| Research Article | ||
Open Vet. J.. 2025; 15(5): 1947-1957 Open Veterinary Journal, (2025), Vol. 15(5): 1947-1957 Research Article Clinical cases of contagious caprine pleuropneumonia in sheep: Retrospective clinical, sonographic, and pathological investigationsMohamed Tharwat1, Abdulrahman A. Alkheraif2 and Haytham Ali3*1Department of Clinical Sciences, College of Veterinary Medicine, Qassim University, Buraidah, Saudi Arabia 2Department of Pathology and Laboratory Diagnosis, College of Veterinary Medicine, Qassim University, Buraidah, Saudi Arabia 3Department of Animal and Veterinary Sciences, College of Agricultural and Marine Sciences, Sultan Qaboos University, Muscat, Sultanate of Oman *Corresponding Author: Haytham Ali. Department of Animal and Veterinary Sciences, College of Agricultural and Marine Sciences, Sultan Qaboos University, Muscat, Sultanate of Oman. Email: h.ali [at] squ.edu.om Submitted: 05/12/2024 Revised: 26/03/2025 Accepted: 16/04/2025 Published: 31/05/2025 © 2025 Open Veterinary Journal
AbstractBackground: Contagious caprine pleuropneumonia (CCPP) is a highly contagious respiratory disease caused by Mycoplasma capricolum subsp. capripneumoniae. It affects goats, sheep, and wild ruminants. Aim: This study investigated the clinical, sonographic, and pathological findings in sheep with serologically confirmed CCPP, highlighting the potential of ultrasound as a diagnostic tool. Methods: Thirty-four sheep were serologically confirmed to have CCPP by latex agglutination test (LAT) and underwent clinical examination and sonographic imaging of the lungs, pleura, urinary and digestive systems, peritoneum, and liver. Pleural effusion was aspirated under ultrasound guidance to alleviate dyspnea. Necropsy and histopathological examination were performed to correlate imaging findings with pathological changes. Results: Clinical signs included weight loss, fever, tachycardia, polypnea, dyspnea, cough, nasal discharge, and bluish mucous membranes. Pulmonary ultrasound showing consolidated lung parenchyma with a hepatic-like texture. Pleural effusion, the most prominent sonographic finding, exhibited echogenicity ranging from anechoic to hyperechoic, with fibrin networks and precipitates of varying severity. Dyspnea significantly improved after pleural effusion aspiration. Necropsy revealed typical CCPP lesions, including unilateral sero-fibrinous pleuropneumonia with lung consolidation and pleural effusion varying from straw-colored to bloody or turbid. Histopathology revealed bronchiolar and alveolar obliteration by fibrin and inflammatory cells, predominantly neutrophils and macrophages. Conclusion: Ultrasound effectively identified sero-fibrinous pleuropneumonia in CCPP-infected sheep, which was in agreement with postmortem findings. This technique aids in the early detection and isolation of infected animals and is a valuable tool until culture or molecular testing provides definitive results. Keywords: Contagious caprine pleuropneumonia, Mycoplasma, Pathology, Sheep, Ultrasound. IntroductionContagious caprine pleuropneumonia (CCPP) is listed by the World Organization of Animal Health (WOAH) as a notifiable serious highly contagious respiratory disease in goats, sheep, and wild ruminants (Iqbal Yatoo et al., 2019). It is caused by Mycoplasma capricolum subsp. capripneumoniae (Mccp) (El-Manakhly and Tharwat, 2016; Tharwat and Al-Sobayil, 2017; Ahaduzzaman, 2021; Ali et al., 2024). A morbidity rate of 80%–100% and a mortality rate of up to 100% were reported due to progressive fibrinous pleuropneumonia (Ahmad et al., 2021). Therefore, CCPP causes a huge financial crisis among small ruminant farmers worldwide (Nicholas and Churchward, 2012; Renault et al., 2019). In goats, CCPP poses a significant threat and is commonly observed as a clinical disease (Tharwat and Al-Sobayil, 2017; Ali et al., 2024). According to WOAH, CCPP has been reported in 40 countries, principally in the Middle East, Asia, and Africa, where most of the global goat population is reared (El-Manakhly and Tharwat, 2016; Tharwat and Al-Sobayil, 2017; Elhassan and Salama, 2018; Teshome et al., 2019; Ali et al., 2024). In sheep, CCPP is typically considered a subclinical disease (Asmare et al., 2016), and both sheep and wild ruminants can contract the infection from diseased goats. The Mccp bacteria were successfully isolated from sheep in Kenya (Litamoi et al., 1990), Uganda (Bölske et al., 1995), and Ethiopia (Shiferaw et al., 2006). Anti-Mccp antibodies were identified in sheep (Mbyuzi et al., 2014), indicating an immune response and replication of Mccp in animals. Additionally, the presence of these antibodies in significant amounts, capable of causing agglutination, has also been observed under field conditions (Iqbal Yatoo et al., 2019). Older goats and sheep (over 4 years of age) raised in groups of more than 100 animals with shared water and feeding sources are at an increased risk of contracting CCPP (Selim et al., 2021). Additionally, CCPP is highly prevalent in goats and sheep, especially in Asia and Africa, highlighting the need for strict control measures (Ahaduzzaman, 2021). Although the occurrence and fatality rates of CCPP were significantly lower in sheep than in goats, CCPP was diagnosed in sheep using culture and polymerase chain reaction (PCR) techniques (Abd-Elrahman et al., 2020). The reported clinical manifestations in goats with CCPP include depression, anorexia, weakness, fever, polypnea, dyspnea, mouth breathing, cough, and nasal discharge (Tharwat and Al-Sobayil, 2017; Iqbal Yatoo et al., 2019). The diagnosis of CCPP is primarily based on clinical symptoms and necropsy findings. Laboratory diagnostic methods include the culture of pleural fluid, serological