| Research Article | ||
Open Vet. J.. 2025; 15(9): 4578-4591 Open Veterinary Journal, (2025), Vol. 15(9): 4578-4591 Research Article Cryopreserved Indonesian Simmental bull semen: Effect of L-arginine supplementation in Tris egg yolk extender on sperm qualityAulia Firmawati1,2, Habib Asshidiq Syah1, Dikky Eka Mandala Putranto3, Widjiati Widjiati4, Sri Wahjuningsih5 and Trinil Susilawati5*1Doctoral Student, Faculty of Animal Science, Universitas Brawijaya, Malang, Indonesia 2Departemen of Reproduction, Faculty of Veterinary Medicine, Brawijaya University, Malang, Indonesia 3Artificial Insemination Centre, Teaching Farm, Faculty of Veterinary Medicine, Airlangga University, Gresik, Indonesia 4Departement of Anatomy and Embryology, Faculty of Veterinary Medicine, Airlangga University, Surabaya, Indonesia 5Department of Animal Reproduction and Breeding, Faculty of Animal Science, Universitas Brawijaya, Malang, Indonesia *Corresponding Author: Trinil Susilawati. Jl. Veteran, Malang, Indonesia. Email: tsusilawati [at] ub.ac.id Submitted: 24/03/2025 Revised: 15/08/2025 Accepted: 25/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
AbstractBackground: The Indonesian government aims to encourage the beef cattle population to increase livestock numbers through artificial insemination (AI). However, this initiative encounters challenges due to the low quality of semen following cryopreservation. Aim: This study aimed to evaluate the influence of L-arginine supplementation in the Tris egg yolk extender on the motility, kinematic movement, viability, acrosome integrity, and lipid peroxidation of cryopreserved Simmental bull spermatozoa. Methods: The experiment involved four groups with 20 replicates each: the control group (CG), Group 1 (G1) using 0.05 M L-arginine, Group 2 (G2) using 0.1 M L-arginine, and Group 3 (G3) using 0.2 M L-arginine in a Tris-Yolk extender. Observations included spermatozoa motility and kinematic movement (computer-assisted sperm analysis), viability, acrosome integrity, and lipid peroxidation measured by Malondialdehyde (MDA) (thiobarbituric acid test). Data were analyzed using post hoc analysis of variance, Duncan’s test, with significance p < 0.05. Results: The findings of this study indicate that the supplementation of 0.05 M L-arginine (G1) in extender significantly (p < 0.05) enhanced motility and kinematic movement parameters, viability, and acrosome integrity while reducing lipid peroxidation, as measured by MDA levels. Furthermore, supplementation with 0.05 M L-arginine had a positive effect on motility and kinematic movement both before freezing and post-thawing. In contrast, the addition of L-arginine at higher concentrations (G2 and G3) did not benefit the observed parameters in this study. Conclusion: A low dose of L-arginine (G1) effectively enhances sperm motility, viability, and acrosome integrity and reduces lipid peroxidation levels. In contrast, higher doses (G2 and G3) had no beneficial effects compared with GC and G1. Keywords: Acrosome integrity, Lipid peroxidation, Motility, Tris-egg yolk, Viability. IntroductionThe beef cattle industry in Indonesia is crucial to national food security and rural economic development. According to Statistics Indonesia (Badan Pusat Statistik, 2023), the national beef cattle population reached approximately 11.7 million heads, with over 90% raised by smallholder farmers (Hilmiati et al., 2024). However, domestic beef production meets only about 60%–70% of national consumption, resulting in continued reliance on imports. This production deficit undermines the government’s efforts to stabilize prices, enhance farmer competitiveness, and achieve beef supply self-sufficiency. The Indonesian government emphasizes increasing the beef cattle population through artificial insemination (AI) to increase the livestock population. However, this initiative faces challenges due to reduced semen quality after cryopreservation. Although semen cryopreservation is a highly valuable technique for long-term storage, it may negatively impact sperm quality, potentially affecting their fertilization capacity. Afsar et al. (2024) reported that spermatozoa dying during freezing are relatively high, with losses ranging from 20% to 80%. Various strategies have been explored to mitigate these effects, including incorporating antioxidants into cryoprotectant media and using alternative cryoprotectants, such as L-arginine (Paoli et al., 2019; Afsar et al., 2024). Optimizing semen quality through optimized cryopreservation protocols not only enhances conception rates but also supports the wider distribution of superior genetic material. This contributes to greater genetic diversity within herds, which is essential for livestock populations to improve resilience, adaptability, and long-term productivity (Leroy et al., 2011; Jakop et al., 2023). L-arginine functions as an extracellular antioxidant, improving sperm motility and acrosome integrity while suppressing lipid peroxidation (Afsar et al., 2024). During cryopreservation, L-arginine, a semi-essential amino acid, protects spermatozoa from oxidative stress. These amino acids increase the production of nitric oxide, which plays a vital role in maintaining cell metabolism and protecting spermatozoa from oxidative damage, thus increasing the viability of thawed semen (Peña et al., 2019; Susilowati et al., 2021). Nitric oxide helps reduce lipid peroxidation, stabilizes sperm membranes, and supports adenosine triphosphate (ATP) production during the freezing and thawing phases, which are essential for maintaining sperm motility and viability (O’Flaherty et al., 2004; Khodaei et al., 2016; Badr et al., 2020). Incorporating L-arginine into Tris Egg Yolk extenders improves sperm motility and reduces acrosome and sperm plasma membrane integrity (Afsar et al., 2024). This protective effect is essential for maintaining the integrity of the sperm plasma acrosome and membrane, thereby improving the quality of the sperm before freezing (Peña et al., 2019). Optimizing extenders with antioxidants, such as L-arginine, can significantly improve the quality of frozen semen, thereby increasing the efficacy of AI programs in Indonesia (Susilowati et al., 2021; Baharun et al., 2023). Previous studies have investigated the supplementation of L-arginine into Tris-egg yolk extenders for sub-fertile buffalo semen. Hegazy et al. (2021) reported modest improvements in motility and viability using concentrations between 3 and 6 mM, whereas Badr et al. (2020) and Abd-Allah and Eliraqy (2019) found variable effects depending on the dose and semen condition. To address this issue, this study investigated the optimal L-arginine dosage in Indonesian Simmental semen using the Tris-Egg Yolk extender. Simmental bulls were selected because they are the most widely raised breed of beef in Indonesia. They are valued for their high growth rates, meat quality, and suitability for artificial insemination (AI) programs. L-arginine functions as an extracellular antioxidant, enhancing post-thawing sperm motility, preserving the integrity of the plasma membrane and acrosome, and reducing lipid peroxidation. This study evaluated its effects on acrosome viability, integrity, motility, and kinematic movement parameters using computer-assisted sperm analysis (CASA). Materials and MethodsStudy designThis research design is a true experimental laboratory design using a completely randomized design with four treatment groups and 20 replicates per group (Table 1). This study aimed to evaluate the effect of different concentrations of L-arginine supplementation in a Tris egg yolk extender on the semen quality of Simmental bulls during cryopreservation. After semen collection and preliminary evaluation, the ejaculates were randomly allocated into treatment groups. Comparative analyses were performed to assess sperm performance before freezing and post thawing. Animal materialThis study involved four clinically healthy, 6-year-old Indonesian Simmental bulls weighing 800 and 900 kg. Semen was routinely collected twice per week using an artificial vagina, and libido was optimized with the aid of a teaser animal (Adamczyk et al., 2013; SNI, 2017;Budiyanto et al., 2021; Ratnawati et al., 2023a,b). Ejaculates that met the eligibility criteria were randomly assigned to four experimental groups, each consisting of 20 replicates. The treatments included one control group (CG), which received a Tris-egg yolk extender without L-arginine supplementation, and three treatment groups (G1, G2, G3), each supplemented with different L-arginine concentrations. All groups—control and treatment—underwent the same semen processing protocol. Each ejaculate was divided into four groups: CG (control, no L-arginine), G1 (0.05 M), G2 (0.1 M), and G3 (0.2 M) L-arginine supplementation. This study aimed to comprehensively evaluate the effect of L-arginine supplementation in Tris egg yolk extenders on semen quality by assessing sperm performance at two critical stages: before freezing (pre-freezing) and after thawing (post-thawing). Although the use of antioxidants in semen extenders has been widely studied, few investigations have systematically evaluated the optimal concentration to enhance sperm quality, especially in high-value semen, such as that from Simmental bulls. Excessive antioxidant supplementation may adversely affect spermatozoa cell structure (da Silva, 2017). Therefore, determining the appropriate dosage range for L-arginine supplementation in Tris-Egg Yolk extenders is essential to ensure optimal sperm preservation without compromising post-thaw viability. Adding high doses of L-arginine can cause premature hyperactivity of spermatozoa, resulting in decreased motility before fertilization (Silvestre et al., 2021). Table 1. Experimental groups of Tris-egg yolk extender with L-arginine supplementation.
