| Research Article | ||
Open Vet. J.. 2025; 15(4): 1593-1598 Open Veterinary Journal, (2025), Vol. 15(4): 1593-1598 Research Article Investigation of clinical and genetic study of bacteria during estrus, pregnancy, and post-partum in cowsHayder Abdul-Kareem AL-Mutar*Department of Surgery and Obstetrics, College of Veterinary Medicine, University of Baghdad, Baghdad, Iraq *Corresponding Author: Hayder Abdul-Kareem AL-Mutar. Department of Surgery and Obstetrics, College of Veterinary Medicine, University of Baghdad, Baghdad, Iraq. Email: al_mutar.haydar [at] covm.uobaghdad.edu.iq Submitted: 13/11/2024 Accepted: 09/03/2025 Published: 30/04/2025 © 2025 Open Veterinary Journal
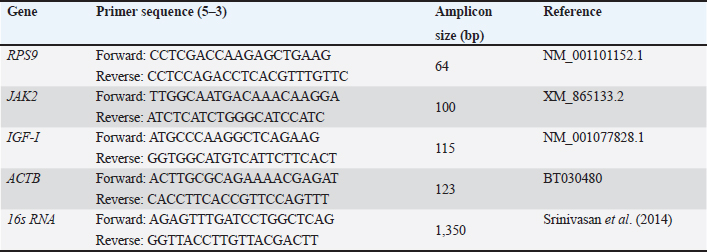
AbstractBackground: Cattle have a high potential for meat and calf production due to their flexibility and high-quality reproduction. Bacterial illnesses, on the other hand, pose a hazard to optimal reproduction. Aim: This study aimed to identify the bacterial type that colonized in the vagina during estrus, pregnancy, and post-partum. Methods: Using normal culture testing, a study was carried out with the secretions of the vagina. Healthy pregnant cross-breed cattle from the farm in order to detect the vaginal microbes. The study included 44 crossbred cows who were 3, 6, and 9 months pregnant, as well as six of each regular cycle (proestrus, estrus, metestrus, and diestrus). Using a syringe and pipette, aseptic cervicovaginal mucus/discharge samples were obtained during pregnancy, non-pregnancy, and postpartum. Gram’s staining and biochemical tests were used to identify the samples after they had been managed for culture isolation on blood agar and MacConkey agar, the time of the experiment is 1 year. Results: All 44 vaginal swabs (100%) from cattle with different physiopathological conditions contained bacteria. During pregnancy, in descending order, were Escherichia coli, Klebsiella pneumonia, Pseudomonas, Bacillus sp., Corynebacterium sp., Salmonella, and Micrococcus. The results showed that the vagina of crossbred cattle with different physiological circumstances had a vast diversity of bacteria. Conclusion: Consequently, it is concluded that the vaginal cavity of pregnant cows demonstrates bacterial isolate dynamics influenced by endocrine conditions, highlighting its importance in the reproductive physiopathology of crossbred cattle. Keywords: Bacteria, Cattle, Pregnancy rate, Vagina Swab. IntroductionSeveral genital tract pathogenic organisms, either specific or general, reduce a cow’s fertility. This is owing to cattle’s adaptability and good reproductive quality, which make them suitable for meat and calf production (Suranjaya et al., 2010). Cervical mucus discharge creates a mechanical barrier against bacteria in the uterus. Farmers and inseminators believe that among the characteristics that inhibit reproductive performance is the discharge of cervical mucus from cows and heifers with irregular estrous cycles (Mahmoudzadeh et al., 2001). Depending on many researches (Fernandez et al., 2006), anaerobic and aerobic bacteria form the majority of the typical vaginal microbiota. Little amounts of Escherichia coli have been found in the urogenital tracts of calves (Otero et al., 2000). However, it is well known that E. coli causes cattle to become infertile (Sheldon et al., 