| Research Article | ||
Open Vet. J.. 2025; 15(5): 2009-2015 Open Veterinary Journal, (2025), Vol. 15(5): 2009-2015 Case Report Multisystemic aspergillosis with unusual vertebral osteomyelitis in a turkey flock in Bordj Bou Arreridj Province, AlgeriaNor El Houda Belalmi1,2*, Nassim Sid1,2, Tahar Sedrati2, Soraya Ouhida3, Omar Bennoune4 and Özlem Özmen51Laboratory of Health and Environment, University Mohamed El Bachir El Ibrahimi of Bordj Bou Arreridj, El Anceur, Algeria 2Faculty of Nature and Life Sciences and Earth and the Universe, University Mohamed El Bachir El Ibrahimi of Bordj Bou Arreridj, El Anceur, Algeria 3Histopathology Laboratory, University Hospital Center Saadna Mohamed Abdennour, Setif, Algeria 4Laboratory of Health, Animal Production and Environment (ESPA), Institute of Veterinary and Agricultural Sciences, University of Batna1, Batna, Algeria 5Department of Pathology, Faculty of Veterinary Medicine, Burdur Mehmet Akif Ersoy University, Burdur, Türkiye *Corresponding Author: Nor El Houda Belalmi. Laboratory of Health and Environment, University Mohamed El Bachir El Ibrahimi of Bordj Bou Arreridj, El Anceur, Algeria. Email: nourelhouda.belalmi [at] univ-bba.dz Submitted: 05/01/2025 Revised: 26/03/2025 Accepted: 07/04/2025 Published: 31/05/2025 © 2025 Open Veterinary Journal
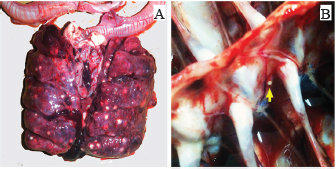
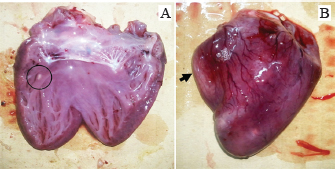
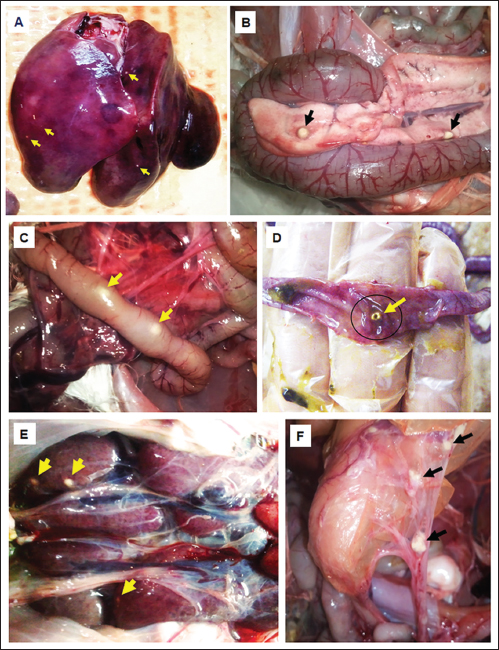
ABSTRACTBackground: Avian aspergillosis is primarily a respiratory disease that can spread to other organs, resulting in systemic aspergillosis. Aim: This report describes the clinical and pathological features of multisystemic aspergillosis caused by Aspergillus fumigatus in a meat turkey flock, including an unusual case of vertebral Aspergillus osteomyelitis. Methods: Five affected turkeys were necropsied from a flock size of 1,000 birds. Gross lesions were recorded, and samples from affected organs were sampled for histopathological examination. Tissue sections were stained with hematoxylin and eosin and periodic acid-Schiff (PAS). Microscopic examination of morphological features was performed to identify the causative agent isolated from fungal cultures on Sabouraud Dextrose Agar (SDA). Results: The affected turkeys exhibited symptoms such as respiratory distress, open-beak breathing, prostration, cyanosis, lethargy, anorexia, and weight loss. Postmortem examinations revealed whitish-yellow caseous nodules in multiple organs, including the lungs, air sacs, thoracic vertebrae, heart, liver, pancreas, spleen, peritoneum, small intestine, proventriculus, gizzard, and kidneys. Histopathological analysis showed granulomas with a necrotic core surrounded by lymphocytes, plasma cells, macrophages, and large multinucleated foreign-body giant cells. PAS staining revealed abundant fungal hyphae within the necrotic center. Fungal culture on SDA and subsequent morphological identification confirmed the presence of A. fumigatus. Conclusion: To our knowledge, this is the first documented case of thoracic vertebral aspergillosis in turkeys. Future studies are needed to assess the prevalence of this mycosis in Algerian turkey flocks. Keywords: Aspergillus fumigatus, Histopathology, Multisystemic aspergillosis, Turkey, Vertebral osteomyelitis. IntroductionAspergillosis is a noncontagious mycotic disease caused by fungal species within the genus Aspergillus. While it primarily affects turkeys, chickens, humans, and other mammals, it is less frequently observed in pigeons, ducks, geese, and other domestic and wild bird species (Arné et al., 2021; Girh and Shaapan, 2024). The warm and humid conditions typical of brooder houses create an ideal environment for the proliferation of this pathogen, which poses a persistent threat to poultry health. In addition to direct losses due to mortality, surviving birds often exhibit