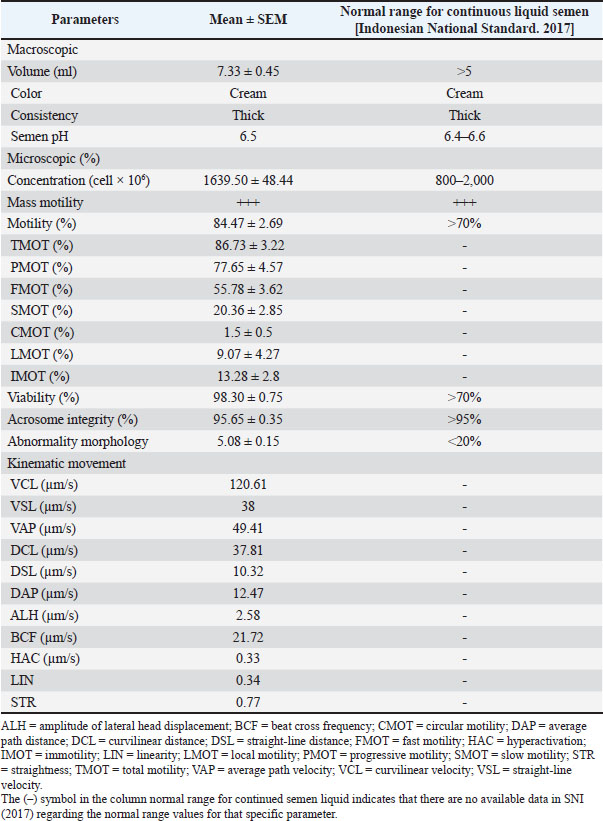
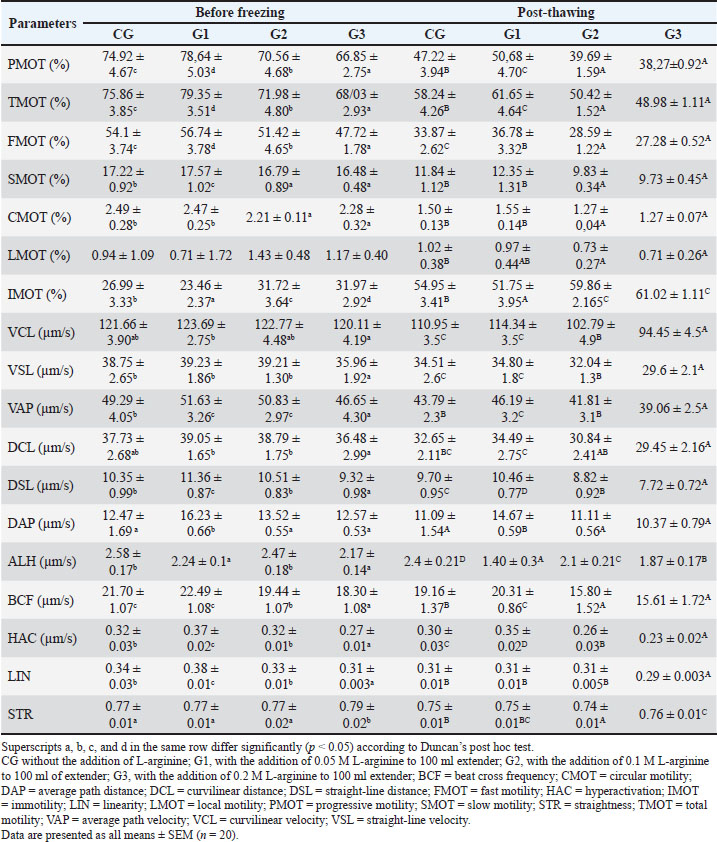
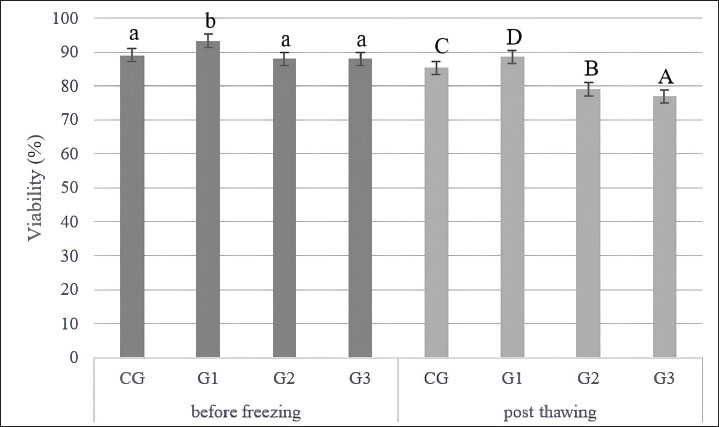
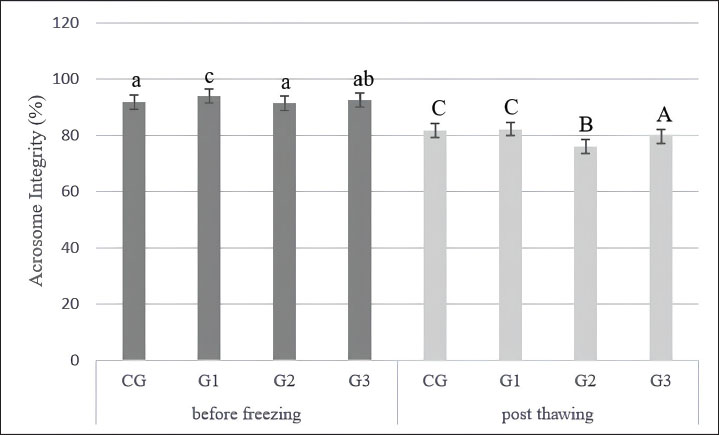
Collection and quality assessment of semenEjaculates were collected from four healthy Indonesian Simmental bulls and handled uniformly throughout the process. All collected ejaculates were pooled in equal proportions before dilution and cryopreservation. This pooling procedure was performed to minimize individual variation and eliminate the specific influence of each bull on the evaluation outcomes. Each ejaculate was individually evaluated before pooling, and only those meeting the minimum quality standards were included in the pooled sample. The eligibility criteria included a progressive motility (PMOT) of at least 70%, sperm concentration ranging from 800 to 2,000 × 106 cells/ml, and mass motility scored at +++, as assessed using a subjective scoring system ranging from + (very low) to ++++ (very high and vigorously swirling). A score of +++ indicates high sperm activity and is considered acceptable for AI according to national standards (Indonesian National Standard, 2017). Following pooling, the semen sample was immediately evaluated for macroscopic characteristics, including color, odor, volume, consistency (viscosity), and pH. Microscopic evaluations were also conducted to assess mass movement, sperm concentration, PMOT, viability (≥80%), morphological abnormalities of the head, midpiece, and tail (≤20%), and plasma membrane integrity (≥60%) (Indonesian National Standard, 2017). This study evaluated spermatozoa performance at two critical stages: before freezing (pre-freezing) and after thawing (post-thawing). The evaluation focused on parameters such as sperm motility and kinematic movement, viability, acrosome integrity, and lipid peroxidation level, measured through MDA concentrations. Supplementation of L-arginine in Tris-egg yolk extenderTris-egg yolk extender was freshly prepared before each freezing session, 1.363 g of Tris aminomethane (Merck, CAS: 108382), 0.762 g of citric acid (Merck Millipore, CAS: 77-92-9), 1.5 g of lactose monohydrate (Merck, CAS: 1.07660), 2.7 g of D-(+)-raffinose pentahydrate (Merck, CAS: 512-69-6), and 0.5 g D-(–)-fructose (Merck, CAS: 57-48-7) were added to 80 ml of double-distilled water. The mixture was homogenized for 15 minutes using a magnetic stirrer for 15 minutes. The osmolarity and pH were measured at 295 mOsm/l and 6.7, respectively (WESCOR model 5500, Inc., USA). The solution was then heated at 100°C for 5–10 minutes, cooled to room temperature, and supplemented with 0.1 g (100 IU) penicillin G and 0.1 g streptomycin sulfate (Meiji Seika Co., Japan). After rehomogenization, 20 ml of egg yolk was added to 80 ml of the extender and centrifuged at 450 rpm for 15 minutes. The supernatant was used, and the sediment was discarded (Bustani and Baiee, 2021; Kamal et al., 2022; Meles et al., 2022). The final Tris-egg yolk extender was divided into two components to facilitate a two-step dilution process. The first component, Diluter A, was used for initial dilution at 37 °C and contained either Tris-egg yolk alone (for the CG) or Tris-egg yolk supplemented with L-arginine (Sigma-Aldrich, CAS: 74-79-3) for the treatment groups G1, G2, and G3. The second component, Diluter B, consisted of Tris-egg yolk supplemented with 5% glycerol (without L-arginine) and was used as a cryoprotectant in all groups. Semen samples were divided into four experimental groups: CG (control, no L-arginine), G1 (0.05 M L-arginine), G2 (0.1 M L-arginine), and G3 (0.2 M L-arginine). Each ejaculate was initially diluted with Diluter A according to its assigned group and then gradually cooled to 5°C. Diluter B was gradually added to all groups after a 4-hours equilibration period at 5°C (Arif et al., 2020; Mokhtassi-Bidgoli et al., 2023) to ensure cryoprotection prior to freezing. This standardized two-step dilution protocol ensured that each group received the same final glycerol concentration (5%) regardless of L-arginine treatment (Málková et al., 2023). Following the equilibration phase, semen samples were cryopreserved by exposure to liquid nitrogen vapor and thawed at 37°C for 30 seconds, following procedures described by Badr et al. (2020). After the 4-hour equilibration period and prior to cryopreservation, prefreezing sperm quality evaluation was conducted. This evaluation was designed to assess the immediate physiological effects of L-arginine supplementation on sperm quality, independent of freeze–thaw-related damage. Acrosome integrityA thin smear of 0.1 l of semen was made on a slide of 0.1 µl of semen, dried, and fixed using 5% formaldehyde (Sigma Aldrich, NOCAT: 606758) in Phosphate Buffer Saline (HIMEDIA) with pH 7.4 for 15 minutes. Wash the slide with phosphate-buffered saline to remove formaldehyde residues. Staining Coomassie Brilliant Blue (CBB) (Sigma Aldrich, NOCAT: 6104-59-2) by mixing in a solution of 10% glacial acid (Rofa Laboratory Center, NOCAT: 1.00063.2500) and 25% methanol (NGI), wait for 5 minutes so that the CBB solution can react with the spermatozoa on the slide (Yániz et al., 2021). The slides were washed with distilled water (HIMEDIA) to remove residual CBB solution and observed under a microscope (Olympus CX-21, Olympus Corporation, Tokyo, Japan) with 1,000× magnification, and the microscope is connected to an OptiLab Advance image capture tool (PT. MICONOS) with five-megapixel photos. Spermatozoa acrosome integrity was assessed on 100 spermatozoa per field of view (Kim et al., 2022). The integrity of the spermatozoa acrosome is evaluated by determining whether or not the acrosome has an intense blue apical cap. An intact acrosome will appear with a fierce blue apical cap due to the reaction of CBB with spermatozoa, whereas damaged acrosomes will not appear blue on the acrosome’s apical cap (Kamal et al., 2022). Counting viable sperm with ImageJ count software determined