2002). During in vitro fertilization and culture, oocytes can develop into morula-stage embryos and is decreased by uterine infection, suggesting that oocyte ability is affected both during and after infection (Dickson et al., 2020). The endometrium may also contribute to the subfertility seen in cattle following uterine health problems, as it is important to note that transplanting embryos from healthy donors to recipients who have previously experienced uterine infection does not heal uterine disease-related pregnancy loss. (Ribeiro et al., 2016; Estrada-Corte et al., 2019; Edelhoff et al., 2020; Rahawy and AL-Mutar, 2021) demonstrated that when embryos are given to recipients after seven days of pregnancy, further pregnancy loss takes place. Pregnancy-related glycoproteins are a broad family of inactive aspartic proteinases expressed in the placenta found in domestic animal species, such as goats, sheep, and cows (Haugejorden et al., 2006). The purpose of the current study is to determine the typical bacterial flora found in pregnant cows’ vaginal discharges and aspirates. Materials and MethodsHousing for the animalsThe study looked at the vaginal aspiration of infertile pregnant crossbred cows in Bagdad’s fields. There were 44 crossbred cows in total, 10 for each regular cycle, and 3, 6, and 9 months pregnant. Clinical evaluation of the cattle during birth, every animal underwent a routine gynecological examination. Non-pregnant cattle were gynecologically examined 90–120 days following their last birth, which included a rectal examination and a vaginal examination. The type of vaginal discharge was identified through a vaginal examination. As previously stated, a flashlight was used to visually inspect the cervix and vagina for any signs and discharge quality (Leutert et al., 2012). To describe vaginal mucous, Williams et al. (2005) developed a 4-point scoring structure: 1 for pure mucus, 2 for mucus with pus flecks, 3 for discharge with fewer than 50% pus, and 4 for discharge with above 50% pus. This method was employed in recent studies (Sheldon et al., 2006; Dubuc et al., 2010; Kaufmann et al., 2010). Clear mucus, or grade 1, was thought to be normal vaginal results, while grades 2–4 were thought to be abnormal within an hour after collection (Šavc et al., 2016), the 42 vaginal samples were prepared for cultural isolation (Kaufmann et al., 2010). Using routine vaginal sample culture on blood-agar and MacConkey-agar plates, the study intended to identify vaginal bacteria in cattle under a range of physio-pathological conditions (Cruickshank, 1965). Using Gram’s staining and biochemical tests including oxidase, potassium hydroxide, and catalase, the isolates were thoroughly identified. Ethical approvalAll the protocols for experiments have been accepted by the Ethics Committee for Animal Studies at the University of Baghdad College of Veterinary Medicine. Table 1. Oligonucleotide primer sequence information used for real-time quantitative polymerase chain reaction (PCR) and PCR assays.
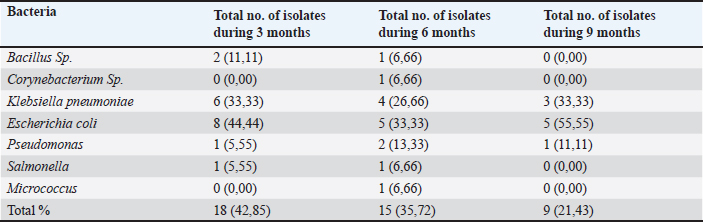
Table 2. Cultural isolates from vaginal aspirates of crossbred cows during different stages of pregnancy.
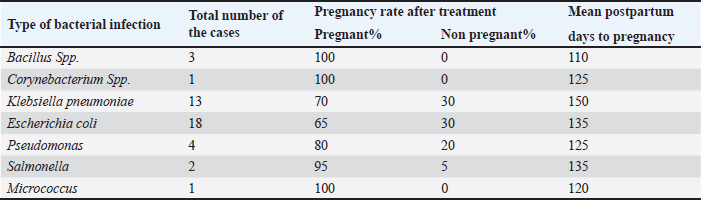
Table 3. Dairy cow fertility measurements (pregnancy rate and days open) associated with various types of bacterial infections.
