| Original Article | ||
Open Vet J. 2020; 10(3): 289-296 doi: 10.4314/ovj.v10i3.7 Open Veterinary Journal, (2020), Vol. 10(3): 289–296 Original Research http://dx.doi.org/10.4314/ovj.v10i3.7 Evaluation of pigment epithelium-derived factor concentration in equine amniotic membrane homogenate and its in-vitro vascular endothelial growth factor inhibition effect in tears of dogs with vascularized ulcerative keratitisTatiane Villar1, Ana L. Pascoli1, Sabal Chaulagain2, Bahaa A. Fadl-Alla2 and Bianca C. Martins1,3*1Department of Veterinary Clinical Medicine, College of Veterinary Medicine, University of Illinois, Urbana-Champaign, IL 61802, USA 2Department of Pathobiology, College of Veterinary Medicine, University of Illinois, Urbana-Champaign, IL 61802, USA 3Current address: Department of Surgical and Radiological Sciences, School of Veterinary Medicine, University of California, Davis, CA 95616, USA *Corresponding Author: Bianca Martins. Department of Surgical and Radiological Sciences, School of Veterinary Medicine, University of California, Davis, CA 95616, USA. Email: bcmartins [at] ucdavis.edu Submitted: 02/03/2020 Accepted: 20/08/2020 Published: 03/09/2020 © 2020 Open Veterinary Journal
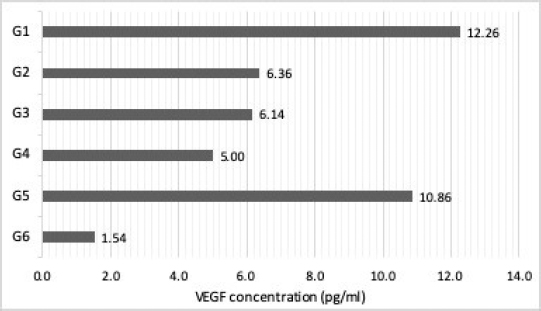
AbstractBackground: Corneal neovascularization can result from many pathological processes affecting the ocular surface leading to disturbances and opacifications that reduce corneal clarity and may impact vision. In veterinary medicine, the use of topical corticosteroid is contraindicated in the presence of ulcerative keratitis, and there is sparse research regarding safe medical alternatives to inhibit corneal neovascularization in dogs to improve visual outcome. Aim: To investigate the pigment epithelium-derived factor (PEDF) concentration in equine amniotic membrane homogenate (EAMH) and its in-vitro vascular endothelial growth factor (VEGF) inhibition in tears of dogs with vascularized ulcerative keratitis. Methods: Homogenates from 10 equine amniotic membranes (AM) were analyzed by sandwich enzyme-linked immunosorbent assay (ELISA) for quantification of equine PEDF and VEGF. Forty tear samples were collected from both eyes of dogs diagnosed with vascularized ulcerative keratitis, and 50 samples from healthy dogs. Samples from affected eyes were allocated to G1 – affected undiluted tears; G2 – affected tears diluted with phosphate-buffer solution; G3 – affected tears treated with low-concentrated EAMH; and G4 – affected tears treated with high-concentrated EAMH. Tears from the unaffected contralateral eyes were composed in G5, while G6 was composed by tears from healthy dogs (control). The presence and levels of VEGF were evaluated in all groups by Western blot and ELISA. Results: The PEDF:VEGF ratio in EAMH was 110:1. An increase in VEGF levels was observed in tears from eyes with vascularized corneal ulcers (G1) as well as in contralateral tears (G5), compared to normal dogs (G6). High-concentrated EAMH provided a greater decrease in VEGF levels in-vitro compared to low-concentrated EAMH. Conclusion: EAMHs exhibited high concentrations of PEDF in comparison to VEGF and were able to partially decrease VEGF levels in tears of dogs with vascularized ulcers, in-vitro. Our results suggest that VEGF concentration is elevated in tears of dogs with active vascularized ulcerative keratitis in both affected and contralateral eyes compared to that of healthy dogs. Keywords: Amniotic membrane, Corneal neovascularization, Dog, PEDF, VEGF. IntroductionPreservation of an avascular state of the cornea is crucial for optical clarity and maintenance of visual acuity. However, many injuries and diseases of the ocular surface promote corneal neovascularization, as this is a normal and expected reparative response in the face of an injury in a variety of tissues (Brooks et al., 2017). In the cornea, neovascularization results in opacification and disturbance of corneal architecture, leading to a significant decrease in vision (Ledbetter and Gilger, 2013). The mechanisms involved in corneal neovascularization are not fully elucidated; however, it is known that multiple forms of insults can trigger inflammatory mediators that stimulate the release of growth factors and upregulate the expression of angiogenic factors, such as vascular endothelial growth factor (VEGF) (Plummer et al., 2009; Qazi et al., 2009; Chang et al., 2012; Brooks et al., 2017). VEGF, also referred to as VEGF-A, is a growth factor peptide and the main regulator of hemangiogenesis, a potent promoter of vascular endothelial cell proliferation, migration, and tube formation (Penn et al., 2008; Chang et al., 2012). In humans, VEGF is extensively found in the epithelium of vascularized corneas secondary to inflammation. Studies on growth factors and clinically relevant models of corneal neovascularization have mainly investigated the role of VEGF in this process (Kvanta, 2006). Pigment epithelium-derived factor (PEDF) is considered as the main endogenous negative regulator of VEGF expression (Penn et al., 2008). This molecule is a glycoprotein that belongs to the family of serine protease inhibitors and was first purified from a conditioned media of human retinal pigment epithelial cells with neuronal differentiation-inducing property (Tombran-Tink et al., 1991). Considered a potent inhibitor of angiogenesis in the mammalian eye (Dawson et al., 