistic regression analysis All 15 variables were analyzed using univariate logistic regression; seven variables demonstrated a significant connection with the incidence of FMD outbreaks. The presence of animals other than bovines, visiting of veterinarians, no separate calf section, mixing young calves with other animal species, and regular disinfection showed a significant association (p < 0.05). The remaining study variables did not show any significant association with the occurrence of FMD (p > 0.05) (Table 1). Table 1. A list of the biologically reasonable variables included in the univariate analysis to determine FMD risk factors in a case−control study in East Java province.
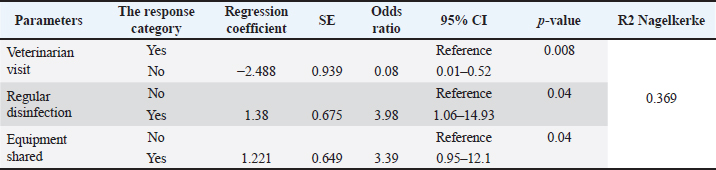
Variables are included in the final model Five of the 15 variables were chosen for inclusion in the multiple logistic regression analysis based on the criterion that the p-value was less than 0.05. Of these five variables, only three variables (visiting of veterinarians on the farms, regular disinfection, and sharing of equipment between farms) were significantly associated with the occurrence of FMD based on p < 0.05 (Table 2). DiscussionIn Indonesia, 2022 marked the beginning of an outbreak of FMD (Chen et al., 2022), a critical transboundary disease that quickly swept through the country (Chen et al., 2022). The virus is highly contagious and has the potential to spread over national and international borders, posing significant risks to the national food supply chain and economy (Ali et al., 2022; Chen et al., 2022; Kabir et al., 2024). There is a paucity of accessible data on the risk factors of cattle farms in Indonesia. Although FMD is expected to re-emerge in 2022, it is expected to cause huge economic losses in terms of morbidity, loss of production, abortion, medication, and mortality. Table 2. Summary of parameters in final mode.
The current study results indicated that the districts of Kesamben Kulon (case=16, control=8) and Gresik (case=20, control=10) were the most highly affected regions during the epidemic with a total of 36 outbreaks, which may have been caused by the existence of the big cattle market in the area involving significant influx of animals into the district. Based on the information from the district research laboratory, serotype “O” was found to be the most prevalent serotype in the recent outbreak of 2022; this predominance of serotype “O” has been observed in a number of Asian countries (Knowles et al., 2005; Abdul-Hamid et al., 2011; Khan et al., 2024). The Indonesian serotype O/ME-SA/Ind-2001 viruses were initially believed to belong to the Ind-2001e sublineage. However, they actually formed their own unique cluster and had an average nucleotide sequence similarity of 95.3% with Ind-2001e viruses from other Asian countries. The source of the virus that disseminated across Indonesia remains undetermined. Nevertheless, in early May 2022, there were reports of disease outbreaks in Aceh and East Java provinces, with symptoms resembling those of FMD. Subsequently, additional cases of FMD were reported in various areas of Java, Sumatra, Kalimantan, and West Nusa Tenggara. In response to this, the monitoring apparatus was strengthened so that tests could be conducted, the epidemiology of the virus could be tracked, and a better understanding of its spread across the country could be achieved (Ludi et al., 2024). There should be some thought into the possibility of adapting the vaccine selection to cover the recently circulating strains of FMDV in the country. We believe that this is the first and only case–control study on livestock farms in East Java, Indonesia, to