| Original Article | ||
Open Vet J. 2023; 13(3): 278-287 Open Veterinary Journal, (2023), Vol. 13(3): 278–287 Original Research Retrospective evaluation of a hand-sewn side-to-side intestinal anastomosis technique in dogs and catsLuca Ciammaichella, Armando Foglia, Sara Del Magno*, Veronica Cola, Stefano Zanardi, Debora Tinto, Ombretta Capitani, Monika Joechler and Luciano PisoniDipartimento di Scienze Mediche Veterinarie, Alma Mater Studiorum, University of Bologna, Bologna, Italy *Corresponding Author: Sara Del Magno. Dipartimento di Scienze Mediche Veterinarie, Alma Mater Studiorum, University of Bologna, Bologna, Italy. Email: sara.delmagno [at] unibo.it. Submitted: 28/10/2022 Accepted: 09/02/2023 Published: 06/03/2023 © 2023 Open Veterinary Journal
AbstractBackground: Hand-sewn intestinal resection and anastomosis are commonly performed in veterinary medicine. The outcome of the hand-sewn side-to-side anastomosis (SSA) technique has never been described and compared to other techniques in dogs and cats. Aim: The study aims to describe the side-to-side hand-sewn anastomosis technique in small animals and to compare it with the end-to-end technique. Methods: A retrospective evaluation of the clinical records of dogs and cats that underwent enterectomy between 2000 and 2020 and were treated with side-to-side or end-to-end anastomosis (EEA) was performed. Results: Of the 52 dogs and 16 cats included in the study, 19 dogs and 6 cats received an SSA, and the remaining received an EEA. No intraoperative complication was reported. However, short-term complication rates were comparable, and mortality rates in the EEA group were higher. At the same time, stenosis was a frequent complication of SSA and was never reported following EEA. Conclusion: End-to-end technique remains the gold standard for hand-sewn intestinal anastomosis in small animals. However, SSA can be considered for selected cases with acceptable morbidity and mortality rates. Keywords: Anastomosis, Bowel, Cat, Dog, Stenosis. IntroductionIntestinal anastomosis is a surgical procedure commonly performed in veterinary medicine (Giuffrida and Brown, 2018; Ellison et al., 2019). Indications for intestinal resection and anastomosis include neoplasia, intestinal wall necrosis due to non-reducible intussusception, foreign body obstruction, intestinal torsion or volvulus, strangulation, stricture, and traumatic intestinal perforation (Giuffrida and Brown, 2018; Mullen et al., 2020). Intestinal anastomosis can be hand-sewn or realized with mechanical staplers (Ullman, 1994; Weisman et al., 1999; White, 2008; Ellison et al., 2019). Different anastomotic configurations have been proposed in humans and horses, depending on the arrangement of the loops and the underlying disease. Anastomotic compositions for intestinal resection generally include proximal-distal bowel side-to-side anastomosis (SSA), end-to-end anastomosis (EEA), and end-to-side anastomosis. By the proximal-distal bowel peristalsis direction, SSA may be divided into two subtypes: antiperistaltic and isoperistaltic orientation (Feng et al., 2018). Regardless of the technique, the most severe complications are the anastomotic leakage and the dehiscence of the anastomosis, resulting in septic peritonitis, whose incidence is frequently used for evaluation and comparison of the various techniques (Ralphs et al., 2003; Grimes et al., 2011; DePompeo et al., 2018). In human medicine, growing interest has been recently raised in the effect of intestinal anastomosis on the postoperative outcome, and several studies suggest that the anastomotic technique may influence anastomotic leak rates. In this regard, end-to-end ileocolic anastomosis after bowel resection for Crohn’s disease seems to be associated with increased anastomotic leak rates. SSA may lead to fewer postoperative complications and shorter hospital stays (Simillis et al., 2007; Feng et al., 2018). Conversely, several studies in equine surgery suggest that EEA presents higher survival rates and lower complication rates, compared to SSA, notably for the small intestine (Proudman et al., 2007; Stewart et al., 2014; Freeman, 2018). Several surgical options for hand-sewn intestinal anastomosis in dogs and cats have been described, with different threads and suture patterns, but concerning only end-to-end technique (Weisman et al., 