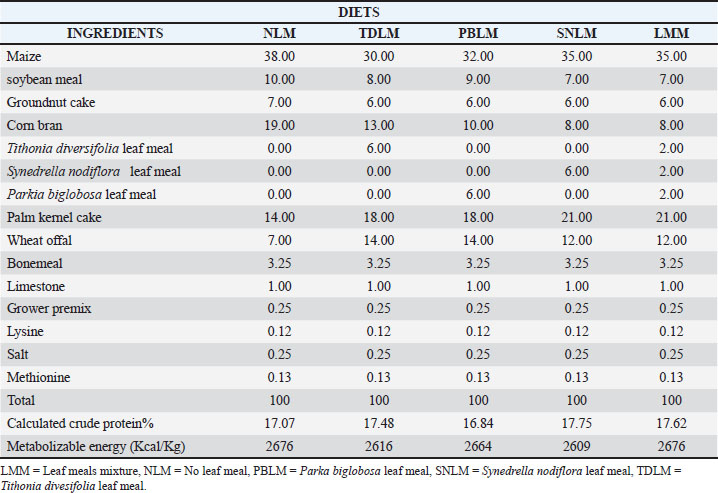
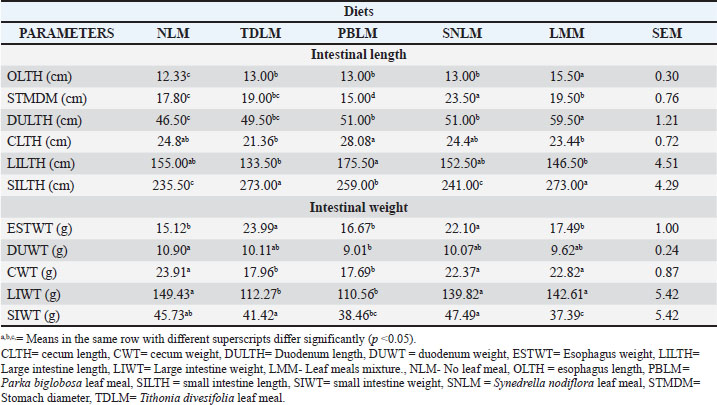
| Research Article | ||
Open Vet. J.. 2025; 15(9): 4709-4715 Open Veterinary Journal, (2025), Vol. 15(9): 4709-4715 Research Article Gut morphometric and duodenal histologic changes caused by dietary inclusions of different plant species in leaf mealsOkeniyi Funmilayo Abimbola1, Olajide Olasunkanmi Peter1,2*, Animashaun Rasaq Adekunle1, Olawoye Samuel Oyewale1, Amodu Opeyemi Temidayo11Animal Science Department, College of Agricultural Science, Landmark University, Omu-Aran, Nigeria 2 Animal Science Department, Sustainable Development Goal (SDG) 12: Responsible Consumption and Production, Landmark University, Omu-Aran, Nigeria *Corresponding Author: Olajide Olasunkanmi Peter, Sustainable Development Goal (SDG) 12: Responsible Consumption and Production, Landmark University, Omu-Aran, Nigeria. Email: olajide.olasunkanmi [at] lmu.edu.ng Submitted: 07/05/2025 Revised: 01/08/2025 Accepted: 14/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
AbstractBackground: Leaf meals are a good potential alternative feed source for livestock production. However, this is limited by the presence of antinutritional components that must be regulated before inclusion in animal diets. Aim: This study evaluated the gut morphometric and duodenal histological morphometric parameters of local rabbits fed a diet that included various leaf meals. Methods: The local rabbits were aged between 6 and 7 weeks and had an average weight of 900 grams, were placed on five diets [D1, D2, D3, D4, and D5] containing 0% NLM (no leaf meal), 6% TDLM (Tithonia diversifolia leaf meal), 6% PBLM (Parkia biglobosa leaf meal), 6% SNLM (Synedrella nodiflora leaf meal), and 6% LMM (2% TD + 2% PB + 2% SN leaf meal mixture). The diets were arranged in a completely randomized design with five treatments, three replicates, and nine animals per replicate for 56 days. Data collected on gut morphometry were subjected to one-way analysis of variance. Results: The results indicated that the length of the small intestine significantly (p < 0.05) increased in rabbits fed TDLM (273.00 cm) and LMM (273.00 cm), while the lowest value was observed in rabbits fed NLM (235.50 cm). The lengths and weights of the ceca were similar for the NLM, SNLM, and LMM groups but significantly (p < 0.05) different from those of rabbits fed TDLM and PBLM groups, which were also comparable. The duodenal histological results demonstrated that intestinal tissues were moderately affected by the inclusion of leaf meals in the diets. Conclusion: In conclusion, leaf meal inclusion in the diet enhances gut morphometry and development. However, leaf meal inclusion must be controlled and adequately processed to avoid negative health implications on the rabbits based on the observed mild necrosis on the mucosal epithelial cells. Keywords: Dietary, Duodenal, Gut, Leaf meal, Mucosal, Necrosis, Histology. IntroductionThe local rabbit (Oryctolagus cuniculus) is an offshoot of the wild rabbit, initially considered a pest due to its destructive activities on crops (Ayeni et al., 2023; Siudak and Kowalska, 2024). They are naturally herbivores and are very active in the dark and at night, depending mainly on plants (Abd-Elghany et al., 2021). The