determine risk factors associated with FMD epidemics. The findings of the current study revealed that the presence of animals other than bovines, regular visits of veterinarians on the farm, no seprate calf section, mixing young calves with other animal species, and the sharing of equipment between farms were risk factors that showed a significant association (p < 0.05) with the spread of FMD in the East Java province of Indonesia. The findings of the current study revealed that visiting veterinarians on farms is among the most significant factors influencing the likelihood of FMD outbreaks in 2022. The risk of an FMD outbreak was considerably higher on farms with veterinary doctor visits (OR=0.08, 95% CI=0.01–0.52, p=0.008) compared to the farms with no veterinary doctor visits. Our results were consistent with earlier research by Muroga et al. (2013) and Rojanasthien et al. (2006), which found that veterinary doctor visits on farms were significantly (p=0.04) related to the likelihood of FMD disease outbreaks. The findings of the present study revealed that outbreaks on farms where recently arrived animals were added to the herd showed a significant association (p < 0.05) compared to farms whose owners prohibited bringing in new animals to their herds. These farms had a significantly higher incidence of FMD. These findings are consistent with those found in previous studies conducted in Iran (Ilbeigi et al., 2018), Ecuador (Hernandez, 2007), Cameroon (Bronsvoort et al., 2004), Thailand (Sansamur et al., 2020), Laos (Miller et al., 2018), and Pakistan (Ali et al., 2022; Ullah et al., 2023) in which the researchers reported that adding cattle having no FMD vaccination record that had just been purchased from a market to an existing herd enhanced the probability of an FMD outbreak (Ali et al., 2022). According to another study conducted in Uganda, the incidence of FMD outbreaks was found to be more common in regions where sick animals were moved without first undergoing pre-movement screening (Ayebazibwe et al., 2010; Ali et al., 2024). During the early phases of FMD in East Java province, animals were frequently moved between livestock farms without being screened or given health certificates. Additionally, because the animals are typically not isolated, there is a higher chance that freshly acquired animals will transmit FMDV to farm animals that are susceptible to it. Therefore, additional control measures, such as the rigorous implementation of biosecurity standards under the supervision of experts, are essential. FMDV is a silent enemy for livestock farming, and it can spread to farms by infected animals, fomites, and even aerosols. Biosecurity measures have been demonstrated that biosecurity measures minimize the danger of FMD epidemics in the United Kingdom (Nerlich and Wright, 2006; Rehman et al., 2024). In the final model of the current study, it was revealed that FMD occurrence was about three times higher (OR=3.98, 95% CI=1.06–14.93, p=0.04) at farms where regular disinfection practices were performed compared to those farms where no regular disinfection (not changing the disinfectant frequently enough, using a disinfectant that cannot kill FMDV, or not performing disinfection correctly). However, ineffective disinfection practices can create a false sense of security, potentially leading to the relaxation of other biosecurity measures. This could explain the higher OR for disinfecting farms. A similar phenomenon was observed during the COVID outbreak. According to Rojanasthien et al. (2006), farms that frequently disinfect their animals and have a disinfectant pool had a significantly higher