| Research Article | ||
Open Vet. J.. 2025; 15(9): 4700-4708 Open Veterinary Journal, (2025), Vol. 15(9): 4700-4708 Research Article Increasing the fertility of Sapudi sheep semen at 5°C storage temperature with the addition of oxytocin in a diluent of skim milk, egg yolk, and citrateSri Mulyati1*, Imam Mustofa1, Suherni Susilowati1, Aswin Rafif Khairullah2, Riza Zainuddin Ahmad2, Adeyinka Oye Akintunde3, Bima Putra Pratama4, Latifah Latifah5, Ulvi Fitri Handayani5 and Lili Anggraini51Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 2Research Center for Veterinary Science, National Research and Innovation Agency (BRIN), Bogor, Indonesia 3Department of Agriculture and Industrial Technology, Babcock University, Ilishan Remo, Nigeria 4Research Center for Agroindustry, National Research and Innovation Agency (BRIN), South Tangerang, Indonesia 5Research Center for Animal Husbandry, National Research and Innovation Agency (BRIN), Bogor, Indonesia *Corresponding Author: Sri Mulyati. Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: sri-m [at] fkh.unair.ac.id Submitted: 07/05/2025 Revised: 07/08/2025 Accepted: 24/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
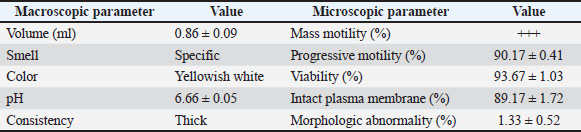
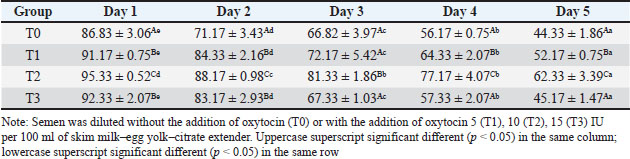
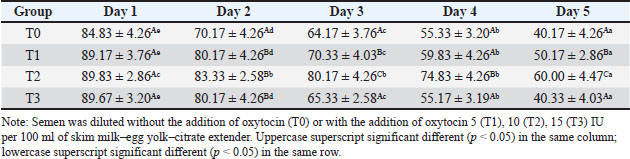
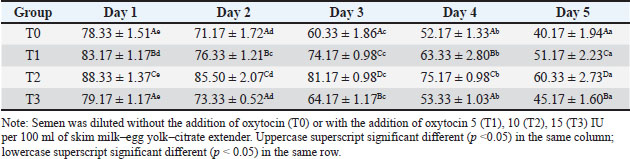
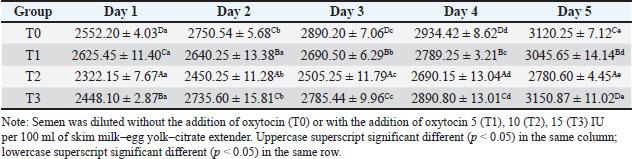
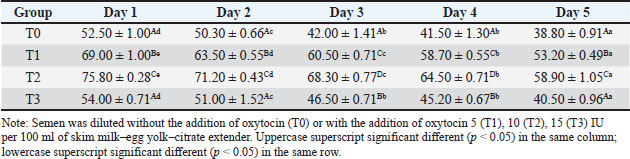
ABSTRACTBackground: Artificial insemination in sheep is one of the important reproductive technologies that accelerate the increase in the population of Sapudi sheep. The success of fertilization is higher with the addition of oxytocin in semen than with exogenous oxytocin administration. The semen diluents used were milk and egg yolk citrate. Aim: This study aimed to determine the effect of oxytocin inclusion in extender on viability, motility, plasma membrane integrity, malondialdehyde (MDA) levels, and catalase levels in Sapudi sheep semen stored at 5°C. Methods: Semen was collected with an artificial vagina and examined macroscopically and microscopically. Each ejaculate was divided into four parts according to the treatment group. The control group (T0) consisted of fresh semen diluted in a skim milk–egg yolk–citrate extender without oxytocin addition. The treatment groups received the same extender supplemented with oxytocin at different concentrations: T1 with 5 IU/100 ml, T2 with 10 IU/100 ml, and T3 with 15 IU/100 ml of extender. All treatments were stored at 5°C and evaluated daily for 5 days. Results: Group T2 had the highest viability, motility, intact plasma membrane, and catalase enzyme levels (p < 0.05) and the lowest MDA levels (p < 0.05). In group T3, the viability, motility, intact plasma membrane, and catalase enzyme levels decreased (p < 0.05), and the MDA levels increased (p < 0.05) compared with the dose in group T2. Conclusion: This study indicated that the inclusion of 10 IU/100 ml of oxytocin in skim milk–egg yolk–citrate extender can maintain the quality of Sapudi ram semen with a motility of more than 40% after 5 days of refrigerated storage. Keywords: Extenders, Catalase, Undernutrition, Motility, Viability. IntroductionIncreasing the population of Sapudi sheep is essential for supporting the Free Nutritional Meal Program in Indonesia (Nilamcaya et al., 2024). Sapudi sheep have good meat and milk quality and high nutritional value (Khasanah et al., 2025). Increasing sheep populations can provide a source of quality animal protein for the community, especially for children and vulnerable groups who need optimal nutritional intake for growth and development (Khasanah et al., 2025). The Free Nutritional Meal Program aims to reduce stunting and malnutrition among children (Sarjito, 2024). One strategy for ensuring the availability of nutritious meat as part of a balanced diet is to utilize Sapudi sheep. In addition, Sapudi sheep have good adaptability to the local environment, so they can be raised in various regions, especially in rural areas that have the potential for livestock development (Mudawamah et al., 2022). Increasing the population of Sapudi sheep offers not only nutritional benefits but also significant economic benefits for livestock communities (Mirella et al., 2022). Enhancing sheep numbers and quality enables farmers to generate additional income from meat and milk production, thereby supporting household welfare. This initiative contributes to food security while simultaneously strengthening the local economy (Mudawamah et al., 2022). Strong collaboration between the government, farmers, and the wider community is essential to ensure the effective and sustainable implementation of the Free Nutritious Meal Program (Pambudi, 2025). Artificial insemination (AI) in sheep is one of the important reproductive technologies that accelerate the increase in the population of Sapudi sheep (Madrigali et al., 2021). With AI, males with superior genetics can produce a much higher number of offspring. However, an important reason why AI is not widely used in sheep today is the high cost of AI and the low quality of sheep spermatozoa after the storage process (Gibbons et al., 2019). Fertilization success is higher with the addition of oxytocin in semen than with exogenous oxytocin (Kandemir, 2023). Oxytocin administration can increase contractions of the female