nutritional intake of local rabbits depends on the availability and quality of feed. During inclement periods, they experience malnourishment, emaciation, and ill health, which affects their gastrointestinal (GIT) health (Animashahun et al., 2024). The competition between humans and animals for available food sources has become a serious issue in rabbit farming (Alabi et al., 2023). However, the local rabbit’s uniqueness is its ability to feed on non-competitive feed materials, such as leaf meals, which do not compete with the conventional food sources of interest to humans (Animashahun et al., 2024). This ability to use leaf meals in their diets can improve feed intake efficiency and possibly benefit local rabbit gut health and general well-being (Abd-Elghany et al., 2021). Studies have shown the potential of leaf meals as a source of nutritional and gut improvement essential for optimum nutrient assimilation and immune functioning (Abdelli et al., 2021; Wang et al., 2024). The GIT tract plays a crucial role in food breakdown, absorption, and total well-being. Therefore, dietary inclusions that aid the GIT health can improve the efficiency of the rabbit (Alabi et al., 2021; Zhang et al., 2022). It is necessary to investigate the dietary effect of leaf meal inclusion on the histological status and profile of the GIT to develop sustainable feeding approaches that will enhance the growth and health status of the local rabbit. This study aimed to evaluate how the inclusion of selected leaf meal in local rabbit diets affects the gut and histological status, thereby contributing to the wider knowledge of using alternative feed sources in rabbit rearing. Materials and MethodsStudy areaThe study was conducted at the Landmark University Teaching and Research farms in Omu-Aran, Kwara State, Nigeria. Experimental animals and their managementThe experiment was conducted in the Rabbit Unit of the Landmark University Teaching and Research Farm. Forty-five unsexed growing rabbits of local breeds between 6 and 7 weeks of age, and with an average weight of 900 g, were allotted to 5 dietary groups of 9 animals per group in a completely randomized design. The rabbits were housed individually in cages measuring 35 cm x 36 cm × 45 cm. The cages were raised one meter above the ground for easy cleaning. The room was cleaned and fumigated a week before the arrival of the rabbits. Thereafter, the room was cleaned daily. Rabbits were dewormed using a broad-spectrum dewormer. The rabbits were acclimatized for 2 weeks before the start of the trial. Feeding and managementThe leaves of Tithonia diversifolia, Parkia biglobosa, and Synedrella nodiflora were collected from across the Landmark University Campus, rinsed thoroughly under running water, and spread in a well-ventilated space to air-dry with daily turning to avoid fungal growth, and allow for constant dried weight. The diets formulated included: 0% NLM (no leaf meal), and 6% of T. diversifolia leaf meal (TDLM), P. biglobosa leaf meal (PBLM), S. nodiflora leaf meal (SNLM) (Liu et al., 2019; Biasato et al., 2025), and LMM (2% TD + 2% PB + 2% SN leaf meal mixture), and were used in a feeding trial for 56 days. Feed was offered to the rabbits twice daily at 8.00 am and 4.00 pm, and water was provided ad libitum. Experimental