incidence of FMD than farms that did not. These results are in contrast to research from Afghanistan and Japan, which revealed that routine disinfection lowers FMD risk. Our findings demonstrated a significant (p=0.04) (Table 2) association between the prevalence of FMD on farms that shared equipment with other farms as opposed to farms that did not. It has been revealed in past reports that infected vehicles and equipment are involved in the dissemination of FMD (Gibbens et al., 2001; Sutmoller et al., 2003; Ward et al., 2004). A previous study conducted by Muroga et al. (2013) and Sirdar et al. (2024) found that the percentage of case farms in which farm equipment was shared with other farms was much greater than the percentage of control farms in which it was not shared. A study conducted by Fèvre et al. (2006) and Porphyre et al. (2020) reported that transporting infected animals and sharing contaminated equipment are two of the most common pathways for the dissemination of infectious diseases from one farm to another. It was discovered that farms that mixed young calves with other animal species had significant (p=0.026) connections with FMD spread compared with farms that did not. According to Rojanasthien et al. (2006), Osmani et al. (2021), and Li et al. (2024), mixing of animals on farms was significantly correlated with the occurrence of FMD. In this particular research project, we found that vaccination was associated with the onset of the pandemic. As noted at the outset, vaccination was not required for 36 years after the country successfully maintained its FMD-free status. In the majority of farms, farmers do not adhere to basic biosecurity measures that guarantee that sick animals are kept on the farm. In addition, the farmers are not aware of sharing equipment with other animals on the farm, which might spread disease. LimitationsThe vaccination teams had vaccinated a higher number of animals at some district levels of the province, whereas in other parts of the province, they had vaccinated a lesser number of animals. Therefore, animals in regions with low vaccination percentages were more susceptible than those in regions with the highest vaccination portages. Case farms and control farms were chosen not based on the results of serological investigations but rather on clinical indications that were reported to relevant veterinary authorities. The questionnaire may have induced recall bias. The Director General of Livestock and Animal Health Services (DGLAHS) was not informed at the time of each outbreak, and the DGLAHS did not make all of its data available to the researchers conducting the study. In addition, precise statistics for the farm at the specific geographic site where the outbreaks developed were not accessible; as a result, only the linked village area was evaluated in each district. ConclusionFMD became a national animal crisis for Indonesia in 2022. Instead of carrying out widespread culling, the Indonesian government opted to control the disease through the education of farmers, the administration of mass vaccinations, and the imposition of mobility restrictions across the provinces within the country and across the districts within a province. According to the results of a recent study, visiting veterinary doctors, regular disinfection, and sharing equipment between healthy and infected farms were highly connected with FMD outbreaks that occurred in 2022 across a number of districts of East Java province. These findings are important for enhancing the strategies used to control FMD throughout Indonesia. AcknowledgmentsWe would like to express our profound gratitude to the Faculty