reproductive tract, but the dose must be right so that it can increase the pregnancy rate (Viudes-de-Castro et al., 2009). The sheep spermatozoa are more vulnerable than those of other species. This means that sheep sperm cells (spermatozoa) are more sensitive or fragile compared with the sperm of other animal species, such as cattle, goats, or pigs, which is primarily attributed to their plasma membrane composition (Yánez-Ortiz et al., 2022). Sheep sperm membranes contain a higher proportion of PUFAs and a lower cholesterol-to-phospholipid ratio, resulting in increased membrane fluidity but reduced structural stability during cooling, freezing, or thawing. This lipid composition makes the membrane more susceptible to cold shock and oxidative stress, thereby lowering their resilience compared to species with higher cholesterol content in sperm membranes (Castro et al., 2025). Dilution of sheep semen is needed to increase the dose and extend the life span of the sheep (Zhang et al., 2024). Live spermatozoa do not necessarily show progressive movement. The success of artificial insemination also depends on the percentage of live and movement of spermatozoa (Dcunha et al., 2022). The semen extenders used were milk and egg yolk citrate. Milk contains carbohydrates, whereas egg yolk contains lecithin, which protects spermatozoa from cold shock (Bustani and Baiee, 2021). Studies on the use of milk and egg yolk citrate extenders with the addition of oxytocin have never been conducted on semen from Sapudi sheep. Therefore, this study aimed to detect the role of oxytocin in extenders on viability, motility, plasma membrane integrity, malondialdehyde levels, and post-thawing catalase levels. Materials and MethodsResearch designThis study was conducted in June–November 2024 at the experimental animal cage of the Faculty of Veterinary Medicine, Airlangga University, Mulyorejo, Surabaya, Indonesia, in June−November 2024. Six male Sapudi sheep aged 21.55 ± 4.32 months with a body weight of 47.23 ± 6.51 kg were used. Semen was routinely taken from the sheep for professional student practicums twice a week on Monday and Thursday. The feed was 10% body weight of green fodder and 1% body weight of concentrate (Susfeed, CPPETINDO, Indonesia) with a protein content of 15% given twice a day at 06.00 am and 04.00 pm. Water was provided ad libitum. Sapudi sheep semen storageSemen volume was measured using a graduated collection tube (Cat. No. 12345, Eppendorf, Hamburg, Germany). Sperm motility was evaluated using a light microscope (CX43, Olympus Corporation, Tokyo, Japan) at 400× magnification with a warm stage (37°C). We assessed the viability of the samples using eosin–nigrosin staining solution (Cat. No. E4009, Sigma-Aldrich, St. Louis, MO, USA). Catalase activity was determined using a Catalase Assay Kit (Cat. No. ab83464, Abcam, Cambridge, UK) according to the manufacturer’s instructions. Semen was collected twice a week from sexually mature Balb/c rams using a standard artificial vagina (Model AV-1, IMV Technologies, L’Aigle, France) specifically designed for small ruminants. The artificial vagina was prepared by filling the jacket with water at 42°C–45°C to maintain an internal temperature of approximately 40°C–41°C, and the internal liner was lubricated with sterile non-spermicidal lubricant. Before collection, each ram was allowed brief visual and physical contact with a teaser ewe in estrus to stimulate libido. The ejaculates were collected according to the procedure described by Salamon and Maxwell (2000). Sapudi sheep semen was collected using an artificial vagina and subsequently examined both macroscopically and microscopically within 1 hour after collection. Macroscopic evaluation included volume, color, odor, consistency, and pH, whereas microscopic assessment included mass motility, individual progressive motility, viability, and sperm concentration. Ejaculates that met the processing criteria—defined as individual motility and viability ≥70%—were selected for further analysis. Semen was collected from six healthy and reproductively mature Sapudi rams, with each ram subjected to semen collection twice a week for three consecutive weeks, resulting in 36 ejaculates. Each qualified ejaculate was divided equally into four parts according to the respective treatment groups (Miranda et al., 2023). Egg yolk milk, thinner, and oxytocin addedThe egg yolk milk extender was made by heating skim milk in 100 ml of water, heated to 92°C−95°C, then cooled to 20°C−27°C. The diluent was prepared by adding 5% egg yolk (5 ml per 100 ml of milk) and supplementing it with penicillin (1,000 IU/ml) and streptomycin (1 mg/ml) (Susilowati et al., 2022). Oxytocin was added to the milk–egg yolk–citrate extender at a concentration of 10 IU per 100 ml, following the protocol of Chankitisakul et al. (2024), who reported that this dosage improved sperm quality and fertility potential during semen storage. Control group (T0): egg yolk milk diluent + sheep semen (3 × 106 motile spz), Group I (T1): egg yolk milk diluent + oxytocin 5 IU/100 ml diluent in + sheep semen (3 × 106 motile spz), Group II (T2): egg yolk milk diluent + 10 IU/100 ml diluent in + sheep semen (3 × 106 motile spz), and Group III (T3): egg yolk milk diluent + 15 IU/100 ml diluent + sheep semen (3 × 106 motile spz). All treatments were cooled to 5°C and evaluated