| Research Article | ||
Open Vet. J.. 2025; 15(7): 3087-3096 Open Veterinary Journal, (2025), Vol. 15(7): 3087-3096 Research Article Effects of sildenafil and metformin on endothelin-1 in the progression of pulmonary hypertensionAgus Cahyono1,2, Irwanto3*, Mahrus Abdur Rahman3 and Widjiati Widjiati41Doctoral Program of Medical Science, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia 2Department of Clinical Medicine, Faculty of Medicine, Universitas Surabaya, Surabaya, Indonesia 3Department of Child Health, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia 4Department of Veterinary Science, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia *Corresponding Author: Irwanto. Department of Child Health, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: irwanto [at] fk.unair.ac.id Submitted: 12/03/2025 Revised: 16/06/2025 Accepted: 18/06/2025 Published: 31/07/2025 © 2025 Open Veterinary Journal
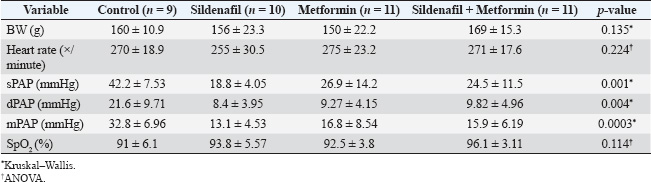
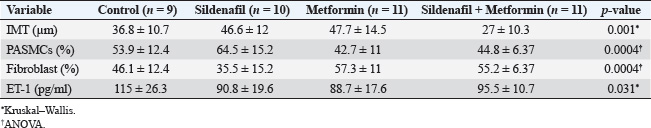
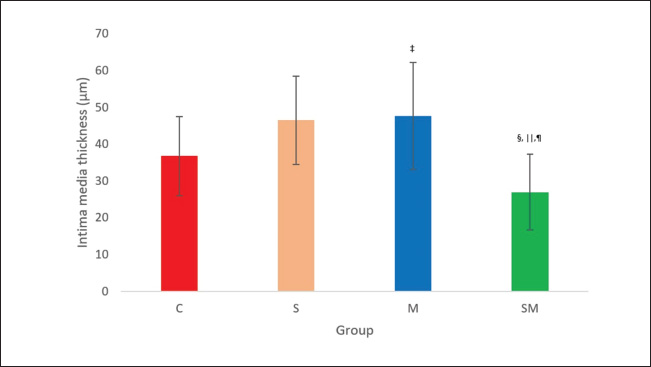
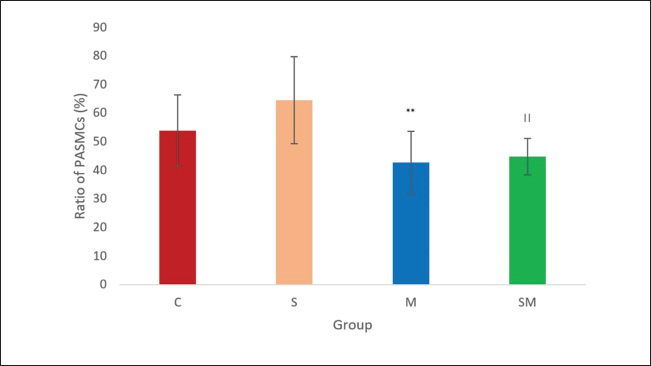
ABSTRACTBackground: Pulmonary hypertension (PH) is a progressive and potentially fatal condition if left untreated. Currently, only sildenafil and bosentan have been approved by the Food and Drug Administration for the medical treatment of pediatric patients. The availability of bosentan, in a developing country, such as Indonesia, is a challenge. Aim: To evaluate the effect of sildenafil and metformin on endothelin (ET)-1 levels in the progression of PH. Methods: Forty-eight, 12-week-old, male Wistar rats, weighing 117–193 g, were injected by monocrotaline (MCT) [60 mg/kg body weight (BW)] subcutaneously. The rats were then randomly assigned to four groups (control, sildenafil, metformin, and sildenafil plus metformin). One day after MCT injection, sildenafil (5 mg/kg BW) and metformin (100 mg/kg BW) were administered once daily for 21 days. Right heart catheterization was performed on day 22 after anesthesia with ketamine 50 mg/kg BW and xylazine 5 mg/kg BW intramuscularly. Blood was taken intracardially for approximately 3.5 ml, and the rats were euthanized. The serum was stored in −20° refrigerator before ELISA analysis. The lung was harvested and paraffin-blocked for later histology analysis. Blood serum was analyzed for ET-1, and a section of paraffin-blocked lung was stained with hematoxylin-eosin and α-smooth muscle actin. Intima media thickness (IMT) of 50–200 µm artery and ratio of pulmonary artery (PA) smooth muscle cells/fibroblast were analyzed. Results: The mean PA pressure (mPAP) and ET-1 of the treatment group were significantly lower than those of the control group. IMT was significantly lower in the sildenafil plus metformin group than in the other groups. Conclusion: Sildenafil and metformin alone or in combination improve mPAP and ET-1 levels. Sildenafil plus metformin prevents artery remodeling in the PH model. Keywords: Sildenafil; Metformin; Endothelin-1; Pulmonary; Hypertension. IntroductionPulmonary hypertension (PH), albeit rare its consequences, may lead to fatality when not well treated. It is defined as resting mean pulmonary arterial pressure (mPAP) of >20 mm Hg by right heart catheterization (Humbert et al., 2023). A study revealed its prevalence of 15–50 cases/million in adults and 2–16 cases/million in children. In the higher-risk groups, such as children with congenital heart disease, this number is higher (Hansmann, 2017). Nowadays, progression has been made in PH medical treatment (Guglielmi et al., 2025). However, for pediatric patients, only sildenafil (PDE-5 inhibitor) and bosentan (endothelin receptor antagonist) are approved by the Food and Drug Administration (FDA) and the European Medical Agency (EMA) (Wang et al., 2019; Avitabile et al., 2020). In developing countries, such as Indonesia, sildenafil is easier to obtain than bosentan. Sildenafil, as a single drug, has limitation in its clinical use for children with bronchopulmonary dysplasia (BPD), congenital diaphragmatic hernia (CDH), and Down syndrome. The survival rate and lowering of mPAP in these patients are still unsatisfactory (Beghetti et al., 2017; Cohen et al., 2019). Metformin is an alternative to bosentan which has similarity in its action to lower endothelin-1 (ET-1) (Yoshida et al., 2020). Endothelin is one of the important keys in development of PH (Alqarni et al., 2023). One of its substance, ET-1, is a potent and the strongest vasoconstrictor (Yanagisawa et al., 1988). It plays an important role in the pathobiology of PH, not only because of its vasoconstriction action but also because of its mitogenic properties (Barton and Yanagisawa, 2019; Chester and Yacoub, 2014). Research aims to understand ET-1 and its effect on PH is growing (Parikh et al., 2016; Low et al., 2018; Feriel et al., 2024). Sildenafil inhibits pulmonary artery (PA) remodeling by lowering ET-1A receptor and plasma ET-1 (Liu et al., 