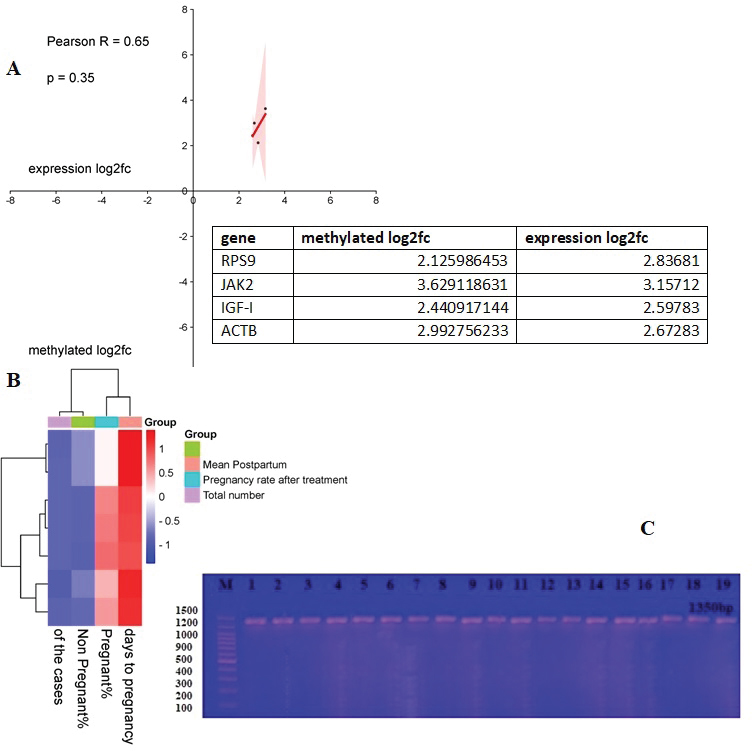
Validation of the gene-specific m6A qPCRUsing reverse transcription (RT)-qPCR, five genes with differentially methylated sites identified by m6A-seq were investigated. A sample of fragmented messenger RNA was retained as an input control. Beads coated with anti-m6A antibodies were combined with the resting RNA. Following immunoprecipitation, the m6A-containing RNAs emerged from beads. RT-qPCR was performed on the m6A-IP and input control samples using specific genes of primers. The specific genes of qPCR analysis primers are listed below (Table 1). Analysis of statisticsThe acquired data were statistically examined. The results were analyzed statistically using Statistical Package for the Social Sciences. The results of the reproductive groups and the types of bacteriological illnesses were evaluated using an analysis of variance. A p value < 0.05 indicated statistical significance. ResultsBacterial isolates from vagina of pregnant cowsBacillus Spp. (11,11%), Klebsiella (33,33%), Salmonella and Pseudomonas (5,55% each), and Proteus (4.55%) were the bacteria isolated from vaginal aspirates of 3-month-pregnant cows, accounting for 42.85% of the total bacterium isolates. Another 35.72 percent of the bacteria identified were from 6-month-old pregnant cows, with Bacillus Spp., Corynebacterium (6.66% each), Klebsiella pneumonia (26.66%), E. coli (33.33%), Pseudomonas (13.33%), Salmonella, and Micrococcus (6.66%). Furthermore, the vagina of nine-month pregnant cattle was displayed. Klebsiella pneumonia (33.33%), E. coli (55.55%), and Pseudomonas (11.11%), accounting for 21.43 percent of the total bacterial isolates (Tables 2 and 3). The pregnancy rate was elevated in instances of infections including Bacillus spp., Corynebacterium spp., and Micrococcus, reaching 100%. On the other hand, in combined infections with E. coli and Klebsiella, this rate decreased to moderate levels, at 50% and 70%, respectively. The pregnancy prevalence was higher than average in cases of combined infection with Pseudomonas (80%) besides Salmonella (95%). The current study discovered that some bacteria, such as those found in combination infections with E. coli and Staphylococcus, as well as Klebsiella and E. coli, function synergistically to promote pregnancy. Conjoint analysis of m6A-RNA binding protein immunoprecipitation (RIP)-seq and RNA-seq data of samplesCross-analysis of m6A-Seq and RNA-Seq data revealed a strong connection between differentially methylated m6A peaks and gene expression levels among pregnant and non-pregnant samples (Fig. 1a and b). Among 210 elevated m6A sites revealed by m6A-Seq, we identified 10 targets with up-regulated Messenger RNA (mRNA) a different version of the gene (fold change > 2, p < 0.05), known as “hyper-up”. We found that four genes had “hyper-down” (fold change > 2, p < 0.05) Messenger RNA transcripts and hyper-methylated m6A sites. In contrast, 11 out of 359 genes exhibited down-regulated Messenger RNA a different version of the gene (fold change > 2, p < 0.05), referred to as “hypo-down,” while four genes with hypo-methylated m6A sites had up-regulated Messenger RNA a different version of the gene (fold change > 2, p < 0.05). With relation to the overall number of cases in cattle, both pregnant and not. A heat map of genes with differential expression is displayed in Figure 1C. Submission of local Iraq isolate in National Center for Biotechnology Information (NCBI)Following interactions with the NCBI, the bacteria supplied during cow pregnancy utilizing the 16S ribosomal RNA gene were registered, assigned an accession