| Research Article | ||
Open Vet. J.. 2025; 15(5): 2103-2111 Open Veterinary Journal, (2025), Vol. 15(5): 2103-2111 Research Article A comparative study of intramuscular versus epidural administration of follicle-stimulating hormone for embryo production in Wagyu CattleAbdullah Baharun1, Athhar Manabi Diansyah2*, Ristika Handarini1, Sikin Sikin3, Putri Indah Ningtias3, Weni Kurniati3, Hikmayani Iskandar4, Erni Damayanti2, Adiba Kanza Arasya1 and Annisa Rahmi11Department of Animals Science, Faculty of Agriculture, Universitas Djuanda, Bogor, Indonesia 2Faculty of Animal Science, Hasanuddin University, Makassar, Indonesia 3Animal Embryo Hall, Directorate General of Animal Husbandry and Animal Health, Ministry of Agriculture, Bogor, Indonesia 4Research Center for Applied Zoology, National Research and Innovation Agency (BRIN), Bogor, Indonesia *Corresponding Author: Athhar Manabi Diansyah. Faculty of Animal Science, Hasanuddin University, Makassar, Indonesia. Email: athhar.md [at] unhas.ac.id Submitted: 20/01/2025 Revised: 28/03/2025 Accepted: 29/04/2025 Published: 31/05/2025 © 2025 Open Veterinary Journal
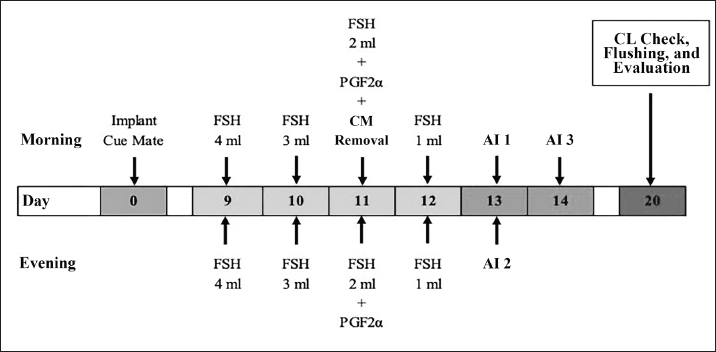
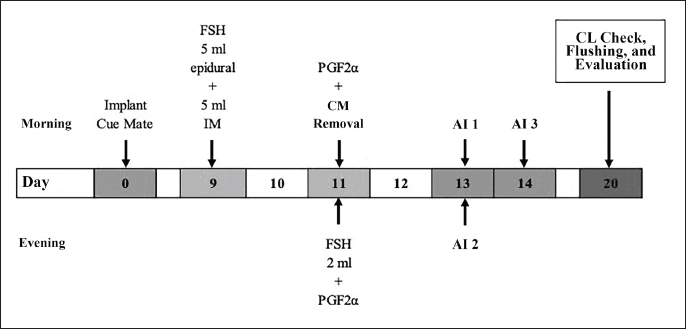
ABSTRACTBackground: The increasing demand for high-quality livestock products necessitates the optimization of reproductive technologies, particularly superovulation and embryo transfer, to enhance genetic improvement and productivity. Superovulation increases embryo availability and accelerates genetic enhancement. The success of these techniques depends on factors such as hormone protocols, donor-recipient selection, and environmental conditions. Aim: This study aimed to compared two superovulation protocols—conventional intramuscular injection (P1) and combined epidural-intramuscular injection (P2)—in Wagyu donor cows to evaluate their effects on corpus luteum (CL) formation, embryo yield, and quality. Methods: The study was conducted from May to November 2024. Twelve cows were divided into two treatment groups (n=6 per group), with follicle-stimulating hormone administered either through multiple intramuscular injections (P1) or a single-dose epidural-intramuscular injection (P2). Selection criteria included superior genetics, normal reproduction, regular estrus cycles (18–24 days), high fertility, prior calving, and disease-free status. Donor cows had a Body Condition Score of 2.5–3.5 and were confirmed to have normal reproductive organs via rectal examination. They were housed in a free-stall system and fed a controlled diet. Evaluated reproductive parameters included CL formation, embryo recovery rates (ERRs), and embryo quality. Results: There were no statistically significant differences between P1 and P2 in CL formation (p=0.480), ERRs (p=0.810), or embryo quality (p=0.871). Both protocols supported comparable follicular development, embryo recovery, and transferable embryos across developmental stages (morula, early blastocyst, and blastocyst). While P2 produced slightly more CL and blastocysts, the differences were not significant. Both protocols yielded similar proportions of unsuitable embryos, indicating no adverse effects on fertilization or embryonic viability. Conclusion: The findings suggest that the combined epidural-intramuscular protocol (P2) is a viable alternative to the conventional intramuscular protocol (P1), providing comparable reproductive outcomes while potentially reducing handling stress and labor intensity. Future research should address the pharmacokinetics of hormonal delivery, its long-term reproductive impacts, and its applicability across other breeds to further optimize superovulation strategies for sustainable livestock production and genetic improvement. Keywords: Embryo, Epidural, Intramuscular, Superovulation, Wagyu. IntroductionThe increasing global demand for high-quality livestock products necessitates the adoption of advanced reproductive technologies that enhance genetic improvement and reproductive efficiency. Superovulation and embryo transfer have become indispensable tools in this effort, allowing the production of multiple embryos from genetically superior donor animals (Engdawork et al., 2024). These technologies are particularly valuable for high-value breeds such as Wagyu cattle (Bos taurus), which are renowned for their superior meat quality and exceptional marbling, tenderness, and flavor. The success of these techniques relies heavily on optimizing superovulation protocols to maximize embryo yield and quality while minimizing inefficiencies and animal welfare concerns (Tian et al., 2024). Superovulation involves the administration of exogenous follicle-stimulating hormone (FSH) to induce the development of multiple ovarian follicles (Vega et al., 2019). The conventional protocol, which requires repeated intramuscular injections of FSH over several days, has proven effective, but it presents logistical and welfare challenges (Cizmeci et al., 2022). Increased labor