2007). Its anti-inflammatory effects are by decreasing proinflammatory cytokine (TNF-α, IL-6, and IFN- γ) and increasing anti-inflammatory cytokine (TGF-β and IL-10) (Zych et al., 2019; Di Luigi et al., 2020; Kniotek et al., 2021). Metformin decreases ET-1, IL-1, IL-6, TNF-α, and increasing IL-10 (Kang et al., 2013; Ye et al., 2018; Ranchoux et al., 2019; Vazifehkhah et al., 2020; Yoshida et al., 2020). The combination of these drugs may inhibit PH progression. This combination may result in further decreasing of ET-1. This study aimed to evaluate the effects of sildenafil and metformin treatment in a PH model focusing on histology profile and ET-1. Materials and MethodsStudy design and settingThis experimental study was conducted from April 2024 to June 2024 at the Animal Laboratory, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, East Java, Indonesia. The study used Rattus norvegicus strain Wistar as the model for PH disease. After inducing the model using monocrotaline (MCT), the rats were randomly assigned to 4 groups (control, sildenafil, metformin, and sildenafil + metformin). One day later, the rats were treated for 21 days. On the 22nd day, right heart catheterization was performed, and the rats were euthanized. Animal preparation and samplingRattus norvegicus strain Wistar was obtained from PT Riset, AIRC, Indonesia. The minimum sample calculation for this study was nine per group. To anticipate 20% dropout, the animal requirement was 12 rats per group. Animal eligibility and randomizationHealthy, 12-week-old, male Wistar rats with a range of body weight (BW) 117–193 g were included in the study. Rats that died during the study were excluded from the analysis. After MCT injection, the rats were assigned to four groups by simple random allocation. Each group was differed by symbols (C for control, S for sildenafil, M for metformin, and SM for sildenafil + metformin). Animal acclimatization and housingRats were acclimatized for 7 days before the intervention. The rats were roomed under standard conditions (24°C room temperature, 50%–70% humidity) on a 12-hour light/12-hour dark cycle and supplied with standard laboratory food and water. Each rat was housed individually. Animal model and interventionRats were injected by 60 mg/kg of BW MCT (MedChemExpress, catalog number HY-N0750) subcutaneously to induce PH. One milliliter of hydrochloric acid was poured into MCT and mixed with 1 M Sodium hydroxide to the desired pH of 7.4. After MCT injection, the rats were randomly assigned to 4 groups (control, sildenafil, metformin, and metformin + sildenafil). For sildenafil, we purchased Revatio oral suspension (10 mg/ml) from Pfizer, and metformin (Glucophage) was purchased from Merck. The dose used for sildenafil was 5 mg/kg BW, and metformin was 100 mg/kg BW. Treatment was given on the 1st day after MCT injection for 21 days. At day 22nd after MCT injection, pulmonary arterial pressure was evaluated by right heart catheterization. Intramuscular anesthesia using ketamine 50 mg/kg BW and xylazine 5 mg/kg BW was administered before the catheterization procedure. The rats were placed in the supine position. Surgical procedure was performed to make the heart silhouette seen after shaved fur. After washing with heparin, intravenous catheter no. 22G was slipped under the 4th parasternal intercostalis space and directed to the right ventricle. Pulsatile dark red blood will come out of the right ventricle. The catheter was then connected to the transducer and Mac 51 cable. The PA could be reached by pushing approximately 5 mm and maneuvering the catheter. The correct position of the catheter was confirmed by the waveform seen on the DASH 4000 monitor. Systolic pulmonary arterial pressure (sPAP), diastolic pulmonary arterial pressure (dPAP), and mPAP were recorded when the pressure was stable. Other clinical data, such as heart rate and peripheral oxygen saturation (SpO2), were also obtained. After catheterization, blood was exsanguinated intracardially for approximately 3.5 ml. The euthanasia was completed by cutting the aorta. The lungs were harvested, submerged in formalin, and embedded in paraffin. A refrigerated centrifuge (Kubota K3520) was used to centrifuge blood (at 3,500 rpm and 4°C) for 10 minute. The serum was stored in a refrigerator (at −20°C) before ELISA analysis. Histological analysisFormalin-fixed, paraffin-embedded blocks of the right and left lung were cut to 5 µm thickness. These sections were deparaffinized by washing with xylene for 5 minutes three times. The xylene was removed using methanol, and the sample was washed for 1 minute in water. After the procedure, the samples were stained with hematoxylin for 10 minutes and eosin for 6 minutes at room temperature. Small to medium pulmonary arteries ranging from 50 to 200 µm in diameter were assessed using a light microscope at ×400 magnification. Four pulmonary arteries for each lung were randomly selected. For each