| Research Article | ||
Open Vet. J.. 2025; 15(5): 2004-2008 Open Veterinary Journal, (2025), Vol. 15(5): 2004-2008 Research Article The effect of cryoprotectant on the quality of post-warming goat oocytes by examining SOD levelsMeisa Zalfa Adisti1, Widjiati Widjiati2, Agus Sunarso3, Epy Muhammad Luqman2, Tri Wahyu Suprayogi4, Sri Pantja Madyawati4, Suzanita Utama4 and Aulia Firmawati51Student of Postgraduate Biology Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Mulyorejo, Indonesia 2Departement of Veterinary Science, Faculty of Veterinary Medicine, Universitas Airlangga, Mulyorejo, Surabaya, Indonesia 3Departement of Veterinary Parasitology, Faculty of Veterinary Medicine, Universitas Airlangga, Mulyorejo, Surabaya, Indonesia 4Departement of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Mulyorejo, Surabaya, Indonesia 5Departement of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Brawijaya, Dieng, Malang, Indonesia *Corresponding Author: Widjiati Widjiati. Department of Veterinary Medicine, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, 60115, Indonesia. Email: widjiati [at] fkh.unair.ac.id Submitted: 05/01/2025 Revised: 19/03/2025 Accepted: 12/04/2025 Published: 31/05/2025 © 2025 Open Veterinary Journal
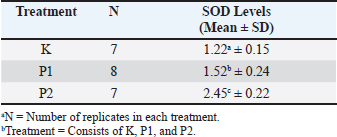
ABSTRACTBackground: Vitrification of oocytes can lead to the formation of reactive oxygen species during the in vitro maturation and warming processes. In this study, there is a comparison between the use of commercial cryoprotectant and ethylene glycol + sucrose to observe the antioxidant activity that arises during oxidative stress, namely superoxide dismutase (SOD). SOD is produced to counteract free radicals that are damaging to cells; the higher the level of SOD in a cell, the further the cell is from damage. Improving oocyte cryopreservation techniques contributes to greater reproductive efficiency in livestock, which plays an important role in supporting sustainable and effective food production. Aim: The aim of this study is to determine whether different cryoprotectant use can affect the intracellular SOD levels in oocytes. Methods: This study is an experimental laboratory study using goat oocytes divided into 3 treatment groups: control (K) goat oocytes without vitrification, Treatment 1 (P1) goat oocytes vitrified using a commercial cryoprotectant, and Treatment 2 (P2) goat oocytes vitrified using ethylene glycol and 1-M sucrose. All groups were vitrified for 1 week, followed by a warming process. Then, the SOD levels were calculated using SOD ELISA kit. Result: The results showed that this study has a significance level (p < 0.05) with the control group K having the lowest SOD levels and P2 having the highest SOD levels. Conclusion: Therefore, it can be concluded that the use of ethylene glycol as a cryoprotectant can maintain SOD levels in oocyte cells. Keywords: Ethylene Glycol, Oocyte, ROS, SOD, Food production. IntroductionThe use of experimental animals in the cryopreservation of oocyte cells offers an opportunity to deepen the understanding of the principles of oocyte cryobiology (Chang et al., 2022). Compared to the slow freezing method, vitrification has demonstrated higher efficiency in maintaining the viability of preserved embryos (Inaba et al., 2016). Despite advancements in slow freezing protocols, Edgar and Gook (2012) reported that embryos derived from oocytes preserved via slow freezing experience developmental and implantation issues compared to their control counterparts. Consequently, even though slow freezing of oocytes can be considered successful, vitrification is more widely used due to its higher rates of survival, implantation, and pregnancy (Boldt, 2011). Vitrification emerges as an advantageous alternative, offering practicality, lower costs compared to slow freezing, and high efficiency (Campos et al., 2016). Nevertheless, the vitrification process requires solutions with high viscosity and high concentrations of cryoprotectants (Leonel et al., 2019). Cryoprotectants are agents that protect oocytes undergoing