dietT1(NLM)=Rabbit mash containing 0% leaf meal (LM). T2 (TDLM)=Rabbit mash containing 6% Tithonia diversifolia (TD) leaf meal. T3 (PBLM)=Rabbit mash containing 6% Parkia biglobosa (PB) leaf meal. T4 (SNLM)=Rabbit mash containing 6% Synedrella nodiflora (SN) leaf meal. T5 (LMM)=Rabbit mash containing 6% leaf meal mixture (2% TD + 2% PB + 2% SN). Table 1 shows the composition of the diets with the different leaf meal inclusions. The leaf meal was included in diets 2, 3, and 4. Diet 5 contains all the leaf meals in the same ratio. Diet 1 serves as the control without any leaf meal inclusion. Histology and gut morphometric sampling and measurementsFifteen rabbits were taken off feed for 24 hours were sacrificed for the experiment. The rabbits were fixed on a dissecting board for anatomy, followed by a midline incision in the rabbit’s abdomen. A portion of the duodenum was cut and placed in 10% formalin for histological processing in the laboratory. The gut morphometry was measured using a measuring tape, a metric rule, and a 2-decimal-place sensitive balance. All lengths were measured in centimeters (cm), and weights were measured in grams (g). Statistical analysesThe collected data were subjected to statistical analysis of variance using SPSS (Version 2019), and Duncan’s multiple range test was used to test different means. Ethical approvalThe study was approved by the Landmark University Animal Care Committee, granting the ethical approval number LUAC/BCH/2024/002A. ResultsGastrointestinal tract morphometry in rabbits fed with the experimental dietsThe composition of the trial diets [D1, D2, D3, D4, and D5] containing 0% NLM (no leaf meal), 6% TDLM (Tithonia diversifolia leaf meal), 6% PBLM (Parkia biglobosa leaf meal), 6% SNLM (Synedrella nodiflora leaf meal), and 6% LMM (2% TD + 2% PB + 2% SN leaf meal mixture) is presented in Table 1. Table 2 shows the effect of different leaf meals and their mixtures on the GIT tract morphometry of rabbits. The esophagus length (OLTH) values obtained were significantly higher (p < 0.05) in MLM at 15.50 cm and lowest in 0%LM at 12.33 cm. The stomach diameter (STMDM) value obtained was significantly higher (p < 0.05) in the SNLM at 23.5 cm and lowest in the PBLM at 15.00 cm. The duodenum length (DULTH) values obtained were significantly higher (p < 0.05) in MLM at 59.50 cm and lowest in 0%LM at 46.50 cm. The large intestine length (LILTH) values obtained were similar (p > 0.05) in 0%LM and SNLM, while PBLM at 175.5 cm was significantly higher (p < 0.05) for the LILTH. The small intestine length (SILTH) values were significantly higher (p < 0.05) in TDLM and MLM. The esophagus weight (ESTWT) values were significantly higher (p <0.05) in the TDLM and SNLM groups. The duodenum weight (DUWT) values obtained were similar (p > 0.05) in the TDLM, SNLM, and MLM, whereas 0%LM at 10.90 grams was significantly highest (p <0.05) across diets. The large intestine weight (LIWT) values obtained were significantly lower (p < 0.05) in the TDLM and PBLM groups. The SIWT value was significantly least (p < 0.05) in MLM across diets. Table 1. Composition of the experimental diet fed to the local rabbit breed.
Table 2. Effect of different leaf meals and their mixtures on rabbit gastrointestinal tract morphometry.
