| Research Article | ||
Open Vet. J.. 2025; 15(5): 2021-2029 Open Veterinary Journal, (2025), Vol. 15(5): 2021-2029 Research Article Electrophoretic protein profiles in seminal plasma and sperm: a comparative study of Bali and Wagyu BullsTulus Maulana1,2, Panca Andes Hendrawan1, Sutikno Sutikno2, Pajri Anwar1,3, Mokhamad Fakhrul Ulum4, Rudy Priyanto5 and Jakaria Jakaria5*1Graduate School of Animal Production and Technology, Faculty of Animal Science, IPB University, Bogor, Indonesia 2Research Center for Applied Zoology, National Research and Innovation Agency, Bogor, Indonesia 3Department of Animal Science, Universitas Islam Kuantan Singingi, Teluk Kuantan, Riau, Indonesia 4Division of Reproduction and Obstetrics, School of Veterinary Medicine and Biomedical Sciences, IPB University, Bogor, Indonesia 5Department of Animal Production and Technology, Faculty of Animal Science, IPB University, Bogor, Indonesia *Corresponding Author: Jakaria Jakaria, Department of Animal Production and Technology, Faculty of Animal Science, IPB University, Bogor, Indonesia. Email: jakaria [at] apps.ipb.ac.id Submitted: 08/01/2025 Revised: 09/04/2025 Accepted: 21/04/2025 Published: 31/05/2025 © 2025 Open Veterinary Journal
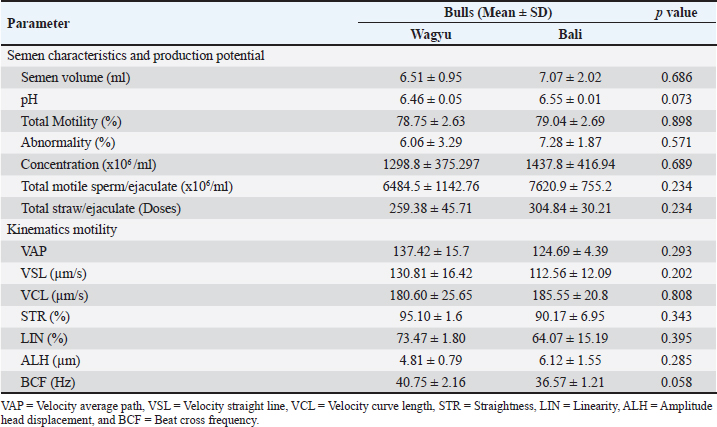
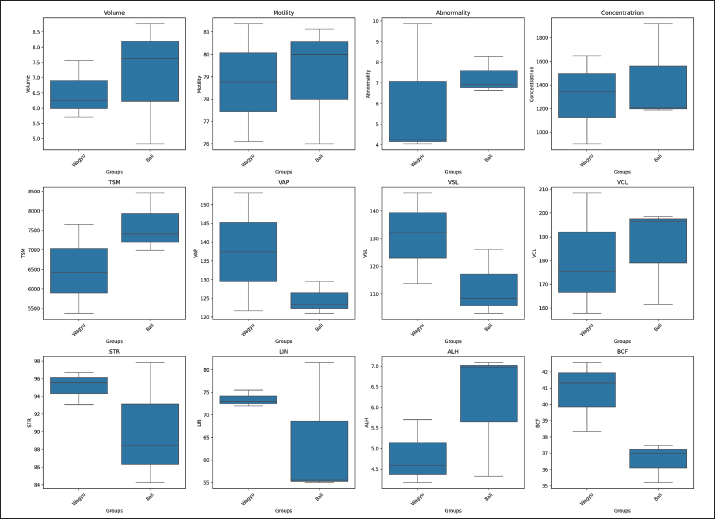
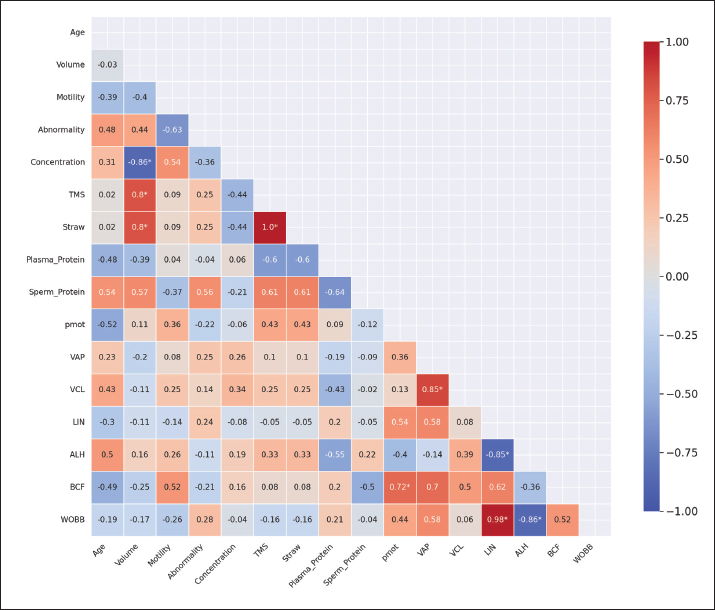
ABSTRACTBackground: Seminal plasma and sperm play vital roles in fertility because they contain various proteins that influence sperm quality and function. Aim: This study aimed to compare the protein profiles of seminal plasma and sperm in Bali and Wagyu cattle and evaluate their correlation with fresh semen quality. Methods: The study was conducted at the Singosari National Artificial Insemination Center using 3 Bali and 3 Wagyu superior bulls aged 5–12 years. Sperm motility parameters were analyzed using computer-assisted sperm analysis (IVOS-Hamilton). Sperm were extracted using the PRO-PREPTM Protein Extraction Solution. Sperm and seminal plasma protein concentrations were measured using the BCA protein assay, and 1D SDS-PAGE was used to analyze protein profiles. The gels were stained with Coomassie Brilliant Blue to visualize protein bands. Results: Comparative analysis of semen characteristics, motility parameters, and kinematic parameters between Wagyu and Bali bulls revealed no significant differences. The seminal plasma protein concentrations in Bali bulls and Wagyu did not show a statistically significant difference. The sperm protein concentration in Bali bulls was significantly higher compared to Wagyu. Seminal plasma analysis of Bali bulls showed a diverse protein profile and Wagyu semen plasma had fewer bands, reflecting breed-specific differences. In contrast, sperm analysis of Bali bulls and Wagyu consistently showed heavy protein bands on sperm function and viability. Conclusion: Bali bulls showed greater variation in spermatozoa protein than Wagyu, with protein differences at molecular weights of 60–63 kDa, 35 kDa, and 18–20 kDa. In addition, a positive correlation was found between the number of motile spermatozoa and the number of straws per ejaculate. Keywords: Bali cattle, electrophoretic, sperm, seminal plasma, wagyu cattle. IntroductionEffective reproduction is a critical factor in the livestock industry, particularly in cattle breeding, for enhancing productivity and genetic quality. Seminal plasma and sperm contain various proteins that influence sperm quality and function, providing insights into fertility potential and reproductive health across breeds. Seminal plasma, a complex fluid derived from the testes, epididymis, and accessory sexual glands, sustains sperm viability and influences key reproductive processes. The protein composition of the sperm varies across species, affecting their functions such as freezability, motility, membrane integrity, protection against reactive oxygen species (ROS), capacitation, and acrosome reaction (Asadpour et al., 2007; Pardede et al., 2020). Correlations between semen characteristics and seminal plasma proteins highlight their roles in reproductive success (Sharma et al., 2015). High levels of these proteins are often linked to greater fertility (Peddinti et al., 2008) because they contribute to energy metabolism, cell communication, and spermatogenesis (Druart et al., 2019). Selecting