suboptimal feed efficiency and growth rates (Seyedmousavi et al., 2015; Horvatek Tomić et al., 2021). The economic impact of aspergillosis is particularly severe in turkey production, especially when the disease occurs later in the growing cycle or affects high-valuable breeder toms (Arné et al., 2021; Kalkayeva et al., 2023). Mortality rates in avian aspergillosis are highly variable, ranging from 4.5% to 90%, depending on factors such as age at exposure (from 3 days to 20 weeks), immune status, and environmental management (Seyedmousavi et al., 2015). Although the disease primarily manifests as pulmonary aspergillosis, systemic or encephalitic forms are also documented (Arné and Lee, 2020). Outbreaks, commonly caused by Aspergillus fumigatus, are frequently reported in chicks and poults (Beernaert et al., 2010; Ulloa-Avellán et al., 2022). This species is the most prevalent isolate in poultry, producing large quantities of airborne conidia during active fungal growth on organic substrates (Munir et al., 2017). The conidia, which are easily dispersed into the environment, can be inhaled and deposited deep within the respiratory tract (Seyedmousavi et al., 2015). In clinical practice, affected birds are often asymptomatic. However, symptomatic cases present with respiratory distress, including dyspnea, gasping, and tachypnea (Charlton et al., 2008). The lungs and air sacs are indeed the primary target sites for Aspergillus spp. infections, typically displaying lesions characterized by granulomas encapsulated by fibrous tissue, congestion, and infiltration of inflammatory cells (Beytut, 2007). Hematogenous dissemination can extend the infection virtually to any organ, with the liver, spleen, and kidneys being frequently affected (Arné and Lee, 2020). However, bone infections caused by Aspergillus spp. are rare. Riddell and Topp (1972) and Bergmann et al. (1980) reported cases of fungal spondylitis characterized by granulomatous vertebral osteomyelitis in broilers. Similarly, Olias et al. (2010) described an unusual case of fungal arthritis caused by A. fumigatus in a turkey, whereas Ghazikhanian (1989) identified fungal granulomas in the sternal bones of turkey poults. The diagnosis of aspergillosis relies on the identification of fungal elements in tissues alongside associated host tissue reactions (Ozmen and Dorrestein, 2003). Although pulmonary aspergillosis remains the most extensively studied manifestation of avian aspergillosis, systemic Aspergillus infections are comparatively underreported (Arulmozhi et al., 2016). Notably, information regarding aspergillosis in Algerian poultry is limited. This study details the pathological findings of a naturally infected turkey flock with severe multisystemic aspergillosis, including an uncommon case of vertebral Aspergillus osteomyelitis. Materials and MethodsFive dead 8-week-old meat turkeys (approximately 2.5-kg body weight), from a flock of 1,000 birds, were submitted for postmortem examinations. The birds originated from a farm in Bordj Bou Arreridj Province, northeast Algeria. The animals were housed under conditions of poor ventilation and unsanitary husbandry practices with elevated humidity (70%) and temperature (29°C). These transient microclimatic conditions, recorded during the 5th week of rearing, fostered an environment conducive to the proliferation and dispersal of Aspergillus spp. spores. Feed was administered manually, while clean tap water was freely available. Feed raw materials were stored in a confined, poorly ventilated area. Birds received vaccines for Newcastle disease, rhinotracheitis, Avian Influenza/Newcastle disease combination, and hemorrhagic enteritis, administered via drinking water and intramuscular injection between 7 and 31 days. The mortality rate in the flock was 10% with respiratory distress being the predominant clinical