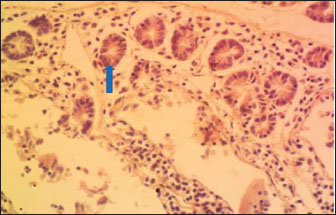
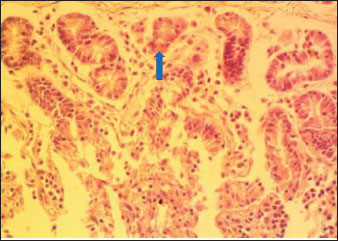
Histology of the duodenum in rabbits fed with the experimental dietsThe histology of the duodenum in local rabbits fed with diet 1 (NLM) is shown in Figure 1. The histological slide indicates that the duodenum in rabbits not incorporating leaf meal in their diet appears normal, with no inflammatory cell infiltration and intestinal tissue necrosis. The histology of the duodenum in rabbits fed with diet 2 (TDLM) is presented in Fig. 2. The slide reveals that the duodenum in rabbits consuming Tithonia diversifolia shows moderate necrosis of the crypts of Lieberkühn and mucosal epithelial cells. The histology of the duodenum in rabbits fed with diet 3 (PBLM) is depicted in Figure 3. The histological slide indicates that the duodenum of rabbits fed with Parkia biglobosa exhibits moderate necrosis of the Lieberkühn crypt and mucosal epithelial cells, accompanied by infiltration of inflammatory cells. The histology of the duodenum in rabbits fed with diet 4 (SNLM) is displayed in Figure 4. The histological slide shows that the duodenum in rabbits consuming Synedrella nodiflora presents moderate necrosis of Lieberkühn crypts and mucosal epithelial cells, with inflammatory cell infiltration. The histology of the duodenum in rabbits fed with diet 5 (LMM) is illustrated in Fig. 5. The histological slide shows that the duodenum in rabbits consuming a leaf meal mixture presents moderate necrosis of Lieberkühn crypts and mucosal epithelial cells, with mild infiltration of inflammatory cells. DiscussionThe morphometry of the GIT of the rabbits was significantly different among the diets, demonstrating the influence of leaf meals as phytogenic feed additives on intestinal function and development (Upadhaya and Kim, 2017). LMM-fed rabbits with a higher esophagus length (OLTH) of 15.50 cm demonstrate improved gut elongation, possibly due to their fiber and biologically active compounds (Zhang et al., 2022). However, it was the shortest in the NLM (12.33 cm). This pattern is reflected in, but LMM-fed rabbits had significantly longer segments, signifying enhanced digestive surface area and potential nutrient absorption capacity (Ayodele et al., 2016). The SNLM remarkably increased the stomach diameter (STMDM: 23.50 cm), whereas it was lower in the PBLM (15.00 cm), possibly reflecting differential gut motility or development stimulation (Xin et al., 2023). The large intestinal weight (LIWT) was heavier in the LMM and SNLM diets, relating to improved microbial activity (Solans et al., 2019). In particular, the NLM showed the highest duodenal weight (DUWT), suggesting localized tissue adaptation due to antinutritional factors or increased digestion workload (Adegbeye et al., 2020). The LMM and SNLM-fed rabbit’s cecal weight (CWT) was higher, attributable to improved fermentative activity. This trend is shown for the small intestine weight (SIWT) in the SNLM and TDLM, potentially due to enhanced villus development or epithelial hypertrophy (Aderemi, 2017).
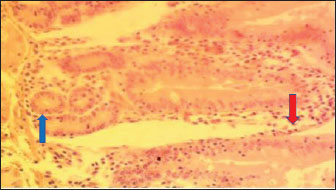
Fig. 1. (NLM): Section of the intestine appearing apparently normal (arrow) (x400; H & E).
Fig. 2. (TDLM): Section of the intestine showing moderated necrosis of Crept of Lieberkühn (arrow) and mucosal (arrow head) epithelial cells (x400; H & E).
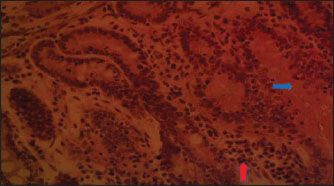
Fig. 3. (PBLM): Section of the intestine showing moderated necrosis of Crept of Lieberkühn (blue arrow) and mucosal (arrow head) epithelial cells, with infiltration of inflammatory cells (red arrow) (x400; H & E).
Fig. 4. (SNLM): Section of the intestine showing moderated necrosis of Crept of Lieberkühn (blue arrow) and mucosal (arrow head) epithelial cells, with infiltration of inflammatory cells (red arrow) (x400; H & E).