1999), PEDF is found in high levels in the basement membrane of the human amniotic membrane (Shao et al., 2004). Amniotic membrane (AM) has been used as graft or as a topical suspension for the treatment of various ocular disorders in humans and animals (Kim and Tseng, 1995; Choi et al., 1998; Kim et al., 2000; Kamiya et al., 2005; Lassaline et al., 2005; Jiang et al., 2006; Barequet et al., 2008; Shahriari et al., 2008; Choi et al., 2009; Liang et al., 2009; Plummer et al., 2009; Rauz and Saw, 2010; Guo et al., 2011). A previous study has demonstrated that the human AM decreases corneal neovascularization by releasing anti-angiogenic factors, including interleukin-1 receptor antagonist, tissue inhibitors of metalloproteinase, collagen 18, interleukin-10 (IL-10), thrombospondin-1, and PEDF (Plummer et al., 2009). Recent studies in humans and animal models have shown that the topical use of AM suspensions have comparable effects to AM grafting in promoting epithelialization, decreasing inflammation, and suppressing corneal neovascularization (Jiang et al., 2006; Shahriari et al., 2008; Choi et al., 2009; Liang et al., 2009; Guo et al., 2011). In veterinary medicine, despite indications supporting the beneficial properties of the equine AM graft, there remains a paucity of studies evaluating the equine amniotic membrane homogenate (EAMH) properties and the role of VEGF in corneal neovascularization in the canine species. The goals of this study are: (1) to provide an initial investigation on the concentration of PEDF in the EAMH; (2) to verify the presence of VEGF in the tear film of dogs with corneal neovascularization secondary to ulcerative keratitis; and (3) to assess the inhibition of VEGF by EAMH, in-vitro, in tears of dogs with vascularized corneal ulcers. The authors hypothesize that: (1) EAMH will maintain a high concentration of PEDF after the homogenization process; (2) VEGF concentrations will be higher in tears of dogs with vascularized ulcerative keratitis than in tears from the contralateral eye and healthy dogs; and (3) that EAMHs will decrease the concentration of VEGF, in vitro, in the tears of dogs with vascularized ulcerative keratitis. The authors believe that the evaluation of anti-angiogenic properties of the EAMH is another step in assessing its usefulness and efficacy as a medical treatment. A better understanding of its properties and mechanisms may explain the benefits observed in clinical studies and further support the use of this promising topical formulation. Material and MethodsThe bioethical handling of all animals involved in this study followed the rules of the Association for Research in Vision and Ophthalmology (ARVO) and were conducted under an approved protocol by the Institutional Animal Care and Use Committee (IACUC) of the University of Illinois. Preparation of EAMHFresh equine AM (n=10) were collected from placentas of normal full-term deliveries at the University of Illinois Veterinary Teaching Hospital. The desired portion of the amniochorion placenta was sectioned, washed three times in phosphate-buffer saline (PBS 1x ULTRA PURE GRADE, VWR®, Los Angeles, CA), and placed in an antibiotic-antimycotic solution bath (ANTIBIOTIC-ANTIMYCOTIC (100×) 15240062, Gibco®, Grand Island, NY) for a period of at least 2 hours. The amniotic membrane was then separated from the chorion by blunt dissection, washed again three times in PBS (PBS 1x ULTRA PURE GRADE, VWR®, Los Angeles, CA), sectioned in smaller pieces, and placed in 15-ml conical tubes containing Hanks balanced salt solution (HBSS, Gibco®, Grand Island, NY) with 0.3 mg/ml gentamicin, which were then stored under −80°C until use. Homogenates were later prepared by resuspending 600 mg of amniotic tissue in 3 ml of PBS, homogenized using a tissue homogenizer (OMNI TISSUE HOMOGENIZER, Omni International, Kennesaw, GA) while doing up-and-down movements and centrifuged at 10,000 rpm for 15 minutes. At last, the supernatant (i.e., homogenate) was separated in new sterile tubes and stored in −80°C until analysis. Determination of VEGF and PEDF concentration in EAMHConcentrations of VEGF and PEDF were determined in 10 EAM homogenate samples by commercially available sandwich enzyme-linked immunosorbent assay (ELISA) for equine VEGF (Horse VEGF ELISA kit MBS007358, Mybiosource Inc., San Diego, CA) and equine PEDF (Horse PEDF ELISA kit MBS030959, Mybiosource Inc., San Diego, CA). All assays were performed in duplicate and according to the manufacturer’s instructions for tissue homogenate. Absorbance was measured at 450 nm in a microplate reader (SPECTRAMAX PLUS 384 Microplate Reader, Molecular Devices Corporation, San Jose, CA). Tear collection and group distributionA total of 40 tear samples were collected from dogs presenting with vascularized ulcerative keratitis to the Ophthalmology Service of the Veterinary Medical Teaching Hospital at the University of Illinois. Tear samples were collected from the affected eye and contralateral unaffected eye. All samples were obtained prior to any diagnostic procedure or application of medications. Inclusion criteria for the experimental groups consisted of the presence of any form of ulcerative keratitis accompanied by corneal vascularization. Fifty tear samples were also collected from both eyes of 25 systemically healthy dogs without ocular alterations (normal tears – G6) confirmed by a full