number, and established themselves as a global reference in Iraq and the Middle East. Ongoing work will contribute to this set when more type strains are published; it is accessible for download from ID: PP907044.1 to ID: PP907057.1. DiscussionEl-Kader and Shehata (2001) and Jadon et al. (2005) reported 92% and 89% of antibiotic-resistant bacteria, samples were positive in pregnant cattle, respectively. Likewise, El-Jakee et al. (2008) found a decrease in the incidence of vaginal isolates as gestation continued. They also discovered more isolates in the early stage (42%), compared to the late stage (16%). According to Patel et al. (2019), proliferating bacteria are typically seen in a cow’s vaginal region. The microbial flora of a healthy cow’s reproductive system includes aerobic microorganisms, facultative anaerobes, and obligatory anaerobes. Corynebacterium is found in tiny numbers in bovine vaginal microbiota; however, E. coli is among the most common (Otero et al., 2000). El-Kader and Shehata (2001) identified E. coli as the most prevalent bacterial isolate from the vaginal tracts of pregnant heifers, then followed by untypable E. coli, Bacillus species, and Klebsiella oxytoca. Similarly, Jadon et al. (2005) reported finding E. coli (18%), Klebsiella species (6%), Staphylococcus (13%), and Bacillus species (9%) in the vaginal tracts of pregnant cows. In our research, we were able to isolate bacteria that aligned with previous studies (Jadon et al., 2005). We found Klebsiella species in 43% of the samples, E. coli in 29%, and Streptococcus species besides Bacillus species in 14% of the samples. These findings reflect those reported in multiple other investigations. The results of DNA extraction from all vaginal samples, along with metagenomic analysis at the phyla, genera, and species levels, were independently reported using the NCBI library (Patel, 2018). The percentage frequency of various isolates collected from several samples in this research was calculated and published as well. These results are similar to those indicated by Singh et al. (2008). It contains some bacteria. According to the current study, cows with corpus luteum on their ovaries have a greater pregnancy rate and fewer days open than cows with an ovarian cyst or no palpable defects. The results of Bonnett et al. (1993), who showed that cows with an early ovarian resumption during the postpartum phase at 26 days in milk had a 10% increase in pregnancy, are somewhat in line with these findings. Cystic ovarian disease may result from elevated PGF2α and cortisol levels caused by a postpartum uterine infection (Etherington et al., 1985). This is also supported by the current study’s findings, which show that some pregnant cows (21% of the cows) have abnormalities on their ovaries while the rest have CLs (Bosu and Peter, 1987).
Fig. 1. Study of m6A-RIP-seq and RNA-seq data together. (A) Total m6A methylation and mRNA expression level are positively correlated (Pearson r=0.1802; p < 0.0001), according to a dot chart of Log2 fold change (FC) (mRNA expression) against Log2 FC (differential m6A methylation). (B) A heat map of the genes shown in (A). (C) The band size of the PCR result. Electrophoresis on 1.5% agarose at 5 volts/cm2 was the final result. 