tests such as enzyme-linked immunosorbent assay, complement fixation test, and latex agglutination test (LAT), and molecular techniques such as real-time PCR for the detection of Mccp and DNA or gene-based molecular tests (El-Manakhly and Tharwat, 2016; Tharawt and Al-Sobayil, 2017; Elhassan and Salama, 2018; Ali et al., 2024). Ultrasound has been proven effective for the diagnosis of several diseases affecting small ruminants (Tharwat et al., 2012a,2024, 2025a,b; Tharwat, 2021a; Sadan et al., 2023; Tharwat and Al-Hawas, 2024a,b; Tharwat and Tsuka, 2024). The methodology played a crucial role in confirming the clinical symptoms and chest pathological lesions of CCPP in goats (Tharwat and Al-Sobayil, 2017). Although bacterial culture and PCR are considered gold standards for confirming CCPP, their application is often limited under field conditions because of resource constraints. In such settings, the LAT is a practical and reliable alternative for rapid diagnosis (WOAH, 2009; Habibur Rahman et al., 2024). However, it is important to note that the LAT may exhibit cross-reactivity with antibodies from other closely related Mycoplasma species, potentially leading to false-positive results. Therefore, combining the LAT with other diagnostic methods, such as sonography and pathological examinations, can enhance diagnostic accuracy and facilitate timely intervention in field clinics. This study aimed to fill this gap by investigating these findings in sheep serologically confirmed to have CCPP, providing a more comprehensive understanding of the disease in this population and supporting the development of improved diagnostic and control strategies. Materials and MethodsAnimal selectionA total of 42 sheep displaying respiratory symptoms were clinically examined and tested for CCPP using the serological LAT for antibody detection (CapriLAT, Animal Health and Veterinary Laboratories Agency, Surrey, UK), following previously established protocols (El-Manakhly and Tharwat, 2016; Tharwat and Al-Sobayil, 2017). Eight animals tested negative for the LAT, while the remaining 34 tested positive. Only LAT-positive patients were included in the study. The 34 infected sheep (25 females, 9 males) had an average age of 3.0 ± 1.6 years and a mean body weight of 43.9 ± 15.0 kg. These animals exhibited signs of partial or complete appetite loss, weight loss, and pulmonary distress. All infected sheep were examined at the University Veterinary Hospital in Qassim, Saudi Arabia, 350 km from Riyadh, between 2014 and 2024. Written informed consent was obtained from the animal owners. A control group of 10 healthy LAT-negative sheep (5 females, 5 males) was selected from a university farm. The animals had an average age of 2.7 ± 1.9 years and the mean body weight of 42.0 ± 17.3 kg. Diseased and healthy sheep were treated based on the Guidelines of Laboratory Animal Control Ethics of Qassim University, which were basically aligned with the Guide for the Use and Care and Laboratory Animals of the National Institutes of Health in the United States (US Department of Health and Human Services, 1996). Clinical and sonographic examinationsImmediately upon presentation, all infected and control animals underwent a thorough clinical examination, which included observing behavior and body condition, auscultating the heart, lungs, rumen, and intestines, and measuring respiratory rate, pulse rate, and rectal temperature. After clipping and shaving the skin, the chest, urinary and digestive systems, peritoneum, and liver were scanned percutaneously in both groups using a 3.5-MHz sector transducer (SonoScape, Sonoscape Medical Corp., China). Cardiopulmonary, gastrointestinal, urinary, and hepatic sonography were performed according to established methodologies for sheep and goats (Tharwat et al., 2012a,2024, 2025a,b; Tharwat and Al-Sobayil, 2017; Tharwat, 2021a; Sadan et al., 2023; Tharwat and Al-Hawas, 2024). Ultrasound-guided aspiration of pleural effusionPleural effusion was aspirated under ultrasound guidance in 12 infected animals to alleviate dyspnea. The procedure involved surgical site preparation and local anesthesia infiltration, following the technique described by Tharwat (2021b). A free-hand ultrasound-guided aspiration technique was applied as reported in large ruminants (Mohamed et al., 2002, 2003a,b,c; Mohamed and Oikawa, 2007, 2008; Tharwat et al., 2012b,c, 2013, 2025c). Postmortem examination and histopathologyPostmortem examinations were conducted on 18 animals (15 females, 3 males). Pulmonary specimens were collected, fixed in 10% neutral buffered formalin, routinely processed, sectioned at 5-μm thickness, and stained with hematoxylin and eosin for histopathological analysis. ResultsOf the 34 LAT-positive animals, the breeds were as follows: Neimi (15), Nagdi (7), Harri (5), Sawakni (4), and Barbari (3). The values of rectal temperature, pulse, and respiratory rates were 40.6ºC ± 1.4ºC (normal 39.2ºC ± 0.6ºC; p=0.02), 110 ± 15 beats/min (normal 88ºC ± 12ºC beats/min; p=0.03), and 55 ± 12 breaths/min (normal 25 ± 7 brath/mim; p=0.005), respectively. Other clinical manifestations included weight loss, polypnea, bluish mucus membranes, dyspnea, cough, nasal discharges, abducted elbows, and open-mouth breathing (Fig. 1). Thoracic auscultation revealed increased breath sounds unilaterally in 23 sheep and bilaterally in 6 sheep, and in the remaining 5 sheep, no sounds were audible. Abnormal cardiopulmonary sounds included pleural friction, wheezing, rales, and pericardial splashing and muffling of the heart. All 34 diseased sheep tested positive for LAT, suggesting Mccp infection, whereas the apparently healthy controls showed normal findings on clinical examination and were LAT-negative.