the acrosome integrity results. Analysis of spermatozoa motilitySperm motion characteristics were evaluated using a CASA system (Androscope, Minitube®, Germany), which was equipped with a heated microscope stage maintained at 37°C to ensure optimal temperature during analysis and support the accuracy and reproducibility of results. In this study, semen samples were diluted at a 1:8 ratio (semen to extender), and 3 µl of the diluted sample was pipetted onto a pre-warmed, 20 µm mini glass disposable counting slide comprising four chambers, each accommodating 3 µl. The preparation was then covered with a pre-warmed cover glass and placed immediately on the heating device. For each sample, a minimum of 100 spermatozoa were analyzed per microscopic field, across three different fields, in accordance with standard CASA protocols (Becherer, 2023). The CASA was configured with a frame rate of 60 Hz and captured 30 frames per field to ensure comprehensive kinematic data acquisition (Maulana et al., 2022; Ratnawati et al., 2023b). Motility and kinematic parameters recorded included total motility (TMOT, %), defined as the percentage of all motile spermatozoa; PMOT, %, referring to spermatozoa moving actively forward along relatively straight trajectories; curvilinear velocity (VCL, µm/s), which denotes the velocity along the actual path taken; straight-line velocity (VSL, µm/s), the velocity measured in a straight line from the starting point to the endpoint; and average path velocity (VAP, µm/s), the mean velocity along a smoothed trajectory. Additional parameters measured included linearity (LIN, %), calculated as the ratio of VSL to VCL and indicative of path straightness (STR); STR, %, the ratio of VSL to VAP; wobble (%), the ratio of VAP to VCL; amplitude of lateral head displacement (ALH, µm), representing the lateral movement width of the sperm head; and beat-cross frequency (BCF, Hz), indicating the frequency at which the sperm head crosses its average path. These parameters provide a comprehensive assessment of sperm motility quality and were measured following validated and reproducible protocols to ensure data reliability and consistency across all samples (Becherer, 2023). Measurement of spermatozoa lipid peroxidation levelsThe thiobarbituric acid method quantified lipid peroxidation levels by measuring MDA concentrations, as previously described by Alyethodi et al. (2021). Spermatozoa and Tris-egg yolk extenders from each group were evaluated both before cryopreservation and after thawing. For analysis, 100 µl of frozen semen was transferred into a microtube, followed by the addition of 550 µl of distilled water and 100 µl of 10% trichloroacetic acid. The contents were thoroughly mixed using a vortex. Subsequently, 250 µl of 1 N HCl and 100 µl of 1% sodium thiosulfate were added to the mixture. The microtubes were vortexed and centrifuged at 4°C and 500 rpm for 10 minutes. The supernatant was collected and transferred into a fresh microtube that was securely sealed with parafilm. The samples were then incubated in a water bath at 100°C for 20 minutes and then allowed to cool to room temperature. The MDA concentration was determined by measuring the absorbance at 530 nm using a spectrophotometer. The quantification was based on a standard curve, with the equation y=198.11x + 0.8522 (Alyethodi et al., 2021; Khaeruddin et al., 2024). ViabilityA semen smear was made on a glass slide by mixing 0.1 µl of semen with 0.5 µl of eosin-nigrosin. Examination of semen smears was performed using a light microscope (Olympus CX-21, Olympus Corporation, Tokyo, Japan) with 400× magnification and a microscope connected to a tool to capture images with OptiLab Advance (PT. MICONOS), with a photo resolution of 5 megapixels. Spermatozoa viability was assessed on 300 spermatozoa per treatment group (Ratnawati et al., 2023a,b). This study was conducted in accordance with Wysokińska et al. (2023) with slight modifications. The method quantified spermatozoa viability as (number of live spermatozoa ÷ total spermatozoa observed [live + dead]) × 100%. Dead and live spermatozoa appeared red and transparent, respectively (Suprayogi et al., 2018; Safa et al., 2025). ImageJ counted viable spermatozoa to generate the final percentage. ParametersThe evaluated parameters encompassed various aspects of sperm motility, kinematic movement, acrosome integrity, and oxidative status. Specifically, motility was assessed through total motility (TMOT), PMOT, fast motility (FMOT), slow motility (SMOT), circular motility (CMOT), local motility (LMOT), and immotility (IMOT). Kinematic parameters were measured using CASA and included VCL, VSL, VAP, curvilinear distance (DCL), straight-line distance (DSL), average path distance (DAP), ALH, beat cross frequency (BCF), hyperactivation (HAC), LIN, and STR. Additionally, acrosome integrity was evaluated, and MDA levels were quantified to indicate lipid peroxidation. Statistical analysisStatistical analyses were performed using R Studio version 4.3.3. Before analysis, the data were tested for normality using the Shapiro-Wilk and homogeneity using the Levene test. If the data followed a normal distribution and were homogenous, they were analyzed using multivariate analysis of variance to identify significant differences among groups. Duncan’s multiple range test was performed for post hoc comparisons when significant differences were found to determine specific differences between treatment groups. Results were considered statistically significant when p < 0.05. All measured parameters, including motility, kinematic movement parameters, viability, acrosome integrity, and lipid peroxidation, were reported as mean ± SD. Ethical approvalA veterinarian supervised the maintenance, semen collection, and frozen semen production processes of Indonesian Simmental bulls. The Animal Care, Use, and Ethics Committee, Brawijaya University, Malang, Indonesia, approved all research procedures (Approval