| Research Article | ||
Open Vet. J.. 2025; 15(5): 1934-1940 Open Veterinary Journal, (2025), Vol. 15(5): 1934-1940 Original Research Oral microflora of Trimeresurus snakes in a biodiversity hotspot of North Eastern IndiaLallianpuii Kawlni1*, Lalchhandama. C2, Lalhruaipuii3 and Tapan Kumar Dutta,31Department of Wildlife Health Management, Wildlife Institute of India, Dehradun, India 2Department of Veterinary Pharmacology and Toxicology, College of Veterinary Sciences and Animal Husbandry, Selesih, Central Agricultural University, Imphal, India 3Department of Veterinary Microbiology, College of Veterinary Sciences and Animal Husbandry, Selesih, Central Agricultural University, Imphal, India *Corresponding Author: Lallianpuii Kawlni. Department of Wildlife Health Management, Wildlife Institute of India, Dehradun, India. Email: kawlni.lalani [at] gmail.com; lallian [at] wii.gov.in Submitted: 29/06/2023 Revised: 21/01/2025 Accepted: 25/04/2025 Published: 31/05/2025 © 2025 Open Veterinary Journal
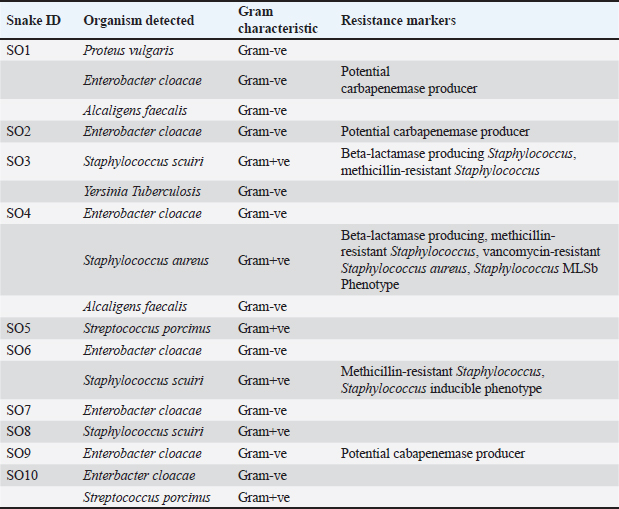
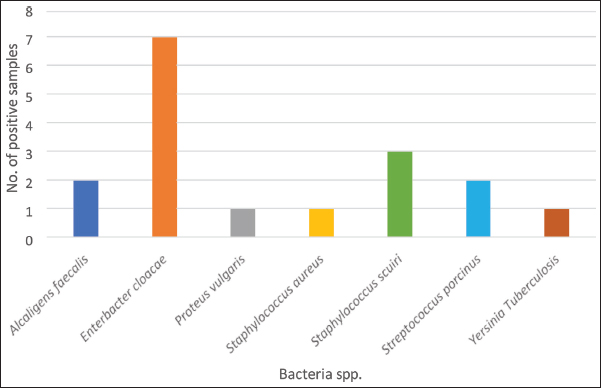
AbstractBackground: A significant number of snake bites are reported every year from Mizoram, the majority of which are considered to be Trimeresurus erythrurus. The occurrence of deaths from deadly venomous to mildly venomous snake bites has also been reflected in records. Even if the bites are not fatal, they cause a significant problem as they are often associated with secondary bacterial infection. This is believed to significantly contribute to complications in addition to the effect of venom. Aim: Here, we attempt to capture aerobic bacterial diversity from the oro-pharyngeal swabs of wild displaced snakes through culture and automated bacterial identification system. Methods: Oro-pharyngeal swabs were collected from 10 snakes (Trimeresurus erythrurus). The swabs were cultured using standard microbiological techniques, and bacterial isolates were identified with BD PHOENIX automated microbiology system (Becton Dickinson Diagnostic Systems, Sparks, Md). Results: The assay showed the presence of Enterobacter cloacae, Staphylococcus sciuri, Staphylococcus aureus, Streptococcus porcinus, Alcaligens faecalis, Yersinia pseudotuberculosis, and Proteus vulgaris in the oral swab culture. Conclusion: The detection of various pathogenic bacteria in the oral swab culture highlights the infectious potential, emphasizes their significance in the management of snakebite wounds, and affirms the importance of administering antibiotic prophylaxis in snakebites. Keywords: Oral microflora, Snakes, Mizoram, Snake bite. IntroductionMizoram harbours almost 50% of the species of snakes recorded from North East India. These species are fairly distributed throughout the state and out of 49 species of snakes recorded, 10 species are reported to be deadly venomous (Lalremsanga et al., 2018). Snake bite is not uncommon in Mizoram, and deaths due to snake bite from both deadly venomous to mildly venomous snakes are not unheard of. Green pit vipers are a large group of venomous snakes, which is responsible for most of the snake bites across the humid and areas of tropical and subtropical Asia (Thakur et al., 2022), and among these vipers, Trimeresurus erythrurus is considered to be responsible for a major portion of the snake bite cases in Mizoram. Snake bite are often associated with secondary infection, which may vary from mild to heavy septic wounds, local necrosis, and gangrene, which may be attributed to the effects of both venom and the oral microflora harboured by the snake (Leon