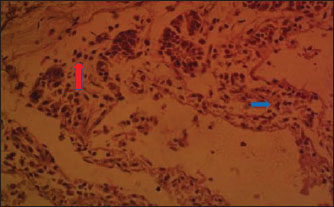
Fig. 5. (LMM) Section of the intestine showing moderated necrosis of Crept of Lieberkühn (blue arrow) and mucosal (arrow head) epithelial cells, with mild infiltration of inflammatory cells (red arrow) (x400; H & E). In the NLM shown in Figure 1, the mucosal lining was complete, with the Lieberkühn villi and crypts properly arranged. The epithelial layer, largely composed of absorptive enterocytes and goblet cells, was well preserved and did not show cellular erosion or necrosis (An et al., 2022). The lamina propria appeared to be free of inflammatory infiltrations, and the make-up was not atrophied or distorted, indicating a healthy mucosal barrier and optimum operational capacity (France and Turner, 2017). The TDLM (Fig. 2) had effects that showed cellular disintegration of the Lieberkühn crypts, signifying epithelial rejuvenation (Liu et al., 2019). At the same time, mucosal epithelial inner layer necrosis, shown by disturbed cellular structure and cytoplasmic degradation, suggests a distorted mucosal barrier that may amplify absorptivity, poor nutrient assimilation, and increased susceptibility to enteric pathogens (Chistiakov et al., 2014; De Medina et al., 2014). These observed changes can be due to the secondary metabolites like saponins, alkaloids, phenolics, and tannins present in Tithonia diversifolia, which are known to cause cytotoxic, anti-proliferative, and membrane-disrupting activities in the GIT tract when taken unprocessed (Abdelsalam and Fathi, 2023). These observations demonstrate the need for detoxification, quantification, and probably fermentation when incorporating TDLM into the diet of local rabbits (Abd-Elghany et al., 2021). Although TDLM provides a good source of nutritious and phytogenic benefits, its raw inclusion must be controlled to avoid compromising gut integrity and performance (Sugiharto et al, no date). In the observed PBLM in Figure 3, the necrosis in the crypt of Lieberkühn shows disturbance of the proliferative region crucial for epithelial regeneration and intestinal homeostasis (Chistiakov et al., 2014). Impairment of the crypts affects enterocyte production, which may cause weakened absorptive and digestive effectiveness in the small intestine (Buckley and Turner, 2018). Concurrently, mucosal epithelial cell degeneration and necrosis show intestinal barrier failure, increasing the danger of luminal antigen translocation and mucosal susceptibility (An et al., 2022). The permeation of inflammatory cells, mainly lymphocytes and macrophages, demonstrates a limited immune response that may be a reaction to epithelial injury caused by phytochemicals in Parkia biglobosa leaves (Franz et al., 2019). These bioactive constituents possess antioxidant and antimicrobial properties and may cause cytotoxic effects at high levels of inclusion in unprocessed form. Tannins and saponins can tamper with membrane integrity, modify gut microflora, and trigger mucosal irritation (De Medina et al., 2014). These effects are consistent with the inflammatory and degenerative changes observed in the duodenal mucosa (Chistiakov et al., 2014; De Medina et al., 2014). The observed necrosis in the crypt that controls epithelial cell production and mucosal renewal in rabbits fed SNLM indicates weakened cellular turnover (Buckley and Turner, 2018). Moreover, the loss of mucosal epithelial cells’ integrity shows that the absorptive and protective barrier is compromised, which is crucial for nutrient uptake and host protection (Liu et al., 2022). The infiltration of inflammatory cells, principally comprising lymphocytes and plasma cells, into the lamina propria, as shown in Figure 4, demonstrates an ongoing immune response that may have been prompted by mucosal or antigenic disturbance from biologically active compounds in Synedrella nodiflora. If the concentration is not