of Veterinary Medicine, Airlangga University, and the Director General of Livestock and Animal Health Services (DGLAHS), East Java, Indonesia, for their cooperation in the data collection process for this study. Conflict of interestAll authors declare that they have no conflicts of interest. FundingThis research was supported by the Faculty of Veterinary Medicine, Universitas Airlangga, Indonesia. The sponsors played no role in the study’s conception, data collection and analysis, publication decision, or writing of the paper. Authors’ contributionsAll authors participated in data analysis, manuscript drafting, and revision, and they consented to assume responsibility for all aspects of this study. Data availabilityData was available from the primary author and will be provided on special request. ReferencesAbdul-Hamid, H., Yarrow, R. and Raney, L. 2011. Silent and lethal: how quiet corruption undermines Africa’s development efforts. Washington, DC: World Bank. Abosrer, M., Farag, E., Mohamed, M., Nour, M. and Al-Dahmoshi, H. 2022. A review of zoonotic diseases and their economic impact on livestock production. Cairo, Egypt: Egyptian Ministry of Agriculture. Ali, M., Ahmed, F. and Khan, S. 2022. Emerging infectious diseases: a multidisciplinary perspective. Islamabad, Pakistan: National Institute of Health Press. Allepuz, A., de la Torre, A., Núñez, J.I. and Muñoz, M.J. 2015. The use of veterinary data for early warning of animal disease outbreaks in Europe. Madrid, Spain: European Veterinary Epidemiology Network. Ayebazibwe, C., Tjørnehøj, K., Mwiine, F.N., Muwanika, V.B., Okurut, A.R., Siegismund, H.R. and Alexandersen, S. 2010. Patterns, risk factors and characteristics of reported and perceived foot-and-mouth disease (FMD) in Uganda. Trop. Anim. Health Prod. 42(7), 1547–1559; doi:10.1007/s11250-010-9605-3. Belsham, G.J. 2005. Translation and replication of FMDV RNA. Curr. Top. Microbiol. Immunol. 288, 43–70; doi:10.1007/3-540-27109-0_3. Belsham, G.J. 2020. Improvement of foot and mouth disease vaccine performance. Acta Vet. Scand. 2(1), 20. doi:10.1186/s13028-020-00519-1. Bronsvoort, B.M., Nfon, C., Hamman, S.M., Tanya, V.N., Kitching, R.P. and Morgan, L.K. 2004. Risk factors for herdsman-reported foot-and-mouth disease in the Adamawa Province of Cameroon. Prev. Vet. Med. 66(1–4), 127–139; doi:10.1016/j.prevetmed.2004.09.010. Can-Sahna, K., Abayli, H., Ilgin, M. and Aksoy, M. 2024. Investigation of some neuroinfectious viral agents in turkish cattle: first detection and molecular characterization of bovine herpesvirus type 5 (BoHV-5). Pak. Vet. J. 44(3), 896–902; doi:10.29261/pakvetj/2024.211. Cao, H., Xu, R., Liang, Y., Li, Q., Jiang, W., Jin, Y., Wang, W. and Yuan, J. 2024. Effects of extreme meteorological factors and high air pollutant concentrations on the incidence of hand, foot, and mouth disease in Jining, China. PeerJ. 12, e17163; doi:10.7717/peerj.17163. Chen, R., Gardiner, E. and Quigley, A. 2022. Foot and mouth disease outbreak in Indonesia: summary and implications. Glob. Biosecurity 4(1), 175; doi:10.31646/gbio.175. Cleland, P.C., Baldock, F.C., Chamnanpood, P. and Gleeson, L.J. 1996. Village-level risk factors for foot and mouth disease in northern Thailand. Prev. Vet. Med. 26(3–4), 253–261. Di Nardo, A., Knowles, N.J. and Paton, D.J. 2011. Combining livestock trade patterns with phylogenetics to help understand the spread of foot and mouth disease in sub-Saharan Africa, the Middle East and Southeast Asia. Rev. Sci. Tech. 30(1), 63–85; doi:10.20506/rst.30.1.2022. Domingo, E., Baranowski, E., Escarmís, C. and Sobrino, F. 2002. Foot-and-mouth disease virus. Compar. Immunol. Microbiol. Infect. Dis. 25(5–6), 297–308; doi:10.1016/s0147-9571(02)00027-9. Fèvre, E.M., Bronsvoort, B.M., Hamilton, K.A. and Cleaveland, S. 2006. Animal movements and the spread of infectious diseases. Trends Microbiol. 