sign. Necropsies were performed on fresh carcasses under aseptic conditions, and tissue samples were collected from the air sacs, lungs, heart, liver, intestines, spleen, pancreas, and kidneys. Histopathological analysis was performed at the Pathology Laboratory of Setif University Hospital. The tissues were fixed in 10% buffered formalin, embedded in paraffin, sectioned at 4 µm, and stained using hematoxylin and eosin (H&E) and periodic acid-Schiff (PAS) methods. The stained sections were examined under a light microscope. Samples from lesioned organs were collected for microbiological analysis and processed at the Microbiology Laboratory, University of Bordj Bou Arreridj. The organ suspensions were inoculated on Sabouraud dextrose agar (SDA) (BioMérieux®, Marcy l’Etoile, France) supplemented with chloramphenicol (0.05 mg/ml) and incubated aerobically at 25°C and 30°C for 48–72 hours. Fungal colonies were stained with lactophenol cotton blue and identified microscopically according to established methods (Quinn et al., 2011). Ethical approvalNot needed for this study. ResultsClinical signsAffected turkeys exhibited pronounced respiratory distress and open-beak breathing. Other clinical signs included prostration, cyanosis, lethargy, anorexia, weight loss, and diarrhea. Necropsy findingsPostmortem examination revealed numerous caseous granulomas on the serosal linings and within the parenchyma of various organs, characteristic of the disease. The lungs were the primary organs affected. They exhibited diffuse congestion and multifocal yellowish-white necrotic nodules measuring 1–8 mm in diameter (Fig. 1A). The thoracic and abdominal air sacs had thickened, opaque walls with yellowish nodules ranging in diameter from 2 to 5 mm. A fungal granuloma was identified in the second thoracic vertebra of one turkey (Fig. 1A). The right ventricle was dilated (Fig. 2B), with a yellowish nodule embedded within the cardiac muscle tissue (Fig. 2A). The liver was enlarged and markedly congested with a few small yellowish-white foci approximately 2 mm in diameter (Fig. 3A). The pancreas showed areas of pallor along with two caseous, yellowish-white nodules measuring 6–8 mm (Fig. 3B). A hypertrophic spleen characterized by numerous small pinpoint granulomas is observed. The small intestine (Fig. 3C and D), proventriculus, and gizzard mucosa displayed multifocal areas of congestion and yellowish-white nodules with central necrosis, measuring 5–6 mm in diameter. The peritoneum was thickened and opaque and contained yellowish, caseous flakes (Fig. 3F). The kidneys exhibited nephritis and congestion, with several small, whitish foci measuring 2–4 mm scattered throughout the caudal lobes (Fig. 3E).
Fig. 1. (A) Numerous yellowish-white caseous granulomas of varying sizes observed on the lung lobes. (B) Vertebral aspergillosis osteomyelitis observed in the second thoracic vertebra (arrow).
Fig. 2. Gross aspergillosis lesion in the heart. (A) Yellowishwhite nodular granuloma in the myocardium (circle). (B) Right ventricular hypertrophy and dilation (arrow).
Fig. 3. Gross findings of aspergillosis in turkey; yellowish-white caseous nodules (arrow) in affected organs, including the liver (A), pancreas (B), intestinal serosa (C), intestinal mucosa (D), kidney (E), and peritoneum (F).
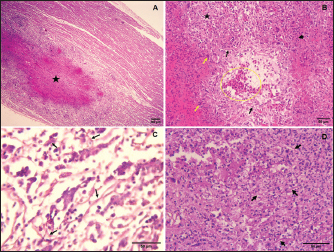
Fig. 4. Histopathological presentations of aspergillosis in turkey. (A) Large granuloma with extended central necrosis in the myocardium (asterisks) (H&E, 100×). (B) Pulmonary aspergillosis, hyperemia in the lung parenchyma (circle), surrounded by numerous giant cells (yellow arrows), heterophils (arrowhead), and lymphocytes (asterisk, H<E, 400×). (C) Numerous septate fungal hyphae observed in the pancreatic parenchyma (arrows, H&E, 400×). (D) Numerous fungal hyphae within the center of a granuloma in the lung (PAS, 400×).