after equilibration for 1 hour. Furthermore, they were checked daily for 5 days for motility and viability, intact plasma membrane, and Malondialdehyde (MDA) and catalase levels. Sperm motilityObservers distinguish sperm motility by comparing progressive forward and non-progressive spermatozoa movement under a microscope with 400× magnification. Sperm viabilitySpermatozoa viability was evaluated by making a smear preparation stained with eosin nigrosin, drying it over a flame, and examining it under a microscope. The percentage of dead and live spermatozoa is calculated by dividing in 100 spermatozoa. The head of live spermatozoa appears transparent, and the plasma membrane of dead spermatozoa is damaged. Permeability increases, and finally, the dye enters the cell and the head appears reddish (Susilowati et al., 2021a). The intact plasma membraneThe integrity of the sperm plasma membrane was assessed using the hypoosmotic swelling test (HOST), following the method described by Revell and Mrode (1994), with slight modifications. The HOST solution was prepared by dissolving 7.35 g of sodium citrate dihydrate (Na3C6H5O7·2H2O) and 13.52 g of fructose in 1,000 ml of distilled water to create a hypoosmotic environment. A total of 0.1 ml of semen was mixed with 1 ml of the HOST solution and incubated at 37°C for 30 minutes. After incubation, the percentage of sperm with coiled tails, indicating intact plasma membranes, was recorded under a microscope. A tail of circular spermatozoa characterizes sperm with intact plasma membranes, while a straight tail characterizes sperm with damaged membranes. Levels of malondialdehydeMalondialdehyde levels in spermatozoa were measured using a modified thiobarbituric acid test method. In this procedure, sperm samples were mixed with trichloroacetic acid to precipitate proteins, followed by the addition of thiobarbituric acid reagent (0.67% w/v in glacial acetic acid). The mixture was then heated in a water bath at 95°C for 30 minutes to allow the formation of a pink MDA-TBA complex. After cooling and centrifugation, the supernatant absorbance was measured at 532 nm using a spectrophotometer. The MDA concentration was calculated based on a standard curve of 1,1,3,3-tetramethoxypropane and expressed in nmol/ml (Susilowati et al., 2022). Catalase activityCatalase activity was measured spectrophotometrically based on the decomposition of hydrogen peroxide (H2O2). The sperm sample was mixed with a phosphate buffer (pH 7.0) and a known concentration of H2O2. The decrease in absorbance at 240 nm was recorded over a fixed period (e.g., 1–3 minute) using a UV-Vis spectrophotometer. The reduction in absorbance corresponds to the breakdown of H2O2 by catalase present in the sample. Catalase activity was expressed in units per milliliter (U/ml), where one unit is defined as the amount of enzyme that decomposes 1 μmol of H2O2 per minute under assay conditions (Mustofa et al., 2023). Data analysisData were analyzed using the general linear model with repeated measures in SPSS version 23.0 for Windows (IBM Corp., Armonk, NY, USA), which is the framework for conducting repeated measures analysis of variance (ANOVA). This model was chosen because observations were recorded over multiple time points on the same experimental units, thus violating the standard two-way ANOVA’s independence assumption. The fixed factors in the model were treatment group and storage time, with their interaction term included. Mauchly’s test of sphericity was used to assess the assumption of sphericity; if the Greenhouse–Geisser correction was violated, it was applied. When a significant main effect or interaction was found (p < 0.05), pairwise comparisons between groups were conducted using Bonferroni’s post hoc test to control for type I error. All results are presented as mean ± standard error of the mean, and statistical significance was set at p < 0.05. Ethical approvalAll procedures involving animals in this study were conducted in accordance with ethical standards and were approved by the Animal Ethics Committee of the Faculty of Dental Medicine Health Research, Universitas Airlangga, under approval number 0536/HRECC.FODM/V/2024 on 6 May 2024. ResultsBased on the macroscopic and microscopic parameters of fresh ram semen defined in this study as ejaculates evaluated within 1 hour after collection to minimize physiological changes before analysis, the samples met the standard criteria for cryopreservation (Table 1). Specifically, semen samples exhibited a volume greater than 0.86 ml, a specific smell, a yellowish white color, a pH, and a thick consistency. Sperm viability exceeding 90%, progressive motility >90%, intact plasma membrane >89%, and morphological abnormalities 2%, indicating high initial semen quality suitable for storage and further processing. Throughout the 5-day storage period at 5°C, there was a statistically significant decline (p < 0.05) in sperm viability, motility, intact plasma membrane integrity, and catalase enzyme activity significantly declined (p 0.05), suggesting progressive cellular damage and oxidative stress during preservation. In contrast, MDA levels, an indicator of lipid peroxidation, increased significantly over time (p < 0.05), supporting the presence of oxidative deterioration (Tables 2–6). Table 1. Macroscopic and microscopic parameters of Sapudi ram fresh semen.
