| Research Article | ||
Open Vet. J.. 2025; 15(5): 1958-1968 Open Veterinary Journal, (2025), Vol. 15(5): 1958-1968 Original Article Clinical and histopathological evaluation of 5-fluorouracil-induced oral mucositis in a rat modelAmbar Kusuma Astuti1,2, Samsul3, Melva Louisa4*, Yuniardini Septorini Wimardhani2, Andi Yasmon5, and Puspita Eka Wuyung6,71Doctoral Program of Biomedical Science, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia 2Oral Medicine Department, Faculty of Dentistry, Universitas Indonesia, Jakarta, Indonesia 3Oral Medicine Residency Program, Faculty of Dentistry, Universitas Indonesia, Jakarta, Indonesia 4Department of Pharmacology and Therapeutics, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia 5Department of Clinical Microbiology, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia 6Department of Anatomical Pathology, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia 7Animal Research Facilities, Indonesian Medical Education and Research Institute, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia *Corresponding Author: Melva Louisa. Department of Pharmacology and Therapeutics, Faculty of Medicine, Universitas Indonesia, Jakarta, Indonesia. Emails: melva.louisa [at] ui.ac.id and melva.louisa [at] gmail.com Submitted: 06/12/2024 Revised: 07/04/2025 Accepted: 19/04/2025 Published: 31/05/2025 © 2025 Open Veterinary Journal
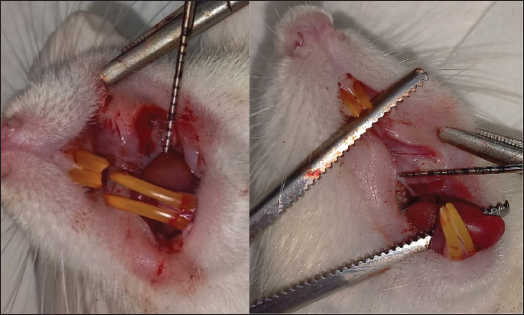
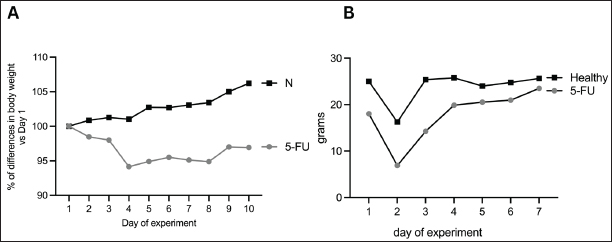
ABSTRACTBackground: Chemotherapeutics like 5-fluorouracil (5-FU) may induce a variety of adverse effects, including oral mucositis (OM), which may necessitate treatment discontinuation in patients with cancer. Currently, only a few models of OM are available for studying many aspects of pathophysiology and treatments. Aim: The current study examined the clinical and histological aspects of 5-fluorouracil-induced oral mucositis (5FU-OM) in rats. Methods: We randomly divided 19 male Sprague-Dawley rats into 8 healthy rats and 11 that received a single dose of 5FU-OM. On the first day of the experiment, the 5FU-OM group was administered an intraperitoneal injection of 5-FU (150-mg/kg BW), whereas the healthy group was not administered the drug. The third day involved scratching the oral mucosa of all rats in both groups. Clinical observations included changes in body weight, food consumption, hair loss, and severity of oral lesions. At the end of the study, we collected cardiac blood and mucosal tissue samples to investigate hematological and histological alterations. Results: Our findings demonstrated that a single intraperitoneal injection of 5-FU and mucosal irritation might result in ulcerative OM. We discovered other clinical toxicities caused by chemotherapy, such as weight loss, red lacrimation, facial edema, epistaxis, and hair loss. 5-FU also produced hematological abnormalities, including anemia and thrombocytopenia. Histopathological changes included ulceration, bleeding, vasodilation, edema, and inflammatory cell infiltration. Conclusion: This simple rat model of OM accurately replicates the clinical and histological mucosal responses to chemotherapy, including its systemic adverse effects. Thus, it can be used in research on OM. Keywords: Chemotherapy, 5-fluorouracil, Hematology, Inflammation, Oral mucositis. IntroductionAlimentary tract mucositis is a common side effect of chemotherapy, affecting around 30%–40% of individuals. Oral mucositis (OM) is one of the most prevalent kinds. After chemotherapy and radiation treatment for head and neck cancer, the risk of OM can increase by up to 90% (Harada et al., 2022). Chemotherapy damages rapidly dividing cells, such as the mouth and gastrointestinal lining. This condition reduces the epithelium’s ability to replace itself, ultimately resulting in atrophy and ulceration. In addition to the epithelium, mucosal tissue can also be implicated (Dahlgren et al., 2021; Harada et al., 2022). There are five stages of OM development: initiation, primary injury response, signal strengthening, ulceration, and healing (Pulito et al., 2020). Mucositis appears immediately after chemotherapy and is characterized by edema, an erythematous area that proceeds into ulceration that may get deeper and has a burning or painful feeling (Da Cruz Campos et al., 2021). Severe OM may cause difficulties in swallowing, impair nutrient intake, and disrupt the cancer treatment strategy (Harada et al., 2022; Shetty et al., 2022; Iovoli et al., 2023). The condition impairs oral activities such as eating, swallowing, and speech and lowers the patient’s quality of life (Abdalla-Aslan et al., 2024). The kidney, liver, cardiac, and salivary gland functions are all disrupted (Harada et al., 2022). Approximately 40%–60% of OM incidence is associated with medicines that modulate DNA synthesis, such as antimetabolites [methotrexate and 5-fluorouracil (5-FU)] and purine analogs (cytarabine) (Pulito et al., 2020). Many chemotherapeutic side effects are linked to 5-FU administration, such as fatigue, appetite loss, and diarrhea (Sougiannis et al., 2019). 5-FU is often used to treat breast, gastrointestinal, or head and neck cancers. It inhibits thymidylate synthase, which may interfere with DNA synthesis and cause apoptosis. The 5-FU-induced oral mucositis (5FU-OM) exhibited notable proinflammatory cytokine and neutrophil (NEU) transmigration (Sobue et al., 2018). Severe OM may interrupt cancer treatment and affect cancer prognosis; therefore, controlling OM is essential. Despite numerous experimental and clinical investigations into the prevention and management of OM, no standard treatment has been established (Pulito et al., 2020). To date, there are limited treatment options with low efficacy. Thus, new strategies for OM prevention and treatment are needed to improve cancer care among chemotherapy patients (Harada et al., 2022). Some experimental models have been developed to explore the mechanisms of OM progression and recovery, as well as novel therapeutic agents (Da Cruz Campos et al., 2021; Kim et al., 2023). Most animal studies were conducted in rodents using an intraperitoneal method (Sangild et al., 2018). The