artery, intima media thickness (IMT) was measured from four of its circular areas. The thickness was calculated by summing and averaging. The samples were also stained with alpha smooth muscle actin (α-SMA, GeneTex, catalog number GTX636885) antibody. The ratio of PA smooth muscle cells (PASMCs) to fibroblasts of each artery was then evaluated. The ratio was quantified by measuring the percentage of PASMCs and fibroblast of eight pulmonary arteries (four for each lung). The percentage was summed and averaged. ELISAThe rat ET-1 competitive ELISA kit (catalog number ER0019, Fine Test) was used to measure serum ET-1 concentrations. The ELISA assay began by thawing frozen serum samples. For ET-1 measurement, the plate was washed twice before the addition of the standard and sample. Each well that contained 50 µl of standard or sample was added by 50 µl of biotin-labeled antibody working solution. After shaking the plate for 1 minute, it was statically incubated at 37°C for 45 minutes. After incubation, it was washed three times and bathed for 1 minute each. Each well was added with 100 µl of horseradish peroxidase-Streptavidin Conjugate working solution. Subsequently, the plate was sealed and statically incubated at 37°C for 45 minutes. After that, it was washed five times and bathed for 1 minute each. The plate was then added with 90 µl 3,3 ‘,5,5 ‘-Tetramethylbenzidine substrate solution. The samples were sealed and incubated at 37°C for 10–20 minutes. At the end, the absorbance was read immediately at 450 nm after 50 µl of stop solution was added. Statistical analysisData are presented as mean ± SD. The normality of data was analyzed using a Shapiro–Wilk test. Differences among multiple groups were analyzed by one-way ANOVA (for normally distributed parameters) followed by Tukey’s post hoc analysis. A Kruskal–Wallis test followed by Mann–Whitney U test was used to analyze nonparametric parameters. Significance was considered at p < 0.05. Statistical analysis was performed using Jamovi v.2.4.14. Ethical approvalThis study was approved by the Animal Care and Use Committee of the Faculty of Veterinary Medicine, Universitas Airlangga No. 2.KEH.161.10.2023. ResultsCharacteristics of the animalsOf 48 rats, 7 rats died (3 in control, 2 in sildenafil, and each 1 in metformin and sildenafil + metformin group) during the study and the remaining 41 rats. Data collected from experimental rats, including BW, heart rate, sPAP, dPAP, mPAP, and SpO2 from four groups, are described in Table 1. Bodyweight, heart rate, and SpO2 of the four groups were homogeneous. However, sPAP, dPAP, and mPAP were significantly lower in the treatment group than in the control group. Post hoc analysis revealed significance between treatment and control groups. The systolic PAP of sildenafil was lowest among the treatment groups but did not reach significance in the metformin (p=0.138) and sildenafil plus metformin (p=0.168) groups. The diastolic PAP of sildenafil was also lowest among the treatment groups, although it did not reach significance when compared with that of metformin (p=0.645) and sildenafil plus metformin (p=0.458) group. Moreover, the mPAP of sildenafil was lowest among the treatment groups but did not reach a significant difference with metformin (p=0.320) and sildenafil plus metformin (p=0.305) groups. Histology and ELISA dataHistology and ELISA data are described in Table 2. There were significant differences among IMT, PASMCs, fibroblasts, and ET-1 on ANOVA and Kruskal–Wllis analysis. Post hoc analysis revealed that the IMT of the sildenafil plus metformin group was significantly thinner than that of the entire group. The IMT of the metformin group was significantly thicker than that of the control (Fig. 1). The ratio of PASMCs in the metformin and sildenafil plus metformin group was significantly lower than that in the sildenafil group (Fig. 2). The ratio of fibroblasts in the metformin and sildenafil plus metformin group was significantly higher than that of the sildenafil group (Fig. 3). ET-1 levels in the treatment groups were significantly lower than in the control group. In the metformin group, ET-1 was the lowest among the treatment groups (Fig. 4). Figure 5 presents a representative of PA histology in which a-d depict a comparison of IMT in each group by hematoxylin-eosin (HE) staining, and Figure 1E–H depicts the comparison of the ratio of PASMCs/fibroblast in each group by α-SMA staining. The intima media was stained purple in HE. The IMT was thicker in the sildenafil and metformin group than in the other groups, and the IMT was thinner in the sildenafil plus metformin group than in the other groups. Comparison of the ratio of PASMCs/fibroblast each group by α-SMA staining can also be observed. In the sildenafil group, PASMCs were more prominent and could be distinguished clearly from other treatment groups. Table 1. Clinical data of the experimental rats.
Table 2. Histology and ELISA data.