1999; Ellison et al., 2019). This study aims to describe the use of SSA hand-sewn anastomosis in dogs and cats and to compare this technique to the EEA hand-sewn technique in terms of complications and outcomes. Materials and MethodsPatient selection and inclusion criteriaThe hospital electronic medical record database (Fenice® 04.70. KA10; ZakSoft S.r.l., Bologna, Italy) was retrospectively reviewed to identify dogs and cats admitted at the “G. Gentile” University Veterinary Hospital of Bologna, from January 2000 to December 2020, and that underwent enterectomy and intestinal anastomosis, using EEA or SSA hand-sewn technique. Animals that received a different anastomosis technique or with incomplete medical records were excluded. Medical records reviewMedical records collected included the patient’s signalment, preoperative clinical conditions, and findings (blood works results, diagnostic imaging, peritoneal fluid culture), reason and description of surgery (intraoperative findings as peritoneal effusion, bowel ischemia, thrombosis, necrosis, perforation or laceration, presence of intestinal mass; site and length of the intestinal excised tract; anastomosis technique; intraoperative complications; duration of anesthesia), histopathological findings, postoperative course (administration of blood products, postoperative short- and long-term complications, length of hospitalization) and outcome. Surgical procedureAnimals were divided into two groups (EEA and SSA) depending on the anastomosis technique performed in each case. The anastomosis technique was chosen according to the surgeon’s preference based on their experience. EEA was performed according to the technique already described (Giuffrida and Brown, 2018). After completing the enterectomy, intestinal edges were juxtaposed by a single-layer, full-thickness simple-interrupted suture pattern, with 3-0 or 4-0 USP monofilament absorbable suture, placing the first and second sutures at the mesenteric and antimesenteric borders, respectively. SSA was performed according to the technique described in equine surgery, which resembles the side-to-side isoperistaltic strictureplasty described in human medicine (Edwards, 1986; Michelassi, 1996). After enterectomy, intestinal stumps were closed with two layers: continuous apposition suture pattern or purse string suture followed by a continuous or interrupted inverting pattern with monofilament absorbable suture material (Fig. 1A). Then, the two stumps were overlapped for approximately an equal length and corresponding to 5–6 times the diameter of the bowel at the site of enterectomy, in an isoperistaltic manner, with antimesenteric side opposed. An incision was made at the antimesenteric side of both parts, of length equal to 2–3 times the diameter of the bowel at the site of enterectomy, to achieve an adequate stoma (Fig. 1B). The incision edges towards the mesenteric side were juxtaposed with an inverting suture, including the seromuscular layers. The submucosa and mucosa were juxtaposed with a continuous layer with 3-0 or 4-0 USP monofilament absorbable suture, including the other edges towards the antimesenteric side, interrupting the suture at the corners (Fig. 1C). Therefore, the anastomosis ended with an inverting suture pattern of the seromuscular layers on the residual edge (Fig. 1D). The interrupted mesentery was then sutured with a continuous appositional pattern. If required, further surgical procedures were performed. A broad-spectrum β-lactamase resistant penicillin was used as the first antimicrobial prophylaxis [ampicillin-sulbactam (Ampicillina + Sulbactam IBI; Welcome Pharma S.p.A., Pomezia (Roma), Italy) 20 mg/kg IV 30 minutes before incision of the skin, then every 120 minutes until the end of the procedure]. In patients with preoperative septic peritonitis, confirmed by cytological examination of peritoneal effusion, antibiotic therapy was empirically implemented with a quinolone [marbofloxacin (Marbocyl F.D. 1%; Vetoquinol Italia, Bertinoro (FC), Italy) 3 mg/kg IV q 24 hours]. Then, the antibiotic treatment was adjusted according to the bacterial culture and sensitivity test results.
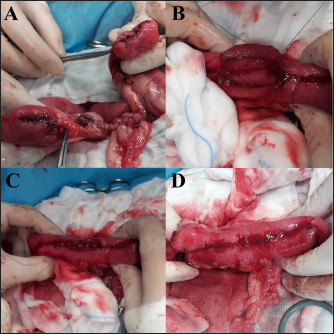
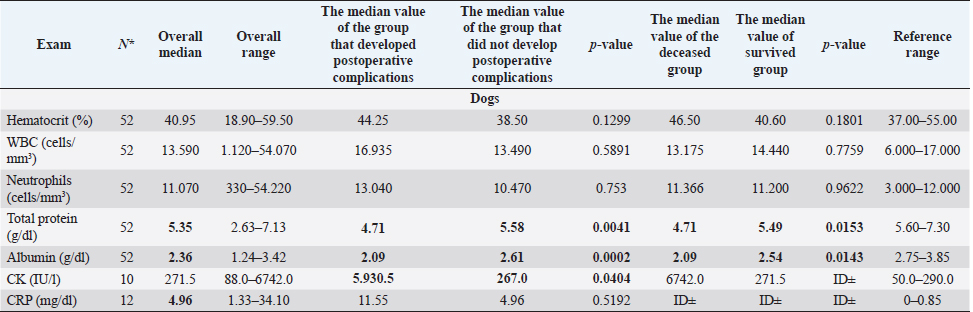
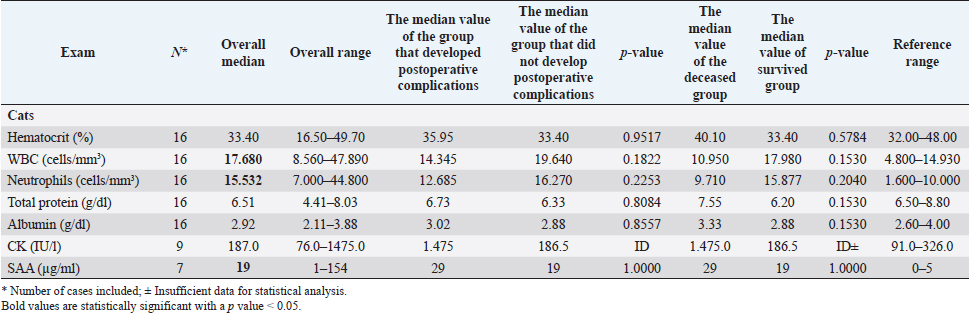
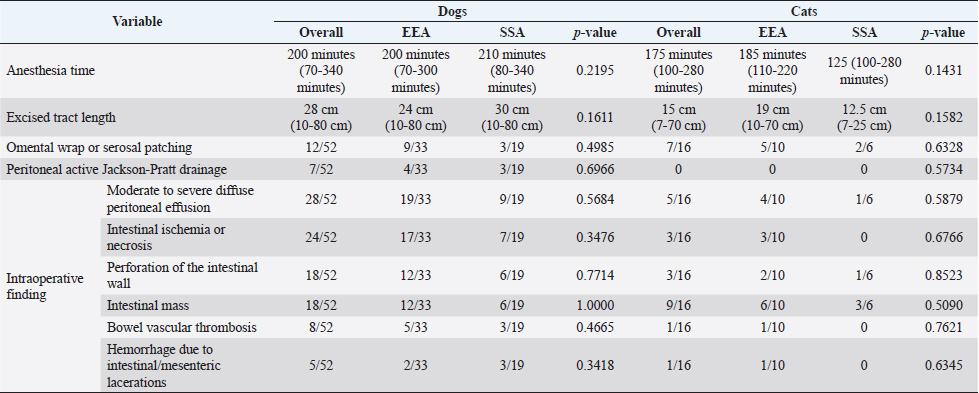