et al., 2017) since secondary bacterial infection are common complications to animal bite wounds (Garg et al., 2009; Zancolli et al., 2015). Certain ecological attributes of the snake such as being a reptilian ground dweller are considered to directly affect its oral microbiota as it interacts directly with the variety of pathogens and zoonosis that the soil hosts. The bacterial flora present in the prey, along with the fecal microbiome, is considered to have a direct impact on the oral microflora of snakes and is transmitted to the bite wound during envenomation when the skin is punctured (Ledbeeter et al., 1969). Here, we report the aerobic bacteria present in the oral cavity of green pit viper in Aizawl district of Mizoram. Though envenomation by viperid snakes may not be immediately life threatening, local tissue damages such as blistering, swelling, haemorrhage, and necrosis of skeletal muscles are the local damages inflicted by envenomation by viperid snakes, and these can often be exacerbated due to mismanagement of bite wounds, which might even lead to septicaemia and death in some cases (Waiddyanatha et al., 2019). Despite the reports of snake venom being antimicrobial and sterile (Al-Asmari et al., 2015; Bocian and and Hus 2020), the presence of bacteria in venom has been reported in various studies (Goldstein et al., 1979; Esmaeilishirazifard et al., 2022). All these factors contribute toward snakes as strong candidates for bacterial infection through bites. Materials and MethodsTen snakes that have been displaced in unsuitable human habituated areas and retrieved from these conflict situations were sampled for oropharyngeal swabs during routine physical examination of the rescue protocol. All the snakes were rescued within Aizawl district of Mizoram (Fig. 2). The snakes are wild and have not been fed or kept by humans. The materials used for the collection of samples and culturing-sterile cotton-tipped swabs, bacteriological media, and antibiotic discs were procured from Hi-Media Laboratories, Mumbai, India. The snakes were physically restrained, and oropharyngeal swabs were collected by gently swabbing the mouth cavity and inner gum line of each snake with sterile cotton-tipped swabs. The swabs were immediately transferred to cold storage at 4°C and transported to the Department of Veterinary Microbiology, College of Veterinary Sciences and Animal Husbandry, Selesih, and processed for identification using the BD PHOENIX automated microbiology system (Becton Dickinson Diagnostic Systems, Sparks, Md). The swabs were transferred to Brain Heart Infusion Broth for enrichment under aerobic conditions at 37°C for 18 hours in an incubator. After incubation, a loop full of each enriched broth was cultured aseptically by the streak plate method on a BHI agar plate and incubated at 37°C for 18 hours. All morphologically distinct colonies were identified and selected to be cultured by the plate streaking method and incubated at 37°C for 18 hours. Gram staining was done, which is a prerequisite for the BD PHOENIX automated microbiology system, after which, a suspension was made for each isolate, maintaining the turbidity of the culture broth as per the protocol given by the BD PHOENIX automated microbiology system. The BD Phoenix automated microbiology system was used for the identification and confirmation of isolates and the reading of resistance markers. Ethical approvalThe sample collection for this study was carried out while doing physical examination on snakes retrieved/rescued from conflict situations as standard protocol