chemotherapy dose can be a single high or low daily dose. The present model is simple to induce intestinal mucositis, but not OM (Bertolini et al., 2017). To induce OM in a murine model, besides administering chemotherapeutic drugs, an additional mechanical or chemical trauma is needed because rats have a mechanical barrier in the form of stratified squamous epithelium (Kim et al., 2023). The reproducibility of the chemotherapy-induced oral mucositis (CIOM) model can be assessed by observing CIOM-related distress and signs of myelosuppression in the animal (Kim et al., 2023). Severe OM is associated with systemic and hematological changes, such as lower hemoglobin (HGB), platelet (PLT), white blood cell (WBC), and lymphocyte (LYM) counts, which are linked to inflammation and the wound healing process (Lorini et al., 2022). The 5-FU direct toxicity to the oral epithelium, as shown in CIOM, is complex and complicated to characterize. The rat model can provide adequate specimens for quantitative analysis in evaluating CIOM (Kim et al., 2023). Therefore, the aim of this study was to evaluate the clinical, hematological, and histopathological changes in a rat model of CIOM after a single high-dose intraperitoneal 5-FU dose with additional mechanical injury. Materials and MethodsEthical approval<AQ1>This research was approved by the Faculty of Medicine Ethics Committee, Universitas Indonesia (No. KET-1368/UN2.F1/ETIK/PPM.00.02/2022. The animal experiments were conducted at the Animal Research Facilities of the Indonesian Medical Education and Research Institute in Jakarta, Indonesia. ChemicalsThe 5-FU 50-mg/ml injection (Curacil®) was obtained from PT. Kalbe Farma Tbk (Indonesia). Ketamine HCl 50 mg/ml was purchased from Bernofarm (Indonesia). Xylazine 2% for injection (Xyla®) was purchased from Interchemie (Holland). Natural Buffered Formalin 10% (Paraform) was purchased from Indopath AKD. All other reagents were of analytical grade. Animals and treatmentsNineteen male Sprague-Dawley rats aged 5 weeks were obtained from the National Agency for Drug and Food Control’s Animal Breeding Facility. Before the experiment, the rats were acclimatized for 2 weeks in a room with a temperature of 25°C–27°C, 45%–65% humidity, 12-hour light and dark cycles, clean bedding, and free access to food and water. The rats were randomly assigned to the healthy and 5-FU-induced oral mucositis (5FU-OM) groups. To induce OM, on the first day of the experiment, the 5FU-OM group was administered an intraperitoneal injection of 5-FU 150-mg/kg BW, as described in a previous study (Bakar et al., 2021). On the third day of the experiment, the healthy and 5FU-OM groups were anesthetized intraperitoneally with ketamine and xylazine to facilitate buccal mucosa scratching. The condition represents the clinical effect of chronic mouth irritation. Anatomical or hemostatic forceps were used to hold the oral cavity, and two horizontal linear scratches were made with a sterile 18-gauge needle in the buccal mucosa (Fig. 1). An anatomical landmark between the second upper molar and buccal commissure was used to create a uniform wound shape. The wound length was recorded with a periodontal probe. OM severity and other clinical effects of chemotherapyThe development of OM lesions and other clinical effects of chemotherapy, such as changes in body weight, complete blood count, hair loss, and other gross toxicity, such as chromodacryorrhea, were also recorded. Hair loss was graded as follows: Type 4 (high hair density, full, and thick fur), Type 3 (moderate hair density with no visible skin area), Type 2 (low hair density, with the visualization of the skin), and Type 1 (uneven hair growth on the test area, skin easily seen) (Hamidi et al., 2023). Clinical oral mucositis severityIn this study, OM was induced by intraperitoneal single 5-FU administration (day 0), followed by mucosal scratching 2 days after 5-FU administration (day 3). The healthy group was scratched only on day 3. The lesion severity was observed, photographed, and evaluated macroscopically by Lima scoring (Lima et al., 2005)—score 0: typical cheek pouch with absent or discrete erythema and hyperemia, no hemorrhagic areas, ulcerations, or abscess; score 1: moderate erythema and hyperemia, no hemorrhagic areas, ulceration, or abscess; score 2: severe erythema and hyperemia, presence of hemorrhagic areas, small ulceration, or scarred tissue, but no abscess; and score 3: severe erythema and hyperemia, presence of hemorrhagic areas, extensive ulcerations, and abscess of the buccal mucosa (Fig. 2). Body weight changes and food intakeThe animal body was also measured as a physiological parameter of 5-FU side effects, and it was performed daily using a digital scale, as described previously (Hamidi et al., 2023). The dose of 5-FU was 150-mg/kg BW (Bakar et al., 2021). Food intake was observed from day 3 to day 9 of treatment. The remaining food pellet in each cage was weighed daily and subtracted from the initial weight of the provided food. The higher the amount of remaining food pellets, the lower the food intake. Changes in the complete blood countIntracardiac blood collection during necropsy was performed to evaluate hematological changes. About 3 ml of blood was drawn, placed in EDTA microtubes, and analyzed using a Vet Auto Hematology analyzer, Onetech Blood Analysis System (China), to determine WBC, LYM, NEU/GRAN, red blood cells (RBC), HGB, hematocrit (HCT), and PLT count. The values obtained calculated the neutrophil: lymphocyte ratio (NLR) and platelet: lymphocyte ratio (PLR). Clinical effects of 5-FU chemotherapyThe other clinical effects of chemotherapy, such as hair loss and red lacrimation around the eyes, were also recorded. Red lacrimal secretion, or chromodacryorrhea hypersecretion of harderian gland fluid behind the orbit, is generally related to nonspecific signs of stress (Ewens et al., 2005; Mancinelli and Capello, 2016). It can be observed visually and might give a deceptive appearance of eyes or nose bleeding (Mancinelli and Capello, 2016).
Fig. 1. Oral mucosal scratching with an 18G needle was performed on the third day of the experiment.