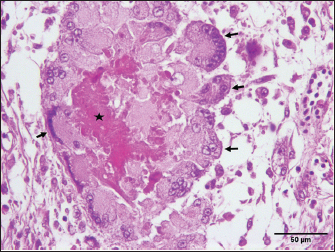
Fig. 5. Pancreatic aspergillosis lesions with necrotic-caseous material (asterisk) surrounded by giant cells (arrows, H&E, 400×). HistopathologyHistopathological examination of H&E-stained sections revealed granulomatous lesions in the parenchyma of various organs in all cases. The granulomas were characterized by a necrotic center surrounded by lymphocytes, plasma cells, macrophages, and large multinucleated foreign-body giant cells (Fig. 4A). PAS staining revealed numerous fungal hyphae within the necrotic centers of the granulomas. The lungs exhibited well-demarcated necrotic foci encapsulated by fibrous tissue. These granulomas caused localized compression of the parenchyma, leading to atelectasis. Extensive congestion and inflammatory cell infiltration were observed throughout the lung tissue (Fig. 4B). PAS staining confirmed the presence of abundant branched, septate fungal hyphae disseminated within the pulmonary parenchyma (Fig. 4D). Severe granulomatous airsacculitis mirrored the pulmonary lesions, featuring necrotic cores encircled by giant multinucleated foreign-body cells. These lesions were encapsulated by fibrous tissue and were accompanied by inflammatory infiltrates and congestion. Additionally, multifocal to coalescing granulomatous myocarditis was observed (Fig. 4A), with intralesional septate hyphae, indicating filamentous fungal infection. Most liver sections displayed polymorphonuclear leukocyte infiltration, disrupting the hepatic architecture. A marked congestion was noted, particularly red blood cell accumulation within the portal vein. Multifocal interstitial heterophilic nephritis was observed, with lesions characterized by inflammatory infiltrates. The pancreatic parenchyma showed numerous interlacing fungal hyphae (Fig. 4C) and contained granulomas of varying sizes, each with a central eosinophilic necrotic area. Multinucleated foreign-body giant cells were scattered throughout the parenchyma adjacent to the granulomas, with an increased concentration around the residual caseous material (Fig. 5). The intestinal tissue exhibited diffuse, moderate, and subacute enteritis with characteristic granulomatous reactions. MycologyFungal colonies developed on SDA exhibited a dark blue-green center with a white periphery and a cream-colored reverse. Microscopic examination of the isolates revealed columnar conidiophores with smooth, flask-shaped vesicles and uniseriate phialides. These phialides produced round, smooth conidia, consistent with the morphological characteristics of A. fumigatus (Johnson and Borman, 2010; Quinn et al., 2011). DiscussionAspergillosis, the most common mycotic infection in avian species, arises from the inhalation of Aspergillus spores, which cause significant economic losses in the poultry industry (Kalkayeva et al., 2023). Although primarily a respiratory disease, aspergillosis can manifest systemically in acute and chronic forms. Acute aspergillosis resulting from the inhalation of massive conidia is typically observed in young birds. Chronic aspergillosis, associated with immunosuppression, occurs sporadically in older birds. A chronic form of aspergillosis can indeed follow an acute episode (Seyedmousavi et al., 2015; Arné and Lee, 2020). Signs, gross lesions, and histopathological findings observed in this study align with those previously reported in the literature (Arné et al., 2011; Timurkaan et al., 2017). Alongside respiratory symptoms such as dyspnea, gasping, cyanosis, and hyperpnea, aspergillosis may also present with nonspecific signs, including anorexia, increased thirst, lethargy, dehydration, polydipsia, polyuria, diarrhea, or sudden death (Arné and Lee, 2020). In this report, a multisystemic form of aspergillosis was observed, characterized by numerous yellowish-white granulomas of varying sizes in multiple organs, including the lungs, air sacs, myocardium, pancreas, liver, spleen, proventriculus, gizzard, small intestine, peritoneum, and kidneys. These findings are consistent with previous research (Arulmozhi et al., 2016; Arné and