1 × TBE buffer for one and a half hours. M: ladder of DNA (100). It has been demonstrated that m6A methylation, the most common internal modification in eukaryotic mRNAs, is crucial for both healthy pregnant and non-pregnant cows (Fu et al., 2014). Nonetheless, the transcriptome-wide patterns of m6A modification in most types of pregnant dairy cows remain unknown, as does the specific role of anomalous m6A modifications in non-pregnant cow pathophysiology (AL-Mutar, 2017; AL-Mutar et al., 2018). In this investigation, we demonstrated global m6A modification patterns in ccRCC samples versus pregnancy, examining gene expression and non-pregnant pathways affected by aberrant m6A RNA modifications (Laith et al., 2018). ConclusionIt is, therefore, concluded that the vaginal cavity of pregnant cows exhibits bacterial isolate dynamics based on endocrine condition, indicating its significance in the physiopathology of reproduction in crossbred cattle. The number of germs that can be isolated from pregnant cows reduces as gestational age increases. Presented the first m6A transcriptome-wide map of a pregnant cow. AcknowledgmentThis study is supported by the College of Veterinary Medicine, University of Baghdad. Conflict of interestThere are no conflicts of interest regarding the publication of this manuscript. Novelty statementThis study introduces an innovative approach to the clinical besides genetic analysis of bacteria during pregnancy of cows and their relationship with prolificacy in improving reproductive efficiency among cows. Authors’ contributionsThis study was designed and written by the author. ReferencesAL-Mutar, H.A. 2017. Investigation the polymorphism of gonadotropin releasing hormone receptor gene in Iraq sheep. Iraqi J. Vet. Med. 41(1), 138–144. Al-Mutar, H.A.K., Younis, H.L. and Khawla, H. 2018. Effect of the point mutation in growth differentiation factor 9 gene in Awassi sheep oocytes on sterility and fertility. J. Pure Appl. Microbiol. 12(4), 2095–2102; doi:10.22207/JPAM.12.4.46 Bonnett, B.N., Martin, S.W. and Meek, A.H. 1993. Associations of clinical findings, bacteriological and histological results of endometrial biopsy with reproductive performance of postpartum dairy cows. Prevent. Vet. Med. 15(2-3), 205–220; doi:10.1016/0167-5877(93)90114-9 Bosu, W. and Peter, A.T. 1987. Evidence for a role of intrauterine infections in the pathogenesis of cystic ovaries in postpartum dairy cows. Theriogenology 28(5), 725–736; doi:10.1016/0093-691X(87)90289-5 Cruickshank, R. 1965. Medical microbiology. 11th ed. The English Language Book Society and E and S Livingstone Ltd., Great Britain. Dickson, M.J., Piersanti, R.L., Ramirez-Hernandez, R., de Oliveira, E.B., Bishop, J.V. and Hansen, T.R. 2020. Experimentally induced endometritis impairs the developmental capacity of bovine oocytes. Biol. Reprod. 103, 508–520; doi:10.1093/biolre/ioaa069 Dubuc, J., Duffield, T.F., Leslie., K.E., Walton, J.S. and LeBlanc., S.J. 2010. Risk factors for postpartum uterine diseases in dairy cows. J. Dairy Sci. 93, 5764–5771; doi:10.3168/jds.2010-3429 Edelhoff, I.N.F., Pereira, M.H.C., Bromfield, J.J., Vasconcelos, J.L.M. and Santos, J.E.P. 2020. Inflammatory diseases in dairy cows: risk factors and associations with pregnancy after embryo transfer. J. Dairy Sci. 103, 11970–11987; doi:10.3168/jds.2020-1907 El-Jakee, J.A., Ahmed, W.M., El-Seedy, F.R. and El-Moez, S.A. 2008. Bacterial profile of the genital tract in female buffaloes during different reproductive stages. Global Vet. 2(1), 7–14. El-Kader, H.A. and Shehata, S.H. 2001. Bacteriological evaluation of vaginal discharges in cows with endometritis and clinically healthy heifers in Assiut governorate. Assiut Univ. Bull. Environ. Res, 4(2), 45-53. Estrada-Corte E, Ortiz, W.G., Chebel, R.C., Jannaman, E.A., Moss, J.I. and De Castro, F.C., 2019. Embryo and cow factors affecting pregnancy per embryo transfer for multiple-service, lactating Holstein recipients. Transl. Anim. Sci. 3, 60–65; doi:10.1093/tas/txz009 