superior bulls from the National Artificial Insemination Center is crucial for enhancing genetic quality and productivity. This process follows the Indonesian Minister of Agricultural Regulation Number 10/Permentan/PK.210/3/2016, involving evaluations of body conformation, testicular health, and internal and external reproductive organs through the Breeding Soundness Examination (BSE) method (Hancock et al., 2016). Such rigorous selection ensures the use of bulls with optimal reproductive potential in artificial insemination programs. Bull fertility hinges on sperm’s ability to fertilize oocytes successfully, yet no single test can definitively assess fertilizing potential. Therefore, researchers are exploring genetic and molecular markers associated with reproductive performance. Seminal proteins have emerged as promising indicators of sperm functionality and fertility potential, influencing spermatogenesis, motility, and overall reproductive health (Singh et al., 2014; Abdulkareem and Musa, 2021; Iskandar et al., 2023). A comprehensive understanding of the protein profiles of bull sperm and their correlation with fertility is crucial for enhancing the efficiency of breeding programs and improving reproductive outcomes. However, despite extensive research on bovine reproduction, comparative analyses of the seminal plasma and sperm proteomes of genetically distinct cattle breeds remain limited. This knowledge gap is particularly relevant for examining breeds with distinct genetic and reproductive traits, such as Bali and Wagyu cattle. Bali cattle are well adapted to tropical environments, exhibiting resilience and high reproductive efficiency (Prastiya et al., 2024), whereas Wagyu cattle are globally renowned for their exceptional meat quality and marbling characteristics (Gotoh et al., 2018). Despite their economic and reproductive significance, the molecular mechanisms underlying the differences in fertility between these breeds remain poorly understood. Investigating the seminal plasma and sperm protein profiles of Bali and Wagyu cattle is therefore essential for identifying key molecular factors that influence fertility, which could contribute to the development of more effective reproductive management strategies and genetic selection programs. This study aimed to compare the protein profiles of seminal plasma and sperm in Bali and Wagyu cattle and assess their correlation with fresh semen quality. By elucidating these relationships, this study is expected to provide deeper insights into the molecular determinants of fertility in both breeds. These findings have significant implications for improving artificial insemination protocols, optimizing breed-specific reproductive management strategies, and refining genetic selection programs to enhance fertility and productivity in cattle breeding systems. Materials and MethodsAnimalsThis research was conducted at the Singosari National Artificial Insemination Center (SNAIC), Singosari, Indonesia, which is a facility certified under SNI ISO 9001:2015 (No. G.01-ID0139-VIII-2019). The study involved six superior bulls, comprising three Bali and three Wagyu bulls, aged between 5 and 12 years, which were maintained under standardized management conditions in strict accordance with SNAIC’s operational protocols. All research activities, including sperm collection and handling, were performed under the direct supervision of licensed veterinarians to ensure animal welfare and procedural integrity. Semen collection and evaluationSemen was collected twice a week using an artificial vagina as a Standard Operating Procedure (SOP) of the SNAIC. Semen quality data were determined in laboratories via direct observation after semen collection. Microscopic evaluations of motility, abnormality, and sperm concentration were recorded. The macroscopic evaluations were volume, pH, and color. Kinematic motility analysis using Computer Assisted Semen Analyzer (CASA) IMV-Hamilton with parameters of total motility, velocity average path (VAP), velocity curve length (VCL), velocity straight length (VSL), straightness (STR), linearity (LIN), amplitude of lateral head (ALH), beat cross frequency (BCF), and wobble (WOBB). According to standard operational procedures (SOP) at SNAIC in a dose of 1 straw (0.25 ml, the total spermatozoa concentration