Fig. 1. Effect of sildenafil and metformin on IMT of PA. The result is presented as mean ± SD. ‡p=0.046 compared with control. §p=0.020 compared with control. ||p=0.001 compared with sildenafil. ¶p=0.001 compared with metformin. C=control; M=metformin; S=sildenafil; SM=sildenafil + metformin. DiscussionAlthough the pathobiology of PH remains unclear, its understanding has been revealed by dedicated research on this topic. Abnormalities can be observed in clinical, pathological, and biomolecular (Humbert et al., 2019). To establish diagnosis of PH, right heart catheterization procedure is a gold standard. Pulmonary arterial pressure more than 20 mm Hg is a confirmation of PH diagnosis (Humbert et al., 2023). With the advancement of PH, remodeling of the PA can become pronounced (Jia et al., 2023). Proinflammatory cytokine (TNF-α, IL-1, and IL-6), vasoconstrictor mediators (ET-1), and PASMCs or fibroblast proliferative agents (ET-1, ERK) are involved in this pathology (Scott et al., 2021; Guo et al., 2022; Alqarni et al., 2023; Guglielmi et al., 2025). Some drugs have been developed to inhibit PH progression in adults. However, in the pediatric population, the use of these drugs has become limited; only sildenafil and bosentan have been approved by the FDA and EMA (Wang et al., 2019; Avitabile et al., 2020). These two drugs when combined, theoretically, have a synergistic effect and will be a hope for those with unsatisfactory results who receive sildenafil for single therapy (BPD, CDH, and Down syndrome) (Beghetti et al., 2017; Cohen et al., 2019). Despite its promising results, the availability of bosentan remains a challenge, especially in Indonesia (Lilyasari et al., 2019). Known for its anti-endothelin, anti-inflammatory, and anti-smooth muscle proliferation properties, metformin is a substitute for bosentan (Yoshida et al., 2020).
Fig. 2. Effect of sildenafil and metformin on PASMCs of PA. The result is presented as mean ± SD. **p=0.00064 compared to sildenafil. ||p=0.002 compared with sildenafil. C=control; M=metformin; S=sildenafil; SM=sildenafil + metformin.
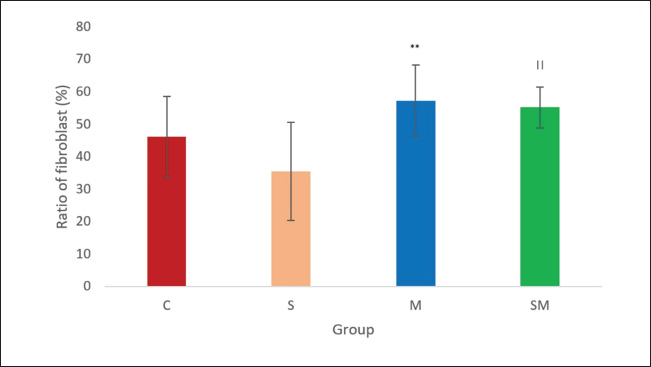
Fig. 3. Effects of sildenafil and metformin on fibroblast of PA. The result is presented as mean ± SD. **p=0.00064 compared to sildenafil. ||p=0.002 compared with sildenafil. C=control; M=metformin; S=sildenafil; SM=sildenafil + metformin.
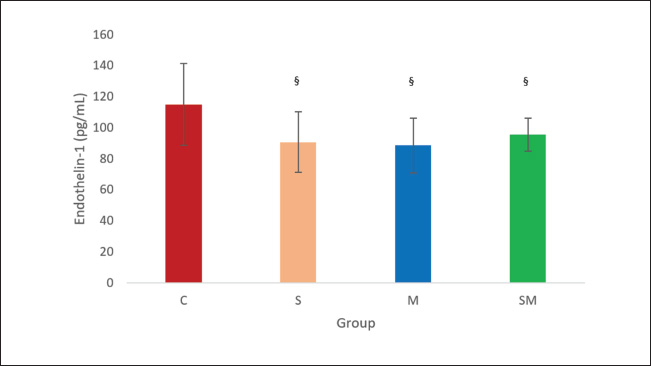
Fig. 4. Effects of sildenafil and metformin on ET-1. The result is presented as mean ± SD. §p=0.033 sildenafil compared with control, §p=0.013 metformin compared with control. §p=0.024 sildenafil + metformin compared with control. C=control; M=metformin; S=sildenafil; SM=sildenafil + metformin. In this study, three groups of treatment showed significant lowering of sPAP, dPAP, and mPAP compared with the control group. It is evident that the drug, either alone or in combination able to improve PH. This fact indicates the drugs have action in improving pulmonary vascular resistance. Previous studies have shown that sildenafil improves pulmonary vascular resistance via its vasodilatory effect by increasing the cyclic guanosine 3′,5′-monophophate level (Itoh et al., 2004). This agent also has action on decreasing expression of Endothelin type 1A receptor effect (Liu et al., 2007). Right ventricular function also improves because of its vasodilatory action (Yoshiyuki et al., 2016). Metformin is able to reduce PA vasoconstriction by enhancing endogenous nitric oxide (NO) phosphorylation (Agard et al., 2009). Moreover, this drug lowers PA pressure by decreasing ET-1 levels (Yoshida et al., 2020). Heart rate and SpO2 were similar in all groups, since MCT caused mild to moderate PH in 3rd–4th week by its administration. Our research evaluated small to moderate pulmonary arteries (50–200 µm) in which IMT is more prominent in PH (Humbert et al., 2019). The data showed that compared with the control group, the IMT of the sildenafil and metformin groups was thicker. However, when the IMT of sildenafil plus metformin was compared between the groups, the IMT value was significantly thinner. This fact showed that a single therapy is not enough to prevent pulmonary arterial remodeling. The intima media is the most affected pulmonary vascular area in patients with pulmonary arterial hypertension (PAH) (PH group 1). In the model, this PH group can be created by inducing rats with MCT (Maarman et al., 2013). The pulmonary vascular intima is lined with endothelial cells, and the medial is occupied by smooth muscle cells (Boucherat et al., 2022). IMT is the end result of works of proliferative mediators (Jia et al., 2023). In our research, the IMT of sildenafil plus metformin was significantly thinner than that of the other groups. This fact shows a combination of two drugs able to inhibit remodeling process of the PA. The sildenafil group, in our research, contrary with the findings of Schermuly et al. (2004), did not show improvement in arterial remodeling. This may have been caused by the relatively short use of sildenafil in our research (21 days) compared with the 42 days in previous research. This research revealed the domination of PASMCs in the sildenafil group and fibroblasts in the other treatment groups. This fact showed that sildenafil has more action on lowering fibroblast proliferation. Metformin, in other way, has more action on lowering PASMC proliferation. However, one should keep in mind that in a normal PA, PASMCs are dominating (Boucherat et al., 2022). Previous studies showed that sildenafil inhibits PASMC proliferation by upregulation of MKP expression and degradation of ERK1/2 phosphorylation (Li et al., 2007). This agent also potentiates antiproliferation of BMP4 in PASMCs. It seems that in the previous research, the antiproliferative effect of sildenafil was dose dependent. In our research, a sildenafil dose 5 mg/kg BW was probably inadequate to show antiproliferative effect. Metformin, in other ways, inhibits PASMC proliferation by decreasing ET-1 levels, which, in sequence, lower Skp2 and increase p27 (Wu et al., 2014; Yoshida et al., 2020). At the gene level, this agent causes PASMC apoptosis by lowering long noncoding RNA (Sun et al., 2019).