intensity, repetitive handling of animals, and stress can adversely affect reproductive outcomes. To address these limitations, alternative approaches, such as a single-dose protocol combining epidural and intramuscular FSH administration, have been developed (El-Shazly et al., 2024). These simplified methods aim to reduce animal handling and operational complexity while maintaining or enhancing superovulation efficiency (Karl et al., 2021). Assessing the effectiveness of superovulation protocols necessitates the evaluation of key reproductive parameters, including the number of corpus luteum (CL) formed, total embryo yield, and embryo quality. High-quality embryos (Grades 1 and 2) are essential for successful cryopreservation and transfer, whereas unsuitable embryos, such as degenerative or unfertilized (UF) embryos, represent process inefficiencies (Rabel et al., 2023). The distribution of embryos across developmental stages, including morula, early blastocyst, and blastocyst, further informs the impact of superovulation methods on embryonic progression and viability. These comprehensive evaluations are critical for identifying protocols that optimize both quantitative and qualitative outcomes (Krisher and Herrick, 2024). This study aimed to compare conventional intramuscular injection protocols with single-dose epidural and intramuscular protocols in Wagyu donor cows. By analyzing CL formation, embryo yield, and quality distribution, this research aims to elucidate the relative efficacy of these methods. The findings aim to support the development of refined, cost-effective, and welfare-oriented superovulation protocols that contribute to sustainable livestock production and genetic improvement programs. Materials and MethodsExperimental animalsThe study was conducted from May to November 2024. The experiment involved 12 Wagyu donor cows, aged 4–5 years, divided equally into two treatment groups. Selection criteria included superior genetic traits, normal reproductive status, regular estrus cycles (18–24 days), high fertility, at least one prior calving, and freedom from strategic infectious animal diseases. The Body Condition Score ranged from 2.5 to 3.5 on a 5-point scale. The normal reproductive organ status was confirmed via rectal examination. Donor cows were maintained in a free-stall system and provided with chopped forage at 10% of their body weight twice daily; they were then concentrated at 1% of their body weight once daily. Progesterone (P4) implant preparationWagyu donor cows were intravaginally implanted with progesterone devices (Cue Mate®, containing 1.56 mg of progesterone in two silicone pods (Bioniche Animal Health (A/Asia) Pty. Ltd., Australia). Implants were inserted during either the luteal or follicular phase, with Day 0 designated as the implantation day. The progesterone device was loaded into an applicator, lubricated with isotonic gel, and inserted intravaginally following cleaning of the external genitalia with alcohol wipes and sterile tissue (Cao et al., 2021). The device was positioned near the cervix and released by pressing the applicator piston. The device has remained in place for 11 days. FSH hormone treatmentTreatment 1: Conventional intramuscular injection FSH injections were initiated on day 9 postprogesterone implantation. A total of 400 mg of FSH was diluted in 20 ml Bacteriostatic Sodium Chloride and administered intramuscularly twice daily for four consecutive days at decreasing doses (4:4, 3:3, 2:2, 1:1 ml morning and evening). The interval between injections was maintained at 8–12 hours. PGF2α (2 ml) was administered intramuscularly on Day 11, both in the morning and evening (Mikkola et al., 2019; Kolling et al., 2021). The progesterone device was removed following the morning injection (Fig. 1). Treatment 2: Single epidural and intramuscular injection In Treatment 2, a single dose of FSH was administered on day 9 after progesterone implantation (Yusuf et al., 2024). A total of 400 mg of FSH was diluted in 10 ml Bacteriostatic Sodium Chloride and injected as 5 ml epidurally and 5 ml intramuscularly. PGF2α was administered in the morning on Day 11, followed by progesterone device removal, with a single evening PGF2α dose administered subsequently (Fig. 2). Insemination and embryo collectionSeven days after the first artificial insemination, rectal palpation was performed to evaluate superovulation by counting the CL on the ovaries (Fig. 3). Embryos were collected using a flushing procedure (Huang et al., 2023). The cows were restrained in a squeeze chute, and the rectum was cleared to maintain hygiene and ease uterine manipulation. Epidural anesthesia (4–6 ml of 2% lidocaine chloride) was administered to the donor cows between the last sacrum and first coccygeal vertebra or between coccygeal vertebrae 1 and 2. Once the anesthesia was effective, as indicated by tail flaccidity, a cervical expander was used to open the cervical lumen, thereby facilitating Foley catheter placement. The Foley catheter was inserted into the uterine horn and inflated using 20 ml of air. The catheter balloon was secured to prevent media leakage during flushing. The flushing system, consisting of a Y-connector, Lactated Ringer solution, and a collection bottle, was used to flush each uterine horn and body (Sonjaya et al., 2025). The media were infused into the uterus and retrieved using palpation to ensure thorough collection. A total of 500 ml of Lactated Ringer solution supplemented with calf serum and antibiotics were used. Flushed media were transported to the laboratory in specialized containers.