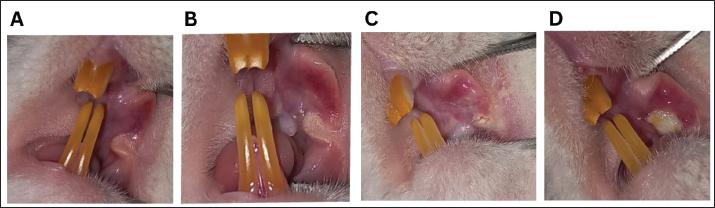
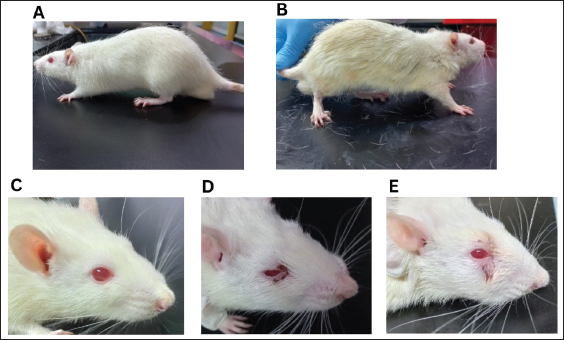
Fig. 2. Mucositis lesion clinical severity. A) Grade 0, B) Grade 1, C) Grade 2, and D) Grade 3. Histopathological examination of 5-FU-OMThe animals were euthanized on the 10th day of the experiment. Rats were euthanized using ketamine (75-mg/kg BW) and xylazine (5-mg/kg BW) intraperitoneally. The severity of the oral mucosal lesions was photographed and measured using a periodontal probe. The buccal mucosal tissues were harvested using a blade no. 11 and tissue scissors and were placed in neutral buffer (10% formalin). Specimens were prepared in paraffin-embedded tissue blocks, and sections of 5-mm thickness were prepared using a microtome. Hematoxylin and eosin (HE) staining was performed on prepared slides, and after mounting, the slides were observed using a Leica DM 1000 LED microscope. The specimens were examined histopathologically using scores of 0–3 from the previous method (Leitão et al., 2007)—score 0: normal epithelium and connective tissue without vasodilatation; absence of or discreet cellular infiltration; and absence of hemorrhagic areas, ulcerations, or abscesses; score 1: discreet vasodilatation and reepithelization areas; discreet inflammatory infiltration with mononuclear prevalence; and absence of hemorrhagic regions, edema, ulcerations, or abscesses; score 2: moderate vasodilatation, areas of hydropic epithelial degeneration, inflammatory infiltration with NEU prevalence, presence of hemorrhagic regions, edema and eventual ulcerations, and absence of abscesses; and score 3: severe vasodilatation, inflammatory infiltration with NEU prevalence, hemorrhagic regions, edema, and extensive ulceration and abscesses. Data analysisVariables are presented as the mean and standard error of the mean. Multiple t-tests were performed using GraphPad Prism version 10.0 (GraphPad Software, La Jolla, CA). A p-value of 0.05 was considered significant. ResultsBody weight changes and food intakeAfter 5-FU administration and mucosal scratching, the body weight of all rats decreased. Rats treated with 5-FU and mucosal scratching on day 3 of experiments showed decreased body weight immediately after the following day (day 4), which began to improve from day 5 to day 10. On the tenth day, most rats lost body weight compared with the first day of the experiment. In contrast, the healthy group exhibited a body weight increase. Marked body weight differences were observed between the healthy and 5-FU groups at the end of the experiment (Fig. 3A). The average food intake in the 5-FU group after mucositis induction observed on day 3 was low but gradually improved at the end of the experiment (Fig. 3B). Oral lesion development and severityTo investigate the impact of 5-FU-induced mucositis in the rat model, 5-FU was administered intraperitoneally on day 1. On day 3, the buccal mucosa was scratched in the rats. In contrast, the healthy group received only a mucosal scratch on day 3 without 5-FU treatment. The cheek pouches were isolated on day 10 to evaluate the severity of OM. The severity of the oral lesions was captured in photographs and assessed using the previous scoring system (Leitão et al., 2007). After the experiment, the 5-FU group had more severe oral lesions than the control group (Fig. 4A). In addition to the development of OM, other significant toxicities in the head and neck area can also be observed, such as red lacrimal secretion or chromodacryorrhea, epistaxis, and facial edema. In the 5-FU group, 2 of 11 rats exhibited grade 2 toxicity in the form of facial edema, whereas 7 of 11 rats demonstrated grade 3 toxicity characterized by facial edema (3/11), profuse epistaxis (1/11), and periorbital hemorrhage (3/11) (Fig. 4B–D).
Fig. 3. (A) Percentage of body weight difference versus day 1 of the experiment in the healthy and 5-FU groups. (B) The amount of food intake in the healthy and 5-FU treated groups.
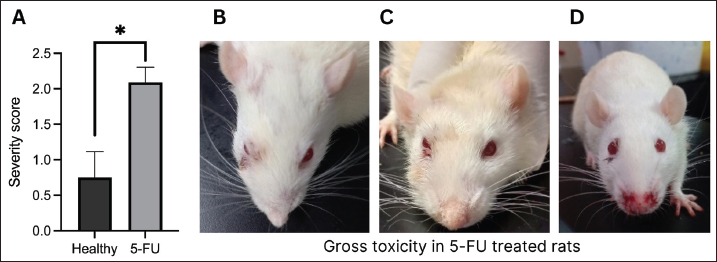
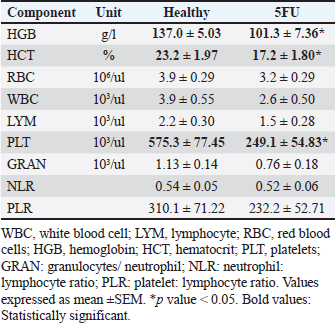
Fig. 4. (A) Oral lesion severity at the end of the experiment (day 10). The 5-FU group exhibited a significantly higher level of lesion severity. *: p < 0.05. B, C, and D show gross toxicity in the 5-FU group in terms of facial edema and chromodacryorrhea (B & C) or epistaxis and chromodacryorrhea (D). Table 1. Hematological components of healthy rats versus 5-FU-induced mucositis.