Fig. 1.Different clinical presentations in six sheep with contagious caprine pleuropneumonia. The ram in image A was admitted with a history of inappetence and polypnea. Image B shows a female sheep with a history of decreased weight gain and seromucoid nasal discharge. Images C and D show 2 females with mucoid and mucopurulent nasal discharge. Image E shows a female sheep with open-mouth breathing. Image F shows a female sheep with purulent nasal discharge and open-mouth breathing. In diseased sheep, ultrasonography of the lungs revealed a consolidated pulmonary parenchyma resembling hepatic texture; the lesions were visualized unilaterally in 28 cases and bilaterally in 6 cases. Pleural effusions were the outstanding sonographic findings in all diseased animals. The echogenicity of the pleural fluid ranged from anechoic to hyperechoic, with imaging showing precipitates in the pleural effusion. Fibrin networks were also visualized in 27 animals that were either mild or massive (Fig. 2). Dyspnea symptoms improved in sheep following pleural effusion aspiration. The volume of aspirated pleural fluid was 50–800 ml (Fig. 3). No other sonographic findings were detected in the abdominal cavity. In control sheep, no sonographic abnormalities were detected on thoracic or abdominal imaging.
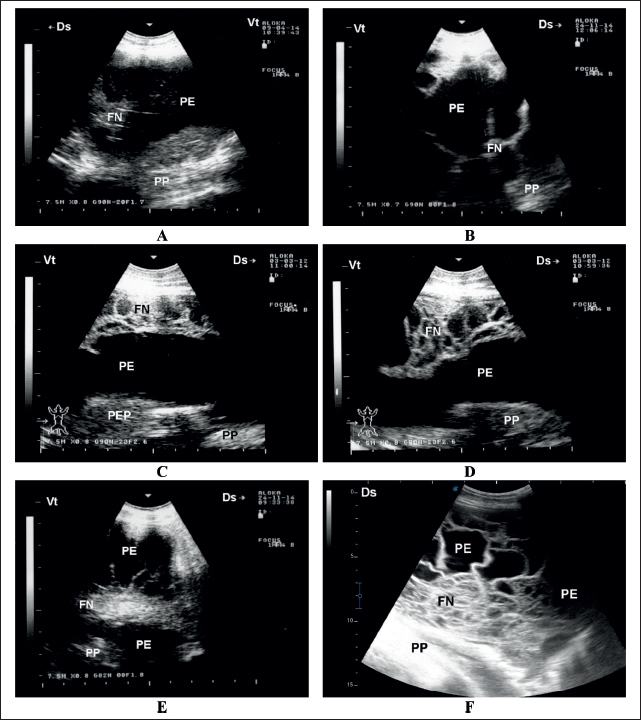
Fig. 2. Sonographic findings in six sheep with contagious caprine pleuropneumonia. Images A and B show PE , mild FN, and compressed PP. Image C shows moderate FN in the absence of PEP. Images D–F show a massive FN in three animals. FN=fibrin network; PE=pleural effusion; PEP=pleural effusion precipitate; PP=pulmonary parenchyma. Postmortem examination revealed unilateral sero-fibrinous pleuropneumonia with a thick yellowish fibrin coat covering the affected lung. Pulmonary hepatization was evident after the removal of the fibrin coat. The cut section showed extensive hepatized and congested parenchyma. A massive amount of fluid exudation was detected within the pleura. The pleural fluid was either straw-colored, bloody, or deep turbid (Fig. 4). Histologically, fibrinous pleuropneumonia was the principal finding. The pleura was greatly thickened with fibrin and leukocytes. The bronchioles and alveoli were obliterated with fibrin threads and inflammatory cells, particularly neutrophils and macrophages. The alveoli were distended with exudate, and congestion of the peri-alveolar vessels was observed. Congestion of the intra-alveolar vessels was observed, with some thrombosis and peri-vascular edema. Multifocal areas of clear tissue carnification were also observed (Fig. 5).
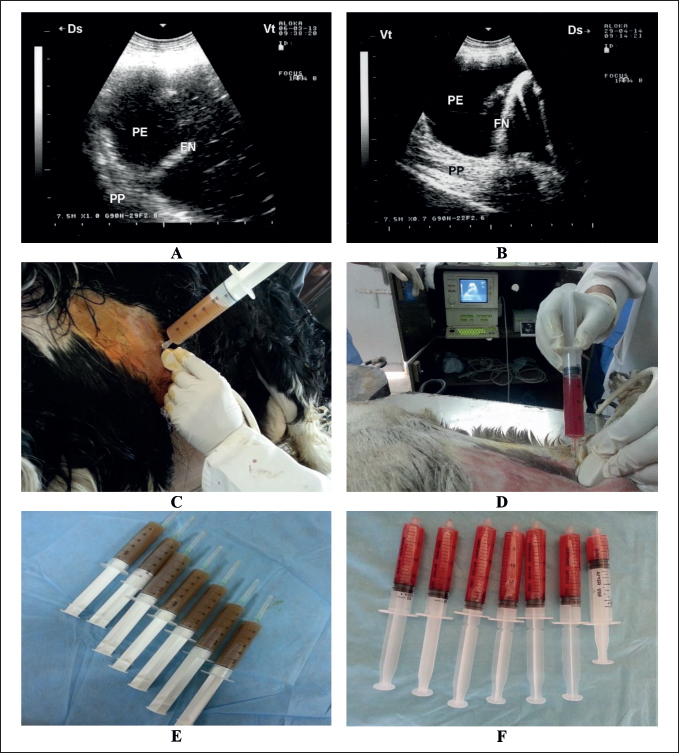
Fig. 3. Ultrasound-guided aspiration of pleuritic fluid in two sheep with contagious caprine pleuropneumonia. Image A shows the sonographic findings of the first case with hyperechoic PE, FN, and compressed PP. Aspiration of the PE was carried out under ultrasound guidance (C), from which approximately 410 ml of deep turbid fluid was collected (E). Image B shows the sonographic findings of the second case with hypoechoic PE, FN, and compressed PP. Aspiration of the PE was carried out under ultrasound guidance (D), in which approximately 127 ml of bloody fluid was collected (F). FN=fibrin network; PE=pleural effusion; PP=pulmonary parenchyma.