and collected by the authors, who are certified veterinarians and have expertise in snake handling. ResultsAll the swabs that were subjected to the aerobic culturing protocol were positive for bacterial growth. From the oro-pharyngeal swabs of 10 snakes, 7 species of bacteria were identified by the BD Phoenix automated bacterial identification system. Enterobacter cloacae (70%) was the most common species isolated, followed by Staphylococcus sciuri (30%), Streptococcus porcinus (20%), Alcaligens faecalis (20%), Staphylococcus aureus (10%), Yersinia pseudotuberculosis (10%), and Proteus vulgaris (10%) were also isolated from the oropharyngeal swabs of the snakes. Resistance markers that were identified by the system were potential carbapenemase producer, Beta-lactamase producing Staphylococcus, methicillin-resistant Staphylococcus, vancomycin-resistant S. aureus, Staphylococcus MLSb Phenotype, and Staphylococcus inducible phenotype (Table 1; Fig. 1). Among these aerobic bacteria that were isolated from 10 Trimeresurus vipers from Mizoram, all have clinical significance; however, not all are recorded to have been recorded in wound culture. DiscussionOro-pharyngeal swabs collected from 10 rescued Green pit vipers from Aizawl district were subjected to culturing for subsequent identification using the BD Phoenix automated system. All isolates were potential or opportunistic pathogens of humans and animals (Shek et al., 2009; Jho et al., 2011; Shaikh et al., 2017). Enterobacter cloacae was the most common bacterial strain isolated (70%). It is ubiquitous in nature and is the most frequently isolated Enterobacter species in human and animals owing to its characteristic as a commensal microflora in the gastrointestinal tracts of animals and humans. Given that it is the most common Enterobacter species isolated in nosocomial infections, its antibiotic resistance characteristics, and its capacity to form biofilms and secrete cytotoxins, has garnered a considerable amount of attention (Mezzatesta et al., 2012; Davin-Regli and Pagès et al., 2015). The presence of a wide variety of resistance determinants in E. cloacae has rendered almost all families of antibiotic drugs ineffective. The production of constitutive AmpC β-lactamase renders intrinsic resistance to ampicillin, amoxicillin, first-generation cephalosporins, and cefoxitin (Davin-Regli and Pagès, 2015). It has been reported to cause fatal human infections (Sanders and Sanders, 1997; Krzymińska et. al., 2009) and previously reported in various studies in snakes (Jho et al., 2011; Abba et al., 2016; Chuang et al., 2022). Staphylococcus sciuri is known primarily as an animal pathogen, but it is becoming increasingly significant in human infections, being reported as a cause of inflammatory conditions, septic shock, and wound and urinary tract infections (Stepanovic et al., 2003). It has been isolated from the skin of pigs suffering from severe exudative epidermitis (Chen et al., 2007). Staphylococcus sciuri inhabit numerous different habitats, including human, animal, and environments, and can serve as a potential source for virulence and antibiotic resistance genes for another Staphylococcus group (Nemeghaire, 2014). No literature has been identified, which documents the presence of S. sciuri in snakes; however, it has been isolated from free living rodents, which indicates the possibility of transfer of the pathogen through rodent prey (Hauschild et al., 2003). Table 1. Species of bacteria isolated from the oro-pharyngeal swab of Green pit vipers.
Fig. 1. Bacterial species isolated.