vitrification from dehydration and damage due to extreme temperatures (Shahsavari et al., 2020). Commonly used cryoprotectants in the vitrification process include ethylene glycol (EG), dimethyl sulfoxide (DMSO), propanediol, and polyvinylpyrrolidone (Sheikhi et al., 2013). The type, concentration, exposure time, and size of the cells to be vitrified impact the outcomes of vitrification since cryoprotectants can be toxic at high concentrations. To mitigate this issue, nonpermeable agents such as sugars are added to reduce the toxicity of cryoprotectants, allowing for lower doses to be used (Pegg, 2005). Cryoprotectants play a crucial role in enabling cells to be processed for storage at vitrification temperatures of −196°C and to be recovered with appropriate functional levels. Although cryoprotectants have various necessary metabolic and biophysical effects, they can also hinder the biological functions of cells. Therefore, if vitrification is to emerge as an alternative cryopreservation method, simplifying the vitrification process by selecting the appropriate cryoprotectant agents is essential (Gloria et al., 2019). Goats are the most prolific ruminants compared to other ruminants (Haldar et al., 2014). Additionally, their oocytes are larger than those of experimental animals such as rats and mice, making them suitable for use in in vitro fertilization (IVF) research (Izquierdo et al., 2019). Among reproductive cells and tissues, oocytes are particularly vulnerable to cryopreservation due to their large size, low surface area-to-volume ratio, high water content, and lack of internal defense mechanisms against thermal shock-induced injuries (Iussig et al., 2019). Enhancing the efficiency of oocyte preservation in goats not only advances reproductive biotechnology but also plays a vital role in increasing livestock productivity to help meet the rising global demand for food. Moreover, subzero temperatures are not physiological conditions for oocytes, as their high water content can turn into ice crystals, leading to severe damage (Rienzi et al., 2015). The maturation process (IVM) can increase the production of reactive oxygen species (ROS) (Cajas et al., 2020). Additionally, the freezing and warming processes disrupt homeostasis, causing oxidative stress and excessive ROS production, ultimately leading to cell damage (Brieger et al., 2012). Enzymatic antioxidants, which serve as the first line of defense against ROS, such as superoxide dismutase (SOD), catalase, and nutritional antioxidants, capture free radicals and function as a free radical scavenging system (Mohammadi, 2019). Prudente et al. (2019) concluded that there is a positive correlation between the use of cryoprotectants during vitrification and enzymatic antioxidant activity, including SOD, CAT, and APX, against cell damage due to homeostasis imbalance from the vitrification process. Therefore, selecting the appropriate cryoprotectant agents for widespread application based on their effectiveness and simpler usage can be achieved by comparing the impact of cryoprotectants on SOD levels. Materials and MethodsThis study used a laboratory experimental method. A completely randomized design was employed, assuming that all treatments were equally derived from sample collection to execution and laboratory conditions. The research utilized goat oocytes as samples. There were four groups: control (K) with fresh oocytes matured in vitro, Treatment 1 (P1) with oocytes matured and then frozen using commercial cryoprotectants, and Treatment 2 (P2) with oocytes frozen using 30% EG and 1-M sucrose. The materials used in this study included collected and matured goat oocytes, phosphate-buffered saline (PBS), cell lysis buffer, commercial cryoprotectant, 30% EG, 1-M sucrose, liquid nitrogen, and an ELISA kit for SOD (ThermoFisher). The equipment included hemi straws, cassettes, mini straws, a liquid nitrogen container, Petri dishes, latex gloves, a microscope, a vortex, a centrifuge, and beakers. The equipment used in this study includes a CO2 incubator (Thermo