No. 177-KEP-UB-2023). This research was conducted from June to September 2024 at the Faculty of Veterinary Medicine, Airlangga University, Gresik, East Java, Indonesia. ResultsEvaluation of fresh semenThe macroscopic and microscopic analysis results are shown in Table 2. The median sperm concentration in this study was 1639.50 × 106 ± 48.44 with an average volume of 7.33 ± 0.45 ml. Average mass motility is +++, with large waves of sperm formed. In addition, the sperm had an average PMOT of 77.65% ± 2.69%. The average sperm viability was 98.30% ± 0.75%, and acrosome integrity was 95.65% ± 0.35%, respectively. In this study, fresh semen evaluation data were within the normal range for bull sperm concentration. Sperm abnormalities in male Simmental bulls range between 0.1% ± 0.01%. The semen is cream-colored and smells fresh, and when the tube is tilted, it appears to have a thick consistency with a semen pH of 6.50 (Susilawati, 2011). Macroscopic and microscopic quality parameters of fresh sperm were assessed (Table 2) to determine the suitability of the ejaculate for processing into frozen semen (Indonesian National Standard, 2017). Sperm motilitySupplementation of 100 ml of Tris-egg yolk extender with 0.05 M L-arginine (G1) significantly improved sperm motility both before and after cryopreservation. Before freezing, the G1 group exhibited significantly higher PMOT, TMOT, FMOT, and SMOT values than the other groups (p < 0.05). Although the CMOT value in G1 was not higher than that of the CG, the ANOVA and Duncan’s post hoc test revealed no significant difference between them. Additionally, G1 exhibited the lowest LMOT and IMOT values, which are considered positive indicators of sperm quality, reflecting a reduced proportion of non-progressive or inefficient sperm movement. Post-thawing, G1 consistently maintained superior motility quality, as indicated by significantly better PMOT, TMOT, FMOT, SMOT, CMOT, and IMOT values compared with those of the other groups (p < 0.05). Although no significant differences in LMOT were observed between G1, G2, and G3, G1 demonstrated the most stable and superior post-thaw performance. Kinematic movementVelocity parametersAlthough G1 and G2 showed numerically higher VCL, VSL, and VAP values before freezing, these differences were not statistically significant and did not improve post-thawing quality in G2 (Table 3). Cryopreservation adversely affected all groups by reducing VCL, VSL, and VAP percentages. However, only G1 demonstrated superior and consistent performance in the post-thawing phase by maintaining significantly higher VCL, VSL, and VAP percentages than G2 and G3 (p < 0.05). Distance parametersG1 exhibited the most favorable values for DCL, DSL, and DAP after freezing, while G2 and G3—despite showing similar pre-freezing values—experienced a decline in post-thaw performance. This indicates that excessive L-arginine (G2 and G3) supplementation may negatively affect sperm motility distance and reduce the overall movement efficiency. Sperm movement parametersSupplementation with L-arginine in G1 and G3 also influenced the ALH values, which were significantly higher than those in the CG (p < 0.05). However, the highest ALH values were observed in the CG, which may reflect uncoordinated or hyperactivated movement patterns. Compared with the other groups, G1 presented moderate ALH with optimal BCF, indicating controlled and efficient motility. BCF differed significantly (p < 0.05), G1 and CG > G2 and G3. Sperm ratio parametersAnalysis of motility ratios revealed that G1 significantly differed in HAC and LIN parameters, both before and after freezing (p < 0.05). Although G3 showed a relatively high STR value, this was not accompanied by improvements in viability, motility, or acrosome integrity, suggesting the presence of hyperactivated or abnormal motility patterns that may not support successful fertilization (Table 3). ViabilityCompared with GC, L-arginine supplementation in the diluent (G1, G2, and G3) positively impacted the viability of Indonesian Simmental bull spermatozoa before freezing (Figs. 1 and 2). The analysis showed a significant difference between GC and other groups (p < 0.05). Furthermore, of the treatment groups, only G1 could sustain a positive impact on post-thawing viability. Acrosome integrityThe supplementation of L-arginine in the various groups showed significant differences both before freezing and after thawing (p < 0.05). G1 had a positive impact on acrosome integrity both before freezing and after thawing. However, G2 and G3 only positively impact some integrity in the post-thawing phase (Fig. 3). Meanwhile, in the CG, before freezing, there was no difference between G2 and G3; post-thawing, the CG value was not different from G1 (p < 0.05) (Fig. 4). Table 2. Quality assessment of fresh Indonesian Simental bull semen.
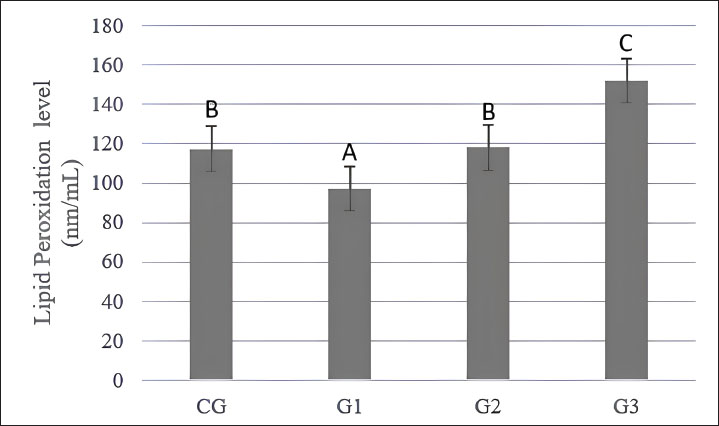
Lipid peroxidation of post-thawing spermatozoaThe level of lipid peroxidation in Indonesian Simmental bull spermatozoa showed significant differences between groups (p < 0.05). Supplementation of 0.05 M L-arginine (G1) in the diluent can reduce the MDA level in the post-thawing phase (Fig. 3). However, supplementation with 0.01 M (G2) and 0.02 M (G3) L-arginine increased MDA levels in spermatozoa. Supplementation with 0.05 M L-arginine (G1) reduced the negative impact of MDA levels in the post-thawing phase on Indonesian Simmental bull spermatozoa (Fig. 5). Although some parameters in G2 and G3 showed numerical increases before freezing, these did not correspond to improvements in post-thaw quality. Higher L-arginine concentrations increased oxidative stress and inconsistent performance across cell viability, motility, and membrane integrity. These findings indicate that only low-dose L-arginine (G1) provided consistent cryoprotective effects. Table 3. Kinematic and movement parameters of Indonesian Simmental spermatozoa before and after freezing and thawing.