Fig. 1. Conventional intramuscular injection procedure.
Fig. 2. Single epidural and intramuscular injection procedure. Embryo evaluationFlushed media were filtered through embryo filters and transferred to marked Petri dishes for evaluation under a stereomicroscope (70× magnification). The detected embryos were collected in round Petri dishes and assessed for developmental stage and morphology. Embryo quality was graded according to the International Embryo Transfer Society classification: grades 1–4 (degenerated), or UF (Sosa et al., 2023). Grades 1 and 2 embryos were deemed suitable for cryopreservation, whereas grade 3 embryos were reserved for fresh transfer or culture in an incubator. Data analysisData are presented as mean ± standard deviation. Statistical analyses were conducted using SPSS version 26. Normality was assessed using the Shapiro–Wilk test. Normally distributed data were analyzed using unpaired t-tests, whereas non-normally distributed data were evaluated using the Mann–Whitney U test.
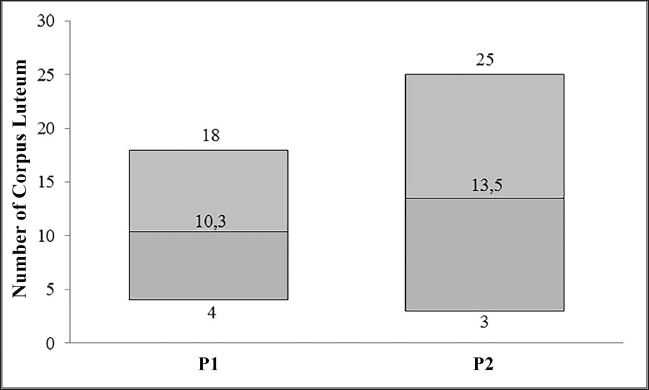
Fig. 3. CL of superovulation results using ultrasound. Ethical approvalThis study adhered to ethical guidelines (SNI 7880.1:2013) and received approval in accordance with ISO 9001:2015 standards, as provided by the Animal Embryo Hall (AEH), Indonesia. ResultsCL collected using different superovulation methodsFigure 4 indicates that both treatments, intramuscular injection and a combination of epidural and intramuscular injections, achieved a total number of CL observed in the P1 group produced a total of 62, with a range of 4–18, while the P2 group produced a higher total number of 81, with a broader range of 3–25.The statistical analysis yielded a p-value of 0.480, indicating no significant difference between the treatments in terms of CL formation. These results suggest that although epidural and intramuscular injections produced a higher total CL volume, the difference compared with intramuscular injection was not statistically significant. Embryos collected on different superovulation methodsThe embryo collection and embryo recovery rates (ERRs) across the two superovulation methods are shown in Table 1. In treatment P1, a total of 62 CLs were observed, yielding 51 collected embryos and an ERR of 82.25%. In treatment P2, 81 CLs were observed, resulting in 61 collected embryos and an ERR of 75.30%. Statistical analysis of the ERR between the two treatments yielded a p-value of 0.810, indicating no significant difference in ERR between P1 and P2. These findings suggest that although P2 produced a greater number of CL and total collected embryos, the efficiency of embryo recovery, as indicated by the ERR, was comparable to that of P1. Embryos collected on different superovulation methodsTable 2 shows that the two superovulation methods evaluated, P1 and P2, yielded comparable outcomes in terms of the quality of embryos suitable for transfer. The statistical analysis revealed no significant difference between the two treatments (p=0.871), suggesting that the efficiency of these methods in producing transferable-quality embryos is similar. This evidence underscores the equivalence of these superovulation protocols in terms of embryo quality, highlighting their interchangeability depending on other factors such as management preferences and resource availability. Table 3 summarizes the distribution of embryos suitable for transfer across the developmental phases of the morula, early blastocyst, and blastocyst (Fig. 5) following two superovulation treatments: P1 and P2. Both treatments produced embryos at all three developmental stages. Although slight numerical differences were observed in the proportions of embryos at each stage, statistical analysis revealed no significant differences between the treatments (p > 0.05). The observed similarity in developmental phase distribution suggests that neither superovulation protocol exerts a differential impact on embryonic progression to transferable stages. These findings provide evidence for the equivalence of the two methods in supporting embryonic development, enabling flexibility in selecting protocols based on other practical considerations. Further studies should investigate whether comparable distributions translate into similar implantation and pregnancy outcomes to optimize embryo transfer success rates.
