| Research Article | ||
Open Veterinary Journal, (2024), Vol. 14(1): 525-533 Original Research Effects of 5-fluorouracil, thymoquinone, and mammary stem cells’ exosomes on in vitro cultured breast cancer cellsAhmed Abdelfattah-Hassan1,2*, Doaa Ibrahim3, Ayman A. Saleh4, Asmaa T.Y. Kishawy3, Reham H.A. Mohamed5 and Safaa I. Khater51Department of Anatomy and Embryology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 2Biomedical Sciences Program, University of Science and Technology, Zewail City of Science and Technology, Giza, Egypt 3Department of Nutrition and Clinical Nutrition, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 4Department of Animal Wealth Development, Genetics and Genetic Engineering, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt 5Department of Biochemistry, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt *Corresponding Author: Ahmed Abdelfattah-Hassan. Department of Anatomy and Embryology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt. Email: abdelfattah [at] zewailcity.edu.eg; aabdelfattah [at] vet.zu.edu.eg © 2024 Open Veterinary Journal
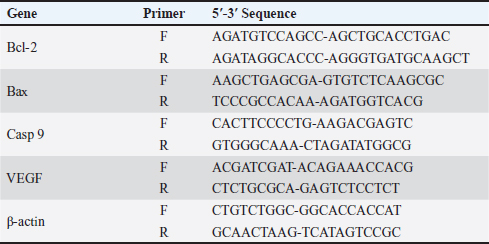
AbstractBackground: 5-fluorouracil (5-FU) is an antimetabolic agent used for treating slowly growing solid tumors like breast and ovarian carcinoma. Thymoquinone (TQ) is the main biologically active constituent of Nigella sativa, it has been found to demonstrate anticancerous effects in several preclinical studies, and this is because TQ possesses multitarget nature. Stem cells-derived exosomes are in the spotlight of research and are promising tissue regenerative and anticancer cell-derived nanovesicles. Aim: Herein, we studied the antineoplastic effects of Exosomes derived from mammary stem cells (MaSCs-Exo) on breast cancer cells, alone or combined with TQ when compared to a breast cancer chemotherapeutic agent; 5-FU. Methods: Our approach included performing viability test and measuring the expression of pro-apoptotic gene (Bax), anti-apoptotic gene (BCL-2) and angiogenic gene (VEGF) on Human MCF-7 cells (breast adenocarcinoma cells), the MCF-7 cells were cultured and incubated with medium containing 5-FU (25 μg/ml), TQ (200 μg/ml), MaSCs-Exo (100 μg protein equivalent), a combination of TQ (200 μg/ml) and MaSCs-Exo (100 μg). Results: Our obtained results show that TQ and MaSCs-Exo each can effectively inhibit breast cancer cell line (MCF-7) proliferation and growth. Also, the results show that the combination of TQ and MaSCs-Exo had higher cytotoxic effects on MCF-7 breast cancer cells than TQ or 5-FU, alone. Conclusion: The present study shows a promising anticancer potential of exosomes isolated from mammary stem cells; this effect was potentiated by adding TQ with MaSCs-derived exosomes. Keywords: Mammary stem cells, 5-fluorouracil, Apoptosis, Breast cancer, Anti-tumor. IntroductionBreast cancer existed in ancient age and reference to this disease can be detected dating back to around 1,500 years BC, in an Egyptian papyrus (Ebeid, 1999; Akram and Siddiqui, 2012). It relates to cancer arising within mammary tissue, frequently from the milk-ducts lining or milk-lobules lining. It is the highest diagnosed in females and the second leading cause of worldwide cancer-related mortalities in females (Siegel et al., 2018). Is also, the 5th most common cause of all cancer-related mortalities and forms one-third of all malignancies in females (Siegel et al., 2018). One from each eight females is predisposed to be diagnosed with breast cancer during her life (Harris et al., 1992). Chemotherapeutic approaches are recognized standard protocol of therapy for breast cancer patients. The antimetabolic agents are used in practice, and 5-fluorouracil (5-FU) is the most commonly used chemotherapy agents in clinically treating breast cancer cases. There are selected therapy regimens in which 5-FU is incorporated with other therapies, however, 5-FU is better used as monotherapy for cancer patients showing less value of the combination therapy of 5-FU and different medicines (Wehler et al., 2013). Thymoquinone (TQ), was acknowledged as a main constituent in N. sativa essential oil. It was shown to interfere with various tumorigenesis processes and was also found to counter the development of cancer and its malignancy, angiogenesis, migration, invasion, and progression (Mostofa et al., 2017). Furthermore, TQ was found to increase cancer-cell sensitivity to other conventional cancer therapy (such as, radiotherapy, chemotherapy, and immunotherapy) and concurrently reduce toxic side-effects associated with therapy in other non-target and normal body cells (Mostofa et al., 2017). TQ suppresses cancer development in various animal breast cancer models and was demonstrated to hinder the development of many in-vitro cultured cancer cells and in xenografted tumors in-vivo (Kundu et al., 2014). Exosomes are small cell-derived vesicles that are 50–150 nm in diameter on average and are accountable for transferring various intercellular signals (Abdelfattah-Hassan and Ibrahim, 2022). These signals include proteins, nucleic acids, and other cell-derived factors which are considered essential components of cell-to-cell communication (Valadi et al., 2007; Bang and Thum, 2012; Abdelfattah-Hassan and Ibrahim, 2022). They were found as mediators to regulate various cell processes such as migration, proliferation, and even morphological changes (Gurunathan et al., 2019; Sung et al., 2021), as essential players in drug delivery and in regenerative medicine (Lamichhane et al., 2015, Abdelfattah-Hassan and Ibrahim, 2022), and as essential diagnostic tools for various diseases (Meng et al., 2019; Abdelfattah-Hassan and Ibrahim, 2022). The current work aimed to determine the anticancer potential of bovine mammary stem cells’ derived exosomes (MaSCs-Exo) in comparison to known breast cancer treatment (5-FU) and a natural extract (TQ), and detecting their effects on the cell viability, regenerative potential and angiogenesis using a breast cancer cell-line. Material and MethodsIsolation of bovine MaSCs and MaSCs-derived exosomesThe bovine mammary stem cells (Bov-MaSCs) were isolated and characterized from the udder of lactating dairy cows, this was performed following our previously published protocol (Abdelfattah-Hassan et al., 2016). The establishment of mammary organoids using isolated Bov-MaSCs via culturing the isolated cells on ultra-low attachment plates (Corning, USA) as described previously with slight modifications (Yip and Papa, 2021). Cell cultureHuman breast cancer cells of the adenocarcinoma type of cells (MCF-7) were obtained from Vacsera Center (Cairo, Egypt). The cells were cultured in sterile tissue-culture flasks using DMEM medium supplemented with 10%–12% FCS and 1% antibiotic mix solution (penicillin, 100 U/ml, and streptomycin, 100 μg/ml) [this is called complete culture medium, all from Lonza, USA], the cells were cultured in a tissue culture incubator with 5% CO _idGenCharOverride-1 level and temperature of 37°C under humid atmosphere. The attached cells to the flasks were detached using trypsin/EDTA (Lonza, USA). TQ (≥98% pure; 164.2 molecular weight, Sigma Aldrich) was dissolved in DMSO, and a stock preparation was made at a concentration of 20 mmol/L, this was later mixed in a complete culture medium. 5-FU was obtained from EMIC united pharmaceuticals, Egypt. Inhibition of cell viability using TQWhen the cultured cells reached the desired confluence, the cells were passaged. After that MCF-7 breast cancer cells were supplied with ascending concentrations of TQ for 24 hours [0, served as control, 5, 50, 100, 200, and 300 µg/ml]. After 24 hours, 10 μl of the MTT reagent (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide, conc. 0.5 mg/ml) was mixed in each well, and the plate was incubated for additional 4 hours. Finally, a microplate reader was used to measure the obtained absorbance at 570 nm and compared to the control concentration. This step was essential to detect which TQ conc. to use in the later steps, which was 200 μg/ml. Inhibition of cell viability by 5-FU, TQ and MaSCs-ExoTo study the effect of 5-FU, TQ, MaSCs-Exo and their combinations on Human MCF-7 cells (breast adenocarcinoma cells), the MCF-7 cells were cultured and incubated with medium containing 5-FU (25 μg/ml), TQ (200 μg/ml), MaSCs-Exo (100 μg protein equivalent), a combination of TQ (200 μg/ml) and MaSCs-Exo (100 μg). Similar to step 2.2, 10 μl of the MTT reagent (conc. 0.5 mg/ml) was mixed in each well, and the plate was incubated for additional 4 hours, and, a microplate reader was used to measure the obtained absorbance at 570 nm to compare between the different treatments. Real-time quantitative PCR analysisQuantitative RT-qPCR was applied to measure the gene expression of pro-apoptotic, anti-apoptotic and angiogenic genes by using SYBR™ Green PCR Master Mix (ThermoFisher Scientific) on a StepOne™ Real-Time PCR System (Applied Biosystems, USA), as we previously reported (Ibrahim et al., 2021, Alasmari et al., 2022, Ibrahim et al., 2023). Total RNA was extracted using easy-spin™ Total RNA Extraction Kit (iNtRON Biotechnology Inc., Republic of Korea), using kit instructions and the purity of isolated RNA was detected by nanodrop. For reverse transcription a cDNA kit (First-strand cDNA Synthesis Kit, Merck, Germany) was used, following manufacturer’s protocol, to obtain cDNA for the qPCR. After that, the mRNA-transcript quantities of Bax, Bcl-2, Casp-9, and VEGF, relative to the expression levels of the internal control gene β-actin (used primers are presented in Table 1), were assayed using commercial kit SYBR Green qPCR Master Mix (Maxima SYBR Green qPCR Master Mix 2X, Thermo Scientific). Quantitative PCR reactions were prepared in a volume of 25 µl following the kit’s guidelines. Briefly, to each well, 5 µl of template cDNA (50 µg), 12.5 µl of SYBER green mix, 2 µl of forward and reverse primers and 5.5 μl RNAse free water were mixed. After a brief centrifugation, the PCR plate was inserted in the qPCR machine and the following run conditions were followed: (a) initial denaturation at 95°C for 5 minutes; then 40 cycles of the following (bi) denaturation at 95°C for 15 seconds; (bii) annealing/extension at 60°C for 1 minute. All controls and samples were done in triplicates on the StepOne™ Real-Time qPCR System. The data obtained from qPCR was analyzed by the relative threshold (Ct) method, and the fold inductions of samples were compared with the untreated samples. The internal reference gene was β-actin, and it was used as normalization for the expression of studied genes. The results were reported as a ratio of the reference gene to the target gene following the Livak and Schmittgen (2001) standard 2-ΔΔCt method. Table 1. The primers’ sequence of different study genes.