Among the treatment groups, the addition of oxytocin at a dose of 10 IU/100 ml (T2) to the skim milk–egg yolk–citrate extender resulted in significantly better semen quality preservation than the control and other treatments. Although all groups exhibited time-dependent changes during storage, the sperm viability, motility, intact plasma membrane, and catalase activity were consistently higher, and MDA concentrations were lower than those of the other groups at each assessed time point (p < 0.05), indicating comparatively better antioxidative protection and membrane stability during storage. However, a higher dose of oxytocin (T3: 15 IU/100 ml) appeared to have a detrimental effect. In this group, viability, motility, membrane integrity, and catalase levels were significantly reduced (p < 0.05), whereas MDA levels were elevated (p < 0.05), suggesting a possible threshold beyond which oxytocin exerts oxidative or cytotoxic effects on spermatozoa. DiscussionOverall viability, motility, intact plasma membrane, and catalase enzyme levels decreased, but MDA levels increased throughout storage. These results are consistent with those of previous studies on Kacang goat semen (Susilowati et al., 2021a; Susilowati et al., 2021b). The purpose of storing semen at cold temperatures is to reduce the metabolic rate, so that spermatozoa can maintain their quality longer than when stored at room temperature (Saha et al., 2022; Moula et al., 2024). The decrease in quality during storage at cold temperatures (5°C) is caused by decreased energy supplies, increased spermatozoa metabolism (Wang et al., 2025), and reactive oxygen species (ROS) due to cold temperatures (Kameni et al., 2021). The sperm plasma membrane is rich in polyunsaturated fatty acids, which are susceptible to free radicals (Carro et al., 2022). Free radicals cause lipid phosphorylation in spermatozoa membranes, characterized by increased MDA levels. Plasma membrane damage follows decreased motility and spermatozoa death (Wang et al., 2025). The molecular mechanism underlying the direct effect of oxytocin on sheep spermatozoa remains unclear. In vitro studies on human semen showed that the addition of 10 IU/ml oxytocin to semen resulted in a significant increase in the percentage of motility and swimming speed of spermatozoa compared to the addition of saline solution as a control (Fuchs et al., 1989). Furthermore, studies on normospermia, oligozoospermia, asthenozoospermia, and teratozoospermia samples in humans showed that oxytocin and oxytocin receptors were expressed in human spermatozoa. The biological mechanism and oxytocin receptors in sheep spermatozoa are unknown. Oxytocin receptor expression has been identified in the testes and epididymis of horses (Jung and Yoon, 2021). Research on the nucleus accumbens and the caudate putamen of the rat brain shows that when oxytocin binds to its receptor, a number of signaling pathways in the cell are activated. These receptors are generally the type that are coupled to G proteins that are associated with various signaling pathways that are important for cell function (Borroto-Escuela et al., 2022). In this study, semen extended with 10 IU oxytocin per 100 ml milk–egg yolk–citrate extender showed the highest sperm viability, motility, intact plasma membrane percentage, and catalase activity, along with the lowest MDA levels, compared with all other treatment groups. Furthermore, ATP is the main energy source for spermatozoa movement. The activated signaling pathway increases the metabolic capacity of the cell, allowing spermatozoa to move more actively and efficiently (Tourmente et al., 2022). Oxytocin can also affect the concentration of calcium ions in the cell (Holda et al., 1996). The increase in calcium ions in spermatozoa cells contributes to the movement of the spermatozoa flagellum, an important structure for motility. Calcium plays a role in muscle contraction and cell movement, thereby increasing spermatozoa’s ability to move (Yoshida and Yoshida, 2011; Vicente-Carrillo et al., 2023). The actin cytoskeleton of the mouse sperm flagellum is arranged in a helical structure (Gervasi et al., 2018). Actin is an important component in the structure and motility of spermatozoa. Strengthening this structure can increase spermatozoa’s ability to move toward the egg (Breitbart and Finkelstein, 2018). Oxytocin is thought to play a role in increasing spermatozoa motility directly or indirectly (through the induction of greater ATP supply). Studies on murine spermatozoa have demonstrated the presence of oxytocin receptors, which are classified as G-protein-coupled receptors (Tsuchiya et al., 2023). When oxytocin binds to this receptor, signaling pathways are activated that can trigger a series of biochemical reactions that support spermatozoa survival. This activation can lead to changes in metabolic activity that maintain cell viability (McCormack et al., 2020). Oxytocin plays a role in inhibiting the apoptosis process in acute orchitis models (El-Sherbiny et al., 2025). This hormone can induce anti-apoptotic signaling pathways that reduce cell death rates. Thus, oxytocin increases the number of surviving cells (Ghasemnezhad et al., 2015), including spermatozoa, in cold storage. Oxytocin plays a role in maintaining the integrity of the spermatozoa cell membrane in canine spermatozoa (Dalmazzo et al., 2019). A healthy and intact membrane is essential for optimal cell function and spermatozoa viability (Tanga et al., 2021). Oxytocin maintains a good ion balance in pyramidal neurons, which is essential for plasma membrane health. This ion charge regulation also contributes to the membrane potential, which is necessary for cell membrane integrity (Liu et al., 2022). Table 2. Viability (%) of Sapudi ram spermatozoa in skim milk–egg yolk–citrate extender without or with the addition of several doses of oxytocin at 5°C for 5 days.
Table 3. Motility (%) of Sapudi ram spermatozoa in skim milk–egg yolk–citrate extender without or with the addition of several doses of oxytocin at 5°C for 5 days.
Table 4. Intact plasma membrane (%) of Sapudi ram spermatozoa in skim milk–egg yolk–citrate extender without or with the addition of several doses of oxytocin at 5°C for 5 days.
Table 5. Malondialdehyde levels (nmol/mL) of Sapudi ram semen in skim milk–egg yolk–citrate extender without or with the addition of several doses of oxytocin at 5°C for 5 days.
Table 6. Catalase (x 10-3 U/mg) levels (nmol/ml) of Sapudi ram semen in skim milk–egg yolk–citrate extender without or with the addition of several doses of oxytocin at 5°C for 5 days.