ophthalmic examination. Informed consent was obtained from all owners prior to tear collection. Ophthalmic examination was performed immediately after tear collection on each dog by a diplomate of the American College of Veterinary Ophthalmologists (BM), including slit-lamp biomicroscopy (KEELER PSL CLASSIC, Broomall PA), indirect ophthalmoscopy (OMEGA 500 LED BINOCULAR, Heine, Germany), Schirmer tear test I (SCHIRMER TEAR TEST, Merck Animal Health, USA), tonometry (TONOVET, Icare, Vantaa, Finland), and fluorescein staining (BIO-GLO fluorescein strips, Hub Pharmaceuticals, San Francisco, CA). Tear samples were collected from the lower conjunctival fornix by capillary force, using 20 µl non-heparinized capillary glass tubes with an atraumatic tip (J-544 Microhematocrit Tube, Jorgensen Labs, Loveland, CO). All samples were immediately transferred into Eppendorf polypropylene tubes, identified, and stored at −80°C until analysis. After thawing, the samples were pooled accordingly as: affected tears (divided into subsequent experimental groups: G1–G4), contralateral tears (G5), and normal tears (G6). The pool of affected tears was subsequently divided into four different experimental groups: affected undiluted tears (G1), affected tears diluted at 1:1 with phosphate-buffer solution (PBS; G2), affected tears treated at 1:1 with low-concentrated EAMH (0.21 mg/ml of total protein; G3), and affected tears treated at 1:1 with high-concentrated EAMH (0.42 mg/ml total protein; G4). Following addition of a diluent (PBS in G2) or treatment (EAMH in G3 and G4), samples were allowed 30 minutes of incubation at room temperature prior to assay readings. In-vitro inhibition of VEGF in tearsOne single equine AM was randomly chosen and the EAMH was prepared as described above. Once the EAMH was prepared, the total protein concentration was measured and found to be 0.42 mg/ml, which composed the treatment in G4 (i.e., high-concentrated EAMH). PBS was then used for 1:1 dilution in order to achieve the second desired concentration of 0.21 mg/ml, which composed the treatment in G3 (i.e., low-concentrated EAMH). Total protein concentration of EAMH and tear samples was measured by spectrophotometer (NANODROP 1,000, Thermo Fisher Scientific, Waltham, MA). A commercially available canine VEGF sandwich ELISA kit was utilized (Canine VEGF ELISA kit MBS737581, Mybiosource Inc., San Diego, CA) to quantify the VEGF concentration in the tears of all groups. Samples were processed according to the manufacturer’s instruction and all assays were performed in duplicate. Absorbance was measured at 450 nm in a microplate reader spectrophotometer (SPECTRAMAX PLUS 384 Microplate Reader, Molecular Devices Corporation). All groups were also submitted to Western blot analysis for detection of VEGF. Mammalian protein extraction reagent was added to each sample and incubated for 20 minutes. The samples were centrifuged at 13,000 × g for 10 minutes and supernatants were collected. A total of 50-µg protein of each group was loaded to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted to a nitrocellulose membrane. Membranes were blocked 0.1% Tris-Buffered Saline/ 0.1% Tween 20 (TBS-T) buffer containing 5% non-fat skimmed milk powder and incubated with primary anti-VEGF antibody (Anti-VEGF antibody ab9570, Abcam, Eugene, OR) with predicted reactivity to canine. The membrane was developed using horseradish peroxidase conjugated goat anti-mouse secondary antibody (sc-2031, Santa Cruz Biotechnology, Santa Cruz, CA) and chemiluminescent reagent (SUPER SIGNAL WEST FEMTO, Thermo Fischer, Waltham, MA). Images of the membrane were then acquired by an imager (FLUORCHEM R, Proteinsimple, San Jose, CA) for detection of bands within 25–55 kDa range size expected for VEGF monomer or dimer forms. Data analysisThe concentrations of PEDF and VEGF in the EAMHs were compared using a two-sample Student’s t-test with unequal variances and significance was set to p < 0.05. In order to achieve the required tear volume for execution of the laboratory analysis proposed, all tear samples had to be pooled accordingly, which eliminated the possibility of statistical analysis due to lack of variability within or between groups. Thus, a descriptive analysis of the VEGF detection in the tears was performed. Ethical approvalThe bioethical handling of all animals involved in this study followed the rules of the ARVO and were conducted under an approved protocol by the IACUC of the University of Illinois. ResultsQuantification of PEDF and VEGF in EAHMsThe concentration of PEDF was significantly higher than VEGF in the EAMHs (p < 0.0004). The mean concentrations of equine PEDF and equine VEGF were 72.34 ± 7.07 ng/ml (median 71.08 ng/ml, range 56.71–81.33 ng/ml) and 0.658 ± 0.074 ng/ml (median 0.67 ng/ml, range 0.512–0.756 ng/ml), respectively. Taking these values into consideration, the PEDF:VEGF ratio calculated was approximately 110:1. In-vitro evaluation of canine VEGF in the tear film The results of the ELISA analysis revealed VEGF levels of 12.26 pg/ml in affected undiluted tears (G1) and 10.86 pg/ml in contralateral tears (G5), whereas normal tears (G6) had a VEGF level of 1.54 pg/ml. The Levels of VEGF were 6.36 pg/ml, 6.14 pg/ml, and 5.0 pg/ml in affected diluted tears (G2), affected tears treated with low-concentrated EAMH (G3), and with high-concentrated EAMH (G4), respectively (Fig. 1).