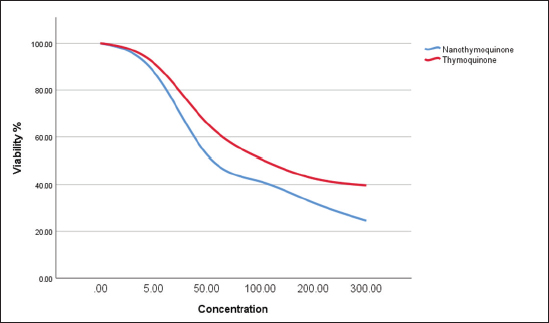
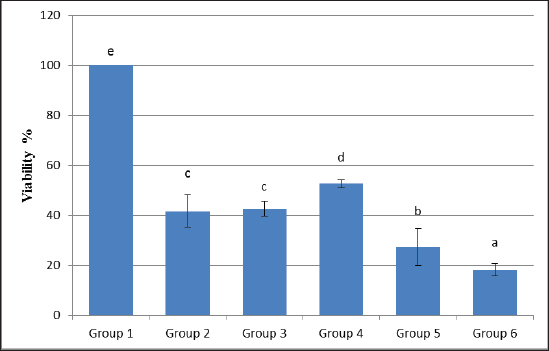
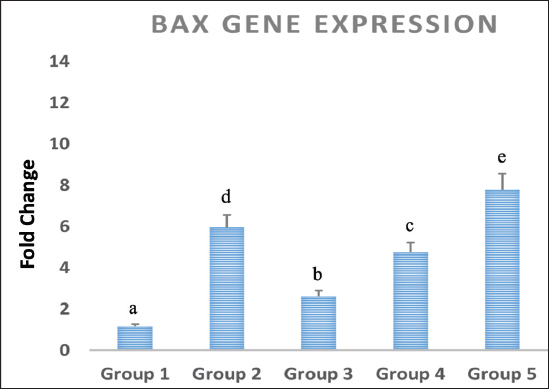
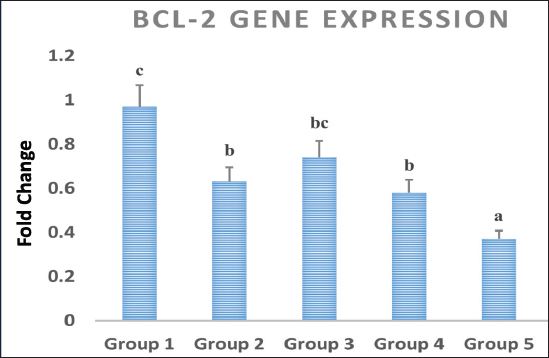
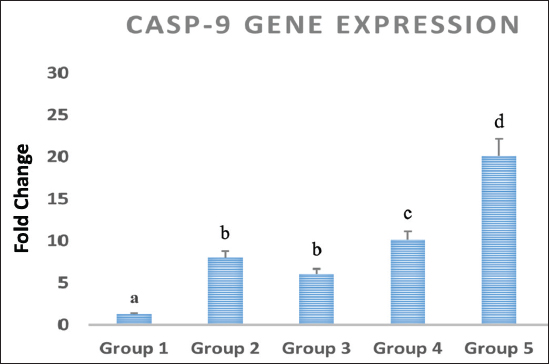
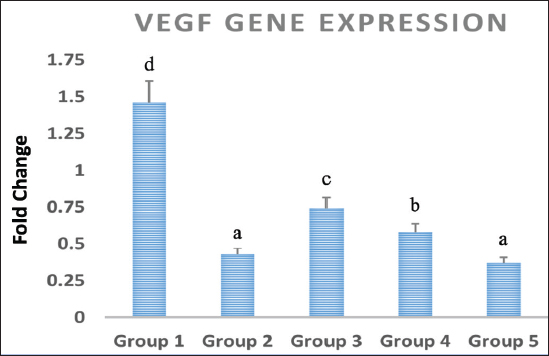
Statistical analysisThe data obtained for all groups (Group 1: Control, Group 2: treatment with 5-FU, Group 3: treated with TQ, Group 4: treated with MaSCs-Exo, and Group 5: treated with TQ and MaSCs-Exo, the differences among different groups were analyzed using one way ANOVA. Following that, a post-hoc test (Duncan multiple range test) was performed. The statistical analyses were made using SPSS version 21 (USA). The data shown represent the Mean ± SD (standard deviation). Ethical approvalNot needed for this study. ResultsInhibitory effect of TQ and MaSCs-Exo on MCF-7The MTT assay results showed that TQ and MaSCs-Exo decreased the viability of MCF-7 cell line. In addition, they inhibit the propagation of MCF-7 breast cancer cells in a dose-dependent mode as shown in Figure 1. Effect of 5-FU, TQ, MaSCs-Exo and their combinations on viability of MCF-7Data for viability in the studied groups is represented in Figure 2. All groups showed significant differences in viability % among each other, except groups treated with 5-FU and TQ alone were non-significantly different. Also, the viability percentage decreased in all treated groups (2–5) compared to the healthy/control group (group 1), nonetheless reached its least percentage in group (5) in which we used a combination of TQ and MaSCs-Exo. Bax gene expressionA significant increase in Bax gene expression was shown in treated groups compared to the healthy/control group (Fig. 3). Also, there is upregulation of Bax in groups (2, 3, 4, and 5) treated with 5-FU, TQ, MaSCs-Exo, and the combined TQ and MaSCs-Exo approach, respectively, compared with the control group. It is noticed that upregulation is higher in group 4 than group 3, and reached to the highest in group 5, treated with the combined TQ and MaSCs-Exo. Bcl-2 gene expressionResults represented in Figure 4 demonstrated that Bcl-2 gene expression showed a significant decrease than the control group in all treated groups. There is downregulation of Bcl-2 gene in groups (2, 3, 4, and 5) treated with 5-FU, TQ, MaSCs-Exo, and combined TQ and MaSCs-Exo, respectively, compared to the control group. It is noticed that downregulation is more in group 4 than group 3, and it decreased the most in group 5, treated with combined TQ and MaSCs-Exo. Caspase-9 gene expressionAs shown in Figure 5, Caspase-9 was poorly expressed in the control group that received no drugs and showed a significant increase in the control group in all treated groups. The increase in the expression of Casp9 in groups (2, 3, 4, and 5) treated with 5-FU, TQ, MaSCs-Exo, and