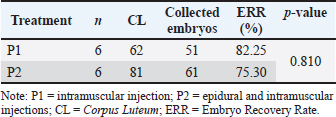
Fig. 4. CL collected using different superovulation methods. Note: P1=intramuscular injection; P2=epidural and intramuscular injections. Table 1. Embryos collected and recovery rates using different superovulation methods.
Table 2. Effect of treatment with the superovulation method on the quality of embryos suitable for transfer.
Table 3. Embryos suitable for transfer based on developmental phase.
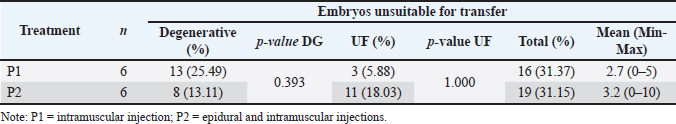
Table 4 demonstrates that embryos unsuitable for transfer, categorized as degenerative or UF (Fig. 5), did not differ significantly between the two superovulation methods (p > 0.05). Both treatments resulted in comparable proportions of total unsuitable embryos, indicating that the choice of the superovulation method did not significantly impact the rate of embryo degeneration or fertilization failure. The variability in the occurrence of unsuitable embryos among individual samples further supports the conclusion that both methods provide consistent outcomes without a significant effect on overall embryo viability.
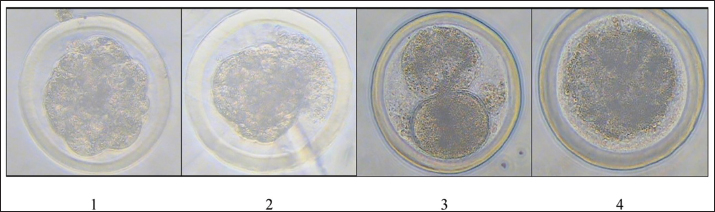
Fig. 5. Embryo quality classification; 1. Grade 1=excellent; 2. Grade 2=fair; 3. Grade 3=degenerative; 4. Grade 4=unfertilized. Table 4. Embryos unsuitable for transfer based on superovulation.
DiscussionThis study provides a comprehensive comparison of two superovulation protocols—conventional intramuscular injection (P1) and combined epidural-intramuscular injection (P2)—in Wagyu donor cows, focusing on CL formation, embryo yield, and quality. The results will contribute to our understanding of the biological and practical implications of these protocols in reproductive biotechnology. The total number of CL was numerically higher in P2 than in to P1, suggesting that the combined epidural and intramuscular protocol may enhance follicular response. Epidural FSH administration is hypothesized to achieve a localized and efficient hormonal distribution near the pelvic region, where it directly interacts with ovarian receptors, potentially stimulating follicular growth more effectively (Prastiya et al., 2022; Wang et al., 2022). However, the lack of statistical significance (p > 0.05) indicates that both methods are equally capable of inducing multiple ovulations. The variability in CL formation among individual cows reflects the interplay of genetic, physiological, and environmental factors (Nevard et al., 2022). Genetic predisposition to superovulation, variability in ovarian reserve, and differences in follicular sensitivity to FSH may all contribute to the observed outcomes (Juengel et al., 2021). The ERR were comparable between the two protocols, with no significant differences observed. Although P1 exhibited a slightly higher ERR, this result may be attributed to the uniform distribution of FSH via repeated intramuscular injections, which ensures a steady stimulation of the ovaries (Raut et al., 2023). In contrast, the single-dose nature of P2 might result in a more concentrated hormonal release, which could lead to suboptimal absorption in some cases. These findings underscore the importance of optimizing hormonal delivery in simplified protocols to further enhance efficiency. The quality and developmental stages of embryos were consistent across both protocols, with no significant differences in the proportions of transferable embryos (Grades 1 and 2). Both P1 and P2 supported embryonic progression to the morula, early blastocyst, and blastocyst stages. This indicates that hormonal stimulation provided by both methods was sufficient to maintain oocyte maturation, fertilization, and early embryonic development (Turathum et al., 2021). Interestingly, a slightly higher proportion of blastocysts was observed in P2, which may be due to the localized effect of epidural administration. Epidural injections can create a more concentrated and stable hormonal environment in the ovarian and uterine regions, facilitating follicular maturation and providing