Oxytocin has antioxidant properties that can protect cells from damage caused by oxidative stress (Alanazi et al., 2020). Oxidative stress can damage membrane lipids, resulting in decreased membrane integrity (Stevenson et al., 2023). Oxytocin helps maintain the integrity and function of the plasma membrane by reducing the effects of oxidative stress. Malondialdehyde is formed as a byproduct of lipid peroxidation, and its levels can indicate the functioning of the antioxidant defense system (Cordiano et al., 2023). Oxytocin plays a role in suppressing the lipid peroxidation process that causes MDA formation by reducing free radical levels (El-Sherbiny et al., 2025). Oxytocin can also stimulate the activity of antioxidant enzymes (Carter et al., 2020). The antioxidant enzymes superoxide dismutase and glutathione peroxidase convert reactive oxygen species (ROS) into non-toxic products, reducing the risk of lipid peroxidation and malondialdehyde (MDA) formation (Afzal et al., 2023). This is important for maintaining spermatozoa health and semen quality. Exogenous oxytocin administration has an antioxidant effect and increases catalase enzyme levels in the placenta (Kouba et al., 2024). Catalase converts hydrogen peroxide (H2O2), a harmful ROS, into water and oxygen, thereby protecting cells from oxidative damage (Anwar et al., 2024). Hypothetically, oxytocin can promote catalase synthesis through certain signaling pathways. When oxytocin binds to G-protein receptors (Tsuchiya et al., 2023), it triggers the activation of signal transduction pathways that can increase the expression of genes encoding the catalase enzyme (Chatterjee et al., 2016). Oxytocin can increase the concentration of calcium ions (Ca2+) in cells (Holda et al., 1996). Increased calcium levels can activate signaling pathways that have a positive impact on catalase enzyme activity, increasing its ability to degrade hydrogen peroxide (Nazıroğlu, 2012). Reducing the levels of reactive oxygen species through increased catalase activity contributes to reducing oxidative stress in semen (Wang et al., 2025). Increased catalase levels increase lipid oxidation and cell damage caused by H2O2, which can be minimized (Rasheed, 2024). The addition of higher oxytocin (15 IU/100 ml of extender) in the present study decreased viability, motility, intact plasma membranes, and catalase enzyme levels and increased MDA levels compared with the optimum dose (10 IU/100 ml of extender). In vitro studies on astrocyte-like cells have shown that oxytocin acts as an antioxidant (Alanazi et al., 2020). These findings imply that while low doses of oxytocin can enhance semen preservation by improving cellular resistance to oxidative stress, excessive doses may have the opposite effect, potentially disrupting sperm function and membrane integrity. The results underline the importance of dose optimization when using hormonal additives in semen extenders for AI programs. Despite the promising findings, this study has several limitations. First, the experiments were conducted on a limited number of Sapudi rams, which may affect the generalizability of the results to a wider population. Second, only a single concentration of oxytocin (10 IU/100 ml extender) was tested; therefore, the potential dose-dependent effects beyond this concentration were not explored. Third, the study evaluated semen quality parameters in vitro at 5°C storage, without assessing actual in vivo fertility outcomes following artificial insemination. Future studies should address these limitations by including more animals, testing multiple doses of oxytocin, and evaluating reproductive performance under field conditions. ConclusionThis study suggests that the addition of 10 IU/100 ml of oxytocin to skim milk–egg yolk–citrate extender can maintain the quality of Sapudi sheep semen with a motility of more than 40% in storage for 5 days. Further research is needed (in silico and in vivo) to prove the existence of oxytocin receptors on the plasma membrane of sheep spermatozoa and its mechanism to maintain the quality of sheep semen in cold or frozen storage. AcknowledgmentsWe thank Agil Ramadan for his technical assistance. Conflict of interest The authors declare no conflict of interest. Funding This research was funded by Airlangga University (contract number: 4190/B/UN3.FKH/PT.01.03/2024). Author’s contributions SM, IM, LL, and ARK: conceived the idea and drafted the manuscript. LA, SS, and BPP: data acquisition, analysis, and interpretation. AOA, RZA, and UFH: critically read and revised the manuscript for intellectual content. All authors have read and approved the final version of the manuscript. All authors have read, reviewed, and approved the final version of the manuscript. Data availability All data are available in the revised manuscript. ReferencesAfzal, S., Manap, A.S.A., Attiq, A., Albokhadaim, I., Kandeel, M. and Alhojaily, S.M. 2023. From imbalance to impairment: the central role of reactive oxygen species in oxidative stress-induced disorders and therapeutic exploration. Front. Pharmacol. 