14(3), 125–131; doi:10.1016/j.tim.2006.01.004. Ghaith, D.M. and Abu Ghazaleh, R. 2021. Carboxamide and N-alkylcarboxamide additives can greatly reduce nonspecific amplification in Loop-Mediated Isothermal Amplification for Foot-and-Mouth disease Virus (FMDV) using Bst 3.0 polymerase. J. Virol. Methods 298, 114284; doi:10.1016/j.jviromet.2021.114284. Gibbens, J.C., Sharpe, C.E., Wilesmith, J.W., Mansley, L.M., Michalopoulou, E., Ryan, J. B. and Hudson, M. 2001. Descriptive epidemiology of the 2001 foot-and-mouth disease epidemic in Great Britain: the first five months. Vet. Rec. 149(24), 729–743. Grubman, M.J. and Baxt, B. 2004. Foot-and-mouth disease. Clin. Microbiol. Rev. 17(2), 465–493; doi:10.1128/CMR.17.2.465-493.2004. Gunasekera, U.C., Sivasothy, A., Wedasingha, N., Thayaparan, S., Rotewewa, B., Muralithas, M.P.O. and Punyapornwithaya, V. 2017. Analyzing the Foot and Mouth Disease outbreak from 2008 to 2014 in cattle and buffaloes in Sri Lanka. Prev. Vet. Med. 148, 78–88; doi:10.1016/j.prevetmed.2017.10.008. Hernandez, J. 2007. Epidemiologic aspects of a foot-and-mouth disease epidemic in cattle in Ecuador. Int. J. Appl. Res. Vet. Med. 5(1), 17–24. Ilbeigi, K., Bokaie, S., Aghasharif, S., Soares Magalhães, R.J. and Rashtibaf, M. 2018. Risk factors for recurrence of FMD outbreaks in Iran: a case-control study in a highly endemic area. Vet Res. 14(1), 253; doi:10.1186/s12917-018-1580-3. Indonesia. 2022. Foot and mouth disease outbreak-emergency plan of action (EPoA), DREF Operation No MDRID024. Reliefweb: International Federation of Red Cross and Red Crescent Societies. Karyza. 2022. Foot-and-mouth could cost country estimated $1.37b a year. The Jakarta Post. Available via https://www.thejakartapost.com/business/2022/05/29/foot-and-mouth-could-cost-country-estimated-1-37b-a-year.html Kabir, A., Kamboh, A.A., Abubakar, M., Baloch, H. and Nizamani, Z. A. 2024. Foot-and-mouth disease virus dynamics in border areas of Pakistan with Afghanistan. Mol. Biol. Rep. 51(1), 370; doi:10.1007/s11033-024-09262-6. Khan, D., Sheikh, I.S., Ullah, A., Kasi, K.K., Mustafa, M.Z., Din, Z.U., Anwar, I., Kakar, N. and Waheed, A. 2024a. Circulation of foot-and-mouth disease serotypes, risk factors, and their effect on hematological and biochemical profiles among cattle and buffalo in Quetta, Balochistan, Pakistan. Vet. World 17(2), 329–336; doi:10.14202/vetworld.2024.329-336. Khan, M.H., Riaz, A., Hou, Z., Qing, Y., Batool, N., Ihsanullah, Bilal, M., Arshad, M.F., Saud, M. and Ma, X. 2024b. Epidemiological survey, molecular characterization, and subtyping of BoHV-1 from healthy and sick cattle and buffaloes from Okara, Pakistan. Pak. Vet. J. 44(1), 111–116; doi:10.29261/pakvetj/2024.140 Knowles, N.J., Samuel, A.R., Davies, P.R., Midgley, R.J. and Valarcher, J.F. 2005. Pandemic strain of foot-and-mouth disease virus serotype O. Emerg. Infect Dis. 11(12), 1887–1893; doi:10.3201/eid1112.050908. Li, Y., Qiu, S., Lu, H. and Niu, B. 2024. Spatio-temporal analysis and risk modeling of foot-and-mouth disease outbreaks in China. Prev. Vet. Med. 224, 106120. Ludi, A.B., Baker, H., Sanki, R., De Jong, R.M.F., Maryan, J., Walker, M., King, D.P., Gubbins, S., Limon, G. and Officer, K. 2024. Epidemiological investigation of foot-and-mouth disease outbreaks in a Vietnamese bear rescue center. Front. Vet. Sci. 11, 1389029; doi:10.3389/fvets.2024.1389029. Lloyd-Jones, K., Mahapatra, M., Upadhyaya, S., Paton, D.J., Babu, A., Hutchings, G. and