regulated, this can lead to a cytotoxic or pro-inflammatory effect on the mucosa of the GIT (Jha et al., 2019). Specifically, saponins are known to disrupt membranes and increase gut penetrability, possibly leading to reactionary inflammation and epithelial damage (Ohimain et al., 2020). Necrosis of the Lieberkühn crypt observed in the LMM (Fig. 5) and damage to the mucosal epithelial cells affect the intestinal epithelium, compromising absorptive and secretory activities crucial for gut health (Buckley and Turner, 2018). This pattern is consistent with that observed in other leaf meals; however, the infiltration of inflammatory cells appeared mild, suggesting a moderate but continuous controlled immune response, possibly to clear away cellular fragments and restore mucosal integrity (Chistiakov et al., 2014; De Medina et al., 2014). This trend aligns with subacute intestinal damage, where soreness is present but not distressing, enabling mucosal recovery if the injurious stimuli are removed (France and Turner, 2017; An et al., 2022). ConclusionThe current study shows that the LMM diet provided better intestinal length and weight, demonstrating a greater possibility for improved gut morphometry and digestive efficiency in local rabbits. SNLM also indicates promising properties in the cecum and duodenum. The effect of leaf meals on the histological structure of the duodenum suggests that although they offer functional benefits in the rabbits’ nutrition, their inclusion must be controlled to mitigate mucosal damage and ensure gut health. Adequate nutrient availability from dietary fiber origin is crucial for maintaining villus height, crypt depth, and mucosal revitalization, contributing to digestion and nutrient absorption efficiency. AcknowledgmentsThe authors acknowledge the proprietor of Landmark University for providing an enabling environment for research. FundingThis work did not receive any funding during the field study. Authors’ contributionsOFA, ARA, and AOT designed the protocol and interpreted the experimental results. OFA, AOT, OOP, and OSO performed experiments and analyzed, and interpreted the data. OFA, AOT, ARA, OOP, and OSO drafted the initial manuscript. All authors have approved the final version of the manuscript. Conflict of interestThe authors declare no conflict of interest. Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesAbd-Elghany, F., El-Gebali, M. and Gad, G. 2021. Influence of papaya leaves and their extract supplementation on growing rabbit performance, physiological traits, immune responses and oxidative enzyme status. Egypt. J. Rabbit Sci. 31(2), 171–198; doi:10.21608/ejrs.2021.205663 Abdelli, N., Solà-Oriol, D. and Pérez, J.F. 2021. Phytogenic feed additives in poultry: achievements, prospective and challenges. Animals 11, 3471; doi:10.3390/ani11123471 Abdelsalam, M. and Fathi, M. 2023. Improving productivity in rabbits by using some natural feed additives under hot environmental conditions — A review. Anim. Biosci. 36(4), 540–554; doi:10.5713/ab.22.0354 Adegbeye, M.J., Elghandour, M.M.M.Y., Faniyi, T.O., Rivero Perez, N., Barbabosa-Pilego, A., Zaragoza-Bastida, A. and Salem, A.Z.M. 2020. Antimicrobial and antihelminthic impacts of black cumin, pawpaw and mustard seeds in livestock production and health. Agrofor. Syst. 94, 1255–1268; doi:10.1007/s10457-018-0337-0 Aderemi, F.A. 2017. Leaf meal effect on rabbits. J. Livest. Sci. 8(8), 28–34. Alabi, O.O., Animashahun, R.A., Oluwafemi, P.T., Shoyombo, A.J., Olawoye, S.O., Okeniyi, F.A. and Falana, B.M. 2021. Histological indices and growth performance of noiler chickens fed dietary inclusion of locust bean leaf meal. Ann. RSCB. 25(6), 16971–16978. Alabi, O.O., Olajide, O.P., Dasaolu, O.S. and Ologbosere, Y.D. 2023. Evaluation of the nutritive qualities of ripe and unripe plantain peels as a potential feed ingredient. JAAT 12(1), 54–69. An, J., Liu, Y., Wang, Y., Fan, R., Hu, X., Zhang, F., Yang, J. and Chen, J. 2022. the role of intestinal mucosal barrier in autoimmune disease: a potential target. Front. Immunol. 