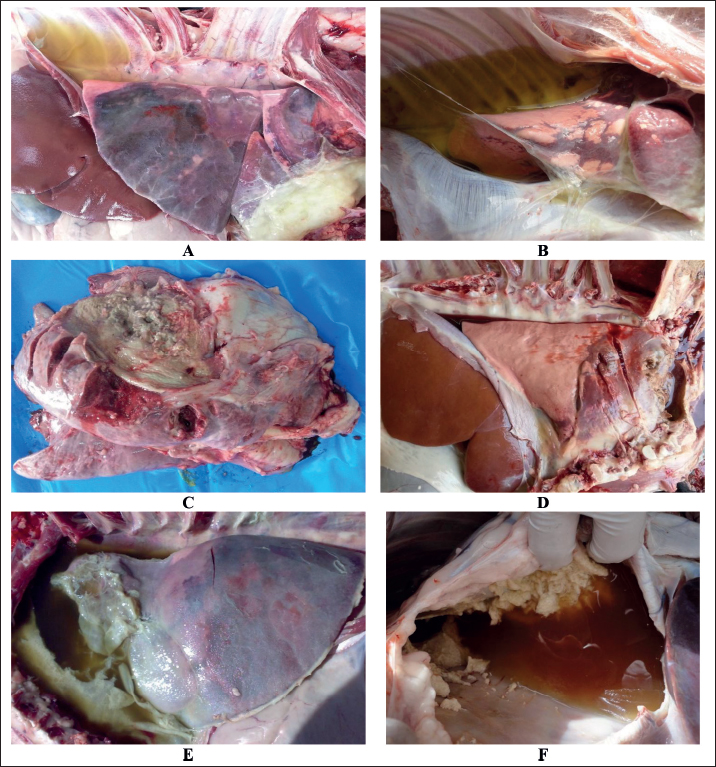
Fig. 4. Postmortem findings in sheep with contagious caprine pleuropneumonia. Images A and B show straw-colored pleural fluid, lung consolidation, and fibrin deposition. Image C shows lung hepatization with fibrinopurulent exudate covering the affected lung. Image D shows consolidated lung with bloody pleuritic fluid. Image E shows consolidated lung, deep turbid pleuritic fluid, and massive fibrin deposition. Image F shows severe fibrin masses with blood-tinged pleuritic fluid.
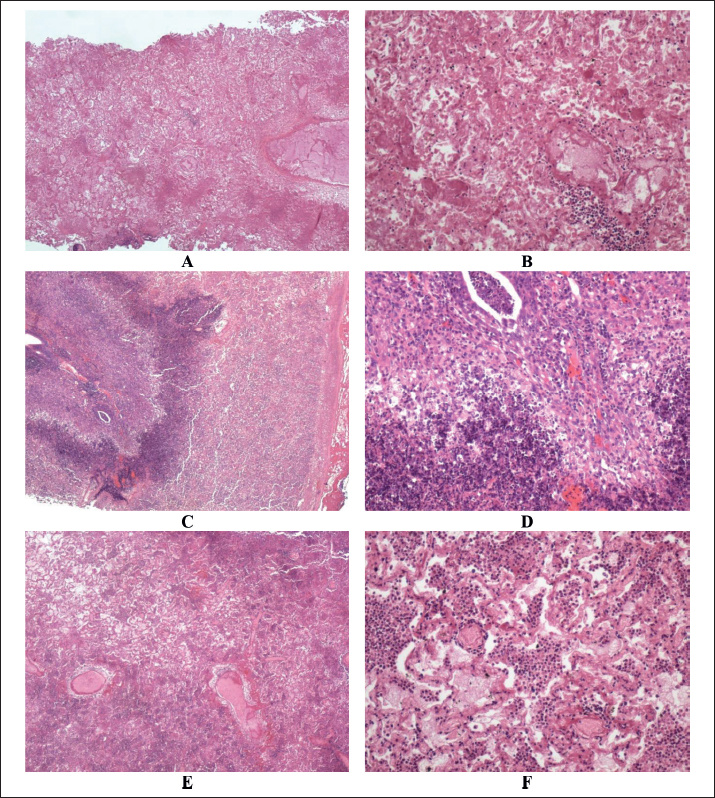
Fig. 5. Histopathological findings in sheep with contagious caprine pleuropneumonia. Images A and B show lung tissue with hemorrhagic infarction and adjacent bronchopneumonia in the residual viable lung parenchyma. Images C and D show lung tissue with marked bronchopneumonia and fibrinous pleurisy in the covering pleura. Images E and F of lung tissue with diffuse bronchopneumonia. A, C, and E (HE, ×100) while B, D, and F (HE, ×400). DiscussionTo the best of our knowledge, this is the first study to document CCPP in sheep in Saudi Arabia caused by Mccp. The study emphasizes initially clinically affected cases in sheep and is supported by verification by ultrasound in conjunction with the pathological changes. Lung affections were observed unilaterally in almost 68% of the diseased sheep and bilaterally in the remaining 18%. These findings correspond well with our previous findings in goats with CCPP (El-Manakhly and Tharwat, 2016; Tharwat and Al-Sobayil, 2017; Tharwat, 2021b). Anorexia, fever, depression, painful breathing, dyspnea, grunting, and painful cough were reported in goats with CCPP (Elhassan and Salama, 2018). It has also been reported that symptoms may vary between animals, with the condition presenting as peracute, acute, or even chronic. This variability poses a challenge in the initial tentative diagnosis of CCPP in small ruminants (Iqbal Yatoo et al., 2019). In humans, lung and pleural sonography allow rapid detection of various respiratory diseases. The ease of performance, repeatability, rapidity, and reliability make thoracic ultrasound the preferred modality for scanning the pleura and lung in an effective, cost-effective, and secure manner. Chest ultrasound is best used alongside other diagnostic approaches to yield an inclusive evaluation of critically diseased patients (Mayo et al., 2019). During the COVID-19 pandemic, lung and pleural abnormalities were also best evaluated by sonography (Smith et al., 2020; Gil-Rodríguez et al., 2022). The procedure is also used in intensive care units to monitor pleural effusion and to guide diagnosis and treatment (Brogi et al., 2017). In veterinary medicine, on-farm chest ultrasonography is a practical and accurate tool for detecting pulmonary affection in bovines (Ollivett and Buczinski, 2016). The procedure has also been found effective in various farm animals with respiratory disorders (Ramirez et al., 2004; Tharwat and Oikawa, 2011; Buczinski et al., 2013; Tharwat and Al-Sobayil, 2016; Tharwat, 2021c). In the current study, chest ultrasonography of sheep with CCPP revealed the presence of consolidated lung that mimics liver parenchyma. The most noticeable findings were the appearance of floating mild-to-massive fibrin threads within anechoic to hyperechoic pleuritic fluids. To alleviate the symptoms of dyspnea, centesis and aspiration of thoracic fluids were also performed under ultrasound guidance; the results agree well with our previous report on goats with CCPP (Tharwat, 2021b). In goats with clinical CCPP, chest ultrasonography revealed hepatized lung parenchyma, pleural effusion, pleural abscesses, and fibrin deposition containing several anechoic fluid spaces with compressed pulmonary tissue and pericardial effusion (Tharwat and Al-Sobayil, 2017). In sheep with pleuropneumonia, pleural ultrasound evaluation showed pleural effusion; moreover, it aided in the aspiration and centesis of this fluid (Braun et al., 1995). In cattle with pleuropneumonia, sonographic examination showed accumulation of hypoechoic to anechoic pleural effusion. Fibrin threads were also imaged between the pulmonary surface and chest wall (Braun et al., 1997). It has been reported that thoracic sonography should be performed in equines with pleuropneumonia to evaluate the resolution or progression of pleural and parenchymal diseases (Reimer et al., 1989). In goats with CCPP, postmortem findings included pulmonary consolidation, turbid pleuritic fluid together with fibrin matrix, pleural abscessation, and fibrinous pericarditis (Tharwat and Al-Sobayil, 2017; Elhassan and Salama, 2018; Iqbal Yatoo et al., 2019). In another study conducted on goats with CCPP, moderate-to-severe pleural adhesions, focal fibrinous pleuritis, and diffuse fibrinous coating of