Fig. 2. Map of Mizoram depicting the study area-Aizawl District. Streptococcus porcinus is commonly associated with pyogenic infections, abortion, and endocarditis in pigs and has been detected in bovine milk and genitourinary tract infection in women (Facklam et al., 1995; Collins et al., 1984; Wessman et al., 1986; Martin et al., 2004; Duarte et al., 2005; Pereira et al., 2013; Wang et al., 2020); however, we could not find any literature of its detection in snakes. Alcaligens faecalis is a saprophyte of soil and water and is multihost opportunistic pathogen, including plants (Tena et al., 2015; Ziedan et al., 2019). Alcaligens faecalis has been isolated from oral swabs of snakes (Panda et al., 2019; Padhi et al., 2020), and its increased resistance to antibiotics in infections has been well documented (Huang, 2020). Among the Staphylococcus species, S. aureus is the most prevalent opportunistic pathogen in humans and can cause a wide spectrum of diseases, ranging from mild skin infections to severe life-threatening conditions, particularly due to the emergence of multidrug-resistant strains (Li, 2018). Its significant impact on animal health, welfare, and magnitude of economic loss it incurs have brought increasing attention to this pathogen (Peton and Le, 2014). Staphylococcus species are predominant in the normal oral and cloaca flora of healthy snakes (Jho et al., 2011) and have been isolated from venom and bite wounds of snakes (Garg et al., 2009). Yersinia pseudotuberculosis, a zoonotic pathogen, is commonly transmitted through contaminated food and water, and it is also capable of infecting both wild and domestic animals as well as birds. It is ubiquitous in nature and has the ability to grow and thrive in refrigeration temperature (˜4°C). It has been commonly isolated from rodents, which strongly suggests that rodents play a significant role as carriers between different reservoirs, particularly in snakes, where rodents are the primary prey for most snake species (Cristi L. Galindo et al., 2011). Proteus spp has been isolated from the oral microflora of Chinese cobra Naja atra, bamboo pit vipers Trimeresurus albolabris, Python bivittatus, and from four different house species of snakes from Southern Africa (Shek et al., 2009; Jho et al., 2011; Shaikh et al., 2017). It is ubiquitous and opportunistic in nature and has been commonly isolated from wound cultures in animal bite cases (Wolff et al., 1998; Abrahamian et al., 2011; Vardanega et al., 2022). This study confirms the presence of multiple pathogenic bacteria in the oral cavity of T. erythrurus snakes. As mentioned above, these bacteria have been reported by several studies to be associated with wound infections and septicaemia. Administration of antibiotics to combat local infection and anti-tetanus prophylaxis should be a routine procedure in snake bite treatment (Gutiérrez et al., 2006). Our findings underline the significance of administering antibiotic prophylaxis in snake bite victims—both humans and animals and highlight that those individuals engaged in activities such as snake research, rescue operations, or veterinary aid are at a higher risk of infection, particularly if they are immunocompromised. In addition to these, it is also important to consider the potential problems related to secondary skin complications and nosocomial infections in victims of snake envenomation. Therefore, despite the opposing views of some authors against prophylactic antibiotic therapy in snake bites, the presence of multiple pathogenic bacteria in the oral cavity of snakes indicates a high risk of secondary bacterial infection in snake bites and reiterates the importance of prophylactic antibiotic therapy in snake bite victims. A comprehensive study of the oral microflora of venomous and nonvenomous