Fig. 1. Main steps of SSA technique. (A): Intestinal stumps closed in two layers with continuous apposition suture pattern followed by the interrupted inverting pattern with monofilament absorbable material. (B): Stumps overlapped with incisions made at the antimesenteric side of both parts. (C): Incision edges juxtaposed with an inverting suture for the seromuscular layers and a continuous appositional layer for the submucosa and mucosa with monofilament absorbable material. (D): Final appearance of the anastomosis after juxtaposition of the seromuscular layers on the residual edge with inverting suture pattern. Postoperative careAll the patients were then hospitalized in the intensive care unit (ICU) for at least 24 hours postoperatively. Analgesia was ensured through opioids [methadone (Semfortan®; Dechra Veterinary Products, Bladel, Netherlands) 0.1–0.2 mg/kg IV q 4 hours, buprenorphine (Temgesic; Indivior Italia S.r.l, Milano, Italy) 10–15 µg/kg IV q 6–8 hours]. Administration of fluid therapy, proton-pump inhibitors, prokinetics, inotropes or vasopressors, and other medications were administered at the discretion of the surgeon and intensivist. US abdominal rechecks and blood exams were performed routinely during the postoperative course. Complications were categorized as intraoperative and postoperative. Postoperative complications were classified as short-term complications, that occurred within one month from surgery, or long-term complications, if they took place more than one month after surgery. Complications were also classified as minor, if responsive to medical therapy, or major, if they required surgical revision or led the patient to death or euthanasia. The follow-up time was calculated as the number of days from the surgery to the patient death or the last clinical recheck. Development of postoperative septic peritonitis was suspected either by clinical, clinic-pathological, or indirect signs at US reassessment, confirmed by cytology of the abdominal effusion and/or bacterial culture. Statistical analysisCollected data were analyzed through medical statistical software (MedCalc®; MedCalc Software Ltd, Ostend, Belgium). Continuous variables were analyzed with the D’Agostino-Pearson test for normality, all summarized by using median and range, while categorical data were described with frequencies and percentages. Fisher exact test was used to compare categorical variables, while the Mann-Whitney U test was performed to confront categorical and continuous data. Statistical significance was set at p ≤ 0.05. Ethical approvalAll patients included in this study were clinically managed according to contemporary standards of care. Informed consent was obtained from pet owners. ResultsEpidemiological data and reason for surgical interventionFifty-two dogs and 16 cats were included. Mixed-breed dogs were the most frequent (16/52), followed by Labrador Retriever (4/52), Boxer (3/52), German Shepherd (3/52), Dachshund (2/52), Dobermann (2/52), Golden Retriever (2/52), Épagneul Breton (2/52), West Highland White Terrier (2/52) and one of each (1/52) of Akita Inu, American Bulldog, American Staffordshire Terrier, Cavalier King Charles Spaniel, Deutscher Drahthaar, English Setter, Flat Coated Retriever, Italian Corso, Maremma Hound, Pomeranian dog, Pug, Rottweiler, Shar-pei, Shi-tzu, Standard Poodle, Swiss Shepherd Dog. The most frequent feline breed was the Domestic shorthair (13/16), followed by Main Coon (2/16) and Siberian (1/16). There were 24/52 male dogs (6/24 neutered) and 28/52 female dogs (16/28 spayed). Seven cats were male (5/7 neutered), and 9/16 were female (7/9 sterilized). The median age was 7 years (range: 1–15 years) for dogs and 6 years (range: 1–15 years) for cats, while the median weight was 17.9 kg (range 3.1–54 kg) for dogs and 4 kg (range: 1.9–6 kg) for cats. Most of the animals included were scheduled for exploratory laparotomy due to intestinal mass (18/52 dogs, 9/16 cats), or they underwent intestinal resection and anastomosis due to vascular damage or bowel wall necrosis caused by foreign body ingestion and occlusion (14/52 dogs, 2/16 cats). Other reasons for surgical intervention were bowel intussusception (8/52 dogs, 2/16 cats), traumatic intestinal lesions (5/52 dogs, 2/16 cats), intestinal herniation with subsequent occlusion (3/52 dogs), volvulus (3/52 dogs), strangulation (1/52 