optimal conditions for embryonic development (Amin et al., 2023). The reduced handling associated with P2 may also minimize stress-related impacts on donor cows (Megahed et al., 2023), which can influence reproductive outcomes by modulating endocrine responses, such as cortisol levels, which are known to affect follicular and oocyte quality (Ramya et al., 2023). Both protocols yielded comparable proportions of unsuitable embryos, including degenerative and UF embryos, with no significant differences observed. This consistency suggests that neither protocol adversely affected fertilization rates nor embryo viability. Degenerative embryos could be attributed to inherent oocyte quality or suboptimal follicular development, factors that are influenced by individual biological variability rather than the superovulation method itself (Pangestu et al., 2024). Similarly, the occurrence of UF embryos may reflect variability in sperm quality, timing of insemination, or minor inconsistencies in the synchronization of ovulation and fertilization processes (Zhang et al., 2023). The observed equivalence in outcomes between the two protocols may be explained by the overall robustness of Wagyu donor cows, which exhibit high fertility and adaptability. Both protocols provided sufficient hormonal stimulation to support follicular recruitment, ovulation, and embryo development, with the differences in administration routes having limited impact on the physiological processes involved (Ata et al., 2021). The lack of significant differences further highlights the importance of individual cow factors, such as ovarian reserve and follicular responsiveness, in determining superovulation success (Lubis et al., 2021). The limited sample size reduces the statistical power to detect subtle differences between the protocols, necessitating larger-scale studies for validation. The absence of detailed hormonal profiling during superovulation restricts the pharmacodynamics of FSH in each protocol. Future research should focus on measuring hormonal levels, including FSH, estradiol, and progesterone, to better understand their role in follicular development and ovulation. Additionally, this study was conducted exclusively on Wagyu cows, limiting the generalizability of the findings to other breeds. Long-term studies evaluating the reproductive performance and fertility of donor cows subjected to these protocols are also warranted to assess their sustainability and broader applicability. This study demonstrated that the combined epidural and intramuscular protocol provides a viable alternative to the conventional intramuscular method, offering comparable outcomes in CL formation, embryo yield, and quality. The scientific rationale behind the observed equivalence lies in the sufficient hormonal stimulation provided by both protocols to support follicular and embryonic development. Future research addressing the identified limitations could further optimize these protocols, contributing to the development of more efficient, welfare-friendly, and sustainable reproductive technologies for cattle breeding. ConclusionThis study compared two superovulation protocols—conventional intramuscular (P1) and combined epidural-intramuscular (P2)—in Wagyu donor cows. The results demonstrated no statistically significant differences between the protocols in terms of CL formation, ERR, or the proportions of transferable (Grades 1 and 2) and unsuitable embryos (degenerative and UF). Both protocols effectively supported follicular development, ovulation, and embryo viability, as evidenced by comparable outcomes in embryo quality and developmental stages. The combined epidural-intramuscular protocol (P2) offers practical benefits, including reduced handling and stress, without compromising efficacy, making it a promising alternative for large-scale reproductive programs. AcknowledgmentsThe authors are also thankful to Animal Embryo Hall, Directorate General of Animal Husbandry and Animal Health, Ministry of Agriculture, Jl. Cipelang, Bogor