snake species of this region is solicited. AcknowledgmentsWe are thankful to the Dean, College of Veterinary Sciences and Animal Husbandry, Selesih, Central Agricultural University. We thank Ms. Akangshya Priya Gogoi and Ms. Prachi Mishra and the lab multitasking staff for their help and support. Conflict of interestThe authors declare that there is no conflict of interest. ReferencesAbba, Y., Ilyasu, Y., Yusoff, M.S.M. and Noordin, M.M. 2016. Bacterial co-infections in a captive Python bivittatus with septicemia. Sokoto J. Vet. Sci. 14(2), 67–71; doi: 10.4314/sokjvs.v14i2.10 Abrahamian, F.M. and Goldstein, E.J. 2011. Microbiology of animal bite wound infections. Clin. Microbiol. Rev. 24(2), 231–246; doi: 10.1128/CMR.00041-10 Al-Asmari, A.K., Abbasmanthiri, R., Osman, N.M.A., Siddiqui, Y., Al-Bannah, F.A., Al-Rawi, A.M. and Al-Asmari, S.A. 2015. Assessment of the antimicrobial activity of few Saudi Arabian snake venoms. Open Microbiol. J. 9, 18; doi: 10.2174%2F1874285801509010018 Bocian, A. and Hus, K.K. 2020. Antibacterial properties of snake venom components. Chem. Pap. 74, 407–419; doi: 10.1007/s11696-019-00939-y Chen, S., Wang, Y., Chen, F., Yang, H., Gan, M. and Zheng, S.J. 2007. A highly pathogenic strain of Staphylococcus sciuri caused fatal exudative epidermitis in piglets. PLoS One 2(1), e147; doi: 10.1371/journal.pone.0000147 Chuang, P.C., Lin, W.H., Chen, Y.C., Chien, C.C., Chiu, I.M., Tsai, T.S. 2022. Oral bacteria and their antibiotic susceptibilities in Taiwanese venomous snakes. Microorganisms 10(5), 951; doi: 10.3390/microorganisms10050951 Collins, M.D., Farrow, J., Katic, V. and Kandler, O. 1984. Taxonomic studies on streptococci of serological groups E, P, U and V: description of Streptococcus porcinus sp. nov. Syst. Appl. Microbial. 5(3), 402–413; doi: 10.1016/S0723-2020(84)80041-7 Davin-Regli, A. and Pagès, J.M. 2015. Enterobacter aerogenes and Enterobacter cloacae; versatile bacterial pathogens confronting antibiotic treatment. Front. Microbiol. 6, 392; doi:10.3389/fmicb.2015.00392 Duarte, R.S., Barros, R.R., Facklam, R.R. and Teixeira, L.M. 2005. Phenotypic and genotypic characteristics of Streptococcus porcinus isolated from human sources. J. Clin. Microbiol. 43(9), 4592–4601; doi: 10.1128/jcm.43.9.4592-4601.2005 Esmaeilishirazifard, E., Usher, L., Trim, C., Denise, H., Sangal, V., Tyson, G.H. and Moschos, S.A. 2022. Bacterial adaptation to venom in snakes and arachnida. Microbiol. Spectrum 10(3), e02408–e02421; doi: 10.1128/spectrum.02408-21 Facklam, R., Elliott, J., Pigott, N. and Franklin, A.R. 1995. Identification of Streptococcus porcinus from human sources. J. Clin. Microbiol. 33(2), 385–388; doi: 10.1128/jcm.33.2.385-388.1995 Garg, A., Sujatha, S., Garg, J., Acharya, N.S. and Chandra Parija, S. 2009. Wound infections secondary to snakebite. J. Infect. Dev. Ctries. 3(3):221–223; doi: 10.3855/jidc.39 Galindo, C.L., Rosenzweig, J.A., Kirtley, M.L. and Chopra, A.K. 2011. Pathogenesis of Y. enterocolitica and Y. pseudotuberculosis in human yersiniosis. J. Pathogens 2011(1), 182051; doi: 10.4061/2011/182051 Goldstein, E.J., Citron, D.M., Gonzalez, H., Russell, F.E. and Finegold, S.M. 1979. Bacteriology of rattlesnake venom and implications for therapy. J. Infect. Dis. 140(5), 818–821; doi: 10.1093/infdis/140.5.818 Gutiérrez, J.M., Theakston, R.D.G. and Warrell, D.A. 2006. Confronting the neglected problem of snake bite envenoming: the need for a global partnership. PLoS Med. 3(6), e150; doi: 10.1371/journal.pmed.0030150 Hauschild, T. and Schwarz, S. 2003. Differentiation of Staphylococcus sciuri strains isolated from free-living rodents and insectivores. J. Vet. Med. Series B 50(5), 241–246; doi: 10.1046/j.1439-0450.2003.00662.x Huang, C. 2020. Extensively drug-resistant Alcaligenes faecalis infection. BMC Infect. Dis. 20(1), 833; doi: 10.1186/s12879-020-05557-8 Jho, Y.S., Park, D.H., Lee, J.H., Cha, S.Y. and Han, J.S. 2011. Identification of bacteria from the oral cavity and cloaca of snakes imported from Vietnam. Lab. Anim. Res. 27(3), 213–217doi: 10.5625/lar.2011.27.213 Krzymińska, S., Mokracka, J., Koczura, R. and Kaznowski, A. 2009. Cytotoxic activity of Enterobacter cloacae human isolates. FEMS Immunol. Med. Microbiol. 