is 25 × 106 cell/ml), bull productivity was assessed from the Total Spermatozoa Motile using the following formula: Total Spermatozoa Motile (x106)=volume × concentration × motility/ejaculate. The estimated number of frozen semen straws produced in one year was calculated using the following formula: number of straws (doses)=Total Spermatozoa Motile/25 × 106. Identification of seminal plasma and spermatozoa proteinSemen was centrifuged at 6500 rpm for 30 minutes to separate sperm and seminal plasma. Pellet sperm were centrifuged at 1800 rpm for 10 minutes using PBS for washing, and sperm pellets were then extracted using PRO-PREPTM Protein extraction solution (iNtRON Biotechnologi, Korea). The seminal plasma and sperm protein concentrations were determined using the bicinchoninic acid (BCA) protein assay (Thermo Scientific™, USA). SDS-PAGE analysis was performed to determine the protein profile based on molecular weight, which was visualized as bands on the gels. Protein separation was performed by 1D SDS-PAGE using SurePAGE™, Bis-Tris, 10 cm × 8 cm, 12 wells, 4%–20% gradient gel (M00656; GenScript) (SurePAGE, Genscript Biotech Corp., Hong Kong) with Broad Multi Color Pre-Stained Protein Standard (M00624; GenScript) with a molecular weight range of ~6.5–240 kDa and Tris-MOPS-SDS Running Buffer (M00138; GenScript) with a voltage of 200 V and a current of 100 mA for 40 minutes. The gel was then stained with Coomassie Brilliant Blue (R-250; Bio-rad, USA). Statistical analysisThe quality of fresh semen and concentration of protein in the seminal plasma and sperm of Bali and Wagyu bulls were analyzed using the Mann–Whitney U test, with statistical significance determined at p < 0.05. All statistical analyses were performed using SPSS version 26 (IBM® Corp., Armonk, NY, US) software. Ethical approvalThe study strictly adhered to the ethical guidelines outlined in SNI ISO 9001:2015, ensuring compliance with best practices in animal research. Ethical approval was granted by the SNAIC Ethics Committee, which reviewed the study protocols to ensure that all procedures minimized animal stress and followed established welfare standards. ResultsFresh semen quality and productivity of Bali and Wagyu bullsThe comparative analysis of semen characteristics and motility parameters between Wagyu and Bali bulls revealed no significant differences p > 0.05 (Table 1). Bali bulls had a slightly higher semen volume (7.07 ± 2.02 ml) than Wagyu bulls (6.51 ± 0.95 ml, p =0.686). Total motility was similar in both breeds (Bali: 79.04 ± 2.69% and Wagyu: 78.75 ± 2.63%, p =0.898). Bali bulls had more abnormal sperm (7.28 ± 1.87%) than Wagyu (6.06 ± 3.29%, p =0.571). Bali bulls also had higher sperm concentrations (1437.8 ± 416.94 × 106/ml) and total motile sperm per ejaculate (7620.9 ± 755.2 × 106/ml), but these differences were not significant (p =0.689, p =0.234). Bali bulls had higher potential straw production per ejaculate (304.84 ± 30.21 doses) than Wagyu (259.38 ± 45.71 doses, p =0.234). Kinematic parameters showed no significant differences, with Wagyu bulls having higher VAP and VSL and Bali bulls having slightly higher VCL. Wagyu bulls had higher STR and LIN percentages, whereas Bali bulls had higher ALH and Wagyu had higher BCF (p =0.058). The boxplots indicate notable differences in the semen quality attributes between the two groups (Fig. 1), suggesting potential variations in reproductive performance or semen characteristics. These differences are reflected in key parameters such as motility, concentration, velocity measures (VAP, VSL, and VCL), and structural features such as ALH and BCF. Such variations may provide insights into the relative fertility or suitability of semen for artificial insemination programs in the studied groups. The correlation matrix revealed a significant positive correlation (p < 0.05) between total motile sperm and number of straw production (TMS-Straw), as well as among VCL-VAP, WOB-LIN, and BCF-progressive motility. Conversely, a significant negative correlation (p < 0.05) was observed between concentration and volume, age and volume, ALH and LIN, and ALH and WOB, indicating an inverse relationship where an increase in one variable corresponds to a decrease in the other (Fig. 2). Table 1. Quality and productivity of fresh semen in Bali and Wagyu bulls.