Lee, 2020). Although several cases of pulmonary aspergillosis have been documented in animals, reports of systemic aspergillosis are rare. In agreement with our findings, systemic aspergillosis has been documented in turkeys (Arulmozhi et al., 2016), chickens (Ahamad et al., 2018), broiler ducks (Chung et al., 2020), dogs (Perez et al., 1996), cats (Barrs and Talbot, 2014), and ruminants (Seyedmousavi et al., 2015). Macroscopically, the lungs were the most severely affected, exhibiting multiple spherical granulomatous nodules of varying sizes throughout the parenchyma. These pulmonary lesions were likely the primary cause of death in the turkeys, as reported in previous studies (Beytut, 2007). Several factors predisposed birds to respiratory aspergillosis. Anatomical features, such as the absence of an epiglottis and a true diaphragm, can hinder the removal of inhaled fungal spores. Furthermore, cellular characteristics such as the limited presence of resident macrophages within airway lumens and the reliance on heterophils that use cationic proteins, hydrolases, and lysozymes instead of myeloperoxidase and oxidative mechanisms to kill fungal hyphae may also contribute to their susceptibility (Femenia et al., 2007; Gulcubuk et al., 2018). Additionally, right ventricular dilatation was observed, consistent with the findings reported by Julian and Goryo (1990), who described pulmonary aspergillosis as a cause of pulmonary hypertension, leading to right ventricular dilatation. This condition can cause ascites and pulmonary congestion due to right ventricular failure. An unusual case of aspergillosis granuloma in the thoracic vertebrae was also identified. However, few cases of bone aspergillosis in poultry have been reported. Riddell and Topp (1972) and Bergmann et al. (1980) reported aspergillosis granulomas in the thoracic vertebrae of chickens, whereas a single case of sternal bone aspergillosis caused by Aspergillus flavus has been described in turkeys (Ghazikhanian, 1989). In humans, fungal osteomyelitis is a rare condition (Asperges et al., 2023). The present study reports a rare case of aspergillosis caused by A. fumigatus in the thoracic vertebrae of meat turkeys, a location not previously reported in the literature. Granulomatous lesions of aspergillosis are typically found in the lower respiratory tract. However, dissemination to other tissues can occur via hematogenous or lymphatic routes. This dissemination is facilitated by the hyphal penetration of lung blood vessels and migration of macrophages carrying viable conidia from the respiratory system. Additionally, direct extension from the air sac wall to contiguous organs or cavities can lead to the development of lesions (Richard and Thurston, 1983; Arne et al., 2021). Histopathological examination revealed characteristic granulomatous lesions indicative of aspergillosis. These lesions were characterized by central necrosis surrounded by a fibrous capsule, infiltrating heterophils, lymphocytes, macrophages, and multinucleated giant cells. Within these granulomas, long septate hyphae consistent with Aspergillus species were observed in the necrotic center. Our findings are consistent with those of previous studies in turkeys and other avian species (Cacciuttolo et al., 2009; Arulmozhi et al., 2016; EL-Shemy et al., 2023). Pathogenic Aspergillus species, such as A. fumigatus, produce various toxins, including gliotoxins, endotoxins, heparin-like factors, and oxalic acid. Birds are particularly susceptible to gliotoxin, which can cause tissue necrosis and immunosuppression (Barathidasan et al., 2013). The definitive diagnosis of aspergillosis can be confirmed through isolation of Aspergillus by culture or using immunohistochemistry or histochemical stains like Grocott or PAS, to detect fungal hyphae in tissue samples (Singh et al., 2009; Timurkaan et al., 2017). In this report, histopathological examination using PAS staining revealed numerous fungal hyphae in the lungs, air sacs, and pancreas. Additionally, A. fumigatus was isolated and identified based on its morphological