56(3), 248–252; doi: 10.1111/j.1574-695X.2009.00572.x Lalremsanga, H.T., Sailo, S. and Hauzel, C. Diversity of snakes (Reptilia: Squamata) and role of environmental factors in their distribution in Mizoram, Northeast India. Proceedings of Advances in Environmental Chemistry (Ed. Diwakari), Published by Excel India Publishers, New Delhi, India, 2018, vol. 079, 265–269. Li, Z. 2018. A review of Staphylococcus aureus and the emergence of drug-resistant problem. Adv. Microbiol. 8(1), 65–76; doi: 10.4236/aim.2018.81006 Martin, C., Fermeaux, V., Eyraud, J.L. and Aubard, Y. 2004. Streptococcus porcinus as a cause of spontaneous preterm human stillbirth. J. Clin. Microbiol. 42(9), 4396–4398; doi: 10.1128/jcm.42.9.4396-4398.2004 Mezzatesta, M.L., Gona, F. and Stefani, S. 2012. Enterobacter cloacae complex: clinical impact and emerging antibiotic resistance. Future Microbiol. 7(7), 887–902; doi: 10.2217/fmb.12.61 Nemeghaire, S., Argudín, M.A., Fessler, A.T., Hauschild, T., Schwarz, S. and Butaye, P. 2014. The ecological importance of the Staphylococcus sciuri species group as a reservoir for resistance and virulence genes. Vet. Microbiol. 171(3-4), 342–356; doi: 10.1016/j.vetmic.2014.02.005 Padhi, L., Panda, S.K., Mohapatra, P.P. and Sahoo, G. 2020. Antibiotic susceptibility of cultivable aerobic microbiota from the oral cavity of Echis carinatus from Odisha (India). Microb. Pathogenesis 143, 104121; doi: 10.1016/j.micpath.2020.104121 Panda, S.K., Padhi, L. and Sahoo, G. 2019. Evaluation of cultivable aerobic bacterial flora from Russell’s viper (Daboia russelii) oral cavity. Microb Pathogenesis 134, 103573; doi: 10.1016/j.micpath.2019.103573 Pereira, N., Powell, A.M., Nyirjesy, P. and Plante, L.A. 2013. Vaginorectal Streptococcus porcinus in pregnancy: an emerging pathogen? J. Lower Genital Tract Dis. 17(4), e18–e21; doi: 10.1097/LGT.0b013e318280407c Clinical and Laboratory Standards Institute (CLSI). 2016. Performance standards for antimicrobial susceptibility testing (26th ed.). CLSI, Wayne, PA. Peton, V. and Le Loir, Y. 2014. Staphylococcus aureus in veterinary medicine. Infect. Genet. Evol. 21, 602–615; doi: 10.1016/j.meegid.2013.08.011 23974078 Shaikh, I.K., Dixit, P.P., Pawade, B.S., Potnis-Lele, M. and Kurhe, B.P. 2017. Assessment of cultivable oral bacterial flora from important venomous snakes of India and their antibiotic susceptibilities. Curr. Microbial. 74, 1278–1286; doi: 10.1007/s00284-017-1313-z Shek, K.C. 2009. Oral bacterial flora of the Chinese cobra (Naia atra) and bamboo pit viper (Trimeresurus albolabris) in Hong Kong SAR, China. Hongkong Med. J. 15(3), 183. Sanders, C.C., Bradford, P.A., Ehrhardt, A.F., Bush, K., Young, K.D., Henderson, T.A. and Sanders, W.E.Jr. 1997. Penicillin-binding proteins and induction of AmpC β-lactamase. Antimicrob. Agents Chemother. 41(9), 2013–2015; doi: 10.1128/aac.41.9.2013 Stepanovic, S., Jezek, P., Vukovic, D., Dakic, I. and Petráš, P. 2003. Isolation of members of the Staphylococcus sciuri group from urine and their relationship to urinary tract infections. J. Clini. Microbial. 