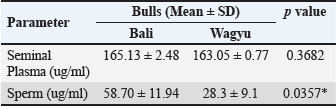
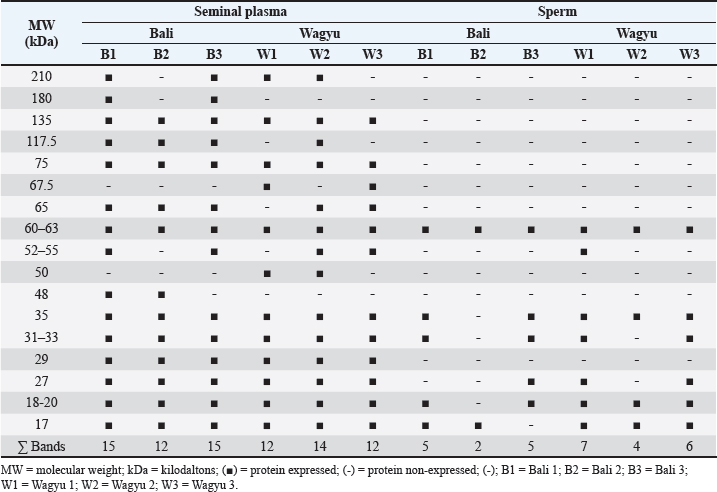
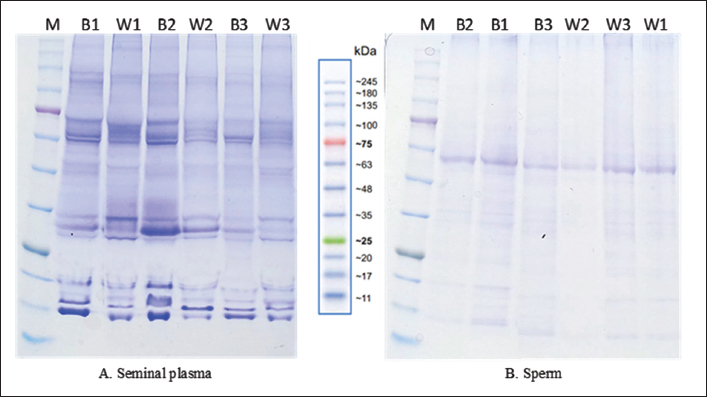
Fig. 1. Distribution of each parameter in the Wagyu and Bali groups. Electrophoretic profiles of seminal plasma and sperm proteinThe seminal plasma protein concentrations in Bali and Wagyu bulls did not show a statistically significant difference ( p=0.3682). However, the sperm protein concentration in Bali bulls (58.70 ± 11.94 µg/ml) was significantly higher than that in Wagyu bulls (28.13 ± 9.10 µg/ml), with a p value of 0.0357 (Table 2). The table presented provides a detailed analysis of protein presence at specific molecular weights (MW) in kilodalton (kDa) within the seminal plasma and sperm of Bali and Wagyu cattle (Table 3). Seminal plasma analysis of Bali cattle revealed a diverse protein profile with MW of 210, 180, 135, 117.5, 75, 65, 60–63, 52–55, 48, 35, 31–33, 29, 27, 18–20, and 17 kDa, with samples B1 and B3 showing the most bands, indicating individual variability or sample processing differences. In contrast, Wagyu cattle’s seminal plasma exhibited proteins at 210, 135, 75, 67.5, 65, 60–63, 50, 35, 31–33, 29, 27, 18–20, and 17 kDa, with fewer bands, reflecting breed-specific differences. Sperm analysis from Bali cattle consistently showed proteins at 60–63, 35, 31–33, 27, 18–20, and 17 kDa across all samples, suggesting their importance for sperm function and viability. Similarly, Wagyu sperm showed proteins at these same MW, with sample W1 displaying a slightly broader range, indicating that these proteins are crucial for sperm functionality across both breeds (Fig. 3). DiscussionThe results of this study indicate that Bali cattle have slightly higher semen volume than Wagyu cattle, although the difference was not statistically significant. This finding may reflect the physiological adaptation of Bali cattle to tropical environments, which affects their overall semen production (Indriastuti et al., 2020; Maulana et al., 2022). Thus, although the difference was not significant, the result provides important insights into the reproductive adaptations of local cattle under tropical conditions.