Fig. 1. Concentration of VEGF in tears of dogs. G1: affected undiluted tears; G2: affected diluted tears; G3: affected tears treated with low-concentrated EAMH; G4: tears treated with high-concentrated EAMH; G5: contralateral tears; G6: normal tears. *EAMH=equine amniotic membrane homogenate; VEGF=vascular endothelial growth factor.
Fig. 2. Image of Western blot gel demonstrating the detection of canine vascular endothelial growth factor (VEGF). Note that G1 has the strongest visible band, whereas its intensity decreases in the other groups and is absent in G6. G1: affected undiluted tears; G2: affected diluted tears; G3: affected tears treated with low-concentrated EAMH; G4: tears treated with high-concentrated EAMH; G5: contralateral tears; G6: normal tears. *EAMH=equine amniotic membrane homogenate; VEGF=vascular endothelial growth factor. When comparing all groups directly to the affected undiluted tears (G1), a decrease in VEGF levels of 59.2% was noted in tears treated with high-concentrated EAMH (G4), and of 48.3% and 49.9% in affected diluted tears (G2), and tears treated with low-concentrated EAMH (G3), respectively. However, if the dilution factor is neutralized and the treated groups (i.e., G3 and G4) are compared to the affected diluted tears (G2), there is a minimal decrease in VEGF levels of 3.4% in G3 and a more pronounced reduction of 21.4% in G4. On Western blot analysis, a specific band against VEGF was detected between 35 and 55 kDa in all groups, except in normal tears (G6) on which no VEGF was detected. Subjectively, the intensity of the band on G1 was stronger than that from the other groups, indicating a higher detection of canine VEGF (Fig. 2). Quantification of VEGF by densitometry was not possible because a control housekeeping protein for tear samples was not available. DiscussionTo the authors’ knowledge, this is the first study to demonstrate that VEGF is present at a minimal concentration in the tear film of ophthalmologically healthy dogs and suggests an important increase in VEGF levels in the tear film of dogs with ulcerative keratitis during active corneal neovascularization. Furthermore, this study also demonstrated that the concentration of VEGF may also be simultaneously elevated in the tear film of the contralateral unaffected eye. The findings of this study provide preliminary data to support the hypothesis that an increase in VEGF expression may play an essential role in corneal neovascularization in canine patients, as observed in humans (Kvanta, 2006; Penn et al., 2008; Chang et al., 2012), and that therapeutic approaches targeting its neutralization, such as by use of PEDF-rich compounds or other anti-angiogenic drugs, should be further investigated (Jin et al., 2010; Matsui et al., 2012; Hsu et al., 2015). Although equine AM grafting has been proven beneficial, several factors preclude its wider application in our veterinary patients, mainly due to the invasive nature of the procedure, which requires general anesthesia. Additionally, the costs involved in the management of corneal diseases and that of surgical procedures may also impose a prohibitive factor for many clients. Therefore, the development of a topical solution of equine AM that could provide biochemical factors to contribute to corneal wound healing, inhibition of proteinases and corneal neovascularization, and decrease of inflammation and scar formation would be highly desirable and beneficial for these cases. This study indicates that the described methodology for processing a homogenate solution of the EAMH is feasible, and the final product is rich on PEDF, a potent anti-angiogenic factor. Several studies have investigated different variations of the amniotic membrane as a topical solution in-vivo. Kamiya et al. (2005) demonstrated that the topical use of human amniotic epithelial cell (HAEC) culture supernatant significantly suppressed corneal neovascularization and inflammatory reactions on surgically injured corneas of mice. Wichayacoop et al. (2009) showed that the use of a topically applied HAEC supernatant in deep corneal ulcers of dogs, following amniotic membrane grafting, resulted in anti-angiogenic outcomes comparable to that of topical corticosteroids, resulting in inhibition of corneal vascularization and minimal scar formation (Wichayacoop et al., 2009). Amniotic membrane extract in combination with traditional medical therapy was also effective in reducing inflammation and promoting epithelialization in humans with corneal chemical burns (Liang et al., 2009). Although these studies have utilized different methodologies for generating a solution-derived product of amniotic membrane, they have all shown to maintain the biochemical benefits of the membrane. The authors believe that the methodology utilized in the current study for the production of EAMH is feasible, low-cost, and easily reproducible at veterinary centers and should, therefore, be further evaluated for the same activities and effects as previously reported for other similar