Parida, S. 2017. Genetic and antigenic characterization of serotype O FMD viruses from East Africa for the selection of suitable vaccine strain. Vaccine 35(49), 6842–6849; doi:10.1016/j.vaccine.2017.10.040. Miller, C.A.J., Young, J.R., Nampanya, S., Khounsy, S., Singanallur, N.B., Vosloo, W., Abila, R., Hamilton, S.A., Bush, R.D. and Windsor, P.A. 2018. Risk factors for emergence of exotic foot-and-mouth disease O/ME-SA/Ind-2001d on smallholder farms in the Greater Mekong Subregion. Prev. Vet. Med. 159, 115–122; doi:10.1016/j.prevetmed.2018.09.007. Ministry of Agriculture, Republic of Indonesia. 2022. Penanggulangan dan tindakan pencegahan qabah PMK [FMD outbreak management and prevention information]. Ministry of Agriculture of the Republic of Indonesia. Available via https://crisiscenterpmk.ditjenpkh.pertanian.go.id/ Mulisa Megersa, A.F., Wondimu, A. and Jibat, T. 2011. Herd composition and characteristics of dairy production in Bishoftu Town, Ethiopia. J. Agric. Ext. Rural Dev. 3(6), 113–117. Muroga, N., Kobayashi, S., Nishida, T., Hayama, Y., Kawano, T., Yamamoto, T. and Tsutsui, T. 2013. Risk factors for the transmission of foot-and-mouth disease during the 2010 outbreak in Japan: a case-control study. Vet. Res. 9, 150; doi:10.1186/1746-6148-9-150. Nerlich, B. and Wright, N. 2006. Biosecurity and insecurity: the interaction between policy and ritual during the foot and mouth crisis. Environ. Values 15(4), 441–462. Osmani, A., Robertson, I.D. and Habib, I. 2021. Seroprevalence and risk factors of foot and mouth disease in cattle of Baghlan Province, Afghanistan. Vet. Med. Sci. 7(4), 1263–1275; doi:10.1002/vms3.477. Ozbek, R., Abayli, H., Tonbak, S. and Sahna, K. 2024. Molecular characterization of important viruses contributing to the bovine respiratory disease complex in Türkiye. Pak. Vet. J. 44(2), 322–329; doi:10.29261/pakvetj/2024.155. Paton, D.J., Di Nardo, A., Knowles, N.J., Wadsworth, J., Pituco, E.M., Cosivi, O., Rivera, A.M., Kassimi, L.B., Brocchi, E., de Clercq, K., Carrillo, C., Maree, F.F., Singh, R.K., Vosloo, W., Park, M.K., Sumption, K.J., Ludi, A.B. and King, D.P. 2021. The history of foot-and-mouth disease virus serotype C: the first known extinct serotype? Virus Evol. 7(1), 009; doi:10.1093/ve/veab009. Porphyre, T., Bronsvoort, B.M.C., Gunn, G.J. and Correia-Gomes, C. 2020. Multilayer network analysis unravels haulage vehicles as a hidden threat to the British swine industry. Transbound Emerg. Dis. 67(3), 1231–1246; doi:10.1111/tbed.13459. Rehman, S., Abuzahra, M., Wibisono, F.J., Effendi, M.H., Khan, M.S., Ullah, S., Abubakar, A.A., Zaman, A., Shah, M.K., Malik, M.I., Rahman, A., Abbas, A. and Nadeem, M. 2024. Identification of risk factors and vaccine efficacy for lumpy skin disease in the Sidoarjo and Blitar districts of East Java, Indonesia. Int. J. Vet. Sci. 13(5), 574–579; doi:10.47278/journal.ijvs/2024.137. Rojanasthien, S., Padungtod, P., Yamsakul, P., Kongkaew, S. and Yano, T. 2006. Risk factors for foot and mouth disease in ruminants in Chiang Mai, Lumphun and Nan. In Proceedings of the 44th Kasetsart University Annual Conference. Bangkok, Thailand: Kasetsart University, pp: 486–493. Sangula, A.K., Siegismund, H.R., Belsham, G.J., Balinda, S.N. and Muwanika, V.B. 2011. Low diversity of foot-and-mouth disease serotype C virus in Kenya: evidence for probable vaccine strain re-introductions in the field. Epidemiol. Infect. 