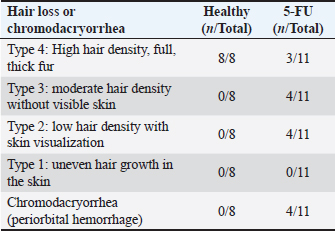
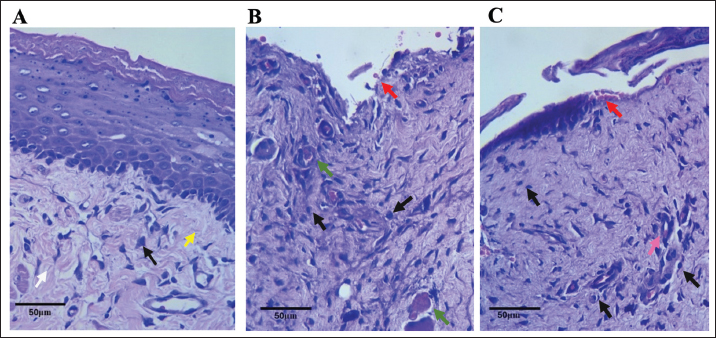
Hematological analysisThe administration of chemotherapy drugs caused hematological changes in the treatment group (Table 1). The HGB, HCT, and PLT levels in the treatment group were below the normal range and significantly lower than those in the control group. Additionally, the RBC, WBC, LYM, granulocyte/NEU count, NLR, and PLR in the 5-FU group also decreased compared with the healthy group, although this decrease was not significant. Furthermore, the RBC levels of the 5-FU rats were also below the reference range. Hair loss and chromodacryorrheaOther clinical changes following chemotherapy, such as hair loss, red lacrimation, and chromodacryorrhea around the eyes, were observed. Hair loss All rats in the normal groups did not experience hair loss (Fig. 5A). In contrast, nearly all rats in the 5-FU group experienced significant hair loss. Most rats treated with 5-FU showed grade 2 hair loss (Table 2). Additionally, the rats in the 5-FU group seemed to have duller fur (Fig. 5B). Chromodacryorrhea Following 5-FU exposure, some rats in the 5-FU group developed chromodacryorrhea (Fig. 5D and E). No rat in the healthy group showed signs of chromodacryorrhea (Fig. 5C). Histopathology examinationTissues were collected from the buccal mucosa of the Sprague-Dawley rats during necropsy on the 10th day of the experiment. Formalin-fixed mucosal tissues were embedded in paraffin blocks and sectioned in 5-μm thickness with microtomes. The prepared slides were stained with HE. Tissues were examined under a Leica DM 1000 LED microscope for morphological changes and the existence of inflammatory infiltrates. The data presented represent the healthy and 5-FU groups (Fig. 6). The healthy group had intact epithelium with stratum basal, stratum spinosum, and stratum corneum. Fibroblasts (white arrow) and collagen fibers (yellow arrow) were also found, as well as a few inflammatory cells (black arrow). In the 5-FU-induced mucositis group, areas of ulceration and thinning of the epithelium were observed. In the ulceration area, the stratum basalis, spinosum, and corneum, including the keratin layer, are affected. Erythrocytes (red arrow) were found on the ulceration surface. There was also moderate vasodilation and edematous areas (green arrow). A dense chronic inflammatory cell infiltrate (black arrow) is also observed. According to Leitao’s scoring, these findings were classified as score 2.
Fig. 5. There were no signs of hair loss or chromodacryorrhea in the healthy rat group (A and C), whereas there were evident hair losses (B) and red lacrimal secretion in the 5-FU-treated rats (D and E). Table 2. The appearance of hair loss or chromodacryorrhea in the healthy or 5-FU groups.
DiscussionMucositis during cancer treatment is associated with cellular and molecular events that affect the epithelium and underlying stroma (Hamidi et al., 2023). In both mice and humans, the stages of OM are initiation, primary damage with signal amplification, ulceration, and healing (Saul-McBeth et al., 2021). OM is prevalent among patients with cancer undergoing chemotherapy; however, discovering a suitable pharmacological intervention remains a challenge (Chang et al., 2015). A preclinical animal model of mucositis might provide important insights for developing novel treatments (Mohammed et al., 2023). 5-FU is commonly used to treat colon, breast, skin, and head and neck cancer (Chang et al., 2015; Safarpour et al., 2022). The anticancer effect of 5-FU is exerted by inhibiting thymidylate synthase, protein p53 activation and regulation of cell cycle, and G1/S arrest after metabolic transformation into its active form (Safarpour et al., 2022; Sharma et al., 2024). 5-FU has a limited therapeutic dosage range, making its dose determination varied between patients and proper dosing challenging (Mohammed et al., 2023). This phenomenon contributes to the increased toxicity of 5-FU in normal tissue. The side effects of 5-FU include myelosuppression, mucositis, nausea, diarrhea, and organ toxicity, such as cardiac toxicity, hepatoxicity, and renal toxicity (Safarpour et al., 2022; Sharma et al., 2024). Myelosuppression is among the most common adverse reactions because 5-FU inhibits blood cell synthesis, triggering anemia, neutropenia, and thrombocytopenia. Myelosuppression may impair the immune system and induce infection (Sharma et al., 2024). Approximately 80% of patients receiving 5FU develop OM as a side effect (Chang et al., 2015). This study investigated the pathological changes of a single high-dose intraperitoneal 5FU-OM mouse model via clinical, hematological, and histopathological examination. The severity of the OM lesion was observed along with other side effects of chemotherapy, such as decreased food intake and body weight, changes in complete blood count, hair loss, facial edema, and red lacrimal secretion around the eyes.