the lungs were observed. The lungs exhibited purple-gray discoloration with a moist, extensively congested, and hepatized cut surface. Serosanguineous or straw-colored pleural effusion is noted along with a thickened, rough, and opaque pericardium. Hydropericardium and pericardial adhesions were also noted (El-Manakhly and Tharwat, 2016). Similar lesions have been reported in CCPP-infected sheep, albeit with less severity and lower infection and fatality rates compared with CCPP-infected goats (Abd-Elrahman et al., 2020).In goats showing the peracute form of CCPP, recorded lesions included only pulmonary edema. Unilateral fibrinous pleuropneumonia, hepatization, and marbling appearance of the lungs, fibrinous pleuritis, and hydrothorax were found in cases of acute form of CCPP. However, in the chronic form of lung hepatization, pleural adhesion and progressive hydrothorax were outstanding postmortem findings (Ali et al., 2024). A study conducted on goats with CCPP reported several histological abnormalities, including lung carnification, diffuse thickening of the pleura with stringy fibrinous masses, adhesions between the parietal and visceral pleurae, distended alveoli filled with exudate, and severe congestion of the interalveolar blood vessels. Additional findings included thrombotic blood vessels, perivascular edema, lung infarcts surrounded by an inflammatory zone, organized infarcts, lymphoid cell aggregates, partially necrotic bronchioles, alveoli containing faint pink albuminous material, dark red fibrinous masses, inflammatory cells predominantly consisting of neutrophils, and fibrinous pericarditis (El-Manakhly and Tharwat, 2016). In the current investigation, the most prominent finding was fibrinous pleuritis accompanied by fibrino-necrotic pneumonia. The pleura is severely thickened, with adhesions at certain sites. Some intra-alveolar vessels exhibited thrombosis with associated peri-vascular edema. The intra-alveolar contents consisted of fibrin threads or eosinophilic homogeneous material along with inflammatory cells, primarily neutrophils and macrophages. Additionally, multifocal areas of tissue carnification were observed. In contrast, another study found that in the peracute form of CCPP, the pulmonary alveoli were filled with an eosinophilic homogeneous substance and polymorphonuclear cells. In the acute form, the alveoli were occupied by fibrinous proteinaceous material, along with interstitial and alveolar edema, hemorrhage, capillary congestion, and bronchioles and bronchi filled with fibrin and inflammatory cells, primarily neutrophils. In the chronic form, multifocal necrotic regions with fibrin and macrophages are present in the bronchi and alveolar spaces (Ali et al., 2024). When diagnosing sheep with CCPP using ultrasonography, some limitations can arise. Limited sensitivity during the early stages of CCPP is one of these limitations because ultrasonography may not detect subtle changes in lung tissues during the initial stages of CCPP, as the disease may present with mild clinical signs that are not immediately visible on ultrasound. The second limitation is interpretation challenges, where the interpretation of ultrasonographic images can be subjective and dependent on the operator’s skill and experience. Different clinicians interpret the same images in different ways, leading to inconsistent diagnoses. Poor visualization of deep or dense lesions is a third limitation. By sonography, it may be difficult to visualize lesions in deeper parts of the lungs or those that are densely consolidated, especially in obese or larger sheep, reducing its diagnostic accuracy. The fourth limitation is the limited specificity in which ultrasonographic findings such as pleural effusion or lung consolidation can also be observed in other respiratory diseases, which can make it difficult to differentiate CCPP from other conditions with similar clinical manifestations. These limitations should be taken into consideration when using ultrasonography to diagnose CCPP in sheep. Combining ultrasound with other diagnostic methods (e.g., clinical evaluation, laboratory tests, or culture) may improve diagnostic accuracy. ConclusionThis clinical, sonographic, and pathological study evaluated sheep with suspected CCPP. Ultrasound findings revealed fibrinous pleuropneumonia in all infected sheep, which generally corresponded with postmortem findings in necropsied cases. The pleuritic fluid aspiration technique was unexpectedly effective in alleviating respiratory symptoms, particularly dyspnea. The present study employed a rapid, simple, and convenient field serological test to diagnose CCPP in sheep, which can be easily performed by veterinarians. Chest ultrasonography was also used to monitor disease progression and to help determine whether the