dogs), or stenosis (1/16 cats). There were no differences in terms of breed, gender, age, weight, or reason for enterectomy between the EEA and SSA groups in both species. Preoperative work upIn 48/52 dogs, the unstable clinical condition required hospitalization in the ICU prior to emergency surgical intervention. Four dogs were submitted to elective surgery in 2–5 days (median: 4 days) after the first presentation. Conversely, only 2/16 cats were submitted to emergency surgery in unstable clinical conditions. No difference was found in terms of preoperative clinical conditions between the EEA and SSA groups. Preoperative blood sample results frequently showed hypoproteinemia and hypoalbuminemia in dogs and moderate neutrophilic leukocytosis in cats (Table 1). Moreover, when evaluated, the increase in C-reactive protein and serum amyloid A levels was observed in dogs and cats (Table 1). Preoperative diagnostic imaging was performed in all dogs and cats. US assessment was often used as a unique diagnostic technique (40/52 dogs, 14/16 cats) and eventually combined with radiographic evaluation (8/52 dogs, 2/16 cats). Radiographic examination alone was performed in 12/52 dogs. In addition, US-guided sampling of the peritoneal effusion was performed for cytology and bacterial culture in 10/52 dogs and one cat (1/16). Preoperative septic peritonitis, confirmed by positive bacterial culture, was detected in 9/52 dogs, distributed between EEA (6/9) and SSA (3/9) groups. Escherichia coli was the most frequently isolated bacteria (6/9), alone (4/9), or in association with Pseudomonas aeruginosa (1/9) or Enterococcus spp. (1/9). Other isolated bacteria were Acinetobacter Baumannii (1/9), Klebsiella pneumonia (1/9), and Pseudomonas aeruginosa (1/9). Table 1. Clinic-pathological variables related to complications and outcomes in dogs and cats.
Table 1. Clinic-pathological variables related to complications and outcomes in dogs and cats (cont.)
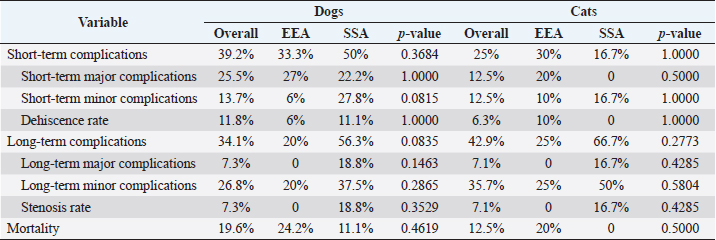
SurgeryEEA was performed in 33/52 dogs and 10/16 cats, while SSA was in 19/52 dogs and 6/16 cats. The jejunum-jejunal anastomosis was the most frequently performed in EEA (23/33 dogs, 8/10 cats) and SSA (14/19 dogs, 6/6 cats) groups. The duodenum-jejunal anastomosis was performed only in 4/33 dogs of the EEA group. The jejunum-ileal anastomosis was performed in 6/33 dogs and 1/10 cats in the EEA group and 3/19 dogs in the SSA group. The jejunum-colic anastomosis was performed in 2/19 dogs of the SSA group and 1/10 cats of the EEA group. Parts of duodenum were removed without ever involving pancreatic or biliary ducts. A single enterectomy and anastomosis was performed in all cases. One dog, presenting a jejunal mass treated with enterectomy and SSA, died intraoperatively from a cardiopulmonary arrest. No other intraoperative complications or decease were reported. Compared to EEA and SSA groups, information regarding intraoperative findings, anesthesia duration, excised tract length, omental wrap, or serosal patching performed, and application of peritoneal drainage connected to an active suction system, are summarized in Table 2. No significant differences were found in surgical findings, site and length of the excised tract, duration of anesthesia, and procedures performed between the two groups. Histopathological assessmentThe excised intestinal tract was submitted for histopathological assessment in 26/52 canine cases and 12/16 feline cases, with no significant differences between the two groups. Histopathological results are summarized in Table 3. Postoperative periodBlood products (packed red blood cells or fresh frozen plasma) were equally administered in EEA (6/33 dogs and 1/10 cats) and the SSA group (10/18 dogs and 1/6 cats). Generally, blood product administration was positively associated with the development of postoperative complications in dogs ( p=0.00009) and cats (p=0.05) and to a worse outcome in dogs ( p=0.00003), regardless of the group. No significant association was found between the type of antibiotics administered, peritoneal fluid culture positivity, bacteria isolated, and outcome between the two groups. Overall median hospitalization time was 5 days (range: 0–30 days) for dogs and 4 days (range: 1–19 days) for cats. In dogs, the median hospitalization time of the EEA group (4 days) was significantly