Fig. 6. Histopathological staining of representative healthy buccal mucosal epithelium of (A) and 5-FU-induced rat buccal mucosal epithelium (B and C) at 40× magnification. White arrow: fibroblast; yellow arrow: collagen fiber; black arrow: inflammatory cells; red arrow: erythrocyte cells; green arrow: vasodilation and edema. Clinically, OM has been successfully developed in the 5-FU group. On average, the 5-FU group developed an ulcerative mucositis lesion; most rats (6/11) experienced severe erythema with the hemorrhagic area, small ulceration, or scarred tissue but no abscess, and 3/11 rats had severe erythema with the hemorrhagic area, extensive ulceration, or scarred tissue and buccal abscess. This experiment also employed mucosal scratching in the healthy group to compare the severity of the mucosal lesions with that of the 5-FU group. The maximum severity of oral lesions in the scratched healthy group was limited to a small number (3/8) of patients with moderate hyperemia without ulceration or abscesses. It has been suggested that 5-FU is a potent OM-inducing chemotherapy agent, as observed in many animal studies (Bertolini et al., 2017; Gupta et al., 2020; Pulito et al., 2020; Bakar et al., 2021; Kim et al., 2023). The experiment by Chang et al. (2015) showed that clinical OM was significantly noticed in the second week and progressed with time. Nevertheless, they found no hemorrhages, extensive ulcers, or abscesses on clinical examination, which might be related to the mice’s smaller size and absence of cheek pouches (Chang et al., 2015). In this experiment, animals receiving 5-FU chemotherapy exhibited a significant decrease in body weight. The average food intake of the 5-FU group immediately after OM induction was also lower than that of the control group. However, it gradually improved and exceeded the normal group at the end of the experiment. However, the higher food intake of the 5-FU group could not facilitate body weight gain because the normal group had significantly higher body weight. OM might induce pain and difficulty in eating, compromising nutritional intake, and eventually decreasing body weight (Bakar et al., 2021). 5-FU might decrease body weight, aggravate symptoms severity, and decrease survival (Sougiannis et al., 2019). In their study, Kim et al. (2023) also found that IP 5-FU at 200 mg/kg in rats decreased WBC counts and rat weight and impaired epithelium proliferation (Kim et al., 2023). Body weight loss could relate to the damage to internal organs such as the liver and intestine, and anaerobic bacteria reduction in the gut (Safarpour et al., 2022). According to Safarpour et al. (2022), body weight change is also a sign of chronic stress, as seen in chemotherapy treatment (Safarpour et al., 2022). Previous studies have confirmed decreased food intake and body weight in 5-FU-induced rats (Bakar et al., 2021; Honda et al., 2022; Safarpour et al., 2022). There were decreases in all hematological parameters in the 5-FU group compared with the healthy group. However, a statistically significant decline was observed only in HGB, HCT, and PLT counts. HGB, RBC, and HCT values in 5FU rats were below the normal range, indicating anemia. Meanwhile, low PLT counts indicate thrombocytopenia. The ability of chemotherapy drugs to induce anemia and thrombocytopenia is well documented. There is a relationship between 5-FU therapy and anemia (Sougiannis et al., 2019). As one of the most widely used chemotherapy agents, 5-FU is known to affect the hematopoietic system directly or indirectly (Sharma et al., 2024). Directly, 5-FU acts upon bone marrow cells and hematopoietic progenitor cells, and 5-FU indirectly influences the hematopoietic system by interacting with cells or factors that control hematopoiesis or affecting the bone marrow microenvironment, such as the cells and cytokines (Gao et al., 2023). Patients receiving 5-FU frequently developed anemia, mainly due to myelosuppression (Berger et al., 2020). Cancer patients treated with chemotherapy may develop thrombocytopenia because of bone marrow inhibition. Functional PLTs are regularly produced from mature megakaryocytes originating from multipotent hematopoietic stem progenitor cells. Approximately 10% of patients receiving 5-FU develop grade 4 thrombocytopenia (Gao et al., 2023). One rat induced with 5-FU in our study demonstrated profuse epistaxis on the tenth day of experimentation, with a thrombocyte count confirmed below standard (individual data not shown). Although the WBC count, LYM level, and NEU count of the 5-FU group were still in the normal range, they were all lower than those of the healthy group. The NLR is determined by dividing the NEU count by the absolute LYM count (Fu et al., 2021). The NLR count of both groups is lower than the healthy values, with the NLR value of the 5-FU group lower than the healthy group, although this difference was not significant. LYM can induce cytotoxic cell death and inhibit tumor cell proliferation and migration. Low LYM counts suggest compromised activation of adaptive immunity (Fu et al., 2021). NEUs are vital to the innate immune response, including releasing cytokines and inflammatory mediators. NLR indicates the balance between the innate and adaptive immune response and serves as a rapid and sensitive indicator of infection, sepsis, inflammation, organ dysfunction, stress response, and disease severity. NLR has been validated in many studies and has a robust prognostic and predictive value (Zahorec, 2021). It is predicted that rats in both groups will experience stress responses to oral mucosal scratching, which will also cause pain and difficulty in eating. The 5-FU group with chemotherapy had a lower NLR value, which might be related to further organ dysfunction. The PLR is a marker of inflammation derived from the ratio of absolute and absolute LYM counts. It has been used to treat cardiovascular and autoimmune diseases (Ravindra et al., 2022). As