characteristics. Aspergillus species are opportunistic pathogens, with A. fumigatus being the primary causative agent of aspergillosis in animals, although other Aspergillus species have also been implicated (Seyedmousavi et al., 2015). They cause disease due to predisposing factors such as stress, immunodeficiency, and adverse environmental conditions, including poor ventilation, dusty air, exposure to toxins, and inappropriate temperature and humidity (Khosravi and Mostofi, 2021). Our findings suggest that suboptimal breeding conditions and favorable environmental factors likely contributed to the development of aspergillosis in this flock. Hot and humid conditions, along with specific microclimatic parameters, increase the risk of aspergillosis in poultry production (Sajid et al., 2006; Horvatek Tomić et al., 2021). Contaminated feed and litter are significant reservoirs of Aspergillus spp. on poultry farms (Arné et al., 2011). In this case, the birds likely ingested spores from contaminated litter. Given the lack of effective treatment, preventive measures should focus on enhancing immune function in the flock and improving husbandry practices, particularly those related to environmental control (Girh and Shaapan, 2024). ConclusionIn conclusion, multisystemic aspergillosis in meat turkey flock attributed to A. fumigatus was confirmed through histological analysis and fungal isolation. Notably, this case report represents the first documented case of aspergillosis affecting the thoracic vertebrae in turkeys. Further studies are needed to investigate the prevalence of this mycosis in Algerian turkeys and other avian species. AcknowledgmentThe authors would like to express their sincere gratitude to Dr. Omar Aissi and Dr. Moussa Chougui veterinarians, as well as the dedicated staff of the Histopathology Laboratory at the Saadna Mohamed Abdennour University Hospital Center in Setif, Algeria. Conflict of interestThe authors declare that they have no conflicts of interest that could have influenced the writing or interpretation of this work. FundingThe research described in this paper was conducted without external funding. Authors’ contributionsNEHB performed pathological examination of autopsy specimens, established the diagnosis, and drafted the manuscript. NS performed autopsy, established the diagnosis, and drafted the manuscript. SO performed pathological examination. TS and OB revise and edit the manuscripts. ÖÖ took part in preparing and critically checking the manuscript. All authors have read and approved the final manuscript. Data availabilityThe data used in this study are available upon request from the corresponding author. ReferencesAhamad, D.B., Ranganathan, V., Punniyamurthy, N., Sivaseelan, S. and Puvarajan, B. 2018. Pathology of systemic aspergillosis in a desi chicken. Shanlax Int. J. Vet. Sci. 5(4), 36–42. Arné, P. and Lee, M.D. 2020. Fungal infections. In Diseases of poultry, 14th ed. Ed., Swayne D.E. Hoboken, NJ: John Wiley & Sons, Inc., pp: 1109–1133. Arné, P., Risco-Castillo, V., Jouvion, G., Le Barzic, C. and Guillot, J. 2021. Aspergillosis in wild birds. J. Fungi 7(3), 241. Arné, P., Thierry, S., Wang, D., Deville, M., Le Loc′h, G., Desoutter, A., Féménia F., Nieguitsila, A., Huang, W., Chermette, R. and Guillot, J. 2011. Aspergillus fumigatus in poultry. Int. J. Microbiol. 1, 746356. Arulmozhi, A., Balasubramaniam, A. and Balasubramaniam, G.A. 2016. Multisystemic aspergillosis in turkeys. Indian J. Vet. Pathol. 40(4), 378–380. Asperges, E., Albi, G., Truffelli, F., Salvaderi, A., Puci, F., Sangani, A., Zuccaro, V., Scotti V., Orsolini, P., Brunetti, E. and Bruno, R. 2023. Fungal osteomyelitis: a systematic review of reported cases. Microorganisms 11(7), 1828. Barathidasan, R., Singh, S.D., Saini, M., Sharma, A.K. and Dhama, K. 2013. The first case of angioinvasive pulmonary aspergillosis in a Himalayan Griffon Vulture (Gyps himalayensis). Avian Biol. Res. 6(4), 302–306. Barrs, V.R. and Talbot, J.J. 2014. Feline aspergillosis. Vet. Clin. North Am. Small Anim. Pract. 