combined TQ and MaSCs-Exo, respectively, compared to the control group, although it is noticed that the increase is higher in group 4 than group 3, and reached to the highest in group 5, treated with combined TQ and MaSCs-Exo. VEGF gene expressionData represented in Figure 6 show that there is a significant decrease in the expression level of the VEGF gene than the control group in all treated groups. There is a reduction in VEGF gene expression in groups (2, 3, 4, and 5) treated with 5-FU, TQ, MaSCs-Exo, and combined TQ and MaSCs-Exo, respectively, compared to the control group. It is noticed that reduction is higher in group 4 than group 3, and reached the lowest levels in group 2 and 5, treated with 5-FU and combined TQ and MaSCs-Exo, respectively.
Fig. 1. The effect of different concentrations of thymoquinone (red line) and MaSCs-Exo (blue line) on viability of MCF-7 cell line.
Fig. 2. Data analysis of the viability percentage in each group after treatment with 5-FU, Thymoquinone, MaSCs-Exo and Thymoquinone + MaSCs-Exo (groups 2–5, respectively). Letters on columns denote statistical significance (p < 0.05) using Duncan’s multiple range test. DiscussionCancer progression involves advanced multistep processes where normal body cells regain characters which allow their transformation into abnormal cells and the initiate tumors and malignancy. Such characters are known as “the hallmarks of cancer” and were first defined by Hanahan and Weinberg in 2000 and then modified in 2011 (Hanahan and Weinberg, 2011). Therapeutic agents which can interfere with one or more of these cancer hallmarks became regarded as futuristic anti-cancer therapies. One of these agents is TQ, which possesses an excellent capacity for translation into the clinic, believing that it influences most of the known cancer hallmarks. The discovery of novel tumor-toxic therapies that have lower side-effects on the immune system is considered a valuable aspect of the recent pharmacological studies (Cragg and Newman, 2005).
Fig. 3. Data analysis of expression of Bax gene in different study groups, treatment with 5-FU, Thymoquinone, MaSCs-Exo and Thymoquinone + MaSCs-Exo (groups 2–5, respectively). Letters on columns denote statistical significance (p < 0.05) using Duncan’s multiple range test.
Fig. 4. Data analysis of Bcl-2 gene expression in different treated groups, treatment with 5-FU, Thymoquinone, MaSCs-Exo and Thymoquinone + MaSCs-Exo (groups 2–5, respectively). Letters on columns denote statistical significance (p < 0.05) using Duncan’s test.
Fig. 5. Data analysis of Caspase-9 (CASP-9) gene expression in different treated groups, treatment with 5-FU, Thymoquinone, MaSCs-Exo and Thymoquinone + MaSCs-Exo (groups 2–5, respectively). Letters on columns denote statistical significance (p < 0.05) using Duncan’s test.