affected sheep should undergo treatment or be euthanized. This procedure is valuable for detecting pulmonary and pleural changes and assessing prognosis, if needed. This technique will help large-scale sheep breeders detect and isolate infected animals until more definitive results from culture and advanced molecular methods, such as PCR, are available. However, it should be noted that PCR is a more specific diagnostic tool with limitations, such as high cost and the need for specialized laboratory equipment, which may limit its widespread use in field settings. Further studies are recommended to explore the potential of ultrasound-guided aspiration as a treatment method for sheep, particularly in the early stages of the disease. AcknowledgmentsNone. Conflict of interestThe authors declare no conflict of interest. FundingThis research received no specific grant. Authors’ contributionMT: conceived the experiments, designed the practical work, wrote the manuscript draft, and prepared the figures. AAA: Editing and revision of the manuscript. HA: We performed histopathologic work and revised the manuscript draft. All authors have approved the manuscript for publication. Data availabilityAll data supporting the findings of this study are available in the manuscript, and no additional data sources are required. ReferencesAbd-Elrahman, A.H., Khafaga, A.F. and Abas, O.M. 2020. The first identification of contagious caprine pleuropneumonia (CCPP) in sheep and goats in Egypt: molecular and pathological characterization. Trop. Anim. Health Prod. 52(3), 1179–1186. Ahaduzzaman, M. 2021. Contagious caprine pleuropneumonia (CCPP): a systematic review and meta-analysis of the prevalence in sheep and goats. Transbound. Emerg. Dis. 68, 1332–1344. Ahmad, F., Khan, H., Khan, F.A., Carson, B.D., Sadique, U., Ahmad, I., Saeed, M., Rehman, F.U. and Rehman, H.U. 2021. The first isolation and molecular characterization of Mycoplasma capricolum subsp. capripneumoniae Pakistan strain: a causative agent of contagious caprine pleuropneumonia. J. Microbiol. Immunol. Infect. 54, 710–717. Ali, H., El-Neweshy, M., Al Mawly, J., Heller, M., Weber, M. and Schnee, C. 2024. A molecular epidemiological investigation of contagious caprine pleuropneumonia in goats and captive Arabian sand gazelle (Gazella marica) in Oman. BMC Vet. Res. 20, 155. Asmare, K., Abayneh, T., Mekuria, S., Ayelet, G., Sibhat, B., Skjerve, E., Szonyi, B. and Wieland, B. 2016. A meta-analysis of contagious caprine pleuropneumonia (CCPP) in Ethiopia. Acta Trop. 158, 231–239. Bölske, G., Johansson, K.E., Heinonen, R., Panvuga, P.A. and Twinamasiko, E. 1995. Contagious caprine pleuropneumonia in Uganda and isolation of Mycoplasma capricolum subspecies capripneumoniae from goats and sheep. Vet. Rec. 137(23), 594. Braun, U., Flückiger, M., Sicher, D. and Theil, D. 1995. Suppurative pleuropneumonia and a pulmonary abscess in a ram: ultrasonographic and radiographic findings. Schweiz. Arch. Tierheilkd. 137(6), 272–278. Braun, U., Pusterla, N. and Flückiger, M. 1997. Ultrasonographic findings in cattle with pleuropneumonia. Vet. Rec. 141(1), 12–17. Brogi, E., Gargani, L., Bignami, E., Barbariol, F., Marra, A., Forfori, F. and Vetrugno, L. 2017. Thoracic ultrasound for pleural effusion in the intensive care unit: a narrative review from diagnosis to treatment. Crit. Care. 21, 325. Buczinski, S., Tolouei, M., Rezakhani, A. and Tharwat, M. 2013. Echocardiographic measurement of cardiac valvular thickness in healthy cows, cows with bacterial endocarditis, and cows with cardiorespiratory diseases. J. Vet. Cardiol. 15, 253–261. Elhassan, M.A. and Salama, A.O. 2018. Clinical and laboratory diagnosis of contagious caprine pleuropneumonia in Qassim region, Saudi Arabia: a comparative study. Trop. Biomed. 35, 67–75. El-Manakhly, E.M. and Tharwat, M. 2016. Correlation between latex agglutination test positivity and contagious caprine pleuropneumonia chronicity. J. Agric. Vet. Sci. 9, 121–135. Gil-Rodríguez, J., Pérez de Rojas, J., Aranda-Laserna, P., Benavente-Fernández, A., Martos-Ruiz, M., Peregrina-Rivas, J.A. and Guirao-Arrabal, E. 2022. Ultrasound findings of lung ultrasonography in COVID-19: a systematic review. Eur. J. Radiol. 