shorter than that of the SSA group (8 days) ( p=0.0044). Median follow-up time was 34 days (range: 3–2,190 days) in dogs and 23.5 days (range: 2–350 days) in cats and did not differ between EEA and SSA groups. Complications and outcomeIn the EEA group, short-term complications were reported in 11/33 dogs and 3/10 cats. Minor complications occurred in 2/33 dogs and 1/10 cats: mild nonseptic peritonitis was reported in 1/33 dogs, while 1/33 dogs and 1/10 cats developed hypothermia, hypotension (systolic blood pressure ≤90 mmHg), and altered mentation, responsive to medical treatment. In 9/33 dogs and 2/10 cats, major complications occurred. Five dogs (5/33) and one cat (1/10) died postoperatively after developing severe sepsis and subsequent septic shock, without US suspicion of anastomosis dehiscence, probably due to the previous septic condition secondary to bowel perforation and septic peritonitis. Four dogs (4/33) and one cat (1/10) developed septic peritonitis and sepsis, with US indirect signs of anastomosis dehiscence (i.e., presence of free peritoneal fluid/gas, loss of intestinal wall stratigraphic detail, focal peritonitis at the site of anastomosis), which was confirmed on cytological evaluation of the abdominal effusion. The cat was euthanized for financial constraints, while a surgical revision was performed in all four dogs. During the second surgery, dehiscence of the intestinal anastomosis was confirmed in all cases, and a new enterectomy with EEA was performed. Three of them (3/4) died in the postoperative course due to sepsis and septic shock complications. Long-term complications in the EEA group occurred in 5/25 dogs and 2/8 cats. These were always minor and characterized by mild and occasional gastroenteric symptoms (inappetence, vomiting, or diarrhea). Overall postoperative mortality of the EEA group was 24.2% in dogs (8/33) and 20% in cats (2/10). In the SSA group, short-term complications occurred in 9/18 dogs and 1/6 cats. Minor complications occurred in 5/18 dogs and 1/6 cats: mild nonseptic peritonitis was reported in 4/18 dogs, while 1/18 dogs and 1/6 cats developed hypothermia, hypotension, and altered mentation responsive to medical treatment. Major complications occurred in 4/18 dogs: 2/18 died postoperatively due to septic shock, without US suspicion of dehiscence. In other 2/18 dogs, with clinical signs of septic peritonitis, dehiscence was suspected at the abdominal US and confirmed on cytological evaluation of the effusion. Animals were surgically revised, receiving a new enterectomy and SSA. Long-term complications of the SSA group were reported in 9/16 dogs and 4/6 cats: 6/16 dogs and 3/6 cats developed minor complications consistent with mild and sporadic gastroenteric symptoms. The only major complication, reported in 3/16 dogs and 1/6 cats, was the stricture of the anastomosis site, which required surgical revision with resection and SSA. Overall postoperative mortality in the SSA group was 11.1% in dogs (2/18). No cat in the SSA group died during the follow-up period. No significant differences in terms of complications or outcomes were found between the two groups (Table 4). Short-term complications occurred in 20/51 dogs and 4/16 cats, while long-term complications occurred in 14/41 dogs and 6/14 cats. The overall postoperative mortality rate was 19.6% in dogs (10/51) and 12.5% in cats (2/16). Considering both groups, dogs that developed short-term complications had significantly lower levels of total protein ( p=0.0041) and albumin ( p=0.0002) and higher levels of creatin-kinase (CK) ( p=0.0404) at the admission time (Table 1). Also, dogs that died postoperatively showed significantly lower levels of total protein ( p=0.0153) and albumin ( p=0.0143) (Table 1). In dogs, preoperative septic peritonitis was associated with a higher rate of dehiscence ( p=0.0313). Furthermore, a wider excision was related to a higher chance of developing postoperative complications ( p=0.0280). In cats, any prognostic factor was significantly associated with the development of complications or decease. Table 2. Surgical variables in dogs and cats compared between EEA and SSA group.
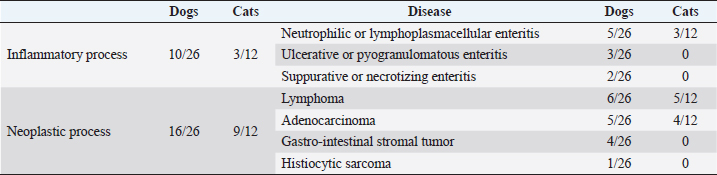
Table 3. Histopathological results in dogs and cats.
Table 4. Complications and outcomes in dogs and cats compared between EEA and SSA group.