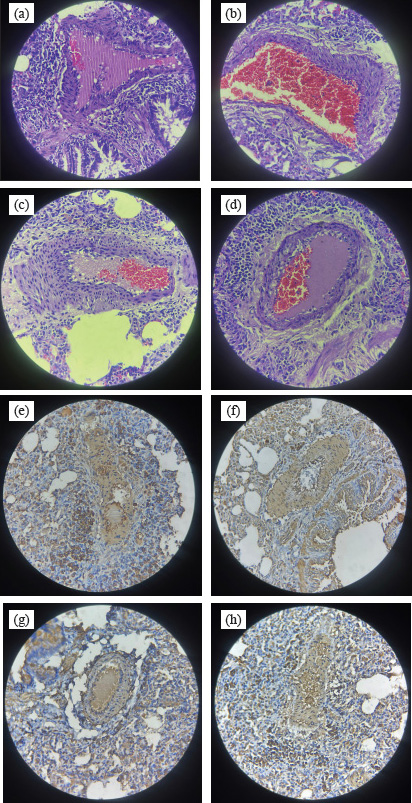
Fig. 5. Representative of PA histology. (a–d) IMT of each group on HE staining (a). control, (b). sildenafil, (c) metformin, (d) sildenafil plus metformin. (e-h) Ratio of PASMCs/fibroblast of each group on α-SMA staining: (e) control, (f) sildenafil, (g). metformin, and (h) sildenafil plus metformin. The PA smooth muscle cells were stained brown. We found a decrease in ET-1 levels in the treatment groups compared with the control group. These findings may inform us that mild–moderate PH, using a single drug, either sildenafil or metformin is enough when the target is to lower ET-1 levels. A previous study showed that sildenafil can reduce ET-1A type receptor and plasma ET-1 (Liu et al., 2007). In patients with PH, sildenafil reduces the ET-1 plasma concentration (Rossi et al., 2008). This substance is important in the progression of PH because it has both vasoconstrictive and proliferative actions (Yanagisawa et al., 1988; Chester and Yacoub, 2014; Barton and Yanagisawa, 2019). Its presence in circulation also reflects the severity of PAH in patients with HIV and congenital heart disease associated PAH (Parikh et al., 2016; Low et al., 2018). In patients with chronic thromboembolic PH, ET-1 elevation contributes to vasculopathy (Feriel et al., 2024). Metformin, in a previous study, was able to lower ET-1 levels (Yoshida et al., 2020). In the sildenafil plus metformin group, IMT was not linier to ET-1 concentration. The higher ET-1 concentration in this group compared with the other treatment groups, although not significantly, could be caused by upregulation expression of inducible and endothelial NO synthase by sildenafil (Salloum et al., 2003). An increase in NO will displace ET-1 from its receptor and cause an increase in ET-1 levels (Wiley and Davenport, 2001). The synergistic action of two drugs to other cytokines may explain IMT reduction (Kang et al., 2013; Ye et al., 2018; Ranchoux et al., 2019; Zych et al., 2019; Di Luigi et al., 2020; Vazifehkhah et al., 2020; Yoshida et al., 2020; Kniotek et al., 2021). This finding is a hope for pediatric patients with a higher risk of developing PAH, such as children with congenital heart disease, for better prognosis (Hansmann, 2017). Further research to prove the efficacy of combination of these two drugs in pediatric patients with PAH is valuable. A clinical study of metformin in adults with PAH showed positive results (improvement in right ventricular function and triglyceride contents) (Brittain et al., 2020). Our study has some limitations. The study used MCT to induce PAH in an animal model. Although it is widely used and has practicality, this approach does not perfectly mimic the pathobiology of PAH in humans. The sample size after mortality was adequate for statistical analysis. Although the more samples would describe the pathobiology better. AcknowledgmentsThe authors thank Anang Murdjito, Vet. Med and Azizah, Vet. Med for providing assistance during the research and Priangga Adi Wiratama, MD, Pathologist for histological analysis. Conflict of interestThere are no conflicts of interest to declare. FundingNo specific financial support was provided for this research. Authors’ contributionsAC, I, and MAR: developing concept and methodology. AC, MAR, and W: performing investigation. MAR and I: performing data curation. AC and MAR wrote the original draft. I and W are reviewing and editing drafts. All authors have reviewed and approved the final manuscript. Data availability All data supporting the findings of this study are available in the manuscript. ReferencesAgard, C., Rolli-Derkinderen, M., Dumas-de-La-Roque, E., Rio, M., Sagan, C., Savineau, J.P., Loirand, G. and Pacaud, P. 2009. Protective role of the antidiabetic drug metformin against chronic experimental pulmonary hypertension. Br. J. Pharmacol. 