Fisher Scientific, USA), dissecting microscope (Olympus, SZ61), inverted microscope (Olympus), laminar air flow, tweezers, beaker glass (500 ml and 250 ml), disposable syringes (1 ml, 5 ml, 10 ml), scissors, syringe filter (0.2 µl), 18-G needles, sterile tissue, side staining jar, sterile Pasteur pipettes (Germany), micro slide Poly-L lysine coating, cover glass, cassettes, liquid nitrogen (N2), containers, and canisters. Medium preparationIVF Universal culture medium was prepared, dropped into the form of drops, covered with mineral oil, and incubated in a 5% CO2 incubator at 38°C, a day before oocyte collection. Oocyte collectionOvaries were cleaned of their attachments and washed with saline at 30°C to 38°C. Oocytes were aspirated using a 5-cc syringe and an 18-G needle and then placed in a Petri dish on a hot plate at 38°C. Oocytes with a thick cumulus complex layer were collected under a microscope. IVM of oocytesCollected oocytes were washed and transferred to the maturation medium prepared the day before. Each drop of medium contained 10 oocytes, and there were 6 drops in each Petri dish. The dishes were incubated in a 5% CO2 incubator at 38°C for 22 hours (Kasman et al., 2020). Vitrification of oocytesOocytes were exposed to commercial cryoprotectant, intracellular cryoprotectant EG 30%, and extracellular cryoprotectant 1-M sucrose. The procedures for each group were as follows. For Group P1, a vitrification plate with equilibration solution (ES), vitrification solution 1 (VS1), and vitrification solution 2 (VS2) was prepared at 25°C–27°C, along with a cooling box containing liquid nitrogen, mini straws, and 0.5-mm straws. Matured oocytes were evaluated for post-maturation quality. Oocytes were transferred to the ES solution using a Pasteur pipette and held for 15 minutes before moving to the next solution. Oocytes were then transferred to VS1 for 30 seconds and VS2 for 10 seconds before being carefully transferred to mini straws with a Pasteur pipette. Mini straws were plunged into the cooling box containing liquid nitrogen along with 0.5-mm straws and cassettes. Mini straws were inserted into 0.5-mm straws, sealed with heated forceps for fixation, and placed into cassettes. This process was conducted in the cooling box containing liquid nitrogen. Cassettes with straws were transferred from the cooling box to the liquid nitrogen container using forceps and stored for one week. After one week, warming was performed by preparing a warming plate with commercial warming solutions: thawing solution (TS), diluting solution (DS), washing solution 1 (WS1), and warming solution 2 (WS2), and a cooling box with liquid nitrogen. Cassettes containing straws were retrieved from the container and placed into the cooling box. The seal of the straw was cut, and mini straws were retrieved. Mini straws were immersed in TS for 1 minute, DS for 3 minutes, WS1 for 5 minutes, and WS2 for 1 minute. Warmed oocytes were collected into fresh IVF Universal media drops. For Group P2, a vitrification plate, EG 30% solution, sucrose 1-M solution, and a cooling box with liquid nitrogen were prepared. Matured oocytes were evaluated for post-maturation quality. Oocytes were transferred to the EG 30% solution using a Pasteur pipette and held for 15 minutes before moving to the next solution. Oocytes were transferred to the 1-M sucrose solution for 30 seconds and then to the 1-M sucrose-2 solution for 10 seconds before being carefully transferred to mini straws with a Pasteur pipette. Mini straws were plunged into the cooling box containing liquid nitrogen along with 0.5-mm straws and cassettes. Mini straws were inserted into 0.5-mm straws, sealed with heated forceps for fixation, and placed into cassettes. This process was conducted in the cooling box containing liquid nitrogen. Cassettes with straws were transferred from the cooling box to the liquid nitrogen container using forceps and stored for one week. After one week, warming was performed by preparing a warming plate with sucrose solutions (0.25M, 0.5M, and 1M) and a cooling box