13, 1–11; doi:10.3389/fimmu.2022.871713 Animashahun, R., Okeniyi, F., Alabi, O., Olawoye, S., Oluwafemi, P., Odhe, P., Akintola, O. and Animashahun, A. 2024. Impact of Tithonia, Parkia and Synedrella leaf meals in kit rabbits’ diets: growth performance, carcass indices and meat quality. Adv. Anim. Vet. Sci. 12(9), 1731–1739; doi:10.17582/journal.aavs/2024/12.9.1731.1739 Ayeni, M.D., Adewumi, M.O., Bello, M.A., Adiadi, K.F. and Osungade, A.A. 2023. Effects of rabbit production on income and livelihood of rural households in Nigeria. Heliyon 9(8), e18568; doi:10.1016/j.heliyon.2023.e18568 Ayodele, S.O., Oloruntola, O.D. and Agbede, J.O. 2016. Effect of Alchornea cordifolia leaf meal inclusion and enzyme supplementation on performance and digestibility of rabbits. World Rabbit Sci. 24(3), 201–206; doi:10.4995/WRS.2016.3933 Biasato, I., Renna, M., Cerutti, G.L., Maccagno, F., Lussiana, C., Ferrocino, I., Bellezza Oddon, S., Caimi, C., Xiccato, G., Trocino, A., Pauciullo, A., Brugiapaglia, A. and Gasco, L. 2025. Use of mulberry leaf meal in fattening diets for rabbits: a comprehensive approach from animals to consumers. animal 19(7), 101546; doi:10.1016/j.animal.2025.101546 Buckley, A. and Turner, J.R. 2018. Cell biology of tight junction barrier regulation and mucosal disease. Cold Spring Harbor Perspect. Biol. 10(1), a029314–a029317; doi:10.1101/cshperspect.a029314 Chistiakov, D.A., Bobryshev, Y.V., Kozarov, E., Sobenin, I.A. and Orekhov, A.N. 2014. Intestinal mucosal tolerance and impact of gut microbiota to mucosal tolerance. Front. Microbiol. , 1–9; doi:10.3389/fmicb.2014.00781 France, M.M. and Turner, J.R. 2017. The mucosal barrier at a glance. J. Cell Sci. 130(2), 307–314; doi:10.1242/jcs.193482 Franz, C.M., Baser, K.H.C. And Hahn-Ramssl, I. 2019. Herbs and aromatic plants as feed additives: aspects of composition, safety, and registration rules. Feed Additive. pp. 35–56. Academic Press; doi: 10.1016/B978-0-12-814700-9.00003-0 Jha, R., Fouhse, J.M., Tiwari, U.P., Li, L. and Willing, B.P. 2019. Dietary fiber and intestinal health of monogastric animals. Front. Vet. Sci. 6, 00048; doi:10.3389/fvets.2019.00048 Liu, B., Cui, Y., Ali, Q., Zhu, X., Li, D., Ma, S., Wang, Z. and Shi, Y. 2022. Gut microbiota modulate rabbit meat quality in response to dietary fiber. Front. Nutr. 9, 849429; doi:10.3389/fnut.2022.849429 Liu, L., Zuo, W. and Li, F. 2019. Dietary addition of Artemisia argyi reduces diarrhea and modulates the gut immune function without affecting growth performances of rabbits after weaning. J. Anim. Sci. 97(4), 1693–1700; doi:10.1093/jas/skz047 Ohimain, E., Nodu, M. and Imoni, O. 2020. Effect of Alchornea Cordifolia and Costus Afer based diet on gut microbes of male rabbits. NAPAS 3(3a), 20–26; doi:10.46912/napas.181 Sánchez De Medina, F., Romero-Calvo, I., Mascaraque, C. and Martínez-Augustin, O. 2014. Intestinal inflammation and mucosal barrier function. Inflamm. Bowel Dis. 20(12), 2394–2404; doi:10.1097/MIB.0000000000000204 Siudak, Z. and Kowalska, D. 2024. Dietary supplements used in rabbit nutrition and their effect on the fatty acid profile of rabbit meat - a review. J. Anim. Feed Sci. 33, 159–169; doi:10.22358/jafs/172585/2023 Solans, L., Arnal, J.L., Sanz, C., Benito, A., Chacón, G., Alzuguren, O. and Fernández, A.B. 2019. Rabbit enteropathies on commercial farms in the Iberian peninsula: etiological agents identified in 2018–2019. Animals 9(9), 1142; doi:10.3390/ani9121142 SPSS. 2019. Version, S. 26. 