inflammatory markers, NLR and PLR are strongly associated with chemotherapeutic responses and chemoresistance. Fu et al. (2021) found that among all inflammatory markers examined, PLR was the only marker associated with chemotherapy-related effects in colorectal cancer patients. Elevated PLR indicates greater tolerance to chemotherapy; thus, patients can take larger doses over extended periods, explaining the association between increased PLR and effective chemotherapy (Fu et al., 2021). The 5-FU group had a lower PLR number than the chemotherapy group. Some of the 5-FU rats with higher grades of ulcerative mucositis exhibited chromodacryorrhea or red lacrimal secretion, a hypersecretion of harderian gland fluid located behind the eye orbit and generally related to the nonspecific sign of stress, which indicates an underlying disease or a painful state (Ewens et al., 2005; Mancinelli and Capello, 2016). It might also be associated with acute anxiety and muscarinic mechanisms (Santos and Carlini, 1988). Generally, lacrimal secretions from the harderian gland help eye lubrication and pheromone-mediated behaviors, but they are removed during daily grooming. In stressed or injured conditions, secretion dries, leading to deceiving appearances of eyes or nose bleeding (Mancinelli and Capello, 2016). This phenomenon can also occur in rats treated with pilocarpine, oxotremorine, and neostigmine and can be stopped by anticholinergic drugs (Santos and Carlini, 1988). Ewens et al. (2005) observed this phenomenon in rats given 5-FU and leucovorin, with toxicities ranging from minor secretion around the eyeball to immense secretion that stains most of the facial hair (Ewens et al., 2005). The 5-FU rats started to lose hair on day 4 and continued until the last day of the experiment. Meanwhile, no hair loss was observed in the healthy group. Hair loss in rat models is associated with chemotherapy-induced alopecia and is a common condition (Davis et al., 2001; Słonimska et al., 2024). Hair follicles exhibit pronounced proliferation and are vulnerable to damage by chemotherapy. Hair matrix keratinocytes have a high proliferation rate below the hair follicle. Thus, extensive cell death due to chemotherapy occurred in this part. Chemotherapy causes apoptosis and impairs the differentiation of hair follicle keratinocytes (Davis et al., 2001). The sensitivity of hair follicle cells to anticancer drugs depends on their proliferation state. Specific anticancer drugs target certain cell cycle phases and thus exhibit selective toxicity toward dividing cells (Santos and Carlini, 1988). Histopathological findings in the mucositis group showed loss of epithelium, inflammatory cell infiltrates, erythrocyte cells that indicated hemorrhagic area, and moderate vasodilatation without abscess, according to the histopathological scoring by Leitao, of score 2. The direct toxicity of 5-FU in basal and suprabasal epithelial cells may result in cell death and activation of inflammatory pathways during the initial mucositis phase. Inflammation itself can impair basal epithelial cell proliferation, resulting in prolonged ulceration (Kim et al., 2023). Our experiments demonstrated that a single intraperitoneal administration of 5-FU at a 150-mg/kg dose in Sprague-Dawley rats has similar effects on 5-FU toxicities in humans, such as decreased food intake and body weight, anemia, thrombocytopenia, and hair loss. This animal model also successfully created ulcerative OM using additional local irritation. Several rats develop severe ulcerative OM with abscesses. As 5-FU causes myelosuppression, the immune system is disturbed, and susceptibility to infections is increased, resulting in severe infections. If left untreated, sepsis may follow. Other gross toxicities were also observed in this study, such as chromodacryorrhea, facial edema, and epistaxis (Takeuchi et al., 2020). The limitation of this study was that not all 5-FU organ toxicities were assessed. The renal, liver, and cardiac toxicity was not evaluated. The sample size was relatively small, the experimental period was short (only 10 days), and histological and blood samples were only obtained at the end of the experiment. Multiple specimen collection, such as hematological parameters and biopsy specimens at various periods, will allow for a more accurate evaluation of the animal model’s performance and provide important information on the OM course. ConclusionIn addition to describing the severity of mucosal clinical lesions and their histopathological features, the current research also describes hematological alterations and systemic 5-FU toxicity in a CIOM model. Our study showed that a single 5-FU dose of 150 mg/kg and additional mucosal irritation could induce ulcerative OM with other clinical side effects of 5-FU treatments, such as anemia, thrombocytopenia, decreased food intake, and body weight. Other gross toxicities, such as red lacrimation, facial edema, epistaxis, and hair loss, were also noted. This single high-dose intraperitoneal 5-FU rat model is simple and can be performed quickly. However, it can provide sufficient samples for clinical and histopathological examination, evaluate systemic and mucosal responses to cancer treatment, and support the development of new OM management strategies. List of Abbreviations5-FU, 5-fluorouracil; CIOM, chemotherapy-induced oral mucositis; HE, hematoxylin and eosin; OM, oral mucositis; TSB, Tripsic Soy Broth. AcknowledgmentThe authors thank all staff of the Animal Research Facilities, Indonesian Medical Education and Research Institute, for their support in this research. Conflicts of interestAll authors declare that they have no conflicts of interest and are responsible for the content and writing of the article. Authors’ contributionAKA, ML, PEW, and YS designed the protocol and interpreted the experimental results. AKA and SS performed experiments and analyzed and interpreted the data. AKA drafted the initial manuscript. All authors have approved the final version of the manuscript. Data availabilityAll data supporting the study are available upon request to the corresponding author. ReferencesAbdalla-Aslan, R., Keegan, R., Zadik, Y., Yarom, N. and Elad, S. 2024. Recent advances in cancer therapy-associated oral mucositis. Oral. Dis. (in press) 1–16; doi:10.1111/odi.14999 Bakar, A., Ningrum, V., Lee, J., Li, C.