compounds. Corneal neovascularization is a complex pathological process that occurs when angiogenic stimulation produces anti-angiogenic activity (Huang et al., 2005). Neovascularization starts when vasodilation of preexisting vessels, in response to various forms of insult, causes an increase in vascular permeability and leakage of plasma proteins (i.e. fibrin, growth factors, and cytokines) that further activates enzymes (i.e. proteinases) to degrade extracellular matrix. This process also facilitates the migration of endothelial cells and enhances the release of angiogenic factors (i.e. VEGF) promoting the formation of sprouting tubes (Huang et al., 2005; Zhang and Ma, 2007; Qazi et al., 2009). Although the exact mechanisms involved in corneal neovascularization have not yet been completely elucidated, it is strongly suggested that VEGF plays a major role in the process (Kvanta, 2006; Penn et al., 2008). The detection of VEGF receptors has been previously reported in canine ocular tissue during physiologic and pathologic changes (Binder et al., 2012). The increased VEGF levels observed on the tear film of dogs with vascularized corneal ulcer observed in this present study is another evidence of the role this compound plays on corneal neovascularization. Furthermore, corneal neovascularization has been efficiently decreased by inhibition of VEGF in both animal models and clinical trials in humans (Kvanta, 2006; Chang et al., 2012). Although the exact mechanisms of interaction between proteinases and angiogenesis have not yet been fully elucidated, previous studies have demonstrated that microorganisms that produce exogenous collagenase may contribute to the severity of corneal neovascularization in cases of complicated and infected ulcerative keratitis, as commonly observed in our veterinary patients (Huang et al., 2005; Zhang and Ma, 2007). In the present study, we utilized samples from dogs exhibiting both superficial and deep corneal ulcerations, with or without signs of keratomalacia, with or without evidence of infection, providing a reasonable representative population for an initial and broad assessment of the activity of VEGF in the process of corneal neovascularization in canine patients with vascularized ulcerative keratitis. However, more studies are required to further characterize the expression of different growth factors in the tear film in specific disorders of the ocular surface to better elucidate the mechanisms involved and their interactions. These investigations are important to support the development of therapeutic strategies for the control of pathological changes observed in each disease process. PEDF is a glycoprotein that belongs to the family of serine protease inhibitors, and considered as a potent inhibitor of angiogenesis in the mammalian eyes (Dawson et al., 1999). Previous studies in laboratory animals have shown that topical application of PEDF protein solutions downregulates the VEGF expression and inhibited corneal neovascularization, suggesting that PEDF has a therapeutic potential for the management of corneal neovascular diseases (Shao et al., 2004; Penn et al., 2008; Jin et al., 2010; Matsui et al., 2012; Hsu et al., 2015). Studies have also demonstrated inhibitory activity of PEDF on various angiogenic inducers, including VEGF, platelet-derived growth factor, interleukin-8, acidic fibroblast growth factor, and lysophosphatidic acid (Dawson et al., 1999). This molecule has been shown to be highly enriched in the basement membrane of human amniotic membrane and to significantly suppress endothelial cell growth in-vitro, suggesting that PEDF is a major contributor to the anti-angiogenic activity of the amniotic membrane (Shao et al., 2004). In accordance, the present study demonstrated that the EAMH entails a high PEDF:VEGF ratio after processing, which is suggestive of a superior anti-angiogenic property as opposed to angiogenic. Although the decrease in VEGF concentration observed in the ELISA may have been enhanced by dilution of the samples with the additional volume of treatments, the Western blot result supports the observation of a suggestive decrease in VEGF since the method detects the targeted protein within a predetermined amount of total protein loaded in each group (50 µg). The purpose of G2 in this study is solely to account for the dilutional effect of the volume of treatment applied to the treated groups (G3 and G4) with the addition of the EAMH. It is not expected that PBS will have any molecular interaction to reduce VEGF levels. When comparing the ELISA results, a decrease in 21.4% was observed in the final concentration of VEGF in affected tears treated with the high-concentrated EAMH (G4) in comparison to the affected tears diluted with PBS (G2). Thus, the authors believe that the findings of this present study are indicative that EAMH might be able to partially reduce the levels of VEGF in tears of dogs with vascularized corneal ulcers. However, in-vivo assessments are needed to confirm this hypothesis