DiscussionThis is the first study reporting the clinical application of the SSA technique and comparing hand-sewn SSA and EEA in dogs and cats. Both techniques can be applied to enteral anastomosis in dogs and cats. However, stenosis can develop in the long term when the SSA technique is used. Although the differences in complications were not statistically significant in both species, SSA seems to be an adequate anastomosis technique in the early postoperative period, with comparable short-term major complication rates and dehiscence rates. Furthermore, SSA seems to be a safer procedure in the perioperative period, showing a lower mortality rate in this report. Also, in human medicine, SSA was associated with reduced anastomotic leaks and decreased postoperative complications, although it is preferably performed with staplers (Simillis et al., 2007; Zhu et al., 2011; Feng et al., 2018). However, SSA in this report presented higher risks of long-term complications, mainly stenosis, never reported in the EEA group. Indeed, it has already been described in horses that the mechanical conformation of the new bowel segment in SSA did not restore the intestinal anatomic and physiologic relationship: a blind pouch can develop in the proximal segment of the anastomosis, leading to stasis, obstruction, dilatation, necrosis, and peritonitis (Freeman, 2018). Also, the excessive scar reaction at the stoma can lead to stenosis over time (Edwards, 1986; Freeman, 2018). Conversely, SSA performed for Chron’s disease in humans provides a wider anastomotic lumen, reducing the risk of stasis and subsequent obstruction, and ensures minimal compartmentalization after ileocolic resection and anastomosis, with a good long-term outcome (Simillis et al., 2007; Michelassi et al., 2020). EEA, therefore, remains the preferred technique for hand-sewn intestinal anastomosis in small animals. At the same time, SSA can be alternatively considered for selected conditions, like in human medicine, as when intestinal stumps are markedly different in diameter, in the specific intestinal tract (e.g., ileocolic resection), or in revised enterectomies (Simillis et al., 2007). As reported in previous studies, the leading cause for intestinal resection and anastomosis in dogs (34.6%) and cats (56.3%) was to treat of intestinal masses, followed by intestinal lesions caused by foreign bodies transit in dogs (26.9%) (Ralphs et al., 2003; Mouat et al., 2014; Duell et al., 2016). The jejunum-jejunal anastomosis was the most performed in both EEA (69.7% of dogs, 80% of cats) and SSA (73.7% of dogs, 100% of cats) group, and the development of complications was not linked to the location of the enterectomy and subsequent anastomosis. The histopathological evaluation of the excised intestinal tract confirmed neoplasia in 61.5% of dogs and 75% of cats. The most frequent types of tumors found in the animals included in the study were lymphoma (23% in dogs, 41.6% in cats) and adenocarcinoma (19.2% in dogs, 33.3% in cats), as already reported in the literature (Willard, 2012). Overall median hospitalization time (5 days in dogs, 4 days in cats) was the same as reported in previous studies, considering that major complications, such as dehiscence and anastomosis leakage, usually develop in the 3–5 postoperative days (Davis et al., 2018). However, SSA canine patients stayed hospitalized longer (8 days) than EEA patients (4 days), probably because they presented minor short-term complications more frequently. Factors associated with the development of complications in dogs were the presence of preoperative septic peritonitis, hypoalbuminemia, and higher levels of CK at the admission time in both groups. Preoperative septic peritonitis and hypoalbuminemia have already been reported as relevant prognostic factors in dogs (Grimes et al., 2011; Davis et al., 2018). Conversely, CK activity was never investigated in clinical canine studies concerning intestinal surgical diseases. In humans, it is related to different intestinal abnormalities, such as infarction (Fried et al., 1991). CK is found in different cell types, such as muscular, myocardial, and enteric (Aktas et al., 1993). An amount of this enzyme is lost by intestinal-damaged cells and released in systemic blood circulation during various bowel diseases (Aktas et al., 1993). Unfortunately, the small number of samples analyzed for CK might have misrepresented this result. A wider excision seemed to be associated with an increased risk of complications. However, no animal in the study developed complications strictly related to extensive intestinal resection (i.e., short-bowel syndrome), which is reported with excision of more than 50% of the overall intestinal length (Gorman et al., 2006). Few studies investigated this factor in intestinal resection and anastomosis with discording results, and literature is doubtful in explaining this relationship (Gorman et al., 2006; Mouat et al., 2014). It is possible that dogs needing wider resection presented a larger pathologic bowel, with potentially greater ischemia or necrosis and more severe systemic consequences. Also, wider excisions are performed in patients with large intestinal masses, which could have presented more complications for the oncologic disease rather than the length of the excised tract. No significative prognostic factor was found in cats, probably due to the reduced number of cases. In addition, few authors investigated intestinal resection and anastomosis in cats, and prognostic factors are rarely reported (Weisman et al., 