0., IBM Corp. Released. IBM Armonk, NY: IBM Corp. Sugiharto, S., Yudiarti, T., Isroli, I., Widiastuti, E., Wahyuni, H.I. and Sartono, T.A. 2019. Recent advances in the incorporation of leaf meals in broiler diets. LRRD 31(7), 31. Upadhaya, S., D., Kim, I. and H. 2017. Efficacy of phytogenic feed additive on performance, production and health status of monogastric animals - A review. Ann. Anim. Sci. 17(4), 929–948; doi:10.1515/aoas-2016-0079 Wang, J., Deng, L., Chen, M., Che, Y., Li, L., Zhu, L., Chen, G. and Feng, T. 2024. Phytogenic feed additives as natural antibiotic alternatives in animal health and production: a review of the literature of the last decade. Anim. Nutr. 17, 244–264; doi:10.1016/j.aninu.2024.01.012 Xin, M., Zhao, M., Tian, J. and Li, B. 2023. Guidelines for in vitro simulated digestion and absorption of food. Food Front. 4(1), 524–532; doi:10.1002/fft2.186 Zhang, X., Sun, Z., Cao, Y., Chen, Y., Li, S., Wang, G., Zhao, X. and Cao, F. 2022. Effects of dietary inclusion of Xanthoceras sorbifolia Bunge leaves on growth performance, gastrointestinal development, digestive function and gut microbial flora of rabbits. Anim. Feed Sci. Technol. 292, 115438; doi:10.1016/J.ANIFEEDSCI.2022.115438 | ||
| How to Cite this Article |
| Pubmed Style Okeniyi FA, Olajide OP, Animashahun RA, Olawoye SO, Amodu OT. Gut morphometric and duodenal histologic changes caused by dietary inclusions of different plant species in leaf meals. Open Vet. J.. 2025; 15(9): 4709-4715. doi:10.5455/OVJ.2025.v15.i9.75 Web Style Okeniyi FA, Olajide OP, Animashahun RA, Olawoye SO, Amodu OT. Gut morphometric and duodenal histologic changes caused by dietary inclusions of different plant species in leaf meals. https://www.openveterinaryjournal.com/?mno=256772 [Access: January 03, 2026]. doi:10.5455/OVJ.2025.v15.i9.75 AMA (American Medical Association) Style Okeniyi FA, Olajide OP, Animashahun RA, Olawoye SO, Amodu OT. Gut morphometric and duodenal histologic changes caused by dietary inclusions of different plant species in leaf meals. Open Vet. J.. 2025; 15(9): 4709-4715. doi:10.5455/OVJ.2025.v15.i9.75 Vancouver/ICMJE Style Okeniyi FA, Olajide OP, Animashahun RA, Olawoye SO, Amodu OT. Gut morphometric and duodenal histologic changes caused by dietary inclusions of different plant species in leaf meals. Open Vet. J.. (2025), [cited January 03, 2026]; 15(9): 4709-4715. doi:10.5455/OVJ.2025.v15.i9.75 Harvard Style Okeniyi, F. A., Olajide, . O. P., Animashahun, . R. A., Olawoye, . S. O. & Amodu, . O. T. (2025) Gut morphometric and duodenal histologic changes caused by dietary inclusions of different plant species in leaf meals. Open Vet. J., 15 (9), 4709-4715. doi:10.5455/OVJ.2025.v15.i9.75 Turabian Style Okeniyi, Funmilayo Abimbola, Olasunkanmi Peter Olajide, Rasaq Adekunle Animashahun, Samuel Oyewale Olawoye, and Opeyemi Temidayo Amodu. 2025. Gut morphometric and duodenal histologic changes caused by dietary inclusions of different plant species in leaf meals. Open Veterinary Journal, 15 (9), 4709-4715. doi:10.5455/OVJ.2025.v15.i9.75 Chicago Style Okeniyi, Funmilayo Abimbola, Olasunkanmi Peter Olajide, Rasaq Adekunle Animashahun, Samuel Oyewale Olawoye, and Opeyemi Temidayo Amodu. "Gut morphometric and duodenal histologic changes caused by dietary inclusions of different plant species in leaf meals." Open Veterinary Journal 15 (2025), 4709-4715. doi:10.5455/OVJ.2025.v15.i9.75 MLA (The Modern Language Association) Style Okeniyi, Funmilayo Abimbola, Olasunkanmi Peter Olajide, Rasaq Adekunle Animashahun, Samuel Oyewale Olawoye, and Opeyemi Temidayo Amodu. "Gut morphometric and duodenal histologic changes caused by dietary inclusions of different plant species in leaf meals." Open Veterinary Journal 15.9 (2025), 4709-4715. Print. doi:10.5455/OVJ.2025.v15.i9.75 APA (American Psychological Association) Style Okeniyi, F. A., Olajide, . O. P., Animashahun, . R. A., Olawoye, . S. O. & Amodu, . O. T. (2025) Gut morphometric and duodenal histologic changes caused by dietary inclusions of different plant species in leaf meals. Open Veterinary Journal, 15 (9), 4709-4715. doi:10.5455/OVJ.2025.v15.i9.75 |