-T., Hsieh, C.-W., Hong, W.S. and Tsai, M.S. 2021. Therapeutic effect of Cinnamomum osmophloeum leaf extract on oral mucositis model rats induced by 5-fluororacil via influencing IL-1β and IL-6 levels. Processes 9, 615; doi:10.3390/pr9040615 Berger, A.K., Allgäuer, M., Apostolidis, L., Schulze-Schleithoff, A.E., Merle, U., Jaeger, D. and Haag, G.M. 2020. Cancer-related microangiopathic hemolytic anemia in patients with advanced gastric cancer: a retrospective single-center analysis. World J. Gastrointest. Oncol. 12(11), 1288–1295; doi:10.4251/wjgo.v12.i11.1288 Bertolini, M., Sobue, T., Thompson, A. and Dongari-Bagtzoglou, A. 2017. Chemotherapy induces oral mucositis in mice without additional noxious stimuli. Transl. Oncol. 10(4), 612–620; doi:10.1016/j.tranon.2017.05.001 Chang, C.T., Hsiang, C.Y., Ho, T.Y., Wu, C.Z., Hong, H.H. and Huang, Y.F. 2015. Comprehensive assessment of host responses to 5-fluorouracil-induced oral mucositis through transcriptomic analysis. PLoS One 10(8), e0135102; doi:10.1371/journal.pone.0135102 Da Cruz Campos, M.I., Campos, C.N., Corrêa, J.O.A., Aarestrup, F.M. and Aarestrup, B.J.V. 2021. Induced oral mucositis in Wistar rats treated with different drugs: preventive potential in cytokine production. Mol. Clin. Oncol. 14(6), 127; doi:10.3892/mco.2021.2289 Dahlgren, D., Sjöblom, M., Hellström, P.M. and Lennernäs, H. 2021. Chemotherapeutics-induced intestinal mucositis: pathophysiology and potential treatment strategies. Front. Pharmacol. 12, 681417; doi:10.3389/fphar.2021.681417 Davis, S.T., Benson, B.G., Bramson, H.N., Chapman, D.E., Dickerson, S.H., Dold, K.M., Eberwein, D.J., Edelstein, M., Frye, S.V., Gampe, R.T.Jr., Griffin, R.J., Harris, P.A., Hassell, A.M., Holmes, W.D., Hunter, R.N., Knick, V.B., Lackey, K., Lovejoy, B., Luzzio, M.J., Murray, D., Parker, P., Rocque, W.J., Shewchuk, L., Veal, J.M., Walker, D.H. and Kuyper, L.F. 2001. Prevention of chemotherapy-induced Alopecia in rats by CDK inhibitors. Science 291(5501), 134–137. Ewens, A.D., Mihich, E. and Ehrke, M.J. 2005. Fluorouracil plus leucovorin induces submandibular salivary gland enlargement in rats. Toxicol. Pathol. 33(4), 507–515; doi:10.1080/01926230490966265 Fu, Y., Chen, X., Song, Y., Huang, X., Chen, Q., Lv, X., Gao, P. and Wang, Z. 2021. The platelet to lymphocyte ratio is a potential inflammatory marker predicting the effects of adjuvant chemotherapy in patients with stage II colorectal cancer. BMC Cancer. 21(1), 792; doi:10.1186/s12885-021-08521-0 Gao, A., Zhang, L. and Zhong, D. 2023. Chemotherapy-induced thrombocytopenia: literature review. Discov. Oncol. 14(1), 10; doi:10.1007/s12672-023-00616-3 Gupta, N., Ferreira, J., Hong, C.H.L. and Tan, K.S. 2020. Lactobacillus reuteri DSM 17938 and ATCC PTA 5289 ameliorates chemotherapy-induced oral mucositis. Sci. Rep. 10(1), 16189; doi:10.1038/s41598-020-73292-w Hamidi, S.P., Koohi-Hosseinabadi, O., Khaksar, S., Ghanbariasad, A., Dehghanian, A.R., Dehghan, A., Haddadi, Z., Gorgin, R., Farjam, M. and Alipanah, H. 2023. Evaluation of the topical gel and oral administration of Punica granatum Var Pleniflora on oral mucositis induced by 5-fluorouracil in golden hamsters. BMC Complement. Med. Ther. 23(1), 225; doi:10.1186/s12906-023-04053-1 Harada, K., Ferdous, T., Fujiwara, R., Watanabe, K., Mizukami, Y. and Mishima, K. 2022. An elemental diet protects mouse salivary glands from 5-fluorouracil-induced atrophy. Oncol. Lett. 23(6), 178; doi:10.3892/ol.2022.13298 Honda, H., Onda, T., Hayashi, K., Shibahara, T. and Takano, M. 2022. Comparison of topical agents that are effective against oral mucositis associated with chemotherapy using a rat anticancer agent-induced oral mucositis model. J. Oral Maxillofac. Surg. Med. Pathol. 34(4), 445–452; doi:10.1016/j.ajoms.2021.11.010 Iovoli, A.J., Turecki, L., Qiu, M.L., Khan, M., Smith, K., Yu, H., Ma, S.J., Farrugia, M.K. and Singh, A.K. 2023. Severe oral mucositis after intensity-modulated radiation therapy for head and neck cancer. JAMA Netw. Open. 6(10), e2337265; doi:10.1001/jamanetworkopen.2023.37265 Kim, D.H., Choi, J., Lim, Y.S., Huh, H.J., Cho, C.G. and Kim, B.H. 2023. A rat model for oral mucositis induced by a single administration of 5-fluorouracil. In Vivo 37(1), 218–224; doi:10.21873/invivo.13070 Leitão, R.F.C., Ribeiro, R.A., Bellaguarda, E.A.L., Macedo, F.D.B., Silva, L.R., Oriá, R.B., Vale, M.L., Cunha, F.D.Q. and Brito, G.A.C. 2007. Role of nitric oxide on pathogenesis of 5-fluorouracil induced experimental oral mucositis in hamster. Cancer Chemother. Pharmacol. 59(5), 603–612; doi:10.1007/s00280-006-0301-y Lima, V., Brito, G.A., Cunha, F.Q., Rebouças, C.G., Falcão, B.A., Augusto, R.F., Souza, M.L., Leitão, B.T. and Ribeiro, R.A. 2005. Effects of the tumour necrosis factor-alpha inhibitors pentoxifylline and thalidomide in short-term experimental oral mucositis in hamsters. Eur. J. Oral Sci. 113(3), 210–217; doi:10.1111/j.1600-0722.2005.00216.x Lorini, L., Perri, F., Vecchio, S., Belgioia, L., Vinches, M., Brana, I., Elad, S. and Bossi, P. 2022. Confounding factors in the assessment of oral mucositis in head and neck cancer. Support. Care Cancer. 30(10), 8455–8463; doi:10.1007/s00520-022-07128-w Mancinelli, E. and Capello, V. 2016. Anatomy and disorders of the oral cavity of rat-like and squirrel-like rodents. Vet. Clin. North Am. Exot. Anim. Pract. 