Fig. 6. Data analysis of VEGF gene expression in different treated groups, treatment with 5-FU, Thymoquinone, MaSCs-Exo and Thymoquinone + MaSCs-Exo (groups 2–5, respectively). Letters on columns denote statistical significance (p < 0.05) using Duncan’s test. TQ is a major bioactive derivative from N. sativa seeds and oil. Traditional medicine has shown that its seeds were used for various therapeutic benefits such as treating gastrointestinal problems, asthma, digestive problems, headache, hypertension, skin affections and to reduce obesity. The therapeutic and chemo-preventive effects of these “black seeds” were documented in previous research (Padhye et al., 2008). It has been shown that TQ has various anti-proliferative effects regarding different cancer cell lines such as breast, lungs, ovary, colon, laryngeal, osteosarcoma, and leukemia. Chemo-sensitization using TQ has been frequently limited to in-vitro studies, however it shows promising effects as a clinical therapy approach for cancer (Banerjee et al., 2010). 5-FU is an antimetabolite that is extensively used for breast cancer treatment (Fumoleau et al., 2003). However, it is mostly recommended for treating highly aggressive types of breast cancer. Still, 5-FU can be used in the treatment of any stage of breast cancer especially within combination therapies such as CMF (Cyclophosphamide, Methotrexate, and 5-FU), CAF (Cyclophosphamide, Adriamycin, and 5-FU) or CEF (Cytoxan, Ellence, and 5-FU) (Falkson et al., 1992). Immense research has been done to increase the efficacy of the previously mentioned combination strategies for breast cancer treatment. Moreover, combining 5-FU with different recently developed anti-cancer therapies (such as, irinotecan, tomudex, and oxaliplatin) increase their efficiency of response in treating breast cancer (Longley et al., 2003). Drug overdose is still a worldwide common clinical problem which may occur accidentally or intentionally. Therefore, a wide variety of nonspecific therapeutics were industrialized to face such problem (Weinberg et al., 1998). The process of combining TQ with other anticancer drugs that are clinically used can augment their chemotherapy effects. Essentially, TQ has been evidenced to show effective outcomes with advanced anticancer effects with already existing chemotherapeutics. Whilst 5-FU is considered the chemotherapeutic gold standard for different cancers, mainly colorectal cancer and breast cancer. The activity of TQ was shown to have some similarity with 5-FU actions on cell killing and interference with an intercellular metabolic function of SW-626 human colon cancer. Furthermore, the use of TQ combined with 5-FU, TQ led to increasing the pro-apoptotic effects in gastric cancer cells both in vitro and in vivo (Norwood et al., 2007; Lei et al., 2012). Our study demonstrated that TQ and MaSCs-Exo inhibit breast cancer cell line (MCF-7) proliferation which was determined via using the MTT assay, and in a dose-dependent manner both inhibited the fast-growing breast cancer cells and their progression. Different concentrations of TQ and MaSCs-Exo had different cytotoxicity effects on MCF-7 cell after 24 hours of treatment. Increasing concentrations of TQ and Nano-TQ, increased cytotoxicity effect. Also, it was shown for us from this study that, MaSCs-Exo had higher cytotoxicity on breast cancer cells than TQ, when considering the same concentration as TQ concentration. One of the classically used and standardized cytostatic chemotherapy drug is 5-FU which is used for treating cancer (Scheper et al., 1984). Previous studies demonstrated that TQ led to inhibiting the growth of cancer cells and augmented the apoptosis induced by 5-FU in gastric cancer cells both in vitro and in vivo (Lei et al., 2012). Our choice in this study to compare between 5-FU and TQ combined with MaSCs-Exo is due to the fact that combination therapies are much more relevant in treating breast cancer and other types of cancer (Wehler et al., 2013). It was also shown that TQ showed robust antiproliferation effects on breast cancer cells, the effect was more evident when it was in combination with doxorubicin, 5-FU led to increasing its cytotoxic effects as well (Woo et al., 2011). According to our results, breast cancer cell’s viability percentage decreased in the treated groups in comparison to group 1 (control group) but reached the least percentage in group (5) in which we used a combination of TQ and MaSCs-Exo. This means that the combination of TQ and MaSCs-Exo is better than using 5-FU or TQ alone. Our study demonstrated that there is upregulation of Bax and Caspase-9 in groups (2, 3, 4, and 5) treated with 5-FU, TQ, MaSCs-Exo, a combination