| Research Article | ||
Open Vet. J.. 2024; 14(4): 973-979 Open Veterinary Journal, (2024), Vol. 14(4): 973–979 Original Research Pharmaceutical activity of sappan wood extract (Caesalpinia sappan L.) for treating Escherichia coli infection in pigletsKridda Chukiatsiri1, Kittiphong Tippaya1 and Ruttayaporn Ngasaman2*1Faculty of Animal Science and Technology, Maejo University, Chiangmai, Thailand 2Faculty of Veterinary Science, Prince of Songkla University, Songkhla, Thailand *Corresponding Author: Ruttayaporn Ngasaman. Faculty of Veterinary Science, Prince of Songkla University, Songkhla, Thailand. Email: ruttayaporn.n [at] psu.ac.th Submitted: 17/10/2023 Accepted: 05/03/2024 Published: 30/04/2024 © 2024 Open Veterinary Journal
AbstractBackground: Escherichia coli infection is one of the major diarrheal diseases resulting in the loss of pigs at a young age. Aim: This research investigated the antimicrobial activity of Caesalpinia sappan wood extract against E. coli infection as an antibiotic replacement. Methods: E. coli was cultured from diarrheal piglets and then used to find the minimal inhibition concentration (MIC). Caesalpinia sappan wood extract (500 mg/kg) was used for the treatment of diarrheal piglets compared to antibiotics (enrofloxacin 5 mg/kg) by oral administration. Another three groups of diarrheal piglets were used supplemented feed with 1% and 2% extract compared with commercial feed. Subsequently, E. coli enumeration, fecal shape, fecal color, and growth rate were recorded from day 1 to 7. Results: Based on the results, C. sappan wood extract could inhibit E. coli growth at a MIC of 16–34 mg/ml. The number of colonies did not significantly differ between C. sappan wood extract and enrofloxacin treatment groups. A supplemented feed with 1% and 2% C. sappan wood extract could improve the fecal shape and fecal score compared to the control group, albeit only in suckling pigs. There were significant differences from the control group on days 4, 5, 6, and 7 (p < 0.05). However, the average daily gain did not significantly differ among the three groups. Conclusion: The results indicate that C. sappan wood extract could improve diarrheal signs in suckling pigs and can be used as a replacement for antibiotics for organic pig production. Keywords: Escherichia coli, Pharmaceutical activity, Piglets, Sappan wood extract, Treatment. IntroductionEscherichia coli infection, or colibacillosis, in suckling and weaned pigs is one of the major diarrheal diseases in the swine industry, occurring both chronically and sporadically (Castro et al., 2022). It may result in the loss of pigs at a young age during the weaning period. This disease is a financial burden in pig production due to the high mortality rate, retarded growth, high treatment costs, feed vaccination, and feed supplementations and accounts for 11.5%–29.5% of piglet deaths worldwide (Sinha et al., 2018; Wang et al., 2019). Therefore, the pig farming industry widely uses antibiotics to reduce E. coli infections and promote growth. However, the use of antibiotics, in addition to causing drug-resistant infections, also results in drug residues in the meat, putting the consumer at risk. Swine production accounts for a large proportion of the global meat production (Österberg et al., 2016). In China, with large-scale pig farms, β-lactam resistance genes are most prevalent in the eastern region (Li et al., 2021). In Beijing, E. coli resistance rates range between 4.05% and 97.64%, and resistance to tetracyclines, penicillin, and chloramphenicol is most common (Liu et al., 2022). In northern Thailand, the prevalence of E. coli in chicken and swine farms ranges from 36.8% to 47.6% (Hanson et al., 2002). In this context, herbal extracts are increasingly being used as antibiotic replacements for organic animal production. Different herbal extracts have shown antibacterial activity, such as pomegranate rind, guava leaves, cinnamon and sappan wood extract (Mith et al., 2014; Nirmal and Panichayupakaranant, 2015; Chukiatsiri et al., 2021). Sappan is a medicinal plant belonging to the bean family (Fabaceae or Leguminosae), with the scientific name Caesalpinia sappan L. It is commonly found in tropical countries such as India, Sri Lanka, Myanmar, Laos, Vietnam, South China, and Thailand. Its phenolic ingredients such as brazilin (sappan red), xanthone, coumarin, chalcones, and flavonoids, such as tannin and saponin, are active ingredients (Srinivasan et al., 2012; Zhao et al., 2013). Brazilin is the main ingredient in the heartwood of C. sappan and inhibits the growth of methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and multidrug-resistant