41(11), 5262–5264; doi: 10.1128/jcm.41.11.5262-5264.2003 Tena, D., Fernández, C. and Lago, M.R. 2015. Alcaligenes faecalis: an unusual cause of skin and soft tissue infection. Jpn. J. Infect. Dis. 68, 128‒130. Thakur, S., Malhotra, A., Giri, S., Lalremsanga, H.T., Bharti, O.K., Santra, V. and Doley, R. 2022. Venom of several Indian green pit vipers: comparison of biochemical activities and cross-reactivity with antivenoms. Toxicon 210, 66-77; doi: 10.1016/j.toxicon.2022.02.014 Vardanega, J., Smith, L.K., Smith, S. and Hanson, J. 2022. Animal bite wounds and their management in tropical Australia. Int. J. Infect. Dis. 118, 188–195; doi: 10.1016/j.ijid.2022.02.026 Waiddyanatha, S., Silva, A., Siribaddana, S. and Isbister, G.K. 2019. Long-term effects of snake envenoming. Toxins (Basel) 11(4), 193; doi: 10.3390/toxins11040193 Wang, Y., Guo, H., Bai, Y., Li, T., Xu, R., Sun, T. and Song, Q. 2020. Isolation and characteristics of multi-drug resistant Streptococcus porcinus from the vaginal secretions of sow with endometritis. BMC Vet. Res. 16, 1–8; doi: 10.1186/s12917-020-02365-9 Wolff, K.D. 1998. Management of animal bite injuries of the face: experience with 94 patients. J. Oral Maxillofacial Surg. 56(7), 838–843; doi: 10.1016/S0278-2391(98)90009-X Wessman, G.E. 1986. Biology of the group E streptococci: a review. Vet. Microbiol. 12(3), 297–328; doi: 10.1016/0378-1135(86)90081-7 Ziedan, E.H., Khattab, A.E.A., Alamri, S.A. and Hashem, M. 2020. Molecular characterization of Alcaligenes faecalis and Pseudomonas aeruginosa causing root rot of date palm. Intl. J. Agric. Biol. 23, 183‒189. Zancolli, G., Mahsberg, D., Sickel, W. and Keller, A. 2015. Reptiles as reservoirs of bacterial infections: real threat or methodological bias? Microb. Ecol. 70, 579–584; doi: 10.1007/s00248-015-0618-3 | ||
| How to Cite this Article |
| Pubmed Style Kawlni L, Lalchhandama C, Lalhruaipuii , Dutta TK. Oral microflora of Trimeresurus snakes in a biodiversity hotspot of North Eastern India. Open Vet. J.. 2025; 15(5): 1934-1940. doi:10.5455/OVJ.2025.v15.i5.7 Web Style Kawlni L, Lalchhandama C, Lalhruaipuii , Dutta TK. Oral microflora of Trimeresurus snakes in a biodiversity hotspot of North Eastern India. https://www.openveterinaryjournal.com/?mno=159328 [Access: January 24, 2026]. doi:10.5455/OVJ.2025.v15.i5.7 AMA (American Medical Association) Style Kawlni L, Lalchhandama C, Lalhruaipuii , Dutta TK. Oral microflora of Trimeresurus snakes in a biodiversity hotspot of North Eastern India. Open Vet. J.. 2025; 15(5): 1934-1940. doi:10.5455/OVJ.2025.v15.i5.7 Vancouver/ICMJE Style Kawlni L, Lalchhandama C, Lalhruaipuii , Dutta TK. Oral microflora of Trimeresurus snakes in a biodiversity hotspot of North Eastern India. Open Vet. J.. (2025), [cited January 24, 2026]; 15(5): 1934-1940. doi:10.5455/OVJ.2025.v15.i5.7 Harvard Style Kawlni, L., Lalchhandama, . C., Lalhruaipuii, . & Dutta, . T. K. (2025) Oral microflora of Trimeresurus snakes in a biodiversity hotspot of North Eastern India. Open Vet. J., 15 (5), 1934-1940. doi:10.5455/OVJ.2025.v15.i5.7 Turabian Style Kawlni, Lallianpuii, C. Lalchhandama, Lalhruaipuii, and Tapan Kumar Dutta. 2025. Oral microflora of Trimeresurus snakes in a biodiversity hotspot of North Eastern India. Open Veterinary Journal, 15 (5), 1934-1940. doi:10.5455/OVJ.2025.v15.i5.7 Chicago Style Kawlni, Lallianpuii, C. Lalchhandama, Lalhruaipuii, and Tapan Kumar Dutta. "Oral microflora of Trimeresurus snakes in a biodiversity hotspot of North Eastern India." Open Veterinary Journal 15 (2025), 1934-1940. doi:10.5455/OVJ.2025.v15.i5.7 MLA (The Modern Language Association) Style Kawlni, Lallianpuii, C. Lalchhandama, Lalhruaipuii, and Tapan Kumar Dutta. "Oral microflora of Trimeresurus snakes in a biodiversity hotspot of North Eastern India." Open Veterinary Journal 15.5 (2025), 1934-1940. Print. doi:10.5455/OVJ.2025.v15.i5.7 APA (American Psychological Association) Style Kawlni, L., Lalchhandama, . C., Lalhruaipuii, . & Dutta, . T. K. (2025) Oral microflora of Trimeresurus snakes in a biodiversity hotspot of North Eastern India. Open Veterinary Journal, 15 (5), 1934-1940. doi:10.5455/OVJ.2025.v15.i5.7 |