148, 110156. Habibur Rahman, Md., Shahin Alam, Md., Zulfekar Ali, Md., Nurul Haque, Md., Akther, S. and Ahmed, A. 2024. First report of contagious caprine pleuropneumonia (CCPP) in Bangladeshi goats: seroprevalence, risk factors and molecular detection from lung samples. Heliyon 10(23), e40507. Iqbal Yatoo, M., Raffiq Parray, O., Tauseef Bashir, S., Ahmed Bhat, R., Gopalakrishnan, A., Karthik, K., Dhama, K. and Vir Singh, S. 2019. Contagious caprine pleuropneumonia - a comprehensive review. Vet. Q. 39, 1–25. Litamoi, J.K., Wanyangu, S.W. and Simam, P.K. 1990. Isolation of Mycoplasma biotype F38 from sheep in Kenya. Trop. Anim. Health Prod. 22, 260–262. Mayo, P.H., Copetti, R., Feller-Kopman, D., Mathis, G., Maury, E., Mongodi, S., Mojoli, F., Volpicelli, G. and Zanobetti, M. 2019. Thoracic ultrasonography: a narrative review. Intensive Care Med. 45, 1200–1211. Mbyuzi, A.O., Komba, E.V., Kimera, S.I. and Kambarage, D.M. 2014. Sero-prevalence and associated risk factors of peste des petits ruminants and contagious caprine pleuropneumonia in goats and sheep in the Southern Zone of Tanzania. Prev. Vet. Med. 116, 138–144. Mohamed, T., Sato, H., Kurosawa, T. and Oikawa, S. 2002. Echo-guided studies on portal and hepatic blood in cattle. J. Vet. Med. Sci. 64, 23–28. Mohamed, T., Oikawa, S., Nakada, K., Kurwasawa, T., Sawamukai, Y. and Sato, H. 2003a. Percutaneous ultrasound-guided over-the-wire catheterization of the portal and hepatic vessels in cattle. J. Vet. Med. Sci. 65, 813–816. Mohamed, T., Sato, H., Kurosawa, T. and Oikawa, S. 2003b. Transcutaneous ultrasound-guided pancreatic biopsy in cattle and its safety: a preliminary report. Vet. J. 166, 188–193. Mohamed, T., Sato, H., Kurosawa, T. and Oikawa, S. 2003c. Ultrasound-guided biopsy of the pancreas of cattle. Vet. Rec. 153, 467. Mohamed, T. and Oikawa, S. 2007. Ultrasonographic characteristics of abdominal and thoracic abscesses in cattle and buffaloes. J. Vet. Med. A. Physiol. Pathol. Clin. Med. 54, 512–517. Mohamed, T. and Oikawa, S. 2008. Efficacy and safety of ultrasound-guided percutaneous biopsy of the right kidney in cattle. J. Vet. Med. Sci. 70, 175–179. Nicholas, R. and Churchward, C. 2012. Contagious caprine pleuropneumonia: new aspects of an old disease. Transbound. Emerg. Dis. 59, 189–196. Ollivett, T.L. and Buczinski, S. 2016. On-farm use of ultrasonography for bovine respiratory disease. Vet. Clin. North Am. Food Anim. Pract. 32, 19–35. Ramirez, S., Lester, G.D. and Roberts, G.R. 2004. Diagnostic contribution of thoracic ultrasonography in 17 foals with Rhodococcus equi pneumonia. Vet. Radiol. Ultrasound. 45, 172–176. Reimer, J.M., Reef, V.B. and Spencer, P.A. 1989. Ultrasonography as a diagnostic aid in horses with anaerobic bacterial pleuropneumonia and/or pulmonary abscessation: 27 cases (1984-1986). J. Am. Vet. Med. Assoc. 194, 278–282. Renault, V., Hambe, H.A., Van Vlaenderen, G., Timmermans, E., Mohamed, A.M., Ethgen, O. and Saegerman, C. 2019. Economic impact of contagious caprine pleuropneumonia and cost-benefit analysis of the vaccination programmes based on a one-year continuous monitoring of flocks in the arid and semi-arid lands of Kenya. Transbound. Emerg. Dis. 66, 2523–2536. Selim, A., Megahed, A., Kandeel, S. and Alanazi, A.D. 2021. Determination of seroprevalence of contagious caprine pleuropneumonia and associated risk factors in goats and sheep using classification and regression tree. Animals 11, 1165. Shiferaw, G., Tariku, S., Ayelet, G. and Abebe, Z. 2006. Contagious caprine pleuropneumonia and Mannheimia haemolytica-associated acute respiratory disease of goats and sheep in Afar Region, Ethiopia. Rev. Sci. Tech. 25, 1153–1163. Smith, M.J., Hayward, S.A., Innes, S.M. and Miller, A.S.C. 2020. Point-of-care lung ultrasound in patients with COVID-19: a narrative review. Anaesthesia 75, 1096–1104. Sadan, M., Tharwat, M. and El-Deeb, W. 2023. Deep swellings in sheep and goats: clinical, ultrasonographic and post-mortem findings. Int. J. Vet. Sci. 12, 793–801. Teshome, D., Sori, T., Sacchini, F. and Wieland, B. 2019. Epidemiological investigations of contagious caprine pleuropneumonia in selected districts of Borana Zone, Southern Oromia, Ethiopia. Trop. Anim. Health Prod. 51, 703–711. Tharwat, M. and Oikawa, S. 2011. Ultrasonographic evaluation of cattle and buffaloes with respiratory disorders. Trop. Anim. Health Prod. 43, 803–810. Tharwat, M., Al-Sobayil, F., Hashad, M. and Buczinski, S. 2012a. Transabdominal ultrasonographic findings in goats with paratuberculosis. Can. Vet. J. 53, 1063–1070. Tharwat, M., Al-Sobayil, F. and Buczinski, S. 2012b. Ultrasound-guided hepatic and renal biopsy in camels (Camelus dromedarius): technique development and assessment of the safety. Small Rum. Res. 103, 211–219. Tharwat, M., Al-Sobayil, F., Ali, A., Thomas Wittel, T. and Floeck, M. 2012c. Percutaneous ultrasound-guided portocentesis in camels (Camelus dromedarius). J. Camel Pract. Res. 19, 193–196. Tharwat, M., Ali, A., Al-Sobayil, F. and Buczinski, S. 2013. Ultrasound-guided collection of peritoneal fluid in healthy camels (Camelus dromedarius) and its biochemical analysis. Small Rum Res. 113, 307–311. Tharwat, M. and Al-Sobayil, F. 2016. Ultrasonographic findings in camel calves (Camelus dromedarius) with thoracic affections. J. Camel Pract. Res. 23, 287–290. Tharwat, M. and Al-Sobayil, F. 2017. Ultrasonographic findings in goats with contagious caprine pleuropneumonia caused by Mycoplasma capricolum subsp. capripneumoniae. BMC Vet. Res. 13, 263. Tharwat, M. 2021a. Clinical, ultrasonographic, and postmortem findings in sheep and goats with urinary tract disorders. Vet. World. 