158(5), 1285–1294; doi:10.1111/j.1476-5381.2009.00445.x. Alqarni, A.A., Aldhahir, A.M., Alghamdi, S.A., Alqahtani, J.S., Siraj, R.A., Alwafi, H., AlGarni, A.A., Majrshi, M.S., Alshehri, S.M. and Pang, L. 2023. Role of prostanoids, nitric oxide and endothelin pathways in pulmonary hypertension due to COPD. Front. Med. 10, 1275684; doi:10.3389/fmed.2023.1275684. Avitabile, C.M., Vorhies, E.E. and Ivy, D.D. 2022. Drug treatment of pulmonary hypertension in children. Paediatr. Drugs 22(2), 123–147; doi:10.1007/s40272-019-00374-2. Barton, M. and Yanagisawa, M. 2019. Endothelin: 30 years from discovery to therapy. Hypertension 74(6), 1232–1265; doi:10.1161/HYPERTENSIONAHA.119.12105. Beghetti, M., Rudzinski, A. and Zhang, M. 2017. Efficacy and safety of oral sildenafil in children with down syndrome and pulmonary hypertension. BMC Cardiovasc. Disord. 17, 177; doi:10.1186/s12872-017-0569-3. Boucherat, O., Agrawal, V., Lawrie, A. and Bonnet, S. 2022. The latest in animal models of pulmonary hypertension and right ventricular failure. Circ. Res. 130(9), 1466–1486; doi:10.1161/CIRCRESAHA.121.319971. Brittain, E.L., Niswender, K., Agrawal, V., Chen, X., Fan, R., Pugh, M.E., Rice, T.W., Robbins, I.M., Song, H., Thompson, C., Ye, F., Yu, C., Zhu, H., West, J., Newman, J.H. and Hemnes, A.R. 2020. Mechanistic phase II clinical trial of metformin in pulmonary arterial hypertension. J. Am. Heart Assoc. 9(22), e018349; doi:10.1161/JAHA.120.018349. Chester, A.H. and Yacoub, M.H. 2014. The role of endothelin-1 in pulmonary arterial hypertension. Glob. Cardiol. Sci. Pract. 2014(2), 62–78; doi:10.5339/gcsp.2014.29. Cohen, J.L., Nees, S.N., Valencia, G.A., Rosenzweig, E.B. and Krishnan, U.S. 2019. Sildenafil use in children with pulmonary hypertension. J. Pediatr. 205, 29–34. doi:10.1016/j.jpeds.2018.09.067. Di Luigi, L., Sgro, P., Duranti, G., Sabatini, S., Caporosi, D., Del Galdo, F., Dimauro, I. and Antinozzi, C. 2020. Sildenafil reduces expression and release of IL-6 and IL-8 induced by reactive oxygen species in systemic sclerosis fibroblasts. Int. J. Mol. Sci. 21(9), 3161; doi:10.3390/ijms21093161. Feriel, B., Alessandra, C., Deborah, G.J., Corinne, N., Raphaël, T., Mina, O., Ali, A., Jean-Baptiste, M., Guillaume, F., Julien, G., Maria-Rosa, G., Elie, F., Laurent, S., Olaf, M., Ly, T., Marc, H. and Christophe, G. 2024. Exploring the Endothelin-1 pathway in chronic thromboembolic pulmonary hypertension microvasculopathy. Sci. Rep. 14(1), 28308; doi:10.1038/s41598-024-79623-5. Guglielmi, G., Dimopoulos, K. and Wort, S.J. 2025. New therapies in pulmonary arterial hypertension: recent insights. Int. J. Cardiol. Congenit. Heart Dis. 19, 100571; doi:10.1016/j.ijcchd.2025.100571. Guo, M., Zhang, M., Cao, X., Fang, X., Li, K., Qin, L., He, Y., Zhao, J., Xu, Y., Liu, X. and Li, X. 2022. Notch4 mediates vascular remodeling via ERK/JNK/P38 MAPK signaling pathways in hypoxic pulmonary hypertension. Respir. Res. 23(1), 6; doi:10.1186/s12931-022-01927-9. Hansmann, G. 2017. Pulmonary hypertension in infants, children, and young adults. J. Am. Coll. Cardiol. 69(20), 2551–2569; doi:10.1016/j.jacc.2017.03.575. Humbert, M., Guignabert, C., Bonnet, S., Dorfmüller, P., Klinger, J.R., Nicolls, M.R., Olschewski, A.J., Pullamsetti, S.S., Schermuly, R.T., Stenmark, K.R. and Rabinovitch, M. 2019. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur. Respir. J. 53(1), 1801887; doi:10.1183/13993003.01887-2018. Humbert, M., Kovacs, G., Hoeper, M.M., Badagliacca, R., Berger, R.M.F., Brida, M., Carlsen, J., Coats, A.J.S., Escribano-Subias, P., Ferrari, P., Ferreira, D.S., Ghofrani, H.A., Giannakoulas, G., Kiely, D.G., Mayer, E., Meszaros, G., Nagavci, B., Olsson, K.M., Pepke-Zaba, J., Quint, J.K., Rådegran, G., Simonneau, G., Sitbon, O., Tonia, T., Toshner, M., Vachiery, J.L., Vonk Noordegraaf, A., Delcroix, M,. Rosenkranz, S. and ESC/ERS Scientific Document Group. 2023. 2022 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension. Eur. Heart. J. 61, 2200879; doi:10.1183/13993003.00879-2022. Itoh, T., Nagaya, N., Fujii, T., Iwase, T., Nakanishi, N., Hamada, K., Kangawa, K. and Kimura, H. 2004. A combination of oral sildenafil and beraprost ameliorates pulmonary hypertension in rats. Am. J. Respir. Crit. Care. Med. 169(1), 34–38; doi:10.1164/rccm.200303-346OC. Jia, Z., Wang, S., Yan, H., Cao, Y., Zhang, X., Wang, L., Zhang, Z., Lin, S., Wang, X. and Mao, J. 2023. Pulmonary vascular remodeling in pulmonary hypertension. J. Pers. Med. 13(2), 366; doi:10.3390/jpm13020366. Kang, K.Y., Kim, Y.K., Yi, H., Kim, J., Jung, H.R., Kim, I.J., Cho, J.H., Park, S.H., Kim, H.Y. and Ju, J.H. 2013. Metformin downregulates Th17 cells differentiation and attenuates murine autoimmune arthritis. Int. Immunopharmacol. 