19(3), 871–900; doi:10.1016/j.cvex.2016.04.008 Mohammed, A.I., Celentano, A., Paolini, R., Low, J.T., McCullough, M.J., O’Reilly, L.A. and Cirillo, N. 2023. Characterization of a novel dual murine model of chemotherapy-induced oral and intestinal mucositis. Sci. Rep. 13(1), 1396; doi:10.1038/s41598-023-28486-3 Pulito, C., Cristaudo, A., Porta, C.L., Zapperi, S., Blandino, G., Morrone, A. and Strano, S. 2020. Oral mucositis: the hidden side of cancer therapy. J. Exp. Clin. Cancer Res. 39(1), 210; doi:10.1186/s13046-020-01715-7 Ravindra, R., Ramamurthy, P., Aslam, S.S., Kulkarni, A., Suhail, K. and Ramamurthy, P.S. 2022. Platelet indices and platelet to lymphocyte ratio (PLR) as markers for predicting COVID-19 infection severity. Cureus 14(8), e28206; doi:10.7759/cureus.28206 Safarpour, S., Safarpour, S., Pirzadeh, M., Moghadamnia, A.A., Ebrahimpour, A., Shirafkan, F., Mansoori, R., Kazemi, S. and Hosseini, M. 2022. Colchicine ameliorates 5-fluorouracil-induced cardiotoxicity in rats. Oxid. Med. Cell. Longev. 2022, 6194532; doi:10.1155/2022/6194532 Sangild, P.T., Shen, R.L., Pontoppidan, P. and Rathe, M., 2018. Animal models of chemotherapy-induced mucositis: translational relevance and challenges. Am. J. Physiol. Gastrointest. Liver Physiol. 314(2), G231–G246; doi:10.1152/ajpgi.00204.2017 Santos, R. and Carlini, E.A. 1988. Red lacrimal secretion (chromodacryorrhea) induced by cholinergic drugs in rats subjected to the watertank technique. Pharmacology 36(1), 61–68; doi:10.1159/000138347 Saul-McBeth, J., Dillon, J., Lee, A., Launder, D., Kratch, J.M., Abutaha, E., Williamson, A.A., Schroering, A.G., Michalski, G., Biswas, P. and Conti III, S.R. 2021. Tissue damage in radiation-induced oral mucositis is mitigated by IL-17 receptor signaling. Front. Immunol. 12, 687627; doi:10.3389/fimmu.2021.687627 Sharma, A., Chorawala, M.R., Rawal, R.M. and Shrivastava, N. 2024. Integrated blood and organ profile analysis to evaluate ameliorative effects of kaempferol on 5-fluorouracil-induced toxicity. Sci. Rep. 14(1), 2363; doi:10.1038/s41598-024-52915-6 Shetty, S.S., Maruthi, M., Dhara, V., de Arruda, J.A.A., Abreu, L.G., Mesquita, R.A., Teixeira, A.L., Silva, T.A. and Merchant, Y. 2022. Oral mucositis: current knowledge and future directions. Dis. Mon. 68(5), 101300; doi:10.1016/j.disamonth.2021.101300 Słonimska, P., Sachadyn, P., Zieliński, J., Skrzypski, M. and Pikuła, M. 2024. Chemotherapy-mediated complications of wound healing: an understudied side effect. Adv. Wound Care (New Rochelle). 13(4), 187–199; doi:10.1089/wound.2023.0097 Sobue, T., Bertolini, M., Thompson, A., Peterson, D.E., Diaz, P.I. and Dongari-Bagtzoglou, A. 2018. Chemotherapy-induced oral mucositis and associated infections in a novel organotypic model. Mol. Oral Microbiol. 33(3), 212–223; doi: 10.1111/omi.12214 Sougiannis, A.T., VanderVeen, B.N., Enos, R.T., Velazquez, K.T., Bader, J.E., Carson, M., Chatzistamou, I., Walla, M., Pena, M.M., Kubinak, J.L. and Nagarkatti, M. 2019. Impact of 5 fluorouracil chemotherapy on gut inflammation, functional parameters, and gut microbiota. Brain Behav. Immun. 80, 44–55; doi:10.1016/j.bbi.2019.02.020 Takeuchi, I., Kawamata, R. and Makino, K. 2020. A rat model of oral mucositis induced by cancer chemotherapy for quantitative experiments. Anticancer Res. 40(5), 2701–2706; doi:10.21873/anticanres.14241 Zahorec, R. 2021. Neutrophil-to-lymphocyte ratio, past, present and future perspectives. Bratisl. Lek. Listy. 122(7), 474–488; doi:10.4149/bll_2021_078 | ||
| How to Cite this Article |
| Pubmed Style Astuti AK, Samsul S, Louisa M, Wimardhani YS, Yasmon A, Wuyung PE. Clinical and histopathological evaluation of 5-fluorouracil-induced oral mucositis in a rat model. Open Vet. J.. 2025; 15(5): 1958-1968. doi:10.5455/OVJ.2025.v15.i5.10 Web Style Astuti AK, Samsul S, Louisa M, Wimardhani YS, Yasmon A, Wuyung PE. Clinical and histopathological evaluation of 5-fluorouracil-induced oral mucositis in a rat model. https://www.openveterinaryjournal.com/?mno=231804 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i5.10 AMA (American Medical Association) Style Astuti AK, Samsul S, Louisa M, Wimardhani YS, Yasmon A, Wuyung PE. Clinical and histopathological evaluation of 5-fluorouracil-induced oral mucositis in a rat model. Open Vet. J.. 2025; 15(5): 1958-1968. doi:10.5455/OVJ.2025.v15.i5.10 Vancouver/ICMJE Style Astuti AK, Samsul S, Louisa M, Wimardhani YS, Yasmon A, Wuyung PE. Clinical and histopathological evaluation of 5-fluorouracil-induced oral mucositis in a rat model. Open Vet. J.. (2025), [cited January 25, 2026]; 15(5): 1958-1968. doi:10.5455/OVJ.2025.v15.i5.10 Harvard Style Astuti, A. K., Samsul, . S., Louisa, . M., Wimardhani, . Y. S., Yasmon, . A. & Wuyung, . P. E. (2025) Clinical and histopathological evaluation of 5-fluorouracil-induced oral mucositis in a rat model. Open Vet. J., 15 (5), 1958-1968. doi:10.5455/OVJ.2025.v15.i5.10 Turabian Style Astuti, Ambar Kusuma, Samsul Samsul, Melva Louisa, Yuniardini Septorini Wimardhani, Andi Yasmon, and Puspita Eka Wuyung. 2025. Clinical and histopathological evaluation of 5-fluorouracil-induced oral mucositis in a rat model. Open Veterinary Journal, 15 (5), 1958-1968. doi:10.5455/OVJ.2025.v15.i5.10 Chicago Style Astuti, Ambar Kusuma, Samsul Samsul, Melva Louisa, Yuniardini Septorini Wimardhani, Andi Yasmon, and Puspita Eka Wuyung. "Clinical and histopathological evaluation of 5-fluorouracil-induced oral mucositis in a rat model." Open Veterinary Journal 15 (2025), 1958-1968. doi:10.5455/OVJ.2025.v15.i5.10 MLA (The Modern Language Association) Style Astuti, Ambar Kusuma, Samsul Samsul, Melva Louisa, Yuniardini Septorini Wimardhani, Andi Yasmon, and Puspita Eka Wuyung. "Clinical and histopathological evaluation of 5-fluorouracil-induced oral mucositis in a rat model." Open Veterinary Journal 15.5 (2025), 1958-1968. Print. doi:10.5455/OVJ.2025.v15.i5.10 APA (American Psychological Association) Style Astuti, A. K., Samsul, . S., Louisa, . M., Wimardhani, . Y. S., Yasmon, . A. & Wuyung, . P. E. (2025) Clinical and histopathological evaluation of 5-fluorouracil-induced oral mucositis in a rat model. Open Veterinary Journal, 15 (5), 1958-1968. doi:10.5455/OVJ.2025.v15.i5.10 |