14, 1879–1887. Tharwat, M. 2021b. Alterations in acid-base balance, blood gases, and hematobiochemical profiles of whole blood and thoracic fluid in goats with contagious caprine pleuropneumonia. Vet. World. 14, 1874–1878. Tharwat, M. 2021c. Ultrasonography of the thorax in healthy and diseased camels (Camelus dromedarius) – a mini-review. J. Camel Pract. Res. 28, 313–318. Tharwat, M. and Al-Hawas, A. 2024a. Liver diseases in sheep and goats: parallel sonographic and pathologic findings. Int. J. Vet. Sci. 13, 284–290. Tharwat, M. and Al-Hawas, A. 2024b. Suppurative pyelonephritis in a caprine buck: clinical, laboratory, ultrasonographic and pathologic findings. Int. J. Vet. Sci. 13, 479–483. Tharwat, M., Hegazy, Y. and Alkheraif, A. 2024. Discolored urine in sheep and goats: clinical, etiological, hematobiochemical, sonographic and postmortem findings. Open Vet. J. 14, 1059–1071. Tharwat, M. and Tsuka, T. 2024. Diagnostic utility of ultrasonography for thoracic and abdominal bacterial and parasitic diseases in ruminants: a comprehensive overview. Front. Vet. Sci. 11, 1435395. Tharwat, M., Alkheraif, A.A. and Marzok, M. 2025a. Pregnancy toxemia in small ruminants: clinical, sonographic, hematobiochemical and pathologic findings. Int. J. Vet. Sci. 14, 204–211. Tharwat, M., Ali, H. and Alkheraif, A.A. 2025b. Paratuberculosis in sheep and goats: pathogenesis, diagnostic findings, and control strategies. Open Vet. J. 15, 1–7. Tharwat, M., Elmoghazy, H.M.M., Saeed, E.M.A. and Alkheraif, A.A. 2025c. Renal abscessation in dromedary camels: clinical, ultrasonographic, hematobiochemical, and etiological investigations. Open Vet. J. 15, 1289–1303. US Department of Health and Human Services. 1996. Guide for the care and use of laboratory animals; NIH Publication No. 86-23, Revised 1996. Washington, DC: Public Health Services, National Institutes of Health. WOAH. 2009. Contagious caprine pleuropneumonia. Available via https://www.woah.org/app/uploads/2021/03/contagious-caprine-pleuro.pdf (Accessed 26 March 2025). | ||
| How to Cite this Article |
| Pubmed Style Tharwat M, Alkheraif AA, Ali H. Clinical cases of contagious caprine pleuropneumonia in sheep: Retrospective clinical, sonographic, and pathological investigations. Open Vet. J.. 2025; 15(5): 1947-1957. doi:10.5455/OVJ.2025.v15.i5.9 Web Style Tharwat M, Alkheraif AA, Ali H. Clinical cases of contagious caprine pleuropneumonia in sheep: Retrospective clinical, sonographic, and pathological investigations. https://www.openveterinaryjournal.com/?mno=231729 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i5.9 AMA (American Medical Association) Style Tharwat M, Alkheraif AA, Ali H. Clinical cases of contagious caprine pleuropneumonia in sheep: Retrospective clinical, sonographic, and pathological investigations. Open Vet. J.. 2025; 15(5): 1947-1957. doi:10.5455/OVJ.2025.v15.i5.9 Vancouver/ICMJE Style Tharwat M, Alkheraif AA, Ali H. Clinical cases of contagious caprine pleuropneumonia in sheep: Retrospective clinical, sonographic, and pathological investigations. Open Vet. J.. (2025), [cited January 25, 2026]; 15(5): 1947-1957. doi:10.5455/OVJ.2025.v15.i5.9 Harvard Style Tharwat, M., Alkheraif, . A. A. & Ali, . H. (2025) Clinical cases of contagious caprine pleuropneumonia in sheep: Retrospective clinical, sonographic, and pathological investigations. Open Vet. J., 15 (5), 1947-1957. doi:10.5455/OVJ.2025.v15.i5.9 Turabian Style Tharwat, Mohamed, Abdulrahman A. Alkheraif, and Haytham Ali. 2025. Clinical cases of contagious caprine pleuropneumonia in sheep: Retrospective clinical, sonographic, and pathological investigations. Open Veterinary Journal, 15 (5), 1947-1957. doi:10.5455/OVJ.2025.v15.i5.9 Chicago Style Tharwat, Mohamed, Abdulrahman A. Alkheraif, and Haytham Ali. "Clinical cases of contagious caprine pleuropneumonia in sheep: Retrospective clinical, sonographic, and pathological investigations." Open Veterinary Journal 15 (2025), 1947-1957. doi:10.5455/OVJ.2025.v15.i5.9 MLA (The Modern Language Association) Style Tharwat, Mohamed, Abdulrahman A. Alkheraif, and Haytham Ali. "Clinical cases of contagious caprine pleuropneumonia in sheep: Retrospective clinical, sonographic, and pathological investigations." Open Veterinary Journal 15.5 (2025), 1947-1957. Print. doi:10.5455/OVJ.2025.v15.i5.9 APA (American Psychological Association) Style Tharwat, M., Alkheraif, . A. A. & Ali, . H. (2025) Clinical cases of contagious caprine pleuropneumonia in sheep: Retrospective clinical, sonographic, and pathological investigations. Open Veterinary Journal, 15 (5), 1947-1957. doi:10.5455/OVJ.2025.v15.i5.9 |