16(1), 85–92; doi:10.1016/j.intimp.2013.03.020. Kniotek, M., Zych, M., Roszczyk, A., Szafarowska, M. and Jerzak, M.M. 2021. Decreased production of TNF-α and IL-6 inflammatory cytokines in non-pregnant idiopathic RPL women immunomodulatory effect of sildenafil citrate on the cellular response of idiopathic RPL women. J. Clin. Med. 10(14), 3115; doi:10.3390/jcm10143115. Li, B., Yang, L., Shen, J., Wang, C. and Jiang, Z. 2007. The antiproliferative effect of sildenafil on pulmonary artery smooth muscle cells is mediated via upregulation of mitogen-activated protein kinase phosphatase-1 and degradation of extracellular signal-regulated kinase 1/2 phosphorylation. Anesth. Analg. 105(4), 1034–1041; doi:10.1213/01.ane.0000278736.81133.26. Lilyasari, O., Subekti, Y., Atika, N., Dinarti, L.K., Putri, S., Opitasari, C., Anggraini, A.B., Bussabawalai, T. and Teerawattananon, Y. 2019. Economic evaluation of sildenafil for the treatment of pulmonary arterial hypertension in Indonesia. BMC Health Serv. Res. 19(1), 573; doi:10.1186/s12913-019-4422-5. Liu, H., Liu, Z.Y. and Guan, Q. 2007. Oral sildenafil prevents and reverses the development of pulmonary hypertension in monocrotaline-treated rats. Interact. Cardiovasc. Thorac. Surg. 6(5), 608–613; doi:10.1510/icvts.2006.147033. Low, A., George, S., Howard, L., Bell, N., Millar, A. and Tulloh, R.M.R. 2018. Lung function, inflammation, and endothelin-1 in congenital heart disease-associated pulmonary arterial hypertension. J. Am. Heart Assoc. 7(4), e007249; doi:10.1161/JAHA.117.007249. Maarman, G., Lecour, S., Butrous, G., Thienemann, F. and Sliwa, K. 2013. A comprehensive review: the evolution of animal models in pulmonary hypertension research; are we there yet? Pulm. Circ. 3(4), 739–756; doi:10.1086/674770. Parikh, R.V., Ma, Y., Scherzer, R., Heringer, A.S., Macgregor, J.S., Martin, J.N., Deeks, S.G., Ganz, P. and Hsue, P.Y., 2016. Endothelin-1 predicts hemodynamically assessed pulmonary arterial hypertension in HIV infection. PLoS One. 11(1), e0146355; doi:10.1371/journal.pone.0146355. Ranchoux, B., Nadeau, V., Bourgeois, A., Provencher, S., Tremblay, É., Omura, J., Coté, N., Abu-Alhayja’a, R., Dumais, V., Nachbar, R.T., Tastet, L., Dahou, A., Breuils-Bonnet, S., Marette, A., Pibarot, P., Dupuis, J., Paulin, R., Boucherat, O., Archer, S.L., Bonnet, S. and Potus, F. 2019. Metabolic syndrome exacerbates pulmonary hypertension due to left heart disease. Circ. Res. 125(4), 449–466; doi:10.1161/CIRCRESAHA.118.314555. Rossi, R., Nuzzo, A., Lattanzi, A., Coppi, F., Modena, M.G. 2008. Sildenafil improves endothelial function in patients with pulmonary hypertension. Pulm. Pharmacol. Ther. 21(1), 172–177; doi:10.1016/j.pupt.2007.01.008. Salloum, F., Yin, C., Xi, L. and Kukreja, R.C. 2003. Sildenafil induces delayed preconditioning through inducible nitric oxide synthase-dependent pathway in mouse heart. Circ. Res. 92(6), 595–597; doi:10.1161/01.RES.0000066853.09821.98. Schermuly, R.T., Kreisselmeier, K.P., Ghofrani, H.A., Yilmaz, H., Butrous, G., Ermert, L., Ermert, M., Weissmann, N., Rose, F., Guenther, A., Walmrath, D., Seeger, W. and Grimminger, F. 2004. Chronic sildenafil treatment inhibits monocrotaline-induced pulmonary hypertension in rats. Am. J. Respir. Crit. Care Med. 169(1), 39–45; doi:10.1164/rccm.200302-282OC. Scott, T.E., Qin, C.X., Drummond, G.R., Hobbs, A.J. and Kemp-Harper, B.K. 2021. Innovative anti-inflammatory and pro-resolving strategies for pulmonary hypertension: high blood pressure research council of Australia award 2019. Hypertension 78(5), 1168–1184; doi:10.1161/HYPERTENSIONAHA.120.14525. Sun, Z., Liu, Y., Yu, F., Xu, Y., Li, Y. and Liu, N. 2019. Long non-coding RNA and mRNA profile analysis of metformin to reverse the pulmonary hypertension vascular remodeling induced by monocrotaline. Biomed. Pharmacother. 115, 108933; doi:10.1016/j.biopha.2019.108933. Vazifehkhah, S., Khanizadeh, A.M., Mojarad, T.B. and Nikbakht, F. 2020. The possible role of progranulin on anti-inflammatory effects of metformin in temporal lobe epilepsy. J. Chem. Neuroanat. 