44(1), 51–73. Beernaert, L.A., Pasmans, F., Van Waeyenberghe, L., Haesebrouck, F. and Martel, A. 2010. Aspergillus infections in birds: a review. Avian Pathol. 39(5), 325–331. Bergmann, V., Heider G. and Vogel, K. 1980. Mycotic spondylitis as a cause of locomotor disorders in broiler chicken. Monatsh. Veterinarmed. 35, 349–351. Beytut, E. 2007. Immunohistochemical diagnosis of aspergillosis in adult turkeys. Turk. J. Vet. Anim. Sci. 31(2), 99–104. Cacciuttolo E, Rossi G, Nardoni S, Legrottaglie, R. and Mani, P. 2009. Anatomopathological aspect of avian aspergillosis. Vet. Res. Commun. 33(6), 521–527. Charlton, B., Chin R.P. and Barnes H.J. 2008. Aspergillosis. In Diseases of poultry, 12th ed. Eds., Saif Y.M., Fadly A.M., Glisson J.R., McDougald L.R., Nolan L.K. and Swayne D.E. Ames, IA: Iowa State Press, pp: 989–1001. Chung, E.L.T., Reduan, M.F.H., Nordin, M.L., Abdullah, F.F.J., Zairi, N.H.M., Mohd Rajdi, N.Z.I., Kamaruzaman, I.N.A. and Shaharulnizim, N. 2020. A case of aspergillosis outbreak in a broiler duck farm in Kelantan, Malaysia. J. Adv. Vet. Anim. Res. 7(4), 692–697. EL-Shemy, A., Mekky, H., Bosila, M., Elbayoumi, K., Amer, M. and Elaish, M. 2023. Investigation of aspergillosis outbreak in young ducklings: unraveling the role of hatcheries in Aspergillus fumigatus transmission. J. Adv. Vet. Anim. Res. 10(4), 763–772. Femenia, F., Fontaine, J.J., Lair-Fulleringer, S., Berkova, N., Huet, D., Towanou, N., Rakotovao, F., Granet, O.I., Le Loc’h, G., Arné, P. and Guillot, J. 2007. Clinical, mycological and pathological findings in turkeys experimentally infected by Aspergillus fumigatus. Avian Pathol. 36(3), 213–219. Ghazikhanian, G.Y. 1989. An outbreak of systemic aspergillosis caused by Aspergillus flavus in turkey poults. J. Am. Vet. Med. Assoc. 194, 1798. Girh, Z.M. and Shaapan, R.M. 2024. Overview of aspergillosis in poultry-a review. Egypt J. Vet. Sci. 55(2), 407–419. Gulcubuk, A., Erdogan-Bamac, O., Metiner, K., Ozturk, G.Y., Ozgur, Y. and Haktanir, D. 2018. A case of pulmonary aspergillosis in white storks. J. Hellenic Vet. Med. Soc. 69(2), 1004–1009. Horvatek Tomić, D., Ravić, I., Kabalin, A. E., Kovačić, M., Gottstein, Ž. and Ostović, M. 2021. Effects of season and house microclimate on fungal flora in air and broiler trachea. Atmosphere 12(4), 459. Johnson, E.M. and Borman, A.M. 2010. The importance of conventional methods: microscopy and culture. In Aspergillosis: from diagnosis to prevention. Ed., Pasqualotto, A.C. Berlin, Germany: Springer, pp: 54–73. Julian, R.J. and Goryo M. 1990. Pulmonary aspergillosis causing right ventricular failure and ascites in meat-type chickens. Avian Pathol. 19(4), 643–654. Kalkayeva, D., Maulanov, A., Sobiech, P., Michalski, M., Kuzembekova, G., Dzhangabulova, A., Nurkhojayev, N. and Aldayarov, N. 2023. Epidemiological characteristics and financial losses due to avian aspergillosis in households in the Almaty region, Republic of Kazakhstan. Front. Vet. Sci.10, 1141456. Khosravi, A. and Mostofi, S. 2021. Disseminated pulmonary Aspergillosis in a quail flock in Iran. J. Zoonotic Dis. 5(4), 50–54. Munir, M.T., Rehman, Z.U., Shah, M.A. and Umar, S. 2017. Interactions of Aspergillus fumigatus with the respiratory system in poultry. Worlds Poult. Sci. J. 73(2), 321–336. Olias, P., Hauck, R., Windhaus, H., Van Der Grinten, E., Gruber, A.D. and Hafez, H.M. 2010. Articular aspergillosis of hip joints in turkeys. Avian Dis. 54(3), 1098–1101. Ozmen, O. and Dorrestein, M.G. 2004. Observations of aspergillosis in the brains of turkey poults using different histopathological staining techniques. Biotech. Histochem. 79(2), 95–99. Perez, J., Mozos, E., de Lara, F.C.M., Paniagüa, J. and Day, M.J. 1996. Disseminated aspergillosis in a dog: an immunohistochemical study. J. Comp. Pathol. 