14(1), 1269581. Alanazi, M.M., Havranek, T., Bakos, J., Cubeddu, L.X. and Castejon, A.M. 2020. Cell proliferation and anti-oxidant effects of oxytocin and oxytocin receptors: role of extracellular signal-regulating kinase in astrocyte-like cells. Endocr. Regul. 54(3), 172–182. Anwar, S., Alrumaihi, F., Sarwar, T., Babiker, A.Y., Khan, A.A., Prabhu, S.V. and Rahmani, A.H. 2024. Exploring therapeutic potential of catalase: strategies in disease prevention and management. Biomolecules 14(6), 697. Borroto-Escuela, D.O., Cuesta-Marti, C., Lopez-Salas, A., Chruścicka-Smaga, B., Crespo-Ramírez, M., Tesoro-Cruz, E., Palacios-Lagunas, D.A., Perez De La Mora, M., Schellekens, H. and Fuxe, K. 2022. The oxytocin receptor represents a key hub in the GPCR heteroreceptor network: potential relevance for brain and behavior. Front. Mol. Neurosci. 15(1), 1055344. Breitbart, H. and Finkelstein, M. 2018. Actin cytoskeleton and sperm function. Biochem. Biophys. Res. Commun. 506(2), 372–377. Bustani, G.S. and Baiee, F.H. 2021. Semen extenders: an evaluative overview of preservative mechanisms of semen and semen extenders. Vet. World 14(5), 1220–1233. Carro, M., Luquez, J.M., Peñalva, D.A., Buschiazzo, J., Hozbor, F.A. and Furland, N.E. 2022. PUFA-rich phospholipid classes and subclasses of ram spermatozoa are unevenly affected by cryopreservation with a soybean lecithin-based extender. Theriogenology 186(1), 122–134. Carter, C.S., Kenkel, W.M., MacLean, E.L., Wilson, S.R., Perkeybile, A.M., Yee, J.R., Ferris, C.F., Nazarloo, H.P., Porges, S.W., Davis, J.M., Connelly, J.J. and Kingsbury, M.A. 2020. Is oxytocin “nature’s medicine”? Pharmacol. Rev. 72(4), 829−861. Castro, M., Leal, K., Pezo, F. and Contreras, M.J. 2025. Sperm membrane: molecular implications and strategies for cryopreservation in productive species. Animals 15(12), 1808. Chankitisakul, V., Tubtimtong, N., Boonkum, W. and Vongpralub, T. 2024. Effects of gelatin and oxytocin supplementation in a long-term semen extender on boar semen quality and fertility potential. Anim. Biosci. 37(2), 210–217. Chatterjee, O., Patil, K., Sahu, A., Gopalakrishnan, L., Mol, P., Advani, J., Mukherjee, S., Christopher, R. and Prasad, T.S. 2016. An overview of the oxytocin-oxytocin receptor signaling network. J. Cell. Commun. Signal. 10(4), 355–360. Cordiano, R., Di Gioacchino, M., Mangifesta, R., Panzera, C., Gangemi, S. and Minciullo, P.L. 2023. Malondialdehyde as a potential oxidative stress marker for allergy-oriented diseases: an update. Molecules 28(16), 5979. Dalmazzo, A., Losano, J.D.A., Angrimani, D.S.R., Pereira, I.V.A., Goissis, M.D., Francischini, M.C.P., Lopes, E., Minazaki, C.K., Blank, M.H., Cogliati, B., Pereira, R.J.G., Barnabe, V.H. and Nichi, M. 2019. Immunolocalisation and expression of oxytocin receptors and sex hormone-binding globulin in the testis and epididymis of dogs: correlation with sperm function. Reprod. Fertil. Dev. 31(9), 1434–1443. Dcunha, R., Hussein, R.S., Ananda, H., Kumari, S., Adiga, S.K., Kannan, N., Zhao, Y. and Kalthur, G. 2022. Current insights and latest updates in sperm motility and associated applications in assisted reproduction. Reprod. Sci. 29(1), 7–25. El-Sherbiny, M., Elkattawy, H.A., Hadhoud, S., Nasr, A.N., Ibrahim, A.M., Ameer, O.Z., Alsaleebi, N., Asfari, J., Zakari, M.O., Mojaddidi, M.A., Ali, E.K., Almohaimeed, H.M., Abdeen, A., Ali, S.K. and Eldesoqui, M. 2025. Oxytocin ameliorates lipopolysaccharide-induced acute orchitis model: interplay of oxidative stress and inflammatory pathways. Front. Pharmacol. 15(1), 1506777. Fuchs, U., Leipnitz, C. and Lippert, T.H. 1989. The action of oxytocin on sperm motility. In vitro experiments with bull spermatozoa. Clin. Exp. Obstet. Gynecol. 16(4), 95–97. Gervasi, M.G., Xu, X., Carbajal-Gonzalez, B., Buffone, M.G., Visconti, P.E. and Krapf, D. 2018. The actin cytoskeleton of the mouse sperm flagellum is organized in a helical structure. J. Cell Sci. 131(11), jcs215897. Ghasemnezhad, R., Mohammadghasemi, F., Faghani, M. and Bahadori, M.H. 2015. Oxytocin can decrease germ cells apoptotic index in testis under acute ischemia reperfusion in a rat model. Iran. J. Reprod. Med. 13(5), 283–290. Gibbons, A.E., Fernandez, J., Bruno-Galarraga, M.M., Spinelli, M.V. and Cueto, M.I. 2019. Technical recommendations for artificial insemination in sheep. Anim. Reprod. 16(4), 803–809. Holda, J.R., Oberti, C., Perez-Reyes, E. and Blatter, L.A. 1996. Characterization of an oxytocin-induced rise in Ca2+i in single human myometrium smooth muscle cells. Cell Calcium 20(1), 43–51. Jung, Y. and Yoon, M. 2021. Oxytocin receptor expression in stallion testes and epididymides. Domest. Anim. Endocrinol. 74(1), 106562. Kameni, S.L., Meutchieye, F. and Ngoula, F. 2021. Liquid storage of ram semen: associated damages and improvement. Open J. Anim. Sci. 11(3), 473–500. Kandemir, C. 2023. Effect of oxytocin added into sperm on artificial insemination in sheep. Arch. Anim. Breed. 