DiscussionThe Indonesian Simmental bull semen used in this study exhibited good quality and was suitable for further freezing processes (Table 1). This study aimed to determine the optimal concentration of L-arginine supplementation (0.05, 0.1, and 0.2 M) in the semen extender for Indonesian Simmental bulls. The CASA technique was used to evaluate various motility parameters and kinematic movement. CASA provides more accurate information on sperm motility, which is one of the most critical parameters in assessing semen quality (Hoogewijs et al., 2012; Gloria et al., 2013; Amann and Waberski, 2014). This study demonstrates that L-arginine supplementation in the semen extender influences spermatozoa quality both before freezing and after thawing. The appropriate L-arginine dose enhances motility (Table 3), kinematic movement (Table 3), viability (Fig. 1), and membrane integrity (Fig. 2) while reducing lipid peroxidation levels (Fig. 3). Similar improvements in the quality of cryopreserved semen have also been reported using other antioxidants such as vitamin E, glutathione, melatonin, and ferulic acid (Omar et al., 2021; Afsar et al., 2024). However, L-arginine offers a unique mechanism of action as a precursor of nitic oxide (NO), which not only acts as a potent antioxidant but also enhances mitochondrial ATP synthesis and stabilizes sperm membranes. These multifaceted effects provide L-arginine with a broader protective role than conventional antioxidants.
Fig. 1. Viability of Indonesian Simmental bulls before freezing and post thawing. Superscripts a, b, c, and d in the same row differ significantly (p < 0.05) according to Duncan’s post hoc test. CG without the addition of L-arginine; G1, with the addition of 0.05 M L-arginine to 100 ml of extender; G2, with the addition of 0.1 M L-arginine to 100 ml of extender; G3, with the addition of 0.2 M L-arginine to 100 ml of extender.
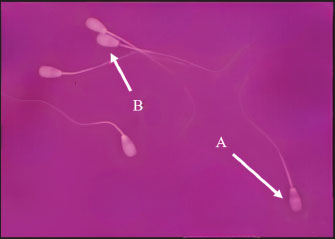
Fig. 2. Assessment of the viability of Indonesian Simmental bull spermatozoa after cryopreservation with L-arginine supplementation. (A) Dead spermatozoa (red staining). (B) Live spermatozoa (white/unstained).
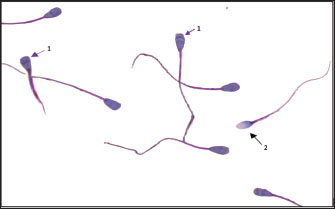
Fig. 3. Acrosome integrity of Indonesian Simmental bull spermatozoa after cryopreservation with various concentrations of L-arginine supplementation: 1) intact acrosome integrity; 2) damaged acrosome integrity.
Fig. 4. Integrity of acrosomes in Indonesian Simmental bulls before freezing and post thawing. Superscripts a, b, c, and d in the same row differ significantly (p < 0.05) according to Duncan’s post hoc test. CG without the addition of L-arginine; G1, with the addition of 0.05 M L-arginine to 100 ml of extender; G2, with the addition of 0.1 M L-arginine to 100 ml of extender; G3, with the addition of 0.2 M L-arginine to 100 ml of extender. L-arginine may enhance sperm motility parameters (PMOT, TMOT, FMOT, SMOT, CMOT, LMOT, and IMOT) at an optimal concentration (0.05 M) through the stimulation of NO synthesis in the seminal plasma, mediated by mitochondrial nitric oxide synthase (NOS). NO supports mitochondrial oxygen uptake and calcium ion transport, facilitating fatty acid β-oxidation and ATP production (Srivastava et al., 2006; Bustani and Baiee, 2021; Meles et al., 2022). In turn, ATP is critical for sustaining flagellar motility. Moreover, NO may activate Cyclic Guanosine Monophosphate-mediated signaling pathways, modulating phosphorylation processes, glucose utilization, and metabolic activity, while also contributing to mitochondrial membrane protection from oxidative damage. Additionally, L-arginine may improve mitochondrial stability by enhancing oxidative phosphorylation and modulating the mitochondrial proteome and reducing apoptotic signaling (Maciel et al., 2018; Bustani and Baiee, 2021). However, higher L-arginine concentrations (0.1 and 0.2 M) exhibit harmful effects. These findings are consistent with those of Silvestre et al. (2021) and Abd-Allah and Eliraqy (2019), who found that excessive antioxidant supplementation can cause oxidative stress, damaging the sperm plasma membrane. These doses induce sperm HAC before freezing and increase the number of immotile spermatozoa after thawing (Peris-Frau et al., 2020; Larasati et al., 2022). Excess NO production at high doses also triggers HAC, acrosome reaction, and capacitation (Adamou et al., 2024) while simultaneously increasing reactive oxygen species (ROS) and reactive nitrogen species (RNS) levels (Verma et al., 2017; Kim et al., 2022). The limited cytoplasmic volume in sperm cells restricts fluid exchange, resulting in osmotic imbalance and oxidative stress (Abd-Allah and Eliraqy 2019). Additionally, antioxidant enzymes such as peroxidase, catalase, superoxide dismutase, and glutathione, stimulated by L-arginine at high doses, may paradoxically contribute to a pro-oxidant state (Satouh and Ikawa, 2018; Omar et al., 2021; Paul et al., 2023). The lack of improvement at higher L-arginine concentrations (0.1 and 0.2 M) may be due to excessive NO production, which triggers oxidative stress through increased ROS/RNS (Srivastava et al., 2006; Verma et al., 2017). This condition can damage sperm membranes and impair motility. High NO levels are associated with premature capacitation and acrosome reactions (Adamou et al., 2024), with altered extender osmolarity and resultant osmotic stress (Abd-Allah and Eliraqy, 2019). Accordingly, optimal dosing is essential: both insufficient and excessive L-arginine compromise sperm function.