Etherington, W., Martin, S., Dohoo, I.R. and Bosu, W. 1985. Interrelationships between postpartum events, hormonal therapy, reproductive abnormalities and reproductive performance in dairy cows: a path analysis. Canadian J. Comparat. Med. 49(3), 261. Fernãndez, M.A., Silveira, P., Enrique, A., Lãpez, R. and Omar, F. 2006. Uterine infections in bovine female. Revista Electrãnica de Veterinaria Redvet® 7, 1695–7504. Available via http://www.veterinaria.org. Fu, Y., Dominissini, D., Rechavi, G. and He, C. 2014. Gene expression regulation mediated through reversible m(6)A RNA methylation. Nat. Rev. Genet. 15(5), 293–306. Haugejorden, G., Waage, S. Dahl, E., Karlbert, K., Beckers, J.F. and Ropstad, E. 2006. Pregnancy associated glycoproteins (PAG) in postpartum cows, ewes, goats and their offspring. Theriogenology 66, 1976–1984. Jadon, R.S. and Dhaliwal, G.S. 2005. Prevalence of aerobic and anaerobic uterine bacteria during peripartum period in normal and dystocia-affected buffaloes. Anim. Reprod. Sci. 88(3–4), 215–224. Kaufmann, T.B., Westermann, S., Drillich, M., Plontzke, J. and Heuwieser, W. 2010. Systemic antibiotic treatment of clinical endometritis in dairy cows with ceftiofur or two doses of cloprostenol in a 14-d interval. Anim. Reprod. Sci. 121, 55–62; doi:10.1016/ j.anireprosci.2010.04.190 Laith, S.Y., Al-Mutar, H.A.K. and Ali, A.A. 2018. Effect of lptin gene polymorphism on reproductive efficiency in Awassi ewes. Adv. Anim. Vet. Sci. 7(1), 18–23; doi:10.17582/journal.aavs/2019/7.1.17.23 Leutert, C., Von Krueger, X., Plontzke, J. and Heuwieser, W. 2012. Evaluation of vaginoscopy for the diagnosis of clinical endometritis in dairy cows. J. Dairy Sci. 95(1), 206–212; doi:10.3168/jds.2011-4603 Mahmoudzadeh, A.R., Tarahomi, M. and Fotoohi, H. 2001. Effect of abnormal vaginal discharge at estrus on conception rate after artificial insemination in cows. Anim. Sci. 72(3), 535–538. Otero, C., Saavedra, L., Silva De Ruiz, C., Wilde, O., Holgado, A.R. and Nader-Macías, M.E. 2000. Vaginal bacterial microflora modifications during the growth of healthy cows. Lett. Appl. Microbiol. 31, 251–254; doi:10.1046/j.1365-2672.2000.00809.x Panangala, V.S., Fish, N.A. and Barnum, D.A. 1978. Microflora of the cervico-vaginal mucus of repeat breeder cows. Canadian Vet. J. 19(4), 83. Patel, C.I., Panchal, M.T., Dhami, A.J., Bhanderi, B.B. and Mathakiya, R.A. 2019. Isolation of bacteria from the vaginal aspirates of cyclic, acyclic, endometritic and pregnant crossbred cows. Int. J. Curr. Microbiol. App. Sci. 8(3), 536–542. Patel, C.I. 2018. Study on reproductive microbiota in cyclic, acyclic and endometritic crossbred cattle. M.V.Sc. Thesis, Anand Agricultural University, Anand, Gujarat, India. Rahawy, M.A. and AL-Mutar, H.A.K. 2021. Association of the KiSS1gene with littersize in Cyprus and Iraqi black goats. Vet. World 14(8), 1995–2001; doi:10.14202/vetworld.2021.1995-2001 Ribeiro, E.S., Gomes, G., Greco, L.F., Cerri, R.L.A., Vieira-Neto, A. and Monteiro, P.L.J. 2016. Carryover effect of postpartum inflammatory diseases on developmental biology and fertility in lactating dairy cows. J. Dairy Sci. 99, 2201–2220; doi:10.3168/jds.2015-10337 Šavc, M., Duane, M., O’Grady, L.E., Somers, J.R. and Beltman, M.E. 2016. Uterine disease and its effect on subsequent reproductive performance of dairy cattle: a comparison of two cow-side diagnostic methods. Theriogenology 86(8), 1983–1988; doi:10.1016/ j.theriogenology.2016.06.018 Sheldon, I.M., Lewis, G.S., LeBlanc, S. and Gilbert, R.O. 2006. Defining postpartum uterine disease in cattle. Theriogenology 65(8):1516–1530Srinivasan, R., Karaoz, U., Volegova, M., MacKichan, J., Kato-Maeda, M., Miller, S., Nadarajan, R., Brodie, E.L. and Lynch, S.V. 2014. Use of 16S rRNA Gene for identification of a broad range of clinically relevant bacterial pathogens. PLoS One 10(2), e0117617; doi:10.1371/journal.pone.0117617.