139(2), 189–196; doi:10.1017/S0950268810000580. Sansamur, C., Arjkumpa, O., Charoenpanyanet, A. and Punyapornwithaya, V. 2020. Determination of risk factors associated with foot and mouth disease outbreaks in dairy farms in Chiang Mai Province, Northern Thailand. Animals 10(3), 512; doi:10.3390/ani10030512. Sarsana, I.N. and Merdana, I.M.J.J.A.P.D.P.K.M. 2022. Feed and mouth disease vaccinations in Bali Cattle in Sanggalangit Village, Gerokgak District, Buleleng Regency, Bali. Altifani J. Res. Commun. Serv. 2(5), 447–452. Sergeant, E.S. 2018. Epitools epidemiological calculators. Available via http://epitools.ausvet.com.au Sirdar, M.M., Fosgate, G.T., Blignaut, B., Heath, L., Lazarus, D.D., Mampane, R.L., Rikhotso, O.B., Du Plessis, B. and Gummow, B. 2024. A comparison of risk factor investigation and experts’ opinion elicitation analysis for identifying foot-and-mouth disease (FMD) high-risk areas within the FMD protection zone of South Africa (2007-2016). Prev. Vet. Med. 226, 106192; doi:10.1016/j.prevetmed.2024.106192. Spickler, A. and Roth, J. 2015. NAHEMS Guidelines: vaccination for contagious diseases, Appendix A: foot-and-mouth disease. Ames, IA: USDA, Animal and Plant Health Inspection Service, Veterinary Services, FAD PReP/NAHEMS Guidelines, Center for Food Security and Public Health, Veterinary Medicine, Iowa State University of Science and Technology. Available via https://dr.lib.iastate.edu/handle/20.500.12876/92410 Sutmoller, P., Barteling, S.J., Olascoaga, R.C. and Sumption, K.J. 2003. Control and eradication of foot-and-mouth disease. Virus Res. 91(1), 101–144. Thomson, G.R., Vosloo, W. and Anderson, A.D.S. 2003. Foot and mouth disease in wildlife. Virus Res. 91(1), 145–161. Tursunov, K., Tokhtarova, L., Kanayev, D., Mustafina, R., Tarlykov, P. and Mukantayev, K. 2024. Evaluation of an in-house ELISA for detecting antibodies against the Lumpy Skin Disease virus in vaccinated cattle. Int. J. Vet. Sci. 13(2), 248–253; doi:10.47278/journal.ijvs/2023.089. Ullah, M., Li, Y., Munib, K., Rahman, H.U. and Zhang, Z. 2023. Sero-epidemiology and associated risk factors of foot-and-mouth disease (FMD) in the northern border regions of Pakistan. Vet. Sci. 10, 356; doi:10.3390/vetsci10050356. Wajid, A., Chaudhry, M., Rashid, H.B., Gill, S.S. and Handlim, S.R. 2020. Outbreak investigation of foot and mouth disease in Nangarhar Province, Afghanistan, 2014. Sci. Rep. 10(1), 13800. Ward, N., Donaldson, A. and Lowe, P. 2004. Policy framing and learning the lessons from the UK’s foot and mouth disease crisis. Environ. Plan. 22(2), 291–306. WHO. 2022. World health statistics 2022: monitoring health for the SDGs. Geneva, Switzerland: World Health Organization. WOAH. 2002. Terrestrial animal health code. Paris, France: World Organisation for Animal Health. World Organization of Animal Health. 2022. WAHIS Database 2022. Available via https://www.woah.org/en/home/ (Accessed 17 January 2023). Wubshet, A.K., Dai, J., Li, Q. and Zhang, J. 2019. Review on outbreak dynamics, endemic serotypes, and diversified topotypic profiles of foot and mouth disease virus isolates in ethiopia from 2008 to 2018. Viruses 11(11), 1076; doi:10.3390/v11111076. Yustendi, D., Rahmazana, S., Yusuf, Y. and Rosa, E. 2022. Management of the transmission of foot and mouth disease (FMD) and lumpy skin disease at the Baitussalam Health Center, Aceh Besar District. In Proceedings of SEMDI-UNAYA (UNAYA Multi-Discipline National Seminar), vol. 1, no. 1, pp 45–52. Zainuddin, N., Susila, E.B., Wibawa, H., Daulay, R.S.D., Wijayanti, P.E., Fitriani, D., Hidayati, D.N., Idris, S., Wadsworth, J., Polo, N., Hicks, H.M., Mioulet, V., Knowles, N. J. and King, D.P. 2023. Genome sequence of a foot-and-mouth disease virus detected in Indonesia in 2022. Microb. Resource Announc. 12(2), e0108122; doi:10.1128/mra.01081-22. Zhang, C., Wang, X., Sun, D., Li, Y., Feng, Y., Zhang, R., Zheng, Y., Kou, Z. and Liu, Y. 2024. Modification effects of long-term air pollution levels on the relationship between short-term exposure to meteorological factors and hand, foot, and mouth disease: a distributed lag non-linear model-based study in Shandong Province, China. Ecotoxicol. Environ. Saf. 272, 116060; doi:10.1016/j.ecoenv.2024.116060. | ||