Fig. 5. Lipid peroxidation level of Indonesian Simmental bulls before freezing and post thawing. Superscripts a, b, c, and d in the same row differ significantly (p < 0.05) according to Duncan’s post hoc test. CG without the addition of L-arginine; G1, with the addition of 0.05 M L-arginine to 100 ml of extender; G2, with the addition of 0.1 M L-arginine to 100 ml of extender; G3, with the addition of 0.2 M L-arginine to 100 ml of extender. In this study, the addition of high doses of L-arginine (G2 and G3) resulted in inconsistencies in kinematic movement, leading to no improvements in semen quality for these groups compared to the control (GC) and low-dose group (G1). VCL is a parameter that measures the total distance traveled by spermatozoa while considering the movement trajectory (Utami et al., 2025). Spermatozoa with high kinematic energy are characterized by high VCL values, which positively influence their motility within the female reproductive tract. Only the G3 group performed worse than the GC group during the before-freezing phase. However, only the G1 group had a positive impact on velocity parameters in the post-thawing phase. The inconsistencies observed in the G2 group and the limited positive effects observed in the G3 group were likely due to the high doses of L-arginine. These elevated levels may disrupt sperm morphology, increase the proportion of abnormal sperm, and lead to changes in motility patterns (Hegazy et al., 2021). G1 demonstrated the highest DCL, DSL, and DAP values (Table 3). This finding is consistent with that of Gilmore et al. (2021), who associated higher DCL with improved sperm penetration ability during fertilization. DCL values are directly proportional to VCL values. A high VCL value indicates a high DCL value (Gilmore et al., 2021). The high DCL in G1 indicates that adding 0.05 M L-arginine to the extender could increase the path of spermatozoa during their movement. Therefore, the distance traveled by spermatozoa along the DAP increased. According to Utami et al. (2025), DSL is the shortest distance between the starting and ending points of spermatozoa movement. Thus, the low DSL value indicates that spermatozoa will have difficulty moving toward the pellucid zone. The G1 spermatozoa kinematic profile—higher BCF and lower ALH—demonstrates more efficient energy utilisation during motility. This profile may enhance the sperm’s ability to reach the oocyte more effectively (Kumar et al., 2016; Vigolo et al., 2022). Additionally, the higher LIN and STR values observed in G1 indicate a more stable and directed movement pattern, reflecting the positive influence of 0.05 M L-arginine supplementation on motility behavior (Broekhuijse et al., 2011; Utami et al., 2025). These motion parameters are strongly associated with the physiological process of capacitation, during which sperm undergo membrane and motility changes to prepare for fertilization. Elevated ALH is typically associated with hyperactivated motility—a hallmark of capacitated sperm—but excessively high ALH may signal premature or dysregulated capacitation, potentially impairing fertilization potential (Utami et al., 2025). In contrast, the moderate ALH values in G1 may reflect a controlled HAC, which is conducive to successful penetration of the zona pellucida. Similarly, optimal BCF values support coordinated flagellar activity, which slightly declines during capacitation but remains essential for propulsion efficiency. Overall, the G1 kinematic profile—moderate ALH, optimal BCF, and elevated LIN and STR—is associated with physiological capacitation whilst maintaining motility efficiency under 0.05 M L-arginine. This highlights its potential as an effective additive in semen extenders for improving the quality of cryopreserved sperm. High-dose L-arginine supplementation also disrupts osmolarity balance in the extender, further compromising sperm quality, motility, and fertilization potential (O’Flaherty et al., 2004; Hegazy et al., 2021). This is particularly evident in the viability (Fig. 1) and integrity of the acrosome (Fig. 4), where the addition of L-arginine at various doses showed no negative impact compared to GC in the before-freezing phase. However, in the post-thawing phase, only group G1 (0.05 M) maintained better viability and membrane integrity than the control (GC). Groups G2 and G3 (0.1 and 0.2 M, respectively) showed significant damage due to oxidative stress and osmolarity imbalance. Fig. 3 illustrates that only G1 could reduce lipid peroxidation levels more effectively than GC. MDA levels serve as an indicator of lipid peroxidation. L-arginine is an antioxidant that protects spermatozoa from lipid peroxidation by enhancing NO production at an appropriate dosage. NO helps suppress lipid peroxidation by neutralizing free radicals (Susilowati et al., 2019). Incorporating 0.05 M L-arginine into semen extenders offers a cost-effective strategy to enhance semen quality in AI programs. This protocol can increase conception rates and improve genetic dissemination efficiency in cattle breeding. Further in vivo trials are recommended to confirm these findings under field conditions. ConclusionBased on the results of this study, the Tris-egg yolk extender containing L-arginine can be an extracellular antioxidant and cryoprotectant that preserves spermatozoa during freezing and storage. The optimal dose of L-arginine is 0.05 M (G1), which can maintain and improve the quality of spermatozoa in motility variables (TMOT, PMOT, FMOT, SMOT, and LMOT), kinematic movement (VCL, VSL, VAP, DCL, DSL, DAP, ALH, BCF, HAC, STR, LIN), viability, CMOT and IMOT, acrosome integrity, and lipid peroxidation by reducing MDA levels in spermatozoa until the post-thawing stage. Supplementation doses of 0.1 M (G2) and 0.2 M (G3) L-arginine did not perform optimally for Indonesian Simmental bull spermatozoa both before freezing and post-thawing. AcknowledgmentsWe sincerely appreciate the support from the director of Research, Technology, and Community Service through the Penelitian Disertasi Doktor (PDD) in 2024. Conflict of interestNo potential conflicts of interest are relevant to the reported article. FundingThis research was financially supported by the Director of Research, Technology, and Community Service and Universitas Brawijaya through the Penelitian Disertasi Doktor (PDD) in 2024 under contract number 00309.138/UN10.A0501/B/PT.01.03.2/2024 Authors' contributionsAulia Firmawati: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Visualization, Writing—original draft, Writing-review and editing, Supervision, Funding acquisition, Habib Asshidiq Syah and Dicky Eka Mandala Putranto: Conceptualization, Methodology, Validation, Writing-review and editing. Trinil Susilawati, Sri Wahjuningsih, Widjiati Widjiati: Conceptualization, Methodology, Validation, Resources, Supervision, Project administration, Funding acquisition. Data availabilityAll data supporting this study’s findings are available within the manuscript. ReferencesAbd-Allah, E. and Eliraqy, E.Z. 2019. Effect of addition L-arginine to extender of poor motile Holstein bulls spermatozoa. J. Prod. Dev. 24(1), 165–179. Adamczyk, K., Makowska, A., Jędraszczyk, J., Hebda, L. and Gil, Z. 2013. Effect of behavior of Holstein-Friesian and Simmental bulls on semen quality. J. Cent. Eur. Agric. 14(4), 500–512. Adamou, M., Baulland, D.N.A., Pierrette, N.B., Herve, T., Momo, C.M.M., Claude, N.K., Mvondo, N.G.E., Nathalie, N., Njeiri, A.Y. and Ferdinand, N. 2024. Influence of aqueous extract of Annona muricata leaves in Tris-egg yolk extender on storage of spermatozoa from West African Dwarf goat (Capra hircus). J. Anim. Reprod. Biotechnol. 