66(1), 61–69. Khasanah, H., Widianingrum, D.C. and Pratiwi, N. 2025. Mineral and vitamin B contents of sapudi and merino-cross Mmeat. Indones. J. Agric. Sci. 30(2), 328–332. Kouba, I., Xue, X., Blitz, M.J., Bracero, L.A. and Metz, C. 2024. The anti-inflammatory and antioxidant effects of exogenous oxytocin on human placental cells. Am. J. Obstet. Gynecol. 230(1), S555–S556. Liu, J.J., Eyring, K.W., König, G.M., Kostenis, E. and Tsien, R.W. 2022. Oxytocin-modulated ion channel ensemble controls depolarization, integration and burst firing in CA2 pyramidal neurons. J. Neurosci. 42(41), 7707–7720. Madrigali, A., Rota, A., Panzani, D., Castellani, S., Shawahina, M., Hassan, A., Di Iacovo, F., Rossignoli, C. and Camillo, F. 2021. Artificial insemination in sheep with fresh diluted semen: comparison between two different semen extenders and management protocols. Trop. Anim. Sci. J. 44(3), 255–260. Mccormack, S.E., Blevins, J.E. and Lawson, E.A. 2020. Metabolic effects of oxytocin. Endocr. Rev. 41(2), 121–145. Miranda, A.S., Suprayogi, T.W., Utomo, B., Susilowati, S. and Dhamayanti, Y. 2023. Impact of green tea (Camellia sinensis) leaf extract in skim milk-goose egg yolk semen extender on the quality of Sapudi sheep spermatozoa stored at 5°C. Ovozoa J. Anim. Reprod. 12(3), 148–156. Mirella, A.A., Mudawamah, M. and Sumartono, S. 2022. Estimation of repeatability and the most probable producing ability (MPPA) based on birth weight and weaning weight for ranking of sapudi sheep. J. Sain. Peternakan. Indones. 17(2), 82–86. Moula, A.B., Hamidallah, N., Badi, A., El Fadili, M. and El Amiri, B. 2024. Adjusting ram semen preservation: exploring the impact of oxygen exposure during liquid storage. Reprod. Domest. Anim. 59(5), e14618. Mudawamah, M., Anwar, M.Z. and Sumartono, S. 2022. Estimation of repeatability and most probable producing ability (MPPA) of sapudi sheep based on daily body weight gain of lambs from birth to pre-weaning and weaning. J. Sain. Peternakan. Indones. 17(3), 149–154. Mustofa, I., Susilowati, S., Suprayogi, T.W., Akintunde, A.O., Oktanella, Y. and Purwanto, D.A. 2023. Epigallocatechin-3-gallate chitosan nanoparticles in an extender improve the antioxidant capacity and post-thawed quality of Kacang goat semen. F1000Research 12(1), 32. Nazıroğlu, M. 2012. Molecular role of catalase on oxidative stress-induced Ca(2+) signaling and TRP cation channel activation in nervous system. J. Recept. Signal Transduct. Res. 32(3), 134–141. Nilamcaya, M., Bastoni, Nurhawaeny, E. and Widyani, R. 2024. Public policy and community empowerment in the development of sheep and goat farming in Indonesia. J. Public Policy 1(5), 237–248. Pambudi, A.W. 2025. Indonesia government’s efforts to eradicate stunting through the free nutritious meal program for children: opportunities and challenges. J. Politikom Indonesiana 9(2), 157–182. Rasheed, Z. 2024. Therapeutic potentials of catalase: mechanisms, applications, and future perspectives. Int. J. Health Sci. 18(2), 1–6. Revell, S.G. and Mrode, R.A. 1994. An omotic resistance test for bovine semen. Anim. Reprod. Sci. 36(1–2), 77–86. Saha, A., Asaduzzaman, M., Akter, S. and Bari, F.Y. 2022. Effect of different preservation time of chilled semen on the fertility of field indigenous ewes. Agric. Sci. Dig. 42(2), 223–227. Salamon, S. and Maxwell, W.M. 2000. Storage of ram semen. Anim. Reprod. Sci. 62(1–3), 77–111. Sarjito, A. 2024. Free nutritious meal program as a human resource development strategy to support national defense. Int. J. Adm. Bus. Organ. 5(5), 129–141. Stevenson, J.R., Mcmahon, E.K., Mcneely, T.L. and Haussmann, M.F. 2023. Oxytocin prevents dysregulation of the acute stress response and glucocorticoid-induced oxidative stress in chronically isolated prairie voles. Psychoneuroendocrinology 153(1), 106121. Susilowati, S., Mustofa, I., Wurlina, W., Hernawati, T. and Oktanella, Y. 2021b. Maintaining the quality of Kacang buck semen in chilled storage with the addition of green tea extract in extender. Trop. Anim. Sci. J. 44(4), 408–414. Susilowati, S., Mustofa, I., Wurlina, W., Hernawati, T., Oktanella, Y., Soeharsono, S. and Purwanto, D.A. 2022. Green tea extract in the extender improved the post-thawed semen quality and decreased amino acid mutation of Kacang buck sperm. Vet. Sci. 9(8), 403. Susilowati, S., Mustofa, I., Wurlina, W., Triana, I.N., Utama, S. and Utomo, B. 2021a. A 150 kDa protein derived from bull seminal plasma extended the survival time of Kacang goat sperm stored at 5°C. Vet. Med. Int. 1(1), 1470209. Tanga, B.M., Qamar, A.Y., Raza, S., Bang, S., Fang, X., Yoon, K. and Cho, J. 2021. Semen evaluation: methodological advancements in sperm quality-specific fertility assessment - a review. Anim. Biosci. 