Fig. 2. Heatmap correlation matrix analysis in Wagyu and Bali bulls. Significant correlation is marked (*). Table 2. Seminal plasma and sperm protein of Bali and Wagyu bulls.
The analysis of sperm quality obtained in this study showed that the motility of fresh semen from both Bali and Wagyu bulls exceeded 70%, with abnormalities below 20%, meeting the standard requirements for frozen semen processing under the Indonesian National Standard (SNI 4869-1:2021). The potential semen production per ejaculate varies among individuals. Bali cattle showed higher frozen semen production per ejaculate, averaging 304.84 straws, compared to Wagyu cattle, exceeding previous findings of 225.52 straws per ejaculate (Iskandar et al., 2022). This advantage indicates that Bali bulls have more significant potential to support artificial insemination programs, thereby enhancing overall livestock reproduction efficiency. The higher straw production per ejaculate in Bali cattle than in Wagyu provides significant benefits for artificial insemination programs. Sperm quality parameters, including concentration and motility, play crucial roles in straw production. Baharun et al. (2023) reported that straw production is strongly influenced by fresh semen quality, such as concentration and motility. Bali and Wagyu cattle were carefully selected at the Artificial Insemination Center based on their semen quality and superior ability to produce frozen semen. This selection was conducted according to the Breeding Soundness Examination (BSE) protocol, which includes assessing male cattle performance, libido, and semen quality (Thundathil et al., 2016). Table 3. Electrophoretic profiles of the seminal plasma and sperm protein of Bali and Wagyu bulls.
Fig. 3. 1D SDS-Page analysis of seminal plasma and spermatozoa protein; kDa: kilodaltons; M: marker; B series=Bali group; W series=Wagyu group. However, semen quality is not always directly correlated with reproductive performance. This highlights the need for molecular evaluations as additional indicators during the selection of male cattle (Baharun et al., 2021). Sperm kinematic parameters showed different patterns between the two cattle breeds. Higher STR, LIN, and BCF values in Wagyu cattle reflect efficient sperm movement in a straight trajectory. In contrast, Bali cattle with higher VCL and ALH exhibit motility patterns with stronger head movements. Fernandez-Novo et al. (2021) reported that spermatozoa with more linear trajectories, higher velocity, and larger head movement amplitudes tend to have better fertility rates. The velocity parameters measured by CASA can also serve as reliable markers of male cattle fertility. A significant positive correlation between total motile sperm and the number of straws produced indicates that higher motility is an important determinant of semen productivity. Higher sperm productivity in Bali cattle is correlated with increased straw production (Tethool et al., 2021; Nugraha et al., 2022). Significant negative relationships between variables such as concentration and volume or ALH and LIN highlight tradeoffs in semen quality parameters. For example, higher semen volume may lead to diluted sperm concentration, whereas increased lateral head displacement (ALH) may reduce linearity, potentially affecting sperm efficiency in reaching the oocyte (Pernas et al., 2023; Hendri et al., 2024). Protein profiles of seminal plasma and sperm revealed significant differences between the two cattle breeds. Although the concentration of seminal plasma proteins does not show significant differences, Bali cattle had significantly higher sperm protein concentrations than Wagyu. This finding suggests that Bali cattle may produce more sperm-associated proteins, which could play an important role in sperm motility, capacitation, and fertilization processes. Iskandar et al. (2023) identified 94 proteins in the seminal plasma of Bali bulls, ranging from <11 to 110 kDa. Proteins, such as spermadhesin 1 (SPADH1), C-type natriuretic peptide (NPPC), clusterin (CLU), apolipoprotein A-II (APOA2), inositol-3-phosphate synthase 1 (ISYNA1), and sulfhydryl oxidase 1 (QSOX1), were identified as important for fertility in Bos javanicus. Sarsaifi et al. (2015) identified