39(3), 179–193. Afsar, M., Soleimanzadeh, A., Khaki, A. and Ayen, E. 2024. Improvement of post-thaw quality and in vivo fertility of Simmental bull spermatozoa using ferulic acid. Vet. Med. Sci. 10(6), 1–10. Alyethodi, R.R., Sirohi, A.S., Karthik, S., Tyagi, S., Perumal, P., Singh, U., Sharma, A. and Kundu, A. 2021. Role of seminal MDA, ROS, and antioxidants in cryopreservation and their kinetics under the influence of ejaculatory abstinence in bovine semen. Cryobiology 98, 187–193. Amann, R.P. and Waberski, D. 2014. Computer-assisted sperm analysis (CASA): capabilities and potential developments. Theriogenology 81(1), 5–17. Arif, A.A., Maulana, T., Kaiin, E.M., Purwantara, B., Arifiantini, R.I. and Memili, E. 2020. Comparative analysis of various step-dilution techniques on the quality of frozen Limousin bull semen. Vet. World J. 13(11), 2422. Badan Pusat Statistik. (2023). Statistik Peternakan dan Kesehatan Hewan 2023. BPS. Available via https://www.bps.go.id/id/statistics-table/2/NDY5IzI=/populasi-sapi-potong-menurut-provinsi.html Badr, M.R., Abdel-Khalek, A.K.E., Sakr, A.M., Hegazy, M.M. and Rawash, Z.M. 2020. Effect of level and time of L-arginine addition to semen extender on the freezability and fertilizing potentials of buffalo spermatozoa. Assiut Vet. Med. J. 66(166), 19–30. Baharun, A., Rahmi, A., Kardaya, D., Said, S., Fahrudin, M., Arifiantini, R.I. and Karja, N.W.K. 2023. Profiling of seminal plasma proteins to identify the fertility of Simmental bull with low semen quality. J. Adv. Vet. Anim. Res. 10(3), 370–377. Becherer, D. 2023. Technical report: how to interpret motility analysis in AndroVision® and AndroScope. Minitube. Available via https://www.minitube.com/userdata/filegallery/original/1712_technical-report_casa-parameter_230406.pdf (Accessed 10 March 2025). Broekhuijse, M.L., Soštarić, E., Feitsma, H. and Gadella, B.M. 2011. Additional value of computer-assisted semen analysis (CASA) compared to conventional motility assessments in pig artificial insemination. Theriogenology 76(8), 1473–1486. Budiyanto, A., Arif, M., Alfons, M.P.W., Fani, R.T., Hafid, A.F., Wicaksono, B., Insani, K.M. and Herdinta, M. 2021. The effect of age and breed on the quality of bull semen in the Regional Artificial Insemination Centre. Acta Vet. Indones. 2021, 132–136. Bustani, G.S. and Baiee, F.H. 2021. Semen extenders: an evaluative overview of preservative mechanisms of semen and semen extenders. Vet. World 14(5), 1220–1233. Da Silva, F.M. 2017. Antioxidant properties of polyphenols and their potential use in improvement of male fertility: a review. Biomed. J. Sci. Tech. Res. 1(3), 612–617. Gilmore, A., Hitit, M., Ugur, M.R., Dinh, T.T.N., Tan, W., Jousan, D., Nicodemus, M., Topper, E., Kaya, A. and Memili, E. 2021. Functional variables of bull sperm associated with cryotolerance. Kafkas Univ. Vet. Fak. Derg. 27(3), 371–379. Gloria, A., Carluccio, A., Contri, A., Wegher, L., Valorz, C. and Robbe, D. 2013. The effect of the chamber on kinetic results in cryopreserved bull spermatozoa. Andrology 1(6), 879–885. Hegazy, M.M., Sakr, A.E.A.M., Abd El-aziz, A.H. and Swelum, A.A. 2021. Effect of adding different concentrations of L-arginine to Tris-yolk extender on the quality of sub-fertile ejaculates in buffalo. Trop. Anim. Health Prod. 53(1), 103. Hilmiati, N., Ilham, N., Nulik, J., Rohaeni, E.S., deRosari, B., Basuki, T., Hau, D.K., Ngongo, Y., Lase, J.A., Fitriawaty, F. and Surya, S. 2024. Smallholder cattle development in Indonesia: learning from the past for an outcome-oriented development model. Int. J. Design Nat. Ecodyn. 19(1), 169–184. Hoogewijs, M.K., De Vliegher, S.P., Govaere, J.L., De Schauwer, C., De Kruif, A. and Van Soom, A. 2012. Influence of counting chamber type on CASA outcomes of equine semen analysis. Equine Vet. J. 44(5), 542–549. Indonesian National Standard. 2017. Frozen semen–Part 1: Cattle (SNI 4869-1:2017). National Standardization Agency of Indonesia. Jakarta, Indonesia. Jakop, U., Engel, K.M., Hürland, M., Müller, P., Osmers, J.H., Jung, M. and Schulze, M. 2023. Lipid alterations by oxidative stress increase detached acrosomes after cryopreservation of semen in Holstein bulls. Theriogenology 197, 37–45. Kamal, M.M., Alam, M.E., Islam, M.A., Gofur, M.R. and Kabir, A. 2022. Effects of Tris (hydroxymethyl) aminomethane and egg yolk on the cryopreservation of buck semen. J. Adv. Vet. Anim. Res. 9(4), 676–683. Khaeruddin, K., Ciptadi, G., Yusuf, M., Iswati, I., Udrayana, S.B. and Wahjuningsih, S. 2024. Effectiveness of butylated hydroxytoluene in maintaining the quality of Gaga chicken sperm in liquid storage for 72 hours. Adv. Anim. Vet. Sci. 12(2), 371–380. Khodaei, H., Chamani, M., Mahdavi, B. and Akhondi, A.A. 2016. Effects of adding sodium nitroprusside to semen diluents on motility, viability and lipid peroxidation of sperm in Holstein bulls. Int. J. Fertil. Steril. 9(4), 521. Kim, S.W., Lee, J.Y., Kim, C.L., Ko, Y.G. and Kim, B. 2022. Evaluation of rooster semen quality using CBB dye-based staining method. J. Anim. Reprod. Biotechnol. 37(1), 55–61. Kumar, L., Yadav, S.K., Kushwaha, B., Pandey, A., Sharma, V., Verma, V., Maikhuri, J.P., Rajender, S., Sharma, V.L. and Gupta, G. 2016. Energy utilization for survival and fertilization—parsimonious quiescent sperm turn extravagant on motility activation in rat. Biol. Reprod. 94(4), 1–9. Larasati, M.D., Lestari, S.W., Hestiantoro, A. and Pangestu, M. 2022. Can cryoprotectant’s modification in spermatozoa cryopreservation be an alternative to improve embryo quality? A review. Int. J. Technol. 13(8), 1755–1767. Leroy, G., Danchin-Burge, C. and Verrier, E. 2011. Impact of the use of cryobank samples in a selected cattle breed: a simulation study. Genet. Sel. Evol. 43(1), 1–8. Maciel, V.L., Caldas-Bussiere, M.C., Silveira, V., Reis, R.S., Rios, A.F.L. and Paes De Carvalho, C.S. 2018. L-arginine alters the proteome of frozen-thawed bovine sperm during in vitro capacitation. Theriogenology 119(1), 1–9. Málková, A., Savvulidi, F.G., Ptáček, M., Machová, K., Janošíková, M., Nagy, S. and Stádník, L. 2023. Glycerol-free equilibration with the addition of glycerol shortly before the freezing procedure: a perspective strategy for cryopreservation of Wallachian ram sperm. Animals 13(7), 1200. Maulana, T., Agung, P.P., Gunawan, M. and Said, S. 2022. Computer-aided semen analysis (CASA) to determine the quality and fertility of frozen-thawed Sumba Ongole sperm supplemented with amino acids. Livest. Anim. Res. 20(2), 194–201. Meles, D.K., Mustofa, I., Hariadi, M., Wurlina, W., Susilowati, S., Amaliya, A., Suparto, S. and Rimayanti, R. 2022. The enriched Y-bearing sperm combined with delayed fixed-time artificial insemination for obtaining male Simmental crossbred offspring. Vet. World 15(1), 102–109. Mokhtassi-Bidgoli, A., Sharafi, M. and Benson, J.D. 2023. Optimizing bull semen cryopreservation media using multivariate statistics approaches. Animals 13(6), 1077. O’Flaherty, C., Rodriguez, P. and Srivastava, S. 2004. L-arginine promotes capacitation and acrosome reaction in cryopreserved bovine spermatozoa. Biochimica Et Biophysica Acta (BBA). - Gen. Subjects 1674(2), 215–221. Omar, A.A., Ahmed, M.N., Asker, A.S., Majeed, A.F. and Awad, R.M. 2021. Effects of addition of melatonin and L-arginine on cooled semen parameter of Iraqi local breed rams in vitro. Indian J. Forensic Med. Toxicol. 