34(8), 1253–1270. Tourmente, M., Sansegundo, E., Rial, E. and Roldan, E.R.S. 2022. Capacitation promotes a shift in energy metabolism in murine sperm. Front. Cell Dev. Biol. 10(1), 950979. Tsuchiya, H., Fujinoki, M., Azuma, M. and Koshimizu, T.A. 2023. Vasopressin V1a receptor and oxytocin receptor regulate murine sperm motility differently. Life Sci. Alliance 6(4), e202201488. Vicente-Carrillo, A., Álvarez-Rodríguez, M. and Rodriguez-Martinez, H. 2023. The cation/calcium channel of sperm (CatSper): a common role played despite inter-species variation?. Int. J. Mol. Sci. 24(18), 13750. Viudes-de-Castro, M.P., Salvador, I., Marco-Jiménez, F., Gómez, E.A. and Silvestre, M.A. 2009. Effect of oxytocin treatment on artificial insemination with frozen-thawed semen in Murciano-Granadina goats. Reprod. Domest. Anim. 44(4), 576–579. Wang, Y., Fu, X. and Li, H. 2025. Mechanisms of oxidative stress-induced sperm dysfunction. Front. Endocrinol. 16(1), 1520835. Yánez-Ortiz, I., Catalán, J., Rodríguez-Gil, J.E., Miró, J. and Yeste, M. 2022. Advances in sperm cryopreservation in farm animals: cattle, horse, pig and sheep. Anim. Reprod. Sci. 246(1), 106904. Yoshida, M. and Yoshida, K. 2011. Sperm chemotaxis and regulation of flagellar movement by Ca2+. Mol. Hum. Reprod. 17(8), 457–465. Zhang, L., Wang, X., Jiang, C., Sohail, T., Sun, Y., Sun, X., Wang, J. and Li, Y. 2024. Effect of different dilution methods and ratios of ram semen on sperm parameters after cryopreservation. Animals 14(6), 907. | ||
| How to Cite this Article |
| Pubmed Style Mulyati S, Mustofa I, Susilowati S, Khairullah AR, Ahmad RZ, Akintunde AO, Pratama BP, Latifah L, Handayani UF, Anggraini L. Increasing the fertility of Sapudi sheep semen at 5°C storage temperature with the addition of oxytocin in a diluent of skim milk, egg yolk, and citrate. Open Vet. J.. 2025; 15(9): 4700-4708. doi:10.5455/OVJ.2025.v15.i9.74 Web Style Mulyati S, Mustofa I, Susilowati S, Khairullah AR, Ahmad RZ, Akintunde AO, Pratama BP, Latifah L, Handayani UF, Anggraini L. Increasing the fertility of Sapudi sheep semen at 5°C storage temperature with the addition of oxytocin in a diluent of skim milk, egg yolk, and citrate. https://www.openveterinaryjournal.com/?mno=256616 [Access: January 02, 2026]. doi:10.5455/OVJ.2025.v15.i9.74 AMA (American Medical Association) Style Mulyati S, Mustofa I, Susilowati S, Khairullah AR, Ahmad RZ, Akintunde AO, Pratama BP, Latifah L, Handayani UF, Anggraini L. Increasing the fertility of Sapudi sheep semen at 5°C storage temperature with the addition of oxytocin in a diluent of skim milk, egg yolk, and citrate. Open Vet. J.. 2025; 15(9): 4700-4708. doi:10.5455/OVJ.2025.v15.i9.74 Vancouver/ICMJE Style Mulyati S, Mustofa I, Susilowati S, Khairullah AR, Ahmad RZ, Akintunde AO, Pratama BP, Latifah L, Handayani UF, Anggraini L. Increasing the fertility of Sapudi sheep semen at 5°C storage temperature with the addition of oxytocin in a diluent of skim milk, egg yolk, and citrate. Open Vet. J.. (2025), [cited January 02, 2026]; 15(9): 4700-4708. doi:10.5455/OVJ.2025.v15.i9.74 Harvard Style Mulyati, S., Mustofa, . I., Susilowati, . S., Khairullah, . A. R., Ahmad, . R. Z., Akintunde, . A. O., Pratama, . B. P., Latifah, . L., Handayani, . U. F. & Anggraini, . L. (2025) Increasing the fertility of Sapudi sheep semen at 5°C storage temperature with the addition of oxytocin in a diluent of skim milk, egg yolk, and citrate. Open Vet. J., 15 (9), 4700-4708. doi:10.5455/OVJ.2025.v15.i9.74 Turabian Style Mulyati, Sri, Imam Mustofa, Suherni Susilowati, Aswin Rafif Khairullah, Riza Zainuddin Ahmad, Adeyinka Oye Akintunde, Bima Putra Pratama, Latifah Latifah, Ulvi Fitri Handayani, and Lili Anggraini. 2025. Increasing the fertility of Sapudi sheep semen at 5°C storage temperature with the addition of oxytocin in a diluent of skim milk, egg yolk, and citrate. Open Veterinary Journal, 15 (9), 4700-4708. doi:10.5455/OVJ.2025.v15.i9.74 Chicago Style Mulyati, Sri, Imam Mustofa, Suherni Susilowati, Aswin Rafif Khairullah, Riza Zainuddin Ahmad, Adeyinka Oye Akintunde, Bima Putra Pratama, Latifah Latifah, Ulvi Fitri Handayani, and Lili Anggraini. "Increasing the fertility of Sapudi sheep semen at 5°C storage temperature with the addition of oxytocin in a diluent of skim milk, egg yolk, and citrate." Open Veterinary Journal 15 (2025), 4700-4708. doi:10.5455/OVJ.2025.v15.i9.74 MLA (The Modern Language Association) Style Mulyati, Sri, Imam Mustofa, Suherni Susilowati, Aswin Rafif Khairullah, Riza Zainuddin Ahmad, Adeyinka Oye Akintunde, Bima Putra Pratama, Latifah Latifah, Ulvi Fitri Handayani, and Lili Anggraini. "Increasing the fertility of Sapudi sheep semen at 5°C storage temperature with the addition of oxytocin in a diluent of skim milk, egg yolk, and citrate." Open Veterinary Journal 15.9 (2025), 4700-4708. Print. doi:10.5455/OVJ.2025.v15.i9.74 APA (American Psychological Association) Style Mulyati, S., Mustofa, . I., Susilowati, . S., Khairullah, . A. R., Ahmad, . R. Z., Akintunde, . A. O., Pratama, . B. P., Latifah, . L., Handayani, . U. F. & Anggraini, . L. (2025) Increasing the fertility of Sapudi sheep semen at 5°C storage temperature with the addition of oxytocin in a diluent of skim milk, egg yolk, and citrate. Open Veterinary Journal, 15 (9), 4700-4708. doi:10.5455/OVJ.2025.v15.i9.74 |