with liquid nitrogen. Cassettes containing straws were retrieved from the container and placed into the cooling box. The seal of the straw was cut, and mini straws were retrieved. Mini straws were immersed in 0.25-M sucrose for 3 minutes, 0.5-M sucrose for 5 minutes, and 1-M sucrose for 1 minute. Warmed oocytes were collected into fresh IVF Universal media drops. SOD MeasurementOocytes were placed into Eppendorf tubes containing cell lysis buffer, vortexed for 5 minutes to break the oocytes and release intracellular SOD, and centrifuged at 3000 rpm for 5 minutes to separate the supernatant. The supernatant was collected for analysis using an ELISA kit. The ELISA plates were prepared by coating them with antibodies and incubating them at 4°C overnight. The plates were washed with 100 µl of PBS and blocked with 50 µl of blocking buffer (PBS-BSA 5%) and then incubated for 2 hours at room temperature or overnight at 4°C. The plates were washed again with PBS, and 100 µl of diluted samples (1:1, 1:4, and 1:8) were added, followed by incubation for 1–2 hours at room temperature or 90 minutes at 37°C. The plates were washed with PBS, and 50 µl of secondary antibody (rabbit anti-goat IgG HRP, diluted 1:4000) was added, followed by 2 hours of incubation at room temperature. After washing the plates again with PBS, 50 µl of TMB substrate was added and incubated for 30 minutes at room temperature. The reaction was stopped with 50 µl of stop solution, and absorbance was read at 450 nm. ResultsBased on the research conducted on the examination of SOD levels in goat oocyte cells vitrified using commercial cryoprotectant and a mixture of 30% EG with 1-M sucrose, quantitative data on SOD levels were obtained from 18 oocyte samples divided into three groups: control (K), P1, and P2. In the K group, oocytes were matured without vitrification, and SOD levels were measured directly. In the P1 group, matured oocytes were vitrified using commercial cryoprotectant, while in the P2 group, matured oocytes were vitrified using a cryoprotectant mixture of 30% EG and 1-M sucrose. The results indicated that the highest SOD levels were found in the P2 group, which used the cryoprotectant mixture of 30% EG and 1-M sucrose, exceeding the SOD levels in the K group. The analysis of SOD levels revealed a significant difference among the treatment groups (p < 0.05). The SOD levels, from highest to lowest, were as follows: P2, P1, and K (Table 1). DiscussionThe results and analysis of SOD levels in the treatment groups using the Kruskal–Wallis test yielded a significant value (p < 0.05), indicating a statistically significant difference. This test was conducted to determine the differences among each group. Based on Table 1, the average SOD concentrations showed significant differences (p < 0.05) between Group K (1.22a ± 0.15), Group P1 (1.52b ± 0.24), and Group P2 (2.45c ± 0.22). Table 1. SOD levels’ examination result.
The findings of this study demonstrate significant differences in SOD levels in vitrified oocytes post-warming using commercial cryoprotectants and a mixture of 30% EG and 1-M sucrose. The commercial cryoprotectant used consisted of DMSO, EG, and trehalose. Analysis revealed significant differences (p < 0.05) between Groups K and P1, K and P2, and P1 and P2, indicating that different cryoprotectants affect SOD levels in vitrified oocytes in each treatment group. Group K, which included matured oocytes that were neither vitrified nor warmed, had the lowest SOD concentration compared to Groups P1 and P2. This is likely because Group K did not undergo vitrification and warming, thereby avoiding oxidative stress caused by homeostasis imbalance, with ROS production occurring only during the IVM stage due to increased ATP consumption and mitochondrial DNA (mtDNA) proliferation, leading to mitochondrial dysfunction and inability to neutralize ROS (Kirillova et al., 2021). Tossetta et al. (2022) reported that increased ROS levels activate the NRF2 signaling pathway to induce the expression of antioxidant enzymes such as haem oxygenase (HO-1), catalase (CAT), glutathione peroxidase (GPx), and SOD. NRF2 binds with the KEAP1/CUL3/RBX1 E3 ubiquitin ligase complex in the cytoplasm, but oxidative stress causes KEAP1 inactivation, releasing NRF2, which then translocates to the nucleus and binds with the antioxidant response element (ARE), thus regulating the expression of antioxidant genes such as SOD, CAT, GPx, HO-1, and others. Consequently, elevated ROS induces higher intracellular SOD levels to neutralize ROS. Group P1, comprising matured oocytes vitrified for one week with commercial cryoprotectants (permeable cryoprotectant DMSO), showed that vitrification and warming cause homeostasis imbalance and oxidative stress, leading to ROS production (Shi et al., 2023). DMSO has a SOD-like mechanism, neutralizing ROS similarly to SOD. DMSO acts as a scavenger for ROS, inhibiting IL-8 formation, which activates the NF-kB pathway, a regulator of ROS production. If IL-8 activates NF-kB, it enhances the expression of genes involved in ROS production, such as NADPH oxidase (DeForge et al., 1992; Santos et al., 2003). Additionally, DMSO reduces NADPH-Oxidase (NOX) activity, limiting ROS formation since NOX primarily generates ROS (Zhang et al., 2020). This reduces ROS levels, preventing SOD induction via the NRF2 pathway. Group P2, which included matured oocytes vitrified for one week using a mixture of 30% EG and 1-M sucrose, had the highest SOD levels compared to Groups K and P1. This may be attributed to the plasma membrane permeability to water and cryoprotectants used in this study. Edashige (2016) investigated the movement of water and cryoprotectants across the plasma membrane. EG passes through the plasma membrane by simple diffusion through the lipid membrane and facilitated diffusion via aquaporin proteins, whereas DMSO also utilizes a DMSO-permeable channel for facilitated diffusion. Vitrification is influenced by exposure duration and temperature; facilitated diffusion primarily affects DMSO, making oocyte exposure duration critical, while exposure temperature has less impact. For EG, which mainly undergoes simple diffusion, both exposure duration and temperature significantly affect plasma membrane permeability. Prolonged exposure increases the risk of cryoprotectant toxicity in oocytes. Naidu et al. (2020) noted that EG has dual effects on oocyte permeability; at low temperatures (below zero), it stabilizes cell membrane proteins, while at room temperature (25°C), it destabilizes cell membranes, leading to ROS accumulation during warming. Higher ROS accumulation in Group P2 activates NRF2 signaling to produce antioxidants like SOD, neutralizing ROS. Thus, higher SOD levels indicate better cell quality due to effective ROS neutralization (Kim et al., 2020). ConclusionBased on the results of this study, it can be concluded that different cryoprotectants used in the vitrification of matured oocytes result in varying SOD levels. The group treated with a mixture of 30% EG and 1-M sucrose exhibited the highest SOD levels, indicating a greater ability of these cells to withstand the harmful effects of free radicals compared to the control (K) and P1 groups. AcknowledgmentsThe authors express gratitude to the academic advisors from the Faculty of Veterinary Medicine, Airlangga University, Surabaya, for their guidance, suggestions, and provision of facilities that supported this research. We also express gratitude to the Directorate General of Higher Education 2023, Research, and Technology; and the Ministry of Education, Culture, Research, and Technology, Indonesia, for funding assistance. Author’s contributionMZA and WW: conceptualization, methodology, validation, formal analysis, investigation, resources, and writing original draft. AS, EML, and AF: visualization, supervision, project administration, and funding acquisition. ND: methodology, validation, investigation, and resources. TWS and SPM: methodology, validation, investigation, and resources. SU: writing original draft, visualization, writing review, and editing. Conflict of interestThe authors declare no conflict of interest. FundingFundamental of this research with funded Directorate General of Higher Education 2023, Research, and Technology; and the Ministry of Education, Culture, Research, and Technology, Indonesia, for funding assistance. Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesBoldt, J. 2011. Current results with slow freezing and vitrification of the human oocyte. Reprod. Biomed. Online 23(3), 314–322. Brieger, K., Schiavone, S., Miller, F J., Jr, and Krause, K. H. 2012. Reactive oxygen species: from health to disease. Swiss. Med. Wkly. 142, w13659. Cajas, Y.N., Cañón-Beltrán, K., Ladrón de Guevara, M., Millán de la Blanca, M.G., Ramos-Ibeas, P., Gutiérrez-Adán, A., Rizos, D. and González, E.M. 2020. Antioxidant nobiletin enhances oocyte maturation and subsequent embryo development and quality. Int. J. Mol. Sci. 21(15), 5340. Campos, A.L., Guedes, J.deS., Rodrigues, J.K., Pace, W.A., Fontoura, R.R., Caetano, J.P. and Marinho, R.M. 2016. Comparison between slow freezing and vitrification in terms of ovarian tissue viability in a bovine model. Rev. Bras. Ginecol. Obstet. 38(7), 333–339. Chang, C.C., Shapiro, D.B. and Nagy, Z.P. 2022. The effect of vitrification on oocyte quality. J. Gynecol. Obstet. Biol. Reprod. 106(2), 16–327. DeForge, L.E., Fantone, J.C., Kenney, J.S. and Remick, D.G. 1992. Oxygen radical scavengers selectively inhibit interleukin 8 production in human whole blood. J. Clin. Invest. 90(5), 2123–2129. Edashige, K. 2016. The movement of water and cryoprotectants across the plasma membrane of mammalian oocytes and embryos and its relevance to vitrification. J. R. Dt. 62(4), 317–321. Edgar, D.H., and Gook, D.A. 2012. A critical appraisal of cryopreservation (slow cooling versus vitrification) of human oocytes and embryos. Hum. Reprod. Update 18(5), 536–554. Gloria, A., Toscani, T., Robbe, D., Parrillo, S., De Amicis, I., and Contri, A. 2019. Cryopreservation of turkey spermatozoa without permeant cryoprotectants. Anim. Reprod. Sci. 211, 106218. Haldar, A., Pal, P., Datta, M., Paul, R., Pal, S.K., Majumdar, D., Biswas, C.K., and Pan, S. 2014. Prolificacy and its relationship with age, body weight, parity, previous litter size and body linear type traits in meat-type goats. A.J.A.S 27(5), 628–634. Inaba, Y., Miyashita, S., Somfai, T., Geshi, M., Doschi, S.M.O. and Nagai, T. 2016. Cryopreservation method affects DNA fragmentation in trophectoderm and the speed of re-expansion in bovine blastocyst. Cryobiology 72(2), 86–92. Iussig, B., Maggiulli, R., Fabozzi, G., Bertelle, S., Vaiarelli, A., Cimadomo, D., Ubaldi, F. M. and Rienzi, L. 2019. A brief history of oocyte cryopreservation: arguments and facts. Acta Obstet. Gynecol. Scand. 98(5), 550–558. Izquierdo, D., Catalá, M.G. and Paramio, M.T. 2019. small ruminants: prepubertal oocyte donors. Methods Mol. Biol. 2006, 155–163. Kirillova, A., Smitz, J.E.J., Sukhikh, G. T. and Mazunin, I. 2021. The role of mitochondria in oocyte maturation. Cells 10(9), 2484. Leonel, E.C.R., Corral, A., Risco, R., Camboni, A., Taboga, S.R., Kilbride, P., Vazquez, M., Morris, J., Dolmans, M.M. and Amorim, C.A. 2019. Stepped vitrification technique for human ovarian tissue cryopreservation. Sci. Rep. 9(1), 20008. Mohammadi, M. 2019. Oxidative stress and polycystic ovary syndrome: a brief review. Int. J. Prev. Med. 10, 86. Naidu, K.T., Rao, D.K. and Prabhu, N.P. 2020. Cryo vs. thermo: duality of ethylene glycol on the stability of proteins. J. Phys. Chem. B. 124(45), 10077–10088. Pegg, D.E. 2005. The role of vitrification techniques of cryopreservation in reproductive medicine. Hum. Fertil. 8(4), 231–239. Prudente, D.O., Paiva, R., Domiciano, D., Souza, L.B., Carpentier, S., Swennen, R., Silva, L.C., Nery, F.C., Máximo, W.P.F. and Panis, B. 2019. The cryoprotectant PVS2 plays a crucial role in germinating Passiflora ligularis embryos after cryopreservation by influencing the mobilization of lipids and the antioxidant metabolism. J. Plant Physiol. 