15(1), 2732–2759. Paoli, D., Pelloni, M., Lenzi, A. and Lombardo, F. 2019. Cryopreservation of sperm: effects on chromatin and strategies to prevent them. Adv. Exp. Med. Biol. 1166, 149–167. Paul, D.R., Talukdar, D., Ahmed, F.A., Lalrintluanga, K., Kalita, G., Tolenkhomba, T.C., Chakravarty, H., Katiyar, R., Khargharia, G. and Deori, S. 2023. Effect of selenium nanoparticles on the quality and fertility of short-term preserved boar semen. Front. Vet. Sci. 10, 1333841. Peña, S.T., Gummow, B., Parker, A.J. and Paris, D.B.B.P. 2019. Antioxidant supplementation mitigates DNA damage in boar (Sus scrofa domesticus) spermatozoa induced by tropical summer. PLos One 14(4), 1–14. Peris-Frau, P., Soler, A.J., Iniesta-Cuerda, M., Martín-Maestro, A., Sánchez-Ajofrín, I., Medina-Chávez, D.A., Fernández-Santos, M.R., García-Álvarez, O., Maroto-Morales, A., Montoro, V. and Garde, J.J. 2020. Sperm cryodamage in ruminants: understanding the molecular changes induced by the cryopreservation process to optimize sperm quality. Int. J. Mol. Sci. 21(8), 2781. Ratnawati, D., Ciptadi, G., Rahayu, S. and Susilawati, T. 2023a. First study of aqueous soybean (Glycine max) extract nanoparticles as a substitute of egg yolk on motility and kinetic parameters spermatozoa of frozen semen. Int. J. Agric. Biol. 30(5), 353–358; doi:10.1016/j.ijab.2008.08.010 Ratnawati, D., Kuswati, K., Yekti, A.P.A., Ciptadi, G., Rahayu, S. and Susilawati, T. 2023b. Effect of modified CEP-3 diluents with aqueous soybean extract on liquid semen quality in Ongole crossbred bull. Vet. World J. 16(5), 1075–1083. Safa, F., Yekti, A.P.A., Utami, P., Syah, H.A., Febrianto, N., Rachmawati, A., Yuswati, A., Amaliya, A., Sulistyowati, D. and Susilawati, T. 2025. Sperm quality, kinematics, membrane integrity, and DNA fragmentation of frozen sexed semen in Holstein-Friesian bulls. Trop. Anim. Sci. J. 48(3), 199–210. Saha, A., Asaduzzaman, M. and Bari, F.Y. 2022. Cryopreservation techniques for ram sperm. Vet. Med. Int. 1(1), 7378379. Satouh, Y. and Ikawa, M. 2018. New insights into the molecular events of mammalian fertilization. Trends Biochem. Sci. 43(10), 818–828. Silvestre, M.A., Yániz, J.L., Peña, F.J., Santolaria, P. and Castelló-Ruiz, M. 2021. Role of antioxidants in cooled liquid storage of mammal spermatozoa. Antioxidants 10(7), 1096. Srivastava, S., Desai, P., Coutinho, E. and Govil, G. 2006. Mechanism of action of L-arginine on the vitality of spermatozoa is primarily through increased biosynthesis of nitric oxide. Biol. Reprod. 74(5), 954–958. Suprayogi, T.W., Hernawati, T. and Susilowati, S. 2018. The addition of L-arginine in capacitation media to motility, viability, and spermatozoa capacity of goats. Int. J. ChemTech. Res. 11(3), 13–18. Susilawati, T. 2011. Spermatology. Malang: Brawijaya University Press, pp. 44–50. Susilowati, S., Mustofa, I., Wurlina, W., Triana, I.N., Utama, S. and Rimayanti, R. 2021. Effect of insulin-like growth factor-1 complex of Simmental bull seminal plasma on post-thawed Kacang buck semen fertility. Vet. World 14(8), 2073–2084. Susilowati, S., Triana, I.N., Wurlina, W., Arimbi, A., Srianto, P. and Mustofa, I. 2019. Addition of L-arginine in skim milk extender maintains goat spermatozoa quality in chilled temperature for five days. Vet. World 12(11), 1784–1789. Utami, P., Yekti, A.P.A., Simbolon, C.N.A., Syah, H.A., Amaliya, A., Siswoyo, T.A., Isnaini, N. and Susilawati, T. 2025. Analysis of kinetic parameters of sexed Holstein-Friesian bull spermatozoa using Percoll density gradient centrifugation with computer-assisted sperm analysis. Vet. World 18(2), 287–295. Verma, N., Singh, A.K. and Singh, M. 2017. L-arginine biosensors: a comprehensive review. Biochem. Biophys. Rep. 12, 228–239. Vigolo, V., Giaretta, E., Da Dalt, L., Damiani, J., Gabai, G., Bertuzzo, F. and Falomo, M.E. 2022. Relationships between biomarkers of oxidative stress in seminal plasma and sperm motility in bulls before and after cryopreservation. Animals 12(19), 2534. Wysokińska, A., Szablicka, D., Dziekońska, A. and Wójcik, E. 2023. Analysis of changes in the morphological structures of sperm during preservation of liquid boar semen in two different seasons of the year. Anim. Reprod. Sci. 256, 107297. Yániz, J.L., Palacín, I., Silvestre, M.A., Hidalgo, C.O., Tamargo, C. and Santolaria, P. 2021. Ability of the ISAS3FUN method to detect sperm acrosome integrity and its potential to discriminate between high and low field fertility bulls. Biol 10(11), 1135. | ||
| How to Cite this Article |
| Pubmed Style Firmawati A, Syah HA, Putranto DEM, Widjiati W, Wahjuningsih S, Susilawati T. Cryopreserved Indonesian Simmental bull semen: Effect of L-argininesup plementation in Tris egg yolk extender on sperm quality. Open Vet. J.. 2025; 15(9): 4578-4591. doi:10.5455/OVJ.2025.v15.i9.64 Web Style Firmawati A, Syah HA, Putranto DEM, Widjiati W, Wahjuningsih S, Susilawati T. Cryopreserved Indonesian Simmental bull semen: Effect of L-argininesup plementation in Tris egg yolk extender on sperm quality. https://www.openveterinaryjournal.com/?mno=249197 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i9.64 AMA (American Medical Association) Style Firmawati A, Syah HA, Putranto DEM, Widjiati W, Wahjuningsih S, Susilawati T. Cryopreserved Indonesian Simmental bull semen: Effect of L-argininesup plementation in Tris egg yolk extender on sperm quality. Open Vet. J.. 2025; 15(9): 4578-4591. doi:10.5455/OVJ.2025.v15.i9.64 Vancouver/ICMJE Style Firmawati A, Syah HA, Putranto DEM, Widjiati W, Wahjuningsih S, Susilawati T. Cryopreserved Indonesian Simmental bull semen: Effect of L-argininesup plementation in Tris egg yolk extender on sperm quality. Open Vet. J.. (2025), [cited January 25, 2026]; 15(9): 4578-4591. doi:10.5455/OVJ.2025.v15.i9.64 Harvard Style Firmawati, A., Syah, . H. A., Putranto, . D. E. M., Widjiati, . W., Wahjuningsih, . S. & Susilawati, . T. (2025) Cryopreserved Indonesian Simmental bull semen: Effect of L-argininesup plementation in Tris egg yolk extender on sperm quality. Open Vet. J., 15 (9), 4578-4591. doi:10.5455/OVJ.2025.v15.i9.64 Turabian Style Firmawati, Aulia, Habib Asshidiq Syah, Dikky Eka Mandala Putranto, Widjiati Widjiati, Sri Wahjuningsih, and Trinil Susilawati. 2025. Cryopreserved Indonesian Simmental bull semen: Effect of L-argininesup plementation in Tris egg yolk extender on sperm quality. Open Veterinary Journal, 15 (9), 4578-4591. doi:10.5455/OVJ.2025.v15.i9.64 Chicago Style Firmawati, Aulia, Habib Asshidiq Syah, Dikky Eka Mandala Putranto, Widjiati Widjiati, Sri Wahjuningsih, and Trinil Susilawati. "Cryopreserved Indonesian Simmental bull semen: Effect of L-argininesup plementation in Tris egg yolk extender on sperm quality." Open Veterinary Journal 15 (2025), 4578-4591. doi:10.5455/OVJ.2025.v15.i9.64 MLA (The Modern Language Association) Style Firmawati, Aulia, Habib Asshidiq Syah, Dikky Eka Mandala Putranto, Widjiati Widjiati, Sri Wahjuningsih, and Trinil Susilawati. "Cryopreserved Indonesian Simmental bull semen: Effect of L-argininesup plementation in Tris egg yolk extender on sperm quality." Open Veterinary Journal 15.9 (2025), 4578-4591. Print. doi:10.5455/OVJ.2025.v15.i9.64 APA (American Psychological Association) Style Firmawati, A., Syah, . H. A., Putranto, . D. E. M., Widjiati, . W., Wahjuningsih, . S. & Susilawati, . T. (2025) Cryopreserved Indonesian Simmental bull semen: Effect of L-argininesup plementation in Tris egg yolk extender on sperm quality. Open Veterinary Journal, 15 (9), 4578-4591. doi:10.5455/OVJ.2025.v15.i9.64 |