115(2), 191–196. Quinn, P.J., Markey, B.K., Leonard, F.C., Hartigan, P., Fanning, S. and Fitzpatrick, E. 2011. Veterinary microbiology and microbial disease, 2ed. Hoboken, NJ: John Wiley & Sons. Edn, pp: 425–429. Richard, J.L. and Thurston, J.R. 1983. Rapid hematogenous dissemination of Aspergillus fumigatus and A. flavus spores in turkey poults following aerosol exposure. Avian Dis. 27, 1025–1033. Riddell, C. and Topp, R. 1972. Mycotic spondylitis involving the first thoracic vertebra in chickens. Avian Dis. 16, 1118–1122. Sajid, M.A., Khan, I.A. and Rauf, U. 2006. Aspergillus fumigatus in commercial poultry flocks, a serious threat to poultry industry in Pakistan. J. Anim. Pl. Sci. 16(3–4), 79–81. Seyedmousavi, S., Guillot, J., Arné, P., de Hoog, G. S., Mouton, J. W., Melchers, W.J. and Verweij, P.E. 2015. Aspergillus and aspergilloses in wild and domestic animals: a global health concern with parallels to human disease. Med. Mycol. 53(8), 765–797. Singh, S., Borah, M.K., Sharma, D.K., Joshi, G.D. and Gogoi, R. 2009. Aspergillosis in turkey poults. Indian J. Vet. Pathol. 33(2), 220–221. Timurkaan, N., Eroksuz, H., Ongor, H., Cevik, A., Karabulut, B., Toraman, Z.A., Eroksuz, Y. and Incili, C.A. 2017. Concurrent occurrence of lower respiratory aspergillosis and pneumoconiosis in a turkey flock. Acta Vet-Beograd. 67(4), 562–571. Ulloa-Avellán, O., Calderón-Hernández, A., Rubí-Chacón, R. and Vargas-Leitón, B. 2022. Aspergillus spp. isolated from lungs of poultry (Gallus gallus) at the Mycology Laboratory, School of Veterinary Medicine, Universidad Nacional, Heredia, Costa Rica between 2008 and 2021 and associated factors. J. Fungi 9(1), 58. | ||
| How to Cite this Article |
| Pubmed Style Belalmi NEH, Sid N, Sedrati T, Ouhida S, Bennoune O, Ozmen O. Multisystemic aspergillosis with unusual vertebral osteomyelitis in a turkey flock in Bordj Bou Arreridj Province, Algeria. Open Vet. J.. 2025; 15(5): 2009-2015. doi:10.5455/OVJ.2025.v15.i5.16 Web Style Belalmi NEH, Sid N, Sedrati T, Ouhida S, Bennoune O, Ozmen O. Multisystemic aspergillosis with unusual vertebral osteomyelitis in a turkey flock in Bordj Bou Arreridj Province, Algeria. https://www.openveterinaryjournal.com/?mno=236194 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i5.16 AMA (American Medical Association) Style Belalmi NEH, Sid N, Sedrati T, Ouhida S, Bennoune O, Ozmen O. Multisystemic aspergillosis with unusual vertebral osteomyelitis in a turkey flock in Bordj Bou Arreridj Province, Algeria. Open Vet. J.. 2025; 15(5): 2009-2015. doi:10.5455/OVJ.2025.v15.i5.16 Vancouver/ICMJE Style Belalmi NEH, Sid N, Sedrati T, Ouhida S, Bennoune O, Ozmen O. Multisystemic aspergillosis with unusual vertebral osteomyelitis in a turkey flock in Bordj Bou Arreridj Province, Algeria. Open Vet. J.. (2025), [cited January 25, 2026]; 15(5): 2009-2015. doi:10.5455/OVJ.2025.v15.i5.16 Harvard Style Belalmi, N. E. H., Sid, . N., Sedrati, . T., Ouhida, . S., Bennoune, . O. & Ozmen, . O. (2025) Multisystemic aspergillosis with unusual vertebral osteomyelitis in a turkey flock in Bordj Bou Arreridj Province, Algeria. Open Vet. J., 15 (5), 2009-2015. doi:10.5455/OVJ.2025.v15.i5.16 Turabian Style Belalmi, Nor El Houda, Nassim Sid, Tahar Sedrati, Soraya Ouhida, Omar Bennoune, and Ozlem Ozmen. 2025. Multisystemic aspergillosis with unusual vertebral osteomyelitis in a turkey flock in Bordj Bou Arreridj Province, Algeria. Open Veterinary Journal, 15 (5), 2009-2015. doi:10.5455/OVJ.2025.v15.i5.16 Chicago Style Belalmi, Nor El Houda, Nassim Sid, Tahar Sedrati, Soraya Ouhida, Omar Bennoune, and Ozlem Ozmen. "Multisystemic aspergillosis with unusual vertebral osteomyelitis in a turkey flock in Bordj Bou Arreridj Province, Algeria." Open Veterinary Journal 15 (2025), 2009-2015. doi:10.5455/OVJ.2025.v15.i5.16 MLA (The Modern Language Association) Style Belalmi, Nor El Houda, Nassim Sid, Tahar Sedrati, Soraya Ouhida, Omar Bennoune, and Ozlem Ozmen. "Multisystemic aspergillosis with unusual vertebral osteomyelitis in a turkey flock in Bordj Bou Arreridj Province, Algeria." Open Veterinary Journal 15.5 (2025), 2009-2015. Print. doi:10.5455/OVJ.2025.v15.i5.16 APA (American Psychological Association) Style Belalmi, N. E. H., Sid, . N., Sedrati, . T., Ouhida, . S., Bennoune, . O. & Ozmen, . O. (2025) Multisystemic aspergillosis with unusual vertebral osteomyelitis in a turkey flock in Bordj Bou Arreridj Province, Algeria. Open Veterinary Journal, 15 (5), 2009-2015. doi:10.5455/OVJ.2025.v15.i5.16 |