Burkholderia cepacia (Xu and Lee, 2004). Protosappanins A (PsA) and B (PsB) can inhibit the growth of MRSA (Zuo et al., 2015) and C. sappan methanol showed antimicrobial activity and the potential to restore the effectiveness of β-lactam antibiotics against MRSA (Kim et al., 2004) and Streptococcus pyogenes (Kaur et al., 2016). In other studies, ethanolic wood extracts of C. sappan (L.) showed activity against Pseudomonas aeruginosa, S. aureus, Salmonella typhi, Enterobacter aeruginosa, Candida albicans, E. coli, Acinetobacter baumannii, and Klebsiella pneumoniae (Temrangsee et al., 2011; Srinivasan et al., 2012). This study investigated the efficiency of C. sappan wood extract in the treatment of E. coli infection in diarrheal pigs by oral treatment compared with antibiotic treatment. Feed supplemented with 1% and 2% C. sappan wood extract was evaluated, and it was expected that the herbal extract could be used as a substitute for antibiotic use in organic pig production. Materials and MethodsCaesalpinia sappan wood extractionCaesalpinia sappan wood extraction followed the protocol of Settharaksa et al (2019) Briefly, one kilogram of C. sappan wood was mixed with 6 l of water and soaked for 30 minutes. Subsequently, the mixture was boiled for 30 minutes (95°C), allowed to cool down, and filtered through cotton wool. The pulp was boiled three times, and the extracts were pooled and analyzed for volume, pH, and percentage of soluble sucrose to get 3% brix. Subsequently, we added Cab-O-Sil at 1.25% w/v, followed by shaking. Drying was performed with a BUCHI Mini Spray Dryer B-290 (Serial no.: 1000278016), which has a spray nozzle hole size of 0.7 mm, at an air pressure of 40 mbar. The drying conditions were as follows: 170°C inlet temperature, 110°C outlet temperature, aspirator 100%, pump 25%, and two nozzle cleaners. The extract powder was kept in a vacuum-laminated sachet, and the packing date was recorded. Figure 1 shows images of the water extract and the powder. Animal selectionDiarrheal pigs from the sucking and weaning group for experiments 1 and 2 were selected on day 2 of showing the clinical signs and weight between 3 and 10 kg. Confirmation of E. coli-caused diarrhea was cultured according to Lupindu (2017). Experiment 1 (direct oral treatment): 34 diarrheal weaned pigs were divided into two groups (17 pigs per group) that were orally treated with enrofloxacin 5 mg/kg and C. sappan wood extract 500 mg/kg. Experiment 2 (feed treatment): diarrheal pigs were chosen from 5 l of suckling (36 pigs) and 5 l of weaning (36 pigs) on day 2 of showing clinical signs and weight between 3 and 5 kg. Both suckling and weaning piglets were divided into three groups, 12 diarrheal piglets per group. Group 1 was fed normal feed, and groups 2 and 3 were fed with 1% and 2% C. sappan wood extract mixed feed, respectively. The feeding process was 7 days starting from day 7 to 14 post-partum, four times per day and water ad libitum. The amount of feed per day, shape, and color of feces was recorded.
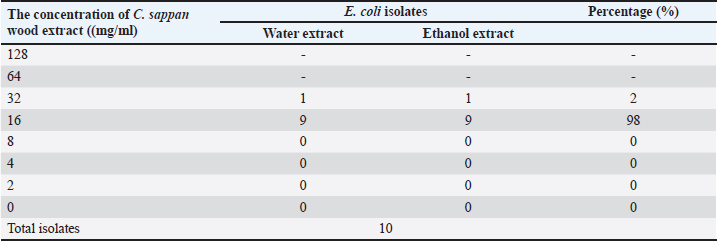
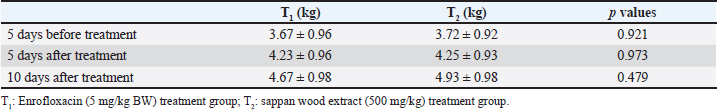
Fig. 1. Images of C. sappan wood, wood extract, and wood powder. Escherichia coli isolation and minimal inhibition concentration (MIC) testIsolates of E. coli were recovered from diarrheal piglets according to Lupindu (2017). Briefly, 1 g of feces was mixed with lauryl sulfate broth and incubated at 37°C for 18 to 24 hours. MacConkey agar (MAC) and eosin methylene-blue (EMB) lactose sucrose agar (EMB) were used to isolate E. coli colonies. Round, medium-sized colonies on MAC and metallic, sheen colonies on EMB were selected and spread on nutrient agar (NA), followed by incubation at 37°C for 18 to 24 hours. Single colonies on NA were tested for confirmation and kept as stock in tryptic soy broth + glycerol 2%. Confirmed isolates were recovered on NA and then cultured in Müller–Hinton broth. Incubation was done at 37°C for 18 to 24 hours, and absorbance was measured at 550 nm for 0.5 McFarland, which yielded an estimation of E. coli at 108 CFU/ml, with a dilution to 107 CFU/ml. Sappan wood extract was prepared by mixing with Müller–Hinton agar (MHA) at concentrations of 128, 64, 32, 16, 8, 4, 2, and 0 mg/ml. Subsequently, 2 μl of E. coli (107 CFU/ml) was dropped on MHA at four points and incubated at 37°C for 18 to 24 hours. After this, the MIC was determined. Antibiotic resistance was interpreted according to CLSI (2008) and BSAC (2011). Enumeration of E. coli in diarrheal pigsBriefly, 1 g of fecal sample was added to trypticase soy broth and diluted to obtain concentrations of 10−4 to 10−9. Then, 0.1 ml of each dilution was spread on EMB and