1999; Ralphs et al., 2003). Short term complication rate, relatively high in dogs, was probably affected by the fact that our canine population consisted of animals mostly unstable upon arrival at the hospital and often submitted to non-elective surgery. Despite this, the canine dehiscence rate was consistent with previously reported rates of 11%–28% (DePompeo et al., 2018; Mullen et al., 2020). Feline patients seem less prone to develop intestinal dehiscence, as reported by other authors, even if this finding might have been affected by the strict number of cases in this study (Ralphs et al., 2003). Also, feline patients were usually stable at first presentation and received elective surgery more often than dogs. Long-term complications occurred not rarely but were predominantly minor and characterized by mild and sporadic gastroenteric symptoms, possibly related to the underlying intestinal disease. The overall mortality rate was consistent with the previous 12%–21% (Ralphs et al., 2003; Duell et al., 2016). Death was always consequent to complications like septic peritonitis or sepsis, preexisting or related to anastomosis dehiscence. This study presents several limitations due to its retrospective nature. The technique performed was chosen discretionally by the surgeon and was not randomized. Moreover, some data in the clinical records might have been missed, and the specialists involved differed. In addition, a small number of patients included, especially in cats, affected statistical analysis. Also, the heterogeneity of intestinal diseases and the variety of clinical conditions and presentations of animals might have impacted the analysis of prognostic factors, complications, and outcomes. In addition, the development of stenosis in both groups could have been underestimated because only symptomatic patients were US reassessed for the development of complications. Lastly, being the first study evaluating the SSA in small animals, the lack of previous literature limited us in assessing complications and prognosis of the technique. The hand-sewn SSA appears safe and effective, presenting a comparable dehiscence rate to the EEA. However, SSA demonstrates inherent complications that might impact the long-term quality of life, like stenosis of the site of anastomosis. EEA remains the gold standard for hand-sewn intestinal anastomosis in dogs and cats. SSA can be used in a few selected cases to overcome stomps discrepancy in specific gastro-intestinal sites or as a rescue technique in multiple revised enterectomies. Conflict of interestThe Authors declare that there is no conflict of interest. Author contributionsConceptualization and writing the original draft: Luca Ciammaichella, Armando Foglia, Sara Del Magno, Veronica Cola, Luciano Pisoni. Investigation: Luca Ciammaichella, Sara Del Magno, Veronica Cola. Data curation: Luca Ciammaichella, Veronica Cola, Stefano Zanardi, Debora Tinto. Statistical analysis: Luca Ciammaichella. Writing review and editing: all Authors. Supervision: Sara Del Magno, Ombretta Capitani, Monika Joechler, Luciano Pisoni. All Authors read and approved the final manuscript. ReferencesAktas, M., Auguste, D., Lefebvre, H.P., Toutain, P.L. and Braun, J.P. 1993. Creatine kinase in the dog: a review. Vet. Res. Commun. 17(5), 353–369. DePompeo, C.M., Bond, L., George, Y.E., Mezzles, M.J., Brourman, J.D., Chandler, J.C., Murphy, S.M., Pike, F. and Mason, D.R. 2018. Intra-abdominal complications following intestinal anastomoses by suture and staple techniques in dogs. J. Am. Vet. Med. Assoc. 253(4), 437–443. Davis, D.J., Demianiuk, R.M., Musser, J., Podsiedlik, M. and Hauptman, J. 2018. Influence of preoperative septic peritonitis and anastomotic technique on the dehiscence of enterectomy sites in dogs: a retrospective review of 210 anastomoses. Vet. Surg. 47(1), 125–129. Duell, J.R., Thieman Mankin, K.M., Rochat, M.C., Regier, P.J., Singh, A., Luther, J.K., Mison, M.B., Leeman, J.J. and Budke, C.M. 2016. Frequency of dehiscence in hand-sutured and stapled intestinal anastomoses in dogs. Vet. Surg. 45(1), 100–103. Edwards, G.B. 1986. Resection and anastomosis of small intestine: current methods applicable to the horse. Equine Vet. J. 18(4), 322–330. Ellison, G.W., Case, J.B. and Regier, P.J. 2019. Intestinal surgery in small animals: historical foundations, current thinking, and future horizons. Vet. Surg. 48(7), 1171–1180. Feng, J.S., Li, J.Y., Yang, Z., Chen, X.Y., Mo, J.J. and Li, S.H. 2018. Stapled side-to-side anastomosis might be benefit in intestinal resection for Crohn's disease: A systematic review and network meta-analysis. Medicine (Baltimore). 97(15), e0315. Freeman, D.E. 2018. Small intestine. In Equine Surgery, 5th ed. Eds., Auer, J.A. and Stick, J.A., Missouri: Elsevier, pp: 536–574. Fried, M.W., Murthy, U.K., Hassig, S.R., Woo, J. and Oates, R.P. 1991. Creatine kinase isoenzymes in the diagnosis of intestinal infarction. Dig. Dis. Sci. 36(11), 1589–1593. Giuffrida, M.A. and Brown, D.C. 2018. Small intestine. In Veterinary Surgery: Small Animals. 2nd ed. Eds., Johnston S.A. and Tobias K.M. Missouri: Elsevier, pp: 1730–1761. Gorman, S.C., Freeman, L.M., Mitchell, S.L. and Chan, D.L. 2006. Extensive small bowel resection in dogs and cats: 20 cases (1998–2004). J. Am. Vet. Med. Assoc. 228(3), 403–407. Grimes, J.A., Schmiedt, C.W., Cornell, K.K. and Radlinksy, M.A.G. 2011. Identification of risk factors for septic peritonitis and failure to survive following gastrointestinal surgery in dogs. J. Am. Vet. Med. Assoc. 