109, 101849; doi:10.1016/j.jchemneu.2020.101849. Wang, Y., Chen, S. and Du, J. 2019. Bosentan for treatment of pediatric idiopathic pulmonary arterial hypertension: state-of-the-Art. Front. Pediatr. 7, 302; doi:10.3389/fped.2019.00302. Wiley, K.E. and Davenport, A.P. 2001. Nitric oxide-mediated modulation of the endothelin-1 signalling pathway in the human cardiovascular system. Br. J. Pharmacol. 132(1), 213–220; doi:10.1038/sj.bjp.0703834. Wu, Y., Liu, L., Zhang, Y., Wang, G., Han, D., Ke, R., Li, S., Feng, W. and Li, M. 2014. Activation of AMPK inhibits pulmonary arterial smooth muscle cells proliferation. Exp. Lung. Res. 40(5), 251–258; doi:10.3109/01902148.2014.913092. Yanagisawa, M., Kurihara, H., Kimura, S., Tomobe, Y., Kobayashi, M., Mitsui, Y., Yazaki, Y., Goto, K. and Masaki, T. 1988. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332(6163), 411–415; doi:10.1038/332411a0 Ye, J., Zhu, N., Sun, R., Liao, W., Fan, S., Shi, F., Lin, H., Jiang, S. and Ying, Y. 2018. Metformin inhibits chemokine expression through the AMPK/NF-kB signaling pathway. J. Interferon Cytokine Res. 38(9), 363–369; doi:10.1089/jir.2018.0061. Yoshida, T., Matsuura, K., Goya, S., Ma, D., Shimada, K., Kitpipatkun, P., Namiki, R., Uemura, A., Suzuki, K. and Tanaka, R. 2020. Metformin prevents the development of monocrotaline-induced pulmonary hypertension by decreasing serum levels of big endothelin-1. Exp. Ther. Med. 20(6), 149–157; doi:10.3892/etm.2020.9278. Yoshiyuki, R., Tanaka, R., Fukushima, R. and Machida, N. 2016. Preventive effect of sildenafil on right ventricular function in rats with monocrotaline-induced pulmonary arterial hypertension. Exp. Anim. 65(3), 215–222; doi:10.1538/expanim.15-0070. Zych, M., Roszczyk, A., Kniotek, M., Kaleta, B. and Zagozdzon, R. 2019. Sildenafil citrate influences production of TNF-α in healthy men lymphocytes. J. Immunol. Res. 2019, 8478750; doi:10.1155/2019/8478750. | ||
| How to Cite this Article |
| Pubmed Style Cahyono A, Irwanto I, Rahman MA, Widjiati W. Effects of sildenafil and metformin on endothelin-1 in the progression of pulmonary hypertension. Open Vet. J.. 2025; 15(7): 3087-3096. doi:10.5455/OVJ.2025.v15.i7.20 Web Style Cahyono A, Irwanto I, Rahman MA, Widjiati W. Effects of sildenafil and metformin on endothelin-1 in the progression of pulmonary hypertension. https://www.openveterinaryjournal.com/?mno=247149 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i7.20 AMA (American Medical Association) Style Cahyono A, Irwanto I, Rahman MA, Widjiati W. Effects of sildenafil and metformin on endothelin-1 in the progression of pulmonary hypertension. Open Vet. J.. 2025; 15(7): 3087-3096. doi:10.5455/OVJ.2025.v15.i7.20 Vancouver/ICMJE Style Cahyono A, Irwanto I, Rahman MA, Widjiati W. Effects of sildenafil and metformin on endothelin-1 in the progression of pulmonary hypertension. Open Vet. J.. (2025), [cited January 25, 2026]; 15(7): 3087-3096. doi:10.5455/OVJ.2025.v15.i7.20 Harvard Style Cahyono, A., Irwanto, . I., Rahman, . M. A. & Widjiati, . W. (2025) Effects of sildenafil and metformin on endothelin-1 in the progression of pulmonary hypertension. Open Vet. J., 15 (7), 3087-3096. doi:10.5455/OVJ.2025.v15.i7.20 Turabian Style Cahyono, Agus, Irwanto Irwanto, Mahrus Abdur Rahman, and Widjiati Widjiati. 2025. Effects of sildenafil and metformin on endothelin-1 in the progression of pulmonary hypertension. Open Veterinary Journal, 15 (7), 3087-3096. doi:10.5455/OVJ.2025.v15.i7.20 Chicago Style Cahyono, Agus, Irwanto Irwanto, Mahrus Abdur Rahman, and Widjiati Widjiati. "Effects of sildenafil and metformin on endothelin-1 in the progression of pulmonary hypertension." Open Veterinary Journal 15 (2025), 3087-3096. doi:10.5455/OVJ.2025.v15.i7.20 MLA (The Modern Language Association) Style Cahyono, Agus, Irwanto Irwanto, Mahrus Abdur Rahman, and Widjiati Widjiati. "Effects of sildenafil and metformin on endothelin-1 in the progression of pulmonary hypertension." Open Veterinary Journal 15.7 (2025), 3087-3096. Print. doi:10.5455/OVJ.2025.v15.i7.20 APA (American Psychological Association) Style Cahyono, A., Irwanto, . I., Rahman, . M. A. & Widjiati, . W. (2025) Effects of sildenafil and metformin on endothelin-1 in the progression of pulmonary hypertension. Open Veterinary Journal, 15 (7), 3087-3096. doi:10.5455/OVJ.2025.v15.i7.20 |