albumin, clusterin, seminal ribonuclease, and cationic trypsin as major proteins in the seminal plasma of Bali bulls that enhance oocyte penetration ability. The diversity of proteins identified in the seminal plasma and sperm of both cattle breeds underscores the importance of these proteins in reproductive function. Certain proteins consistently detected in both breeds, such as those in the molecular weight ranges of 60–63, 35, 31–33, 27, 18–20, and 17 kDa, are likely to play essential roles in sperm function and fertility. Previous studies have linked these proteins to key processes such as sperm motility, membrane integrity, and acrosome reactions, emphasizing their biological significance. Proteins such as Binder of Sperm (BSP) A1/A2, BSP-A3, and BSP-30 (MW 15–30 kDa) are involved in sperm binding and fertility potential (Druart et al., 2019). Furthermore, Recuero et al. (2019) reported that the BSP1, BSP3, and BSP5 protein families in male cattle are correlated with capacitation, sperm reservoirs, and preservation. Proteins with an MW of 50–65 kDa play vital roles in catalytic energy metabolism within mitochondria, which are crucial for sperm motility and function (Özbek et al., 2021). The differences in protein profiles between Bali and Wagyu cattle, particularly the broader range of proteins in Bali cattle, may reflect breed-specific adaptations to environmental or physiological conditions. Moreover, variations in individual protein band patterns suggest that genetic and environmental factors can influence protein expression. Further research is needed to explore the roles of these proteins as fertility biomarkers. ConclusionBali bulls showed greater variation in spermatozoa protein than Wagyu bulls, with protein differences at MW of 60–63 kDa, 35 kDa, and 18–20 kDa. In addition, a positive correlation was found between the number of motile spermatozoa and the number of straws per ejaculate. In contrast, a negative correlation was observed between semen volume and spermatozoa concentration. AcknowledgmentsThe authors gratefully acknowledge the support from the Singosari Center for Artificial Insemination in Malang, East Java, Indonesia. Conflict of interestThe authors declare that there are no conflicts of interest regarding the publication of this paper. Data availabilityAll data were included in the manuscript. FundingThis research received financial support from the National Research and Innovation Agency (BRIN) through the Research and Innovation for Advanced Indonesia (RIIM) Program under contract numbers 18/IV/KS/06/2022 and 4830/IT3.L1/PT.01.03/P/B/2022. Author’s contributionsConceptualization: JK, TM, PAH, PA, S, MFU, and RP. Data curation: JK and TM. Investigation: JK, TM, and PAH. Methodology: JK, PAH, and PA. Resources: JK and TM. Supervision: JK and TM. Writing–original draft: JK, TM, PAH, PA, S, MFU, and RP. Writing–review and editing: JK, TM, PAH, PA, S, MFU, and RP. ReferencesAbdulkareem, T.A. and Musa, K.S. 2021. Protein Profiles in seminal plasma of iraqi buffalo bulls (Bubalus bubalis) associated with fresh and cryopreserved semen quality. IOP Conf Ser: Earth Environ Sci. 7, 1–14. https://doi.org/10.1088/1755-1315/1262/7/072095. Asadpour, R., Alavi–Shoushtri, S.M., Asri Rezaii, S. and Ansari, M.H.K. 2007. SDS-polyacrylamide gel electrophoresis of buffalo bulls seminal plasma proteins and their relation with semen freezability. Anim. Reprod. Sci. 102(2–3), 208–313. https://doi.org/10.1016/j.anireprosci.2007.03.003 Baharun, A., Arifiantini, I.R., Karja, K.W.N. and Said, S. 2021. Seminal plasma protein profile based on molecular weight and the correlation with semen quality of Simmental bull. J. Indones. Trop. Anim. Agric. 46(1), 20–28. https://doi.org/ 10.14710/jitaa.46.1.20-28 Baharun, A., Rahmi, A., Handarini, R., Maulana, T., Said, S., Iskandar, H., Darussalam, I., Nalley, M.M.W. and Arifiantini, I.R. 2023. Semen quality and frozen semen production in Pasundan bulls: a molecular weight perspective on seminal plasma and spermatozoa protein. J. Adv. Vet. Anim. Res. 10(4), 730–737. https://doi.org/ 10.5455/javar.2023.j728 Druart, X., Rickard, J.P., Tsikis, G. and de Graaf, S.P. 2019. Seminal plasma proteins as markers of sperm fertility. J. Theriogenol. 