Regency, for providing samples to carry out the study. Conflicts of interestThe authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript. FundingThe authors did not receive funding from any organization for the submitted work. Authors’ contributionsA Baharun, A. M Diansyah, R Handarini, S Sikin, H Iskandar, and A Rahmi conceptualized and designed the experiment, conducted the literature review, and wrote the first draft of the manuscript. A Baharun, A.M Diansyah, R Handarini, S Sikin, P. I Ningtias, W Kurniati, H Iskandar, E Damayanti, A. K Arasya, and A Rahmi edited and revised the manuscript draft. All authors read and approved the final manuscript. Data availabilityAll data are provided in the manuscript. ReferencesAmin, Y.A., Mahmoud, A.E.Z., Ali, R.A., Fouad, S.S., Shanab, O., Ibrahim, R.M., Farrag, F., Shukry, M., Ibrahim, S.F., Fericean, L. and Mohamed, R.H. 2023. Treatment of inactive ovaries of Holstein dairy cows by epidural injection of GnRH analog (Receptal) and its impact on the reproductive hormones, oxidant/antioxidant profile and micro and macro-elements profile. Animals 13(4), 653; doi:10.3390/ani13040653 Ata, B., Capuzzo, M., Turkgeldi, E., Yildiz, S. and La Marca, A. 2021. Progestins for pituitary suppression during ovarian stimulation for ART: a comprehensive and systematic review including meta-analyses. Hum. Reprod. Update. 27(1), 48–66; doi:10.1093/humupd/dmaa040 Cao, Z., Tang, X., Zhang, Y., Yin, T., Gou, J., Wang, Y. and He, H. 2021. Novel injectable progesterone-loaded nanoparticles embedded in SAIB-PLGA in situ depot system for sustained drug release. Int. J. Pharm. 607, 121021; doi:10.1016/j.ijpharm.2021.121021 Cizmeci, S., Dinç, A., Güler, M., Alkan, H., Kıvrak, M., Takcı, A., Çiftçi, M., Yeşilkaya, Ö.F., Bulut, P., Bulut, S. and Zhunushova, A. 2022. Effects of FSH administered in different ways on superovulation response and blood FSH levels in cows. J. Hellenic Vet. Med. Soc. 73(4), 4739–4746; doi:10.12681/jhvms.27334 El-Shazly, M., Mansour, N., Karen, A., Salama, M., Hijazi, I., El-Ghazaly, M., Sheply, K. and Jaques, S. 2024. Evaluation of a long-acting recombinant bovine FSH for multiple ovulation and embryo transfer in dromedary camels. Anim. Reprod. Sci. 261, 107398; doi:10.1016/j.anireprosci.2023.107398 Engdawork, A., Belayhun, T. and Aseged, T. 2024. The role of reproductive technologies and cryopreservation of genetic materials in the conservation of animal genetic resources, a review. Ecol. Genet. Genomics. 31, 100250; doi:10.1016/j.egg.2024.100250 Huang, F., Niu, P., Wang, J., Liu, B., Yue, C.G., Fang, D., Qian, W. and Gao, Q.H. 2023. Effects of different sources of FSH, injection methods, embryo collection methods, and seasons on the superovulation response in Belgian Blue Cattle. Pak. Vet. J. 43(1), 204–208; doi:10.29261/pakvetj/2023.006 Juengel, J.L., Cushman, R.A., Dupont, J., Fabre, S., Lea, R.G., Martin, G.B., Mossa, F., Pitman, J.L., Price, C.A. and Smith, P. 2021. The ovarian follicle of ruminants: the path from conceptus to adult. Reprod. Fertil. Dev. 33(10), 621–642; doi:10.1071/RD21086 Karl, K.R., Jimenez-Krassel, F., Gibbings, E., Ireland, J.L.H., Clark, Z.L., Tempelman, R.J., Latham, K.E. and Ireland, J.J. 2021. Negative impact of high doses of follicle-stimulating hormone during superovulation on the ovulatory follicle function in small ovarian reserve dairy heifers. Biol. Reprod. 104(3), 695–705; doi:10.1093/biolre/ioaa210 Kolling, A.P.M.L., Brilhante, G.C., Drechmer, J., Santos, L.M., Silva, B.D.M. and Ramos, A.F. 2021. Relationship between superovulation and embryo production and the ovarian follicular population before superovulatory treatment in Brazilian Bergamasca sheep. Arq. Bras. Med. Vet. Zootec. 73, 115–122; doi:10.1590/1678-4162-12105 Krisher, R.L. and Herrick, J.R. 2024. Bovine embryo production in vitro: evolution of culture media and commercial perspectives. Anim. Reprod. 