| How to Cite this Article |
| Pubmed Style Rehman S, Ullah S, Abuzahra M, Effendi MH, Budiastuti B, Kholik K, Munawarah M, Zaman A, Rahman AU, Malik MI, Ullah S, Rustam SA. Determination of risk factors for foot and mouth disease emergence in East Java, Indonesia. Open Vet. J.. 2025; 15(5): 2049-2058. doi:10.5455/OVJ.2025.v15.i5.21 Web Style Rehman S, Ullah S, Abuzahra M, Effendi MH, Budiastuti B, Kholik K, Munawarah M, Zaman A, Rahman AU, Malik MI, Ullah S, Rustam SA. Determination of risk factors for foot and mouth disease emergence in East Java, Indonesia. https://www.openveterinaryjournal.com/?mno=238172 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i5.21 AMA (American Medical Association) Style Rehman S, Ullah S, Abuzahra M, Effendi MH, Budiastuti B, Kholik K, Munawarah M, Zaman A, Rahman AU, Malik MI, Ullah S, Rustam SA. Determination of risk factors for foot and mouth disease emergence in East Java, Indonesia. Open Vet. J.. 2025; 15(5): 2049-2058. doi:10.5455/OVJ.2025.v15.i5.21 Vancouver/ICMJE Style Rehman S, Ullah S, Abuzahra M, Effendi MH, Budiastuti B, Kholik K, Munawarah M, Zaman A, Rahman AU, Malik MI, Ullah S, Rustam SA. Determination of risk factors for foot and mouth disease emergence in East Java, Indonesia. Open Vet. J.. (2025), [cited January 25, 2026]; 15(5): 2049-2058. doi:10.5455/OVJ.2025.v15.i5.21 Harvard Style Rehman, S., Ullah, . S., Abuzahra, . M., Effendi, . M. H., Budiastuti, . B., Kholik, . K., Munawarah, . M., Zaman, . A., Rahman, . A. U., Malik, . M. I., Ullah, . S. & Rustam, . S. A. (2025) Determination of risk factors for foot and mouth disease emergence in East Java, Indonesia. Open Vet. J., 15 (5), 2049-2058. doi:10.5455/OVJ.2025.v15.i5.21 Turabian Style Rehman, Saifur, Shakeeb Ullah, Mutasem Abuzahra, Mustofa Helmi Effendi, Budiastuti Budiastuti, Kholik Kholik, Muhammad Munawarah, Ali Zaman, Atta Ur Rahman, Muhammad Inamullah Malik, Sana Ullah, and Saqib Ali Rustam. 2025. Determination of risk factors for foot and mouth disease emergence in East Java, Indonesia. Open Veterinary Journal, 15 (5), 2049-2058. doi:10.5455/OVJ.2025.v15.i5.21 Chicago Style Rehman, Saifur, Shakeeb Ullah, Mutasem Abuzahra, Mustofa Helmi Effendi, Budiastuti Budiastuti, Kholik Kholik, Muhammad Munawarah, Ali Zaman, Atta Ur Rahman, Muhammad Inamullah Malik, Sana Ullah, and Saqib Ali Rustam. "Determination of risk factors for foot and mouth disease emergence in East Java, Indonesia." Open Veterinary Journal 15 (2025), 2049-2058. doi:10.5455/OVJ.2025.v15.i5.21 MLA (The Modern Language Association) Style Rehman, Saifur, Shakeeb Ullah, Mutasem Abuzahra, Mustofa Helmi Effendi, Budiastuti Budiastuti, Kholik Kholik, Muhammad Munawarah, Ali Zaman, Atta Ur Rahman, Muhammad Inamullah Malik, Sana Ullah, and Saqib Ali Rustam. "Determination of risk factors for foot and mouth disease emergence in East Java, Indonesia." Open Veterinary Journal 15.5 (2025), 2049-2058. Print. doi:10.5455/OVJ.2025.v15.i5.21 APA (American Psychological Association) Style Rehman, S., Ullah, . S., Abuzahra, . M., Effendi, . M. H., Budiastuti, . B., Kholik, . K., Munawarah, . M., Zaman, . A., Rahman, . A. U., Malik, . M. I., Ullah, . S. & Rustam, . S. A. (2025) Determination of risk factors for foot and mouth disease emergence in East Java, Indonesia. Open Veterinary Journal, 15 (5), 2049-2058. doi:10.5455/OVJ.2025.v15.i5.21 |