and evaluate its efficacy. The two concentrations of total protein in EAMHs utilized in this study were aimed based on previous measurements performed by the authors (unpublished data), which indicated a range from 0.15 to 0.65 mg/ml in the stored cryopreserved equine AM. A random selection was used to obtain an equine AM that fell within the median concentration in order to obtain a more realistic representation of the EAMH produced, following our processing method. Western blot analysis was used primarily in this study to detect the presence of canine VEGF, rather than to quantify the concentration, mainly because of limitations of our sample type. Generally, each specific sample type has a standardized housekeeping protein to serve as loading control that allows quantification; however, this has not yet been appropriately established for tear samples. Different loading proteins (Glyceraldehyde 3-phosphate dehydrogenase (GAPDH), α/β tubulin, pan-actin) have been attempted to serve as control for human tear samples; however, they were not detectable in tears collected by capillary tube (You et al., 2012). Additionally, these proteins have band sizes relatively close to the expected range of VEGF, which could potentially interfere with the detection of VEGF in our samples. On the Western blot analysis, a specific band against VEGF was detected between the 35 and 55 kDa lines. The band corresponding to VEGF detection of the affected undiluted tears (G1) was noticeably more intense than that of remaining groups, especially when compared to G6, where no VEGF detection is visible. However, this interpretation is speculative, and no comparative conclusions can be made given the lack of proper quantitative technique and statistical analysis. Nevertheless, the methodology was able to achieve the goal of detecting canine VEGF in the tear film of all groups, except G6 (normal tears). Several limitations were present in this study. The tear sampling technique chosen proved not to be the most efficient in terms of recovering satisfactory volume of each individual, which forced the investigators to pool the samples and analyze them collectively. By doing so, our sample size was reduced to one, which implicated the extinction of variance for statistical analysis. Therefore, our interpretation of the ELISA results is mainly descriptive and no formal conclusions can be drawn without proper statistical proof. Another implication from the pooling of samples is the wide variety of intensity in clinical presentation of vascularized ulcerative keratitis in our canine patients, from simple superficial ulcers to deep stromal and malacic ulcers. Matrix metalloproteinases may play a role in the regulation of angiogenic factors (Huang et al., 2005); however, it remains unknown how the angiogenic index differs in such conditions. Therefore, one could only speculate that keratomalacia would induce a higher angiogenic factor expression into the tears in comparison to non-malacic ulcers. The findings in this study serve as a starting point into the investigation of the VEGF role in corneal neovascularization in the canine patient and further studies are warranted to better characterize the angiogenic drive in each specific clinical corneal pathology. Unfortunately, no loading control was included in the Western blot protocol due to limitations pertaining to our sample type. Therefore, an objective comparison between groups was not possible and any conclusions are based on a subjective interpretation of the intensity of the resulted bands. Analysis of our samples by ELISA appeared to be the most sensitive and efficient for both detection and quantification. Given the final low volume of samples, the laboratory methodologies could not be repeated and improved. For future analysis, the authors believe that the methods applied for tear sampling and evaluation of tear samples, as well as the interaction of PEDF and VEGF, must be further explored and improved. In summary, this study described a methodology for the preparation of a homogenate solution of the equine amniotic membrane to be used topically. It demonstrated that the final homogenate product entails a high concentration of PEDF in comparison to VEGF, which could be beneficial for the management of corneal vascularization. This study provides preliminary indications that the VEGF levels are elevated in tears of dogs with vascularized corneal ulcers, as well as in the tears of contralateral eyes. Furthermore, this study also demonstrated a preliminary assessment of the potential of EAMH in decreasing VEGF levels in tears of dogs, in-vitro. The authors believe that more investigation is needed in order to further characterize the composition, elucidate the biochemical activities, and efficacy of the use of topical EAMHs in-vivo as an additional treatment option for our veterinary patients with corneal vascular diseases. Conflict of interestThe authors declare that there is no conflict of interest. Authors’ contributionTatiane Villar: methodology design, sample collection and processing, data analysis, manuscript preparation, and revision. Ana L. Pascoli: sample collection and processing and data analysis. Sabal Chaulagain: methodology design, sample processing, data analysis, and manuscript revision. Bahaa A. Fadl-alla: methodology design, sample processing, data analysis, and manuscript revision. Bianca C. Martins: study conception, methodology design, data analysis, and manuscript revision. ReferencesBarequet, I.S., Habot-Wilner, Z., Keller, N., Smollan, G., Ziv, H., Belkin, M. and Rosner, M. 2008. Effect of amniotic membrane transplantation on the healing of bacterial keratitis. Invest. Ophthalmol. Vis. Sci. 49, 163–167. Binder, D.R., Herring, I.P., Zimmerman, K.L., Pickett, J.P. and Huckle, W.R. 2012. Expression of vascular endothelial growth factor receptor-1 and -2 in normal and diseased canine eyes. Vet. Ophthalmol. 15, 223–240. Brooks, D.E., Matthews, A. and Clode, A.B. 2017. Diseases of the cornea. In: Equine ophthalmology, 3rd ed. Eds., Gilger, B.C., Ames, I.A. Hoboken, NJ: Wiley-Blackwell, pp: 252–368. Chang, J.H., Garg, N.K., Lunde, E., Han, K.Y., Jain, S. and Azar, D.T. 2012. Corneal neovascularization: an anti-VEGF therapy review. Surv. Ophthalmol. 57, 415–429. Choi, J., Jin, H.J., Jung, S., Yang, E., Choi, J.S., Chung, S.H. and Joo, C.K. 2009. Effects of amniotic membrane suspension in human corneal wound healing in vitro. Mol. Vis. 15, 2230–2238. Choi, Y.S., Kim, J.Y., Wee, W.R. and Lee, J.H. 1998. Effect of the application of human amniotic membrane on rabbit corneal wound healing after excimer laser photorefractive keratectomy. Cornea 17, 389–395. Dawson, D.W., Volpert, O.V., Gillis, P., Crawford, S.E., Xu, H., Benedict, W. and Bouck, N.P. 1999. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Sci. 285, 245–248. Guo, Q., Hao, J., Yang, Q., Guan, L., Ouyang, S. and Wang, L. 2011. A comparison of the effectiveness between amniotic membrane homogenate and transplanted amniotic membrane in healing corneal damage in a rabbit model. Acta Ophthalmol. 89, e315–e319. Hsu, C.C., Chang, H.M., Lin, T.C., Hung, K.H., Chien, K.H., Chen, S.Y., Chen, S.N. and Chen, Y.T. 2015. Corneal neovascularization and contemporary antiangiogenic therapeutics. J. Chin. Med. Assoc. 78, 323–330. Huang, A.J., Li, D.Q., Li, C.H., Shang, T.Y. and Hernandez, E. 2005. Modulation of corneal vascularization. Ocul. Surf. 3, S190–S193. Jiang, A., Li, C., Gao, Y., Zhang, M., Hu, J., Kuang, W., Hao, S., Yang, W., Xu, C., Gao, G., Wang, Z. and Liu, Z. 2006. In-vivo and in-vitro inhibitory effect of amniotic extraction on neovascularization. Cornea 25, S36–S40. Jin, J., Ma, J.X., Guan, M. and Yao, K. 2010. Inhibition of chemical cautery-induced corneal neovascularization by topical pigment epithelium-derived factor eyedrops. Cornea 29, 1055–1061. Kamiya, K., Wang, M., Uchida, S., Amano, S., Oshika, T., Sakuragawa, N. and Hori, J. 2005. Topical application of culture supernatant from human amniotic epithelial cells suppresses inflammatory reactions in cornea. Exp. Eye Res. 80, 671–679. Kim, C.K. and Tseng, S.C.G. 1995. The effect on inhibition of corneal neovascularization after human amniotic membrane transplantation in severely damaged rabbit corneas. Korean J. Ophthalmol. 9, 32–46. Kim, J.S., Kim, J.C., Na, B.K., Jeong, J.M. and Song, C.T. 2000. Amniotic membrane patching promotes healing and inhibits proteinase activity on wound healing following acute corneal alkali burn. Exp. Eye Res. 70, 329–337. Kvanta, A. 2006. Ocular angiogenesis: the role of growth factors. Acta Ophtalmol. Scand. 84, 282–288. Lassaline, M.E., Brooks, D.E., Ollivier, F.J., Komaromy, A.M., Kallber, M.E. and Gelatt, K. 2005. Equine amniotic membrane transplantation for corneal ulceration and keratomalacia in three horses. Vet. Ophthalmol. 8, 311–317. Ledbetter, E.C. and Gilger, B.G. 2013. Equine ophthalmology. In Veterinary ophthalmology, 5th ed. Eds., Gelatt, K.N., Gilger, B.G., Kern, T.J., Ames, I.A. Hoboken, NJ: Wiley-Blackwell, pp: 976–1049. Liang, L., Li, W., Ling, S., Sheha, H., Qiu, W., Li, C. and Liu, Z. 2009. Amniotic membrane extraction solution for ocular chemical burns. Clin. Exp. Ophthalmol. 37, 855–863. Matsui, T., Nishino, Y., Maeda, S. and Yamagishi, S. 2012. PEDF-derived peptide inhibits corneal angiogenesis by suppressing VEGF expression. Microvasc. Res. 84, 105–108. Penn, J.S., Madan, A., Caldwell, R.B., Bartoli, M., Caldwell, R.W. and Hartnett, M.E. 2008. Vascular endothelial growth factor in eye disease. Prog. Retin. Eye Res. 27, 331–371. Plummer, C.E., Ollivier, F., Kallberg, M., Brooks, D., Barrie, K., Utter, M. and Gelatt, K. 2009. The use of amniotic membrane transplantation for ocular surface reconstruction: a review and series of 58 equine clinical cases (2002-2008). Vet. Ophthalmol. 12, 17–24. Qazi, Y., Maddula, S. and Ambati, B.K. 2009. Mediators of ocular angiogenesis. J. Genet. 