21(3), e20240051; doi:10.1590/1984-3143-AR2024-0051 Lubis, A.F., Satyaningtijas, A.S., Lubis, O.P., Kurniati, W. and Boediono, A. (2021, November). Superovulation response of Peranakan Ongole (PO) and Simmental cows after FSH stimulation in multiple ovulation and embryo transfer program. IOP Conf. Ser. Earth Environ. Sci. 902(1), 012044; doi:10.1088/1755-1315/902/1/012044 Megahed, A.A., Bittar, J.H.J., Palomares, R.A., Mercadante, V.R.G. and Dias, N.W. 2023. Evaluation of the stress-reducing effect of trace mineral injection in beef calves. J. Vet. Intern. Med. 37(3), 1278–1285; doi:10.1111/jvim.16721 Mikkola, M., Hasler, J.F. and Taponen, J. 2019. Factors affecting embryo production in superovulated Bos taurus cattle. Reprod. Fertil. Dev. 32(2), 104–124; doi:10.1071/RD19279 Nevard, R.P., Pant, S.D., Broster, J.C., Norman, S.T. and Stephen, C.P. 2022. Maternal behavior in beef cattle: the physiology, assessment and future directions—a review. Vet. Sci. 10(1), 10; doi:10.3390/vetsci10010010 Pangestu, M.S.P., Darodjah, S., Hilmia, N. and Imron, M. 2024. Reproductive performance of Ongole grade, Simmental, and Limousin cattle given superovulation treatment. J. Agripet. 24(2), 168–173; doi:10.17969/agripet.v24i2.32335 Prastiya, R.A., Madyawati, S.P., Sari, S.Y. and Nugroho, A.P. 2022. Effect of follicle-stimulating hormone and luteinizing hormone levels on egg-laying frequency in hens. Vet. World. 15(12), 2890; doi:10.14202/vetworld.2022.2890-2895 Rabel, R.C., Marchioretto, P.V., Bangert, E.A., Wilson, K., Milner, D.J. and Wheeler, M.B. 2023. Pre-implantation bovine embryo evaluation—from optics to omics and beyond. Animals 13(13), 2102; doi:10.3390/ani13132102 Ramya, S., Poornima, P., Jananisri, A., Geofferina, I.P., Bavyataa, V., Divya, M., Priyanga, P., Vadivukarasi, J., Sujitha, S., Elamathi, S., Anand, A.V. and Balamuralikrishnan, B. 2023. Role of hormones and the potential impact of multiple stresses on infertility. Stresses 3(2), 454–474; doi:10.3390/stresses3020033 Raut, S.Y., Fu, K., Taichun, H., Gahane, A., Chaudhari, D., Kushwah, V., Suresh Managuli, R., Hegde, A.R., Jain, S., Kalthur, G., Bandu Joshi, M., Chang, H.I., Dai, N.T. and Mutalik, S. 2023. Engineered nanocarrier systems for the oral targeted delivery of follicle stimulating hormone: development, characterization, and, assessment of in vitro and in vivo performance and targetability. Int. J. Pharm. 637, 122868; doi:10.1016/j.ijpharm.2023.122868 Sonjaya, H., Damayanti, E., Iskandar, H., Baco, S., Hasbi, H., Mujnisa, A., Prahesti, K.I., Khasanah, A.U.N., Firmiaty, S., Nurhidayah, A.F., Kaiin, E.M., Samsudewa, D., Pala, R., Pamungkas, F.J. and Herdis, H.2025. Effects of glutathione on oocyte and embryo development in in vitro fertilization. Asian Pac. J. Reprod. 14 (1), 38–46; doi:10-4103. 10.4103/apjr.apjr_110_24 Sosa, F., Uh, K., Drum, J.N., Stoecklein, K.S., Davenport, K.M., Ortega, M.S., Lee, K. and Hansen, P.J. 2023. Disruption of bovine preimplantation embryos reduces development and affects embryonic gene expression in utero. Reprod. Fertil. 4(2), e230001; doi:10.1530/RAF-23-0001 Tian, R., Mahmoodi, M., Tian, J., Esmailizadeh Koshkoiyeh, S., Zhao, M., Saminzadeh, M., Li, H., Wang, X., Li, Y. and Esmailizadeh, A. 2024. Leveraging functional genomics for understanding beef quality complexities and breeding beef cattle for improved meat quality. Genes 15(8), 1104; doi:10.3390/genes15081104 Turathum, B., Gao, E.M. and Chian, R.C. 2021. The function of cumulus cells in oocyte growth and maturation and in subsequent ovulation and fertilization. Cells 10(9), 2292; doi:10.3390/cells10092292 Vega, V.M.B., Chávez, S.P.J., Franco, C.D.M., Ramos, T.I. and Toledo, J.R. 2019. FSH in bovine superovulation. Bionatura 4(1), 812–816; doi:10.21931/RB/2019.04.01.12 Wang, P., Wang, S.C., Liu, X., Jia, S., Wang, X., Li, T., Yu, J., Parpura, V. and Wang, Y.F. 2022. Neural functions of hypothalamic oxytocin and its regulation. ASN Neurol. 14, 17590914221100706; doi:10.1177/17590914221100706 Yusuf, M., Toleng, A.L., Sahiruddin, Masturi, Hasrin, Mansur, M., Fatem, H. and Koibur, J. 2024. Responses of ovarian activity of dairy Holstein Friesian Heifers after synchronized using Heasynch and select synch protocols. Adv. Anim. Vet. Sci. 12(5), 923–927. doi:10.17582/journal.aavs/2024/12.5.923.927 Zhang, X., Li, L., Zhang, W., Luo, Y., Mao, Y., Du, H. and Li, L.2023. Embryo development and live birth resulted from artificial oocyte activation in microdissection testicular sperm extraction with ICSI in patients with nonobstructive azoospermia. Front. Endocrinol. 14, 1123541; doi:10.3389/fendo.2023.1123541 | ||