of TQ and MaSCs-Exo, respectively, compared with the control group. It is noticed that upregulation is higher in group 4 than group 3, and became highest in group 5. This means that MaSCs-Exo is better than TQ, however, TQ is used in a dose which is 2 folds of MaSCs-Exo used dose, while combination of TQ and MaSCs-Exo is better than using 5-FU or TQ or MaSCs-Exo alone. According to our study, Caspase-9 and VEGF were poorly expressed in the control group that received no drugs and a significant increase than the control group in all treated groups. Also, there is increase in the expression of Caspase-9 in groups (2, 3, 4, and 5) treated with 5-FU, TQ, MaSCs-Exo, a combination of TQ and MaSCs-Exo, respectively, compared to the control group, although it is noticed that the increase is higher in group 4 than group 3, and became the highest in group 5. This means that MaSCs-Exo combination with TQ is better than TQ alone, however, TQ is used in a dose which is 2 folds of MaSCs-Exo used dose. Previous research has shown that MSCs exosomes were internalized by both MCF-7 and MDA-MB-231 breast cancer cell lines (Meng et al., 2019). These exosomes were found to inhibit breast cancer cells’ migration and invasion behaviors by downregulating ZNF367 and upregulation of miR-21-5p (Meng et al., 2019). Other studies showed that exosomes from adipose tissue MSCs can lead to contradictory results and actually promote the invasion and migration of breast cancer cells (Lin et al., 2013; Wang et al., 2019). In accordance with our results, MSCs exosomes were found to have cytotoxic effects and induced apoptosis of MCF-7 breast cancer cells (Mirabdollahi et al., 2019). Newer approaches aiming to avoid this contradiction performed by loading the exosomes with desirable agents (micro-RNA or drugs) to improve their cytotoxic effects and reduce breast cancer progression (Melzer et al., 2019; Khazaei-Poul et al., 2021; Değirmenci et al., 2022). Our results elaborated more on this topic and showed that combining MaSCs exosomes with TQ showed an improved apoptotic response that was more effective than using TQ or MaSCs exosomes alone, and also was much more effective than 5-FU (a known breast cancer chemotherapeutic agent). ConclusionIn conclusion, augmenting the cytotoxic effects of mammary stem cells-derived exosomes (MaSCs-Exo) on breast cancer cells by combining it with TQ showed an improved effect compared to the use of a known breast cancer chemotherapeutic agent (i.e., 5-FU). This was achieved through reducing the viability of breast cancer cells and upregulation of proapoptotic genes Bax and Caspase-9, downregulation of antiapoptotic gene BCL-2, and downregulation of proangiogenic gene VEGF. Our results provide preliminary data on the importance of combination therapy of different therapeutic agents (such as TQ and MaSCs-Exo in our study) in the fight against breast cancer. More research is still needed to elucidate their additional beneficial tissue regenerative and/or anticancer effects. AcknowledgmentsThe authors would like to thank the support of the Egyptian Science, Technology and Innovation Funding Authority and its staff. Conflict of interestThe authors declare that there is no conflict of interest. Author contributionsAll authors contributed equally to this manuscript and approved its final version. FundingThis work was supported by the Egyptian Science, Technology and Innovation Funding Authority (STDF project number 30141). Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesAbdelfattah-Hassan, A. and Ibrahim, D. 2022. Exosomes as a diagnostic tool and stem cells’ exosomes as a promising cell-based cell-free therapeutic tool for ischemic stroke. In regenerative therapies in ischemic stroke recovery. Singapore: Springer Nature Singapore, pp: 239–268. Abdelfattah-Hassan, A., Saadeldin, I.M. and Ghonimi, W. 2016. Optimization of the isolation process of putative bovine mammary stem cells. Japanese J. Vet. Res. 64(Suppl. 2), S65–S71. Akram, M. and Siddiqui, S.A. 2012. Breast cancer management: past, present and evolving. Indian J. Cancer 49(3), 277–282. Alasmari, W.A., El-Shetry, E.S., Ibrahim, D., ElSawy, N.A., Eldoumani, H., Metwally, A.S., Saleh, A.A., Mona, M.M., Abd-Elsalam, M.M., Hendam, B.M. and Essawi, W.M. 2022. Mesenchymal stem-cells’ exosomes are renoprotective in postmenopausal chronic kidney injury via reducing inflammation and degeneration. Free Radic. Biol. Med. 182, 150–159. Banerjee, S., Padhye, S., Azmi, A., Wang, Z., Philip, P.A., Kucuk, O., Sarkar, F.H. and Mohammad, R.M. 2010. Review on molecular and therapeutic potential of thymoquinone in cancer. Nutr. Cancer 62(7), 938–946. Bang, C. and Thum, T. 2012. Exosomes: new players in cell–cell communication. Int. J. Biochem. Cell Biol. 44(11), 2060–2064. Cragg, G.M. and Newman, D.J. 2005. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 100(1–2), 72–79. Değirmenci, N.S., Uslu, M., Kırbaş, O.K., Şahin, F. and Uçar, E.Ö. 2022. Lapatinib loaded exosomes as a drug delivery system in breast cancer. J. Drug Deliv. Sci. Technol. 75, 103584. Ebeid, N.I. 1999. Egyptian medicine in the days of the pharaohs. Cairo, Egypt: General Egyptian Book Organization. Falkson, C.I., Falkson, H.C. and Falkson, G. 1992. Cyclophosphamide, doxorubicin and fluorouracil (CAF) plus the treatment of premenopausal women with metastatic breast cancer. Ann. Oncol. 3(10), 849–853. Fumoleau, P., Bonneterre, J. and Luporsi, E. 2003. Adjuvant chemotherapy for node-positive breast cancer patients: Which is the reference today? J. Clin. Oncol. 21(6), 1190–1191. Gurunathan, S., Kang, M.H., Jeyaraj, M., Qasim, M. and Kim, J.H. 2019. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells 8(4), 307. Hanahan, D. and Weinberg, R.A. 2011. Hallmarks of cancer: the next generation. Cell 144(5), 646–674. Harris, J.R., Lippman, M.E., Veronesi, U. and Willet, W. 1992. Breast cancer (part 3). New Engl. J. Med. 327, 473–480. Ibrahim, D., Al-Khalaifah, H.S., Abdelfattah-Hassan, A., Eldoumani, H., Khater, S.I., Arisha, A.H., Mohamed, S.A., Ismail, T.A. and Tolba, S.A. 2021. Promising role of growth hormone-boosting peptide in regulating the expression of muscle-specific genes and related micrornas in broiler chickens. Animals 11(7), 1906. Ibrahim, D., Khater, S.I., Abdelfattah-Hassan, A., Alqahtani, L.S., Metwally, A.S., Bazeed, S.M., Elgamal, A., Sheraiba, N.I., Hussein, E.M., Alasmary, F.A. and Salem, G.A. 2023. Prospects of new targeted nanotherapy combining liponiosomes with berberine to combat colorectal cancer development: an in vivo experimental model. Int. J. Pharm. 647, 123511. Khazaei-Poul, Y., Shojaei, S., Koochaki, A., Ghanbarian, H. and Mohammadi-Yeganeh, S. 2021. Evaluating the influence of human umbilical cord mesenchymal stem cells-derived exosomes loaded with miR-3182 on metastatic performance of triple negative breast cancer cells. Life Sci. 286, 120015. Kundu, J., Chun, K.S., Aruoma, O.I. and Kundu, J.K. 2014. Mechanistic perspectives on cancer chemoprevention/chemotherapeutic effects of thymoquinone. Mutat. Res-Fund Mol. M. 768, 22–34. Lamichhane, T.N., Sokic, S., Schardt, J.S., Raiker, R.S., Lin, J.W. and Jay, S.M. 2015. Emerging roles for extracellular vesicles in tissue engineering and regenerative medicine. Tissue Eng. Part B Rev. 21(1), 45–54. Lei, X., Lv, X., Liu, M., Yang, Z., Ji, M., Guo, X. and Dong, W. 2012. Thymoquinone inhibits growth and augments 5-fluorouracil-induced apoptosis in gastric cancer cells both in vitro and in vivo. Biochem. Biophys. Res. Commun. 417(2), 864–868. Lin, R., Wang, S. and Zhao, R.C. 2013. Exosomes from human adipose-derived mesenchymal stem cells promote migration through Wnt signaling pathway in a breast cancer cell model. Mol. Cell Biochem. 383, 13–20. Livak, K.J. and Schmittgen, T.D. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4), 402–408. Longley, D.B., Harkin, D.P. and Johnston, P.G. 2003. 5-fluorouracil: mechanisms of action and clinical strategies. Nat. Rev. Cancer 3(5), 330–338. Melzer, C., Rehn, V., Yang, Y., Bähre, H., von der Ohe, J. and Hass, R. 2019. Taxol-loaded MSC-derived exosomes provide a therapeutic vehicle to target metastatic breast cancer and other carcinoma cells. Cancers 11(6), 798. Meng, Y., Sun, J., Wang, X., Hu, T., Ma, Y., Kong, C., Piao, H., Yu, T. and Zhang, G. 2019. Exosomes: a promising avenue for the diagnosis of breast cancer. Technol. Cancer Res. Treat. 18, 1533033818821421. Mirabdollahi, M., Haghjooyjavanmard, S. and Sadeghi-Aliabadi, H., 2019. An anticancer effect of umbilical cord-derived mesenchymal stem cell secretome on the breast cancer cell line. Cell Tissue Bank 20, 423–434. Mostofa, A.G.M., Hossain, M.K., Basak, D. and Bin Sayeed, M.S. 2017. Thymoquinone as a potential adjuvant therapy for cancer treatment: evidence from preclinical studies. Front. Pharmacol. 8, 295. Norwood, A.A., Tucci, M. and Benghuzzi, H. 2007. A comparison of 5-fluorouracil and natural chemotherapeutic agents, EGCG and thymoquinone, delivered by sustained drug delivery on colon cancer cells. Biomed. Sci. Instrum. 43, 272–277. Padhye, S., Banerjee, S., Ahmad, A., Mohammad, R. and Sarkar, F.H. 2008. From here to eternity-the secret of pharaohs: therapeutic potential of black cumin seeds and beyond. Cancer Ther. 6, 495. Scheper, R.J., Vos, A., De Groot, J. and Boerrigter, G.H. 1984. Evaluation of various cytostatic drugs as local immunotherapeutic agents. Investig. New Drugs 2, 221–225. Siegel, R.L., Miller, K.D. and Jemal, A. 2018. Cancer statistics, 2018. CA: Cancer J. Clin. 68(1), 7–30. Sung, B.H., Parent, C.A. and Weaver, A.M. 2021. Extracellular vesicles: critical players during cell migration. Develop. Cell 56(13), 1861–1874. Valadi, H., Ekström, K., Bossios, A., Sjöstrand, M., Lee, J.J. and Lötvall, J.O. 2007. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nature Cell Biol. 9(6), 654–659. Wang, S., Su, X., Xu, M., Xiao, X., Li, X., Li, H., Keating, A. and Zhao, R.C. 2019. Exosomes secreted by mesenchymal stromal/stem cell-derived adipocytes promote breast cancer cell growth via activation of Hippo signaling pathway. Stem Cell Res. Ther. 10, 1–12. Wehler, T.C., Hamdi, S., Maderer, A., Graf, C., Gockel, I., Schmidtmann, I., Hainz, M., Berger, M.R., Theobald, M., Galle, P.R. and Moehler, M. 2013. Single-agent therapy with sorafenib or 5-FU is equally effective in human colorectal cancer xenograft—no benefit of combination therapy. Int. J. Colorectal. Dis. 28, 385–398. Weinberg, G.L., VadeBoncouer, T., Ramaraju, G.A., Garcia-Amaro, M.F. and Cwik, M.J. 1998. Pretreatment or resuscitation with a lipid infusion shifts the dose-response to bupivacaine-induced asystole in rats. J. Am. Soc. Anesth. 88(4), 1071–1075. Woo, C.C., Loo, S.Y., Gee, V., Yap, C.W., Sethi, G., Kumar, A.P. and Tan, K.H.B. 2011. Anticancer activity of thymoquinone in breast cancer cells: possible involvement of PPAR-γ pathway. Biochem. Pharmacol. 82(5), 464–475. Yip, H.Y.K. and Papa, A. 2021. Generation and functional characterization of murine mammary organoids. STAR Protoc. 2(3), 100765. | ||
| How to Cite this Article |
| Pubmed Style Abdelfattah-hassan A, Ibrahim D, Saleh AA, Kishawy AT, Mohamed RH, Khater SI. Effects of 5-fluorouracil, thymoquinone and mammary stem cells’ exosomes on in vitro cultured breast cancer cells. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 525-533. doi:10.5455/OVJ.2024.v14.i1.47 Web Style Abdelfattah-hassan A, Ibrahim D, Saleh AA, Kishawy AT, Mohamed RH, Khater SI. Effects of 5-fluorouracil, thymoquinone and mammary stem cells’ exosomes on in vitro cultured breast cancer cells. https://www.openveterinaryjournal.com/?mno=182418 [Access: July 04, 2025]. doi:10.5455/OVJ.2024.v14.i1.47 AMA (American Medical Association) Style Abdelfattah-hassan A, Ibrahim D, Saleh AA, Kishawy AT, Mohamed RH, Khater SI. Effects of 5-fluorouracil, thymoquinone and mammary stem cells’ exosomes on in vitro cultured breast cancer cells. Open Vet J. 2024; 14((1) (Zagazig Veterinary Conference)): 525-533. doi:10.5455/OVJ.2024.v14.i1.47 Vancouver/ICMJE Style Abdelfattah-hassan A, Ibrahim D, Saleh AA, Kishawy AT, Mohamed RH, Khater SI. Effects of 5-fluorouracil, thymoquinone and mammary stem cells’ exosomes on in vitro cultured breast cancer cells. Open Vet J. (2024), [cited July 04, 2025]; 14((1) (Zagazig Veterinary Conference)): 525-533. doi:10.5455/OVJ.2024.v14.i1.47 Harvard Style Abdelfattah-hassan, A., Ibrahim, . D., Saleh, . A. A., Kishawy, . A. T., Mohamed, . R. H. & Khater, . S. I. (2024) Effects of 5-fluorouracil, thymoquinone and mammary stem cells’ exosomes on in vitro cultured breast cancer cells. Open Vet J, 14 ((1) (Zagazig Veterinary Conference)), 525-533. doi:10.5455/OVJ.2024.v14.i1.47 Turabian Style Abdelfattah-hassan, Ahmed, Doaa Ibrahim, Ayman A. Saleh, Asmaa T.y. Kishawy, Reham H.a. Mohamed, and Safaa I. Khater. 2024. Effects of 5-fluorouracil, thymoquinone and mammary stem cells’ exosomes on in vitro cultured breast cancer cells. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 525-533. doi:10.5455/OVJ.2024.v14.i1.47 Chicago Style Abdelfattah-hassan, Ahmed, Doaa Ibrahim, Ayman A. Saleh, Asmaa T.y. Kishawy, Reham H.a. Mohamed, and Safaa I. Khater. "Effects of 5-fluorouracil, thymoquinone and mammary stem cells’ exosomes on in vitro cultured breast cancer cells." Open Veterinary Journal 14 (2024), 525-533. doi:10.5455/OVJ.2024.v14.i1.47 MLA (The Modern Language Association) Style Abdelfattah-hassan, Ahmed, Doaa Ibrahim, Ayman A. Saleh, Asmaa T.y. Kishawy, Reham H.a. Mohamed, and Safaa I. Khater. "Effects of 5-fluorouracil, thymoquinone and mammary stem cells’ exosomes on in vitro cultured breast cancer cells." Open Veterinary Journal 14.(1) (Zagazig Veterinary Conference) (2024), 525-533. Print. doi:10.5455/OVJ.2024.v14.i1.47 APA (American Psychological Association) Style Abdelfattah-hassan, A., Ibrahim, . D., Saleh, . A. A., Kishawy, . A. T., Mohamed, . R. H. & Khater, . S. I. (2024) Effects of 5-fluorouracil, thymoquinone and mammary stem cells’ exosomes on in vitro cultured breast cancer cells. Open Veterinary Journal, 14 ((1) (Zagazig Veterinary Conference)), 525-533. doi:10.5455/OVJ.2024.v14.i1.47 |