88, 495–515. Rauz, S. and Saw, V.P. 2010. Serum eye drops, amniotic membrane and limbal epithelial stem cells – tools in the treatment of ocular surface disease. Cell Tissue Bank 11, 13–27. Shahriari, H., Tokhmehchi, F., Reza, M. and Hashemi, N.F. 2008. Comparison of the effect of amniotic membrane suspension and autologous serum on alkaline corneal epithelial wound healing in the rabbit model. Cornea 27, 1148–1150. Shao, C., Sima, J., Zhang, J.J., Jin, J., Reinach, P., Wang, Z. and Ma, J.X. 2004. Suppression of corneal neovascularization by PEDF release from human amniotic membranes. Invest. Ophthalmol. Vis. Sci. 45, 1758–1762. Tombran-Tink, J., Chader, C.G. and Johnson, L.V. 1991. PEDF: a pigment epithelium-derived factor with potent neuronal differentiative activity. Exp. Eye Res. 53, 411–414. Wichayacoop, T., Briksawan, P., Tuntivanich, P. and Yibchok-Anun, S. 2009. Anti-inflammatory effects of topical supernatant from human amniotic membrane cell culture on canine deep corneal ulcer after human amniotic membrane transplantation. Vet. Ophthalmol. 12, 28–35. You, J., Hodge, C., Wen, L., McAvoy, J.W., Madigan, M.C. and Sutton, G. 2012. Using soybean trypsin inhibitor as an external loading control for western blot analysis of tear proteins: Application to corneal disease. Exp. Eye Res. 99, 55–62. Zhang, S.X. and Ma, J.X. 2007. Ocular neovascularization: Implication of endogenous angiogenic inhibitors and potential therapy. Prog. Retin. Eye Res. 26, 1–37. | ||
| How to Cite this Article |
| Pubmed Style Villar T, Pascoli AL, Chaulagain S, Alla BAF, Martins BC. Evaluation of pigment epithelium-derived factor (PEDF) concentration in equine amniotic membrane homogenate and its in-vitro vascular endothelial growth factor inhibition effect in tears of dogs with vascularized ulcerative keratitis. Open Vet J. 2020; 10(3): 289-296. doi:10.4314/ovj.v10i3.7 Web Style Villar T, Pascoli AL, Chaulagain S, Alla BAF, Martins BC. Evaluation of pigment epithelium-derived factor (PEDF) concentration in equine amniotic membrane homogenate and its in-vitro vascular endothelial growth factor inhibition effect in tears of dogs with vascularized ulcerative keratitis. https://www.openveterinaryjournal.com/?mno=90220 [Access: July 07, 2025]. doi:10.4314/ovj.v10i3.7 AMA (American Medical Association) Style Villar T, Pascoli AL, Chaulagain S, Alla BAF, Martins BC. Evaluation of pigment epithelium-derived factor (PEDF) concentration in equine amniotic membrane homogenate and its in-vitro vascular endothelial growth factor inhibition effect in tears of dogs with vascularized ulcerative keratitis. Open Vet J. 2020; 10(3): 289-296. doi:10.4314/ovj.v10i3.7 Vancouver/ICMJE Style Villar T, Pascoli AL, Chaulagain S, Alla BAF, Martins BC. Evaluation of pigment epithelium-derived factor (PEDF) concentration in equine amniotic membrane homogenate and its in-vitro vascular endothelial growth factor inhibition effect in tears of dogs with vascularized ulcerative keratitis. Open Vet J. (2020), [cited July 07, 2025]; 10(3): 289-296. doi:10.4314/ovj.v10i3.7 Harvard Style Villar, T., Pascoli, . A. L., Chaulagain, . S., Alla, . B. A. F. & Martins, . B. C. (2020) Evaluation of pigment epithelium-derived factor (PEDF) concentration in equine amniotic membrane homogenate and its in-vitro vascular endothelial growth factor inhibition effect in tears of dogs with vascularized ulcerative keratitis. Open Vet J, 10 (3), 289-296. doi:10.4314/ovj.v10i3.7 Turabian Style Villar, Tatiane, Ana Lucia Pascoli, Sabal Chaulagain, Bahaa A. Fadl Alla, and Bianca C. Martins. 2020. Evaluation of pigment epithelium-derived factor (PEDF) concentration in equine amniotic membrane homogenate and its in-vitro vascular endothelial growth factor inhibition effect in tears of dogs with vascularized ulcerative keratitis. Open Veterinary Journal, 10 (3), 289-296. doi:10.4314/ovj.v10i3.7 Chicago Style Villar, Tatiane, Ana Lucia Pascoli, Sabal Chaulagain, Bahaa A. Fadl Alla, and Bianca C. Martins. "Evaluation of pigment epithelium-derived factor (PEDF) concentration in equine amniotic membrane homogenate and its in-vitro vascular endothelial growth factor inhibition effect in tears of dogs with vascularized ulcerative keratitis." Open Veterinary Journal 10 (2020), 289-296. doi:10.4314/ovj.v10i3.7 MLA (The Modern Language Association) Style Villar, Tatiane, Ana Lucia Pascoli, Sabal Chaulagain, Bahaa A. Fadl Alla, and Bianca C. Martins. "Evaluation of pigment epithelium-derived factor (PEDF) concentration in equine amniotic membrane homogenate and its in-vitro vascular endothelial growth factor inhibition effect in tears of dogs with vascularized ulcerative keratitis." Open Veterinary Journal 10.3 (2020), 289-296. Print. doi:10.4314/ovj.v10i3.7 APA (American Psychological Association) Style Villar, T., Pascoli, . A. L., Chaulagain, . S., Alla, . B. A. F. & Martins, . B. C. (2020) Evaluation of pigment epithelium-derived factor (PEDF) concentration in equine amniotic membrane homogenate and its in-vitro vascular endothelial growth factor inhibition effect in tears of dogs with vascularized ulcerative keratitis. Open Veterinary Journal, 10 (3), 289-296. doi:10.4314/ovj.v10i3.7 |