238(4), 486–494. Michelassi, F. 1996. Side-to-side isoperistaltic strictureplasty for multiple Crohn's strictures. Dis. Colon. Rectum. 39(3), 345–349. Michelassi, F., Mege, D., Rubin, M. and Hurst, R.D. 2020. Long-term results of the side-to-side isoperistaltic strictureplasty in Crohn disease: 25-year follow-up and outcomes. Ann. Surg. 272(1), 130–137. Mouat, E.E., Davis, G.J., Drobatz, K.J. and Wallace, K.A. 2014. Evaluation of data from 35 dogs pertaining to dehiscence following intestinal resection and anastomosis. J. Am. Anim. Hosp. Assoc. 50(4), 254-263. Mullen, K.M., Regier, P.J., Ellison, G.W. and Londoño, L. 2020. A review of normal intestinal healing, intestinal anastomosis, and the pathophysiology and treatment of intestinal dehiscence in foreign body obstructions in dogs. Top. Companion Anim. Med. 41, 100457. Proudman, C.J., Edwards, G.B. and Barnes, J. 2007. Differential survival in horses requiring end-to-end jejunojejunal anastomosis compared to those requiring side-to-side jejunocaecal anastomosis. Equine Vet. J. 39(2), 181–185. Ralphs, S.C., Jessen, C.R. and Lipowitz, A.J. 2003. Risk factors for leakage following intestinal anastomosis in dogs and cats: 115 cases (1991–2000). J. Am. Vet. Med. Assoc. 223(1), 73–77. Simillis, C., Purkayastha, S., Yamamoto, T., Strong, S.A., Darzi, A.W. and Tekkis, P.P. 2007. A meta-analysis comparing conventional end-to-end anastomosis vs. other anastomotic configurations after resection in Crohn's disease. Dis. Colon. Rectum. 50(10), 1674–1687. Stewart, S., Southwood, L.L. and Aceto, H.W. 2014. Comparison of short-and long-term complications and survival following jejunojejunostomy, jejunoileostomy and jejunocaecostomy in 112 horses: 2005–2010. Equine Vet. J. 46(3), 333–338. Ullman, S.L. 1994. Surgical stapling of the small intestine. Vet. Clin. North Am. Small Anim. Pract. 24(2), 305–322. Weisman, D.L., Smeak, D.D., Birchard, S.J. and Zweigart, S.L. 1999. Comparison of a continuous suture pattern with a simple interrupted pattern for enteric closure in dogs and cats: 83 cases (1991-1997). J. Am. Vet. Med. Assoc. 214(10), 1507–1510. White, R.N. 2008. Modified functional end-to-end stapled intestinal anastomosis: technique and clinical results in 15 dogs. J. Small Anim. Pract. 49(6), 274–281. Willard, M.D. 2012. Alimentary neoplasia in geriatric dogs and cats. Vet. Clin. Small Anim. Pract. 42(4), 693–706. Zhu, W.M., Li, Y., Yu, C., Zhang, W., Li, N. and Li, J.S. 2011. Impact of anastomosis type on postoperative recurrence after bowel resection for Crohn disease. Zhonghua wei chang wai ke za zhi. 14(3), 168–170. | ||
| How to Cite this Article |
| Pubmed Style Ciammaichella L, Foglia A, Magno SD, Cola V, Zanardi S, Tinto D, Capitani O, Joechler M, Pisoni L. Retrospective evaluation of an hand-sewn side-to-side intestinal anastomosis technique in dogs and cats. Open Vet J. 2023; 13(3): 278-287. doi:10.5455/OVJ.2023.v13.i3.4 Web Style Ciammaichella L, Foglia A, Magno SD, Cola V, Zanardi S, Tinto D, Capitani O, Joechler M, Pisoni L. Retrospective evaluation of an hand-sewn side-to-side intestinal anastomosis technique in dogs and cats. https://www.openveterinaryjournal.com/?mno=125328 [Access: July 12, 2025]. doi:10.5455/OVJ.2023.v13.i3.4 AMA (American Medical Association) Style Ciammaichella L, Foglia A, Magno SD, Cola V, Zanardi S, Tinto D, Capitani O, Joechler M, Pisoni L. Retrospective evaluation of an hand-sewn side-to-side intestinal anastomosis technique in dogs and cats. Open Vet J. 2023; 13(3): 278-287. doi:10.5455/OVJ.2023.v13.i3.4 Vancouver/ICMJE Style Ciammaichella L, Foglia A, Magno SD, Cola V, Zanardi S, Tinto D, Capitani O, Joechler M, Pisoni L. Retrospective evaluation of an hand-sewn side-to-side intestinal anastomosis technique in dogs and cats. Open Vet J. (2023), [cited July 12, 2025]; 13(3): 278-287. doi:10.5455/OVJ.2023.v13.i3.4 Harvard Style Ciammaichella, L., Foglia, . A., Magno, . S. D., Cola, . V., Zanardi, . S., Tinto, . D., Capitani, . O., Joechler, . M. & Pisoni, . L. (2023) Retrospective evaluation of an hand-sewn side-to-side intestinal anastomosis technique in dogs and cats. Open Vet J, 13 (3), 278-287. doi:10.5455/OVJ.2023.v13.i3.4 Turabian Style Ciammaichella, Luca, Armando Foglia, Sara Del Magno, Veronica Cola, Stefano Zanardi, Debora Tinto, Ombretta Capitani, Monika Joechler, and Luciano Pisoni. 2023. Retrospective evaluation of an hand-sewn side-to-side intestinal anastomosis technique in dogs and cats. Open Veterinary Journal, 13 (3), 278-287. doi:10.5455/OVJ.2023.v13.i3.4 Chicago Style Ciammaichella, Luca, Armando Foglia, Sara Del Magno, Veronica Cola, Stefano Zanardi, Debora Tinto, Ombretta Capitani, Monika Joechler, and Luciano Pisoni. "Retrospective evaluation of an hand-sewn side-to-side intestinal anastomosis technique in dogs and cats." Open Veterinary Journal 13 (2023), 278-287. doi:10.5455/OVJ.2023.v13.i3.4 MLA (The Modern Language Association) Style Ciammaichella, Luca, Armando Foglia, Sara Del Magno, Veronica Cola, Stefano Zanardi, Debora Tinto, Ombretta Capitani, Monika Joechler, and Luciano Pisoni. "Retrospective evaluation of an hand-sewn side-to-side intestinal anastomosis technique in dogs and cats." Open Veterinary Journal 13.3 (2023), 278-287. Print. doi:10.5455/OVJ.2023.v13.i3.4 APA (American Psychological Association) Style Ciammaichella, L., Foglia, . A., Magno, . S. D., Cola, . V., Zanardi, . S., Tinto, . D., Capitani, . O., Joechler, . M. & Pisoni, . L. (2023) Retrospective evaluation of an hand-sewn side-to-side intestinal anastomosis technique in dogs and cats. Open Veterinary Journal, 13 (3), 278-287. doi:10.5455/OVJ.2023.v13.i3.4 |