(1-22) Sheldon, I., Noakes, D. and Rycroft, A. 2002. The vagina on uterine bacterial contamination. Vet. Rec. 151, 531–534. Singh, J., Murray, R., Mshelia, G. and Woldehiwet, Z. 2008. The immune status of the bovine uterus during the peripartum period. Vet. J. 175(3), 301–309; doi:10.1016/j.tvjl.2007.02.003 Srinivasan, R., Karaoz, U., Volegova, M., MacKichan, J., Kato-Maeda, M., Miller, S., Nadarajan, R., Brodie, E.L. and Lynch, S.V. 2014. Use of 16S rRNA Gene for identification of a broad range of clinically relevant bacterial pathogens. PLoS One 10(2), e0117617; doi:10.1371/journal.pone.0117617.(1-22) Suranjaya, I.G., Ardika, I.N. and Indrawati, R.R. 2010.Faktor-Faktor yang Mempengaruhi Produktivitas Sapi Bali di Wilayah Binaan Proyek Pembibitan dan Pengembangan Sapi Bali di Bali. Majalah Ilmiah Peternakan 13(3), 83–87. Williams, E.J., Fischer, D.P., Pfeiffer, D.U., England, G.C., Noakes, D.E., Dobson, H. and Sheldon, I.M. 2005. Clinical evaluation of postpartum vaginal mucus reflects uterine bacterial infection and the immune response in cattle. Theriogenology 63(1), 102–117; doi:10.1016/j.Theriogenology.2004.03.017 | ||
| How to Cite this Article |
| Pubmed Style Hayder Abdul-Kareem AL-Mutar. Investigation of clinical and genetic study of bacteria during estrus, pregnancy, and post-partum in cows. Open Vet. J.. 2025; 15(4): 1593-1598. doi:10.5455/OVJ.2025.v15.i4.10 Web Style Hayder Abdul-Kareem AL-Mutar. Investigation of clinical and genetic study of bacteria during estrus, pregnancy, and post-partum in cows. https://www.openveterinaryjournal.com/?mno=228557 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i4.10 AMA (American Medical Association) Style Hayder Abdul-Kareem AL-Mutar. Investigation of clinical and genetic study of bacteria during estrus, pregnancy, and post-partum in cows. Open Vet. J.. 2025; 15(4): 1593-1598. doi:10.5455/OVJ.2025.v15.i4.10 Vancouver/ICMJE Style Hayder Abdul-Kareem AL-Mutar. Investigation of clinical and genetic study of bacteria during estrus, pregnancy, and post-partum in cows. Open Vet. J.. (2025), [cited January 25, 2026]; 15(4): 1593-1598. doi:10.5455/OVJ.2025.v15.i4.10 Harvard Style Hayder Abdul-Kareem AL-Mutar (2025) Investigation of clinical and genetic study of bacteria during estrus, pregnancy, and post-partum in cows. Open Vet. J., 15 (4), 1593-1598. doi:10.5455/OVJ.2025.v15.i4.10 Turabian Style Hayder Abdul-Kareem AL-Mutar. 2025. Investigation of clinical and genetic study of bacteria during estrus, pregnancy, and post-partum in cows. Open Veterinary Journal, 15 (4), 1593-1598. doi:10.5455/OVJ.2025.v15.i4.10 Chicago Style Hayder Abdul-Kareem AL-Mutar. "Investigation of clinical and genetic study of bacteria during estrus, pregnancy, and post-partum in cows." Open Veterinary Journal 15 (2025), 1593-1598. doi:10.5455/OVJ.2025.v15.i4.10 MLA (The Modern Language Association) Style Hayder Abdul-Kareem AL-Mutar. "Investigation of clinical and genetic study of bacteria during estrus, pregnancy, and post-partum in cows." Open Veterinary Journal 15.4 (2025), 1593-1598. Print. doi:10.5455/OVJ.2025.v15.i4.10 APA (American Psychological Association) Style Hayder Abdul-Kareem AL-Mutar (2025) Investigation of clinical and genetic study of bacteria during estrus, pregnancy, and post-partum in cows. Open Veterinary Journal, 15 (4), 1593-1598. doi:10.5455/OVJ.2025.v15.i4.10 |