| How to Cite this Article |
| Pubmed Style Baharun A, Diansyah AM, Handarini R, Sikin S, Ningtias PI, Kurniati W, Iskandar H, Damayanti E, Arasya AK, Rahmi A. A comparative study of intramuscular versus epidural administration of follicle-stimulating hormone for embryo production in Wagyu cattle. Open Vet. J.. 2025; 15(5): 2103-2111. doi:10.5455/OVJ.2025.v15.i5.28 Web Style Baharun A, Diansyah AM, Handarini R, Sikin S, Ningtias PI, Kurniati W, Iskandar H, Damayanti E, Arasya AK, Rahmi A. A comparative study of intramuscular versus epidural administration of follicle-stimulating hormone for embryo production in Wagyu cattle. https://www.openveterinaryjournal.com/?mno=238725 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i5.28 AMA (American Medical Association) Style Baharun A, Diansyah AM, Handarini R, Sikin S, Ningtias PI, Kurniati W, Iskandar H, Damayanti E, Arasya AK, Rahmi A. A comparative study of intramuscular versus epidural administration of follicle-stimulating hormone for embryo production in Wagyu cattle. Open Vet. J.. 2025; 15(5): 2103-2111. doi:10.5455/OVJ.2025.v15.i5.28 Vancouver/ICMJE Style Baharun A, Diansyah AM, Handarini R, Sikin S, Ningtias PI, Kurniati W, Iskandar H, Damayanti E, Arasya AK, Rahmi A. A comparative study of intramuscular versus epidural administration of follicle-stimulating hormone for embryo production in Wagyu cattle. Open Vet. J.. (2025), [cited January 25, 2026]; 15(5): 2103-2111. doi:10.5455/OVJ.2025.v15.i5.28 Harvard Style Baharun, A., Diansyah, . A. M., Handarini, . R., Sikin, . S., Ningtias, . P. I., Kurniati, . W., Iskandar, . H., Damayanti, . E., Arasya, . A. K. & Rahmi, . A. (2025) A comparative study of intramuscular versus epidural administration of follicle-stimulating hormone for embryo production in Wagyu cattle. Open Vet. J., 15 (5), 2103-2111. doi:10.5455/OVJ.2025.v15.i5.28 Turabian Style Baharun, Abdullah, Athhar Manabi Diansyah, Ristika Handarini, Sikin Sikin, Putri Indah Ningtias, Weni Kurniati, Hikmayani Iskandar, Erni Damayanti, Adiba Kanza Arasya, and Annisa Rahmi. 2025. A comparative study of intramuscular versus epidural administration of follicle-stimulating hormone for embryo production in Wagyu cattle. Open Veterinary Journal, 15 (5), 2103-2111. doi:10.5455/OVJ.2025.v15.i5.28 Chicago Style Baharun, Abdullah, Athhar Manabi Diansyah, Ristika Handarini, Sikin Sikin, Putri Indah Ningtias, Weni Kurniati, Hikmayani Iskandar, Erni Damayanti, Adiba Kanza Arasya, and Annisa Rahmi. "A comparative study of intramuscular versus epidural administration of follicle-stimulating hormone for embryo production in Wagyu cattle." Open Veterinary Journal 15 (2025), 2103-2111. doi:10.5455/OVJ.2025.v15.i5.28 MLA (The Modern Language Association) Style Baharun, Abdullah, Athhar Manabi Diansyah, Ristika Handarini, Sikin Sikin, Putri Indah Ningtias, Weni Kurniati, Hikmayani Iskandar, Erni Damayanti, Adiba Kanza Arasya, and Annisa Rahmi. "A comparative study of intramuscular versus epidural administration of follicle-stimulating hormone for embryo production in Wagyu cattle." Open Veterinary Journal 15.5 (2025), 2103-2111. Print. doi:10.5455/OVJ.2025.v15.i5.28 APA (American Psychological Association) Style Baharun, A., Diansyah, . A. M., Handarini, . R., Sikin, . S., Ningtias, . P. I., Kurniati, . W., Iskandar, . H., Damayanti, . E., Arasya, . A. K. & Rahmi, . A. (2025) A comparative study of intramuscular versus epidural administration of follicle-stimulating hormone for embryo production in Wagyu cattle. Open Veterinary Journal, 15 (5), 2103-2111. doi:10.5455/OVJ.2025.v15.i5.28 |