239, 71–82. doi:10.1016/j.jplph.2019.05.014 Shahsavari, M.H., Alves, K.A., Alves, B.G., de Lima, L.F., Vizcarra, D.A.M., Berrocal, D.J.D., Silva, L.M., da Silva, Y.P., Zelinski, M.B., de Figueiredo, J.R., Moghaddam, G. and Rodrigues, A.P.R. 2020. Impacts of different synthetic polymers on vitrification of ovarian tissue. Cryobiology 94, 66–72. Sheikhi, M., Hultenby, K., Niklasson, B., Lundqvist, M. and Hovatta, O. 2013. Preservation of human ovarian follicles within tissue frozen by vitrification in a xeno-free closed system using only ethylene glycol as a permeating cryoprotectant. Fertil. Steril. 100(1), 170–7.e72. Shi, Y.Q., Zhu, X.T., Zhang, S.N., Ma, Y.F., Han, Y.H., Jiang, Y. and Zhang, Y.H. 2023. Premature ovarian insufficiency: a review on the role of oxidative stress and the application of antioxidants. Front. Endocrinol. 14, 1172481. Tossetta, G., Fantone, S., Montanari, E., Marzioni, D. and Goteri, G. 2022. Role of NRF2 in ovarian cancer. Antioxidants 11(4), 663. Zhang, H., Zhang, X., Dong, C., Zhang, N., Ban, Z., Li, L., Yu, J., Hu, Y. and Chen, C. 2020. Effects of ozone treatment on SOD activity and genes in postharvest cantaloupe. R.S.C Adv. 10(30), 17452–17460. | ||
| How to Cite this Article |
| Pubmed Style Adisti MZ, Widjiati W, Sunarso A, Luqman EM, Suprayogi TW, Madyawati SP, Utama S, Firmawati A. The effect of cryoprotectant on the quality of post-warming goat oocytes by examining SOD levels. Open Vet. J.. 2025; 15(5): 2004-2008. doi:10.5455/OVJ.2025.v15.i5.15 Web Style Adisti MZ, Widjiati W, Sunarso A, Luqman EM, Suprayogi TW, Madyawati SP, Utama S, Firmawati A. The effect of cryoprotectant on the quality of post-warming goat oocytes by examining SOD levels. https://www.openveterinaryjournal.com/?mno=236174 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i5.15 AMA (American Medical Association) Style Adisti MZ, Widjiati W, Sunarso A, Luqman EM, Suprayogi TW, Madyawati SP, Utama S, Firmawati A. The effect of cryoprotectant on the quality of post-warming goat oocytes by examining SOD levels. Open Vet. J.. 2025; 15(5): 2004-2008. doi:10.5455/OVJ.2025.v15.i5.15 Vancouver/ICMJE Style Adisti MZ, Widjiati W, Sunarso A, Luqman EM, Suprayogi TW, Madyawati SP, Utama S, Firmawati A. The effect of cryoprotectant on the quality of post-warming goat oocytes by examining SOD levels. Open Vet. J.. (2025), [cited January 25, 2026]; 15(5): 2004-2008. doi:10.5455/OVJ.2025.v15.i5.15 Harvard Style Adisti, M. Z., Widjiati, . W., Sunarso, . A., Luqman, . E. M., Suprayogi, . T. W., Madyawati, . S. P., Utama, . S. & Firmawati, . A. (2025) The effect of cryoprotectant on the quality of post-warming goat oocytes by examining SOD levels. Open Vet. J., 15 (5), 2004-2008. doi:10.5455/OVJ.2025.v15.i5.15 Turabian Style Adisti, Meisa Zalfa, Widjiati Widjiati, Agus Sunarso, Epy Muhammad Luqman, Tri Wahyu Suprayogi, Sri Pantja Madyawati, Suzanita Utama, and Aulia Firmawati. 2025. The effect of cryoprotectant on the quality of post-warming goat oocytes by examining SOD levels. Open Veterinary Journal, 15 (5), 2004-2008. doi:10.5455/OVJ.2025.v15.i5.15 Chicago Style Adisti, Meisa Zalfa, Widjiati Widjiati, Agus Sunarso, Epy Muhammad Luqman, Tri Wahyu Suprayogi, Sri Pantja Madyawati, Suzanita Utama, and Aulia Firmawati. "The effect of cryoprotectant on the quality of post-warming goat oocytes by examining SOD levels." Open Veterinary Journal 15 (2025), 2004-2008. doi:10.5455/OVJ.2025.v15.i5.15 MLA (The Modern Language Association) Style Adisti, Meisa Zalfa, Widjiati Widjiati, Agus Sunarso, Epy Muhammad Luqman, Tri Wahyu Suprayogi, Sri Pantja Madyawati, Suzanita Utama, and Aulia Firmawati. "The effect of cryoprotectant on the quality of post-warming goat oocytes by examining SOD levels." Open Veterinary Journal 15.5 (2025), 2004-2008. Print. doi:10.5455/OVJ.2025.v15.i5.15 APA (American Psychological Association) Style Adisti, M. Z., Widjiati, . W., Sunarso, . A., Luqman, . E. M., Suprayogi, . T. W., Madyawati, . S. P., Utama, . S. & Firmawati, . A. (2025) The effect of cryoprotectant on the quality of post-warming goat oocytes by examining SOD levels. Open Veterinary Journal, 15 (5), 2004-2008. doi:10.5455/OVJ.2025.v15.i5.15 |