137, 3–-35. https://doi.org/10.1016/j.theriogenology.2019.05.034 Fernandez-Novo, A., Santos-Lopez, S., Barrajon-Masa, C., Mozas, P., de Mercado, E., Caceres, E., Garrafa, A., Gonzalez-Martin, J.V., Perez-Villalobos, N., Oliet, A., Astiz, S. and Perez-Garnelo., S.S. 2021. Effect of extender, storage time and temperature on kinetic parameters (CASA) on bull semen samples. Biology (Basel). 10(8), 1–17. https://doi.org/10.3390/biology10080806h Gotoh, Takafumi, Nishimura, T., Kuchida, K. and Mannen, H. 2018. The Japanese Wagyu beef industry: current situation and future prospects—a review. Asian-Australasian J. Anim. Sci. 31(7), 933–950. https://doi.org/10.5713/ajas.18.0333 Hancock, A.S., Younis, P.J., Beggs, D.S., Mansell, P.D., Stevenson, M.A. and Pyman M.F. 2016. An assessment of dairy herd bulls in southern Australia: 2. Analysis of bull- and herd-level risk factors and their associations with pre- and postmating breeding soundness results. J. Dairy Sci. 99(12), 1–11. https://doi.org/10.3168/jds.2015-10792 Hendri, H., Jaswandi, J., Indriastuti, R. and Ananda, A. 2024. Sperm kinematics of Pesisir bull thawed at different temperatures and times. Bull. Anim. Sci. 48(4), 233–241. https://doi.org/10.21059/buletinpeternakv%vi%i.96459 Indriastuti, R., Ulum, M.F., Arifiantini, R.I. and Purwantara, B. 2020. Individual variation in fresh and frozen semen of Bali bulls (Bos sondaicus). Vet World. 13(5), 840–846. https://doi.org/ 10.14202/vetworld.2020.840-846 Iskandar, H., Andersson, G., Sonjaya, H., Arifiantini, R.I., Said, S., Hasbi, H., Maulana, T. and Baharun, A. 2023. Protein identification of seminal plasma in Bali bull (Bos javanicus). Animals. 13(3), 1–12. https://doi.org/ 10.3390/ani13030514 Iskandar, H., Sonjaya, H., Arifiantini, R. I. and Hasbi H. 2022. The quality of fresh and frozen semen and its correlation with molecular weight of seminal plasma protein in Bali cattle. Trop. Anim. Sci. J. 45(4), 405–412. https://doi.org/10.5398/tasj.2022.45.4.405 Maulana, T., Anwar, S., Volkandari, S., Nur, M. and Said, S. 2022. Seasonal effects on the semen production of Bali bulls (Bos javanicus) in West Nusa Tenggara’s Lelede regional artificial insemination center. Livest. Anim. Res. 20(2), 152–158. https://doi.org/10.20961/lar.v20i2.58677 National Standardization Agency of Indonesia. 2021. Frozen Semen- part 2: Cattle. SNI 4869-2:2021. Jakarta: National Standardization Agency of Indonesia, pp: 1–4. Nugraha, C.D., Herwijanti, E., Novianti, I., Furqon, A., Septian, W.A., Busono, W. and Suyadi, S. 2019. Correlations between age of Bali bull and semen production at national artificial insemination center, Singosari—Indonesia. J. Indonesian Trop. Anim. Agric. 44(3), 258–265. https://doi.org/10.14710/jitaa.44.3.258-265 Özbek, M., Hitit, M., Kaya, A., Jousan, F.D. and Memili E. 2021. Sperm functional genome associated with bull fertility. Front. Vet. Sci. 8(5), 1–17. Pardede, B.P., Agil, M. and Supriatna, I. 2020. Protamine and other proteins in sperm and seminal plasma as molecular markers of bull fertility. Vet. World 13(9), 556–562. https://doi.org/10.3389/fvets.2021.610888 Peddinti, D., Nanduri, B., Kaya, A., Feugang, J.M., Burgess., S.C. and Memili, E. 2008. Comprehensive proteomic analysis of bovine spermatozoa of varying fertility rates and identification of biomarkers associated with fertility. BMC Syst. Biol. 19(2), 1–13. https://doi.org/10.1186/1752-0509-2-19 Pernas, S., Fernandez-Novo, A., Barrajon-Masa, C., Mozas, P., Pérez-Villalobos, N., Martín-Maldonado, B., Oliet, A., Astiz, S. and Pérez-Garnelo, S.S. 2023. Bull semen obtained on beef farms by electroejaculation: sperm quality in the first two hours of storing with different extenders and holding temperatures. Animals. 13(9):1–14. https://doi.org/10.3390/ani13091561 Prastiya, Ragil, Sardjito, T., Saputro, A., Budi, D., Maxdhameta, S., Sulistiyawati, E., Sulistyowati, D., Amaliya, A., Sasi, S. and Haryuni, N. 2024. Quality and kinematic characteristics of Bali bulls frozen semen with purified green tea extract epigallocatechin-3-gallate antioxidant addition in diluent. Open Vet. J. 14(8), 2040–2048. https://doi.org/10.5455/OVJ.2024.v14.i8.33 Recuero, S., Fernandez-Fuertes, B., Bonet, S., Barranco, I. and Yeste, M. 2019. Potential of seminal plasma to improve the fertility of frozen-thawed boar spermatozoa. J. Theriogenol. 137, 36–42. https://doi.org/10.1016/j.theriogenology.2019.05.035 Sarsaifi, K., Haron, W.A., Vijayan, J., Yusoff, R., Hani, H., Omar, A.M., Hong, W.L., Yimer, N., Ju, Y.T. and Othman, M. 2015. Two-dimensional polyacrylamide gel electrophoresis of Bali bull (Bos javanicus) seminal plasma proteins and their relationship with semen quality. J. Theriogenol. 84, 956–968. https://doi.org/10.1016/j.theriogenology.2015.05.035 Sharma, L., Pandey, V., Nigam, R., Saxena, A. and Swain, D.K. 2015. Association of Semen attributes and seminal plasma proteins of buffalo bulls. J. Anim. Res. 5(1), 119–123. https://doi.org/10.5958/2277-940X.2015.00020.0 Singh, A.K., Brar, P.S., Cheema, R.S., Kaur, M. and Bansal, A.K. 2014. Characterization of buffalo bull frozen-thawed sperm proteins through SDSPAGE and their correlation with HOST and in vitro acrosome reaction. Indian J. Anim. Sci. 84(9), 949–953. https://doi.org/10.56093/ijans.v84i9.43514 Tethool, A.N., Ciptadi, G., Wahjuningsih. S., Amaliya, A., Sawitri. W. and Susilawati, T. 2021. The influence of individual factors on the characteristics and production of frozen semen of bali cattle. J. Adv. Vet. Res. 11(3), 162–166. Thundathil, C.J., Dance, L.A. and Kastelic, P.J. 2016. Fertility management of bulls to improve beef cattle productivity. J. Theriogenol. 86, 397–405. https://doi.org/10.1016/j.theriogenology.2016.04.054 | ||
| How to Cite this Article |
| Pubmed Style Maulana T, Hendrawan PA, Sutikno S, Anwar P, Ulum MF, Priyanto R, Jakaria J. Electrophoretic protein profiles in seminal plasma and sperm: a comparative study of Bali and Wagyu Bulls. Open Vet. J.. 2025; 15(5): 2021-2029. doi:10.5455/OVJ.2025.v15.i5.18 Web Style Maulana T, Hendrawan PA, Sutikno S, Anwar P, Ulum MF, Priyanto R, Jakaria J. Electrophoretic protein profiles in seminal plasma and sperm: a comparative study of Bali and Wagyu Bulls. https://www.openveterinaryjournal.com/?mno=236714 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i5.18 AMA (American Medical Association) Style Maulana T, Hendrawan PA, Sutikno S, Anwar P, Ulum MF, Priyanto R, Jakaria J. Electrophoretic protein profiles in seminal plasma and sperm: a comparative study of Bali and Wagyu Bulls. Open Vet. J.. 2025; 15(5): 2021-2029. doi:10.5455/OVJ.2025.v15.i5.18 Vancouver/ICMJE Style Maulana T, Hendrawan PA, Sutikno S, Anwar P, Ulum MF, Priyanto R, Jakaria J. Electrophoretic protein profiles in seminal plasma and sperm: a comparative study of Bali and Wagyu Bulls. Open Vet. J.. (2025), [cited January 25, 2026]; 15(5): 2021-2029. doi:10.5455/OVJ.2025.v15.i5.18 Harvard Style Maulana, T., Hendrawan, . P. A., Sutikno, . S., Anwar, . P., Ulum, . M. F., Priyanto, . R. & Jakaria, . J. (2025) Electrophoretic protein profiles in seminal plasma and sperm: a comparative study of Bali and Wagyu Bulls. Open Vet. J., 15 (5), 2021-2029. doi:10.5455/OVJ.2025.v15.i5.18 Turabian Style Maulana, Tulus, Panca Andes Hendrawan, Sutikno Sutikno, Pajri Anwar, Mokhamad Fakhrul Ulum, Rudy Priyanto, and Jakaria Jakaria. 2025. Electrophoretic protein profiles in seminal plasma and sperm: a comparative study of Bali and Wagyu Bulls. Open Veterinary Journal, 15 (5), 2021-2029. doi:10.5455/OVJ.2025.v15.i5.18 Chicago Style Maulana, Tulus, Panca Andes Hendrawan, Sutikno Sutikno, Pajri Anwar, Mokhamad Fakhrul Ulum, Rudy Priyanto, and Jakaria Jakaria. "Electrophoretic protein profiles in seminal plasma and sperm: a comparative study of Bali and Wagyu Bulls." Open Veterinary Journal 15 (2025), 2021-2029. doi:10.5455/OVJ.2025.v15.i5.18 MLA (The Modern Language Association) Style Maulana, Tulus, Panca Andes Hendrawan, Sutikno Sutikno, Pajri Anwar, Mokhamad Fakhrul Ulum, Rudy Priyanto, and Jakaria Jakaria. "Electrophoretic protein profiles in seminal plasma and sperm: a comparative study of Bali and Wagyu Bulls." Open Veterinary Journal 15.5 (2025), 2021-2029. Print. doi:10.5455/OVJ.2025.v15.i5.18 APA (American Psychological Association) Style Maulana, T., Hendrawan, . P. A., Sutikno, . S., Anwar, . P., Ulum, . M. F., Priyanto, . R. & Jakaria, . J. (2025) Electrophoretic protein profiles in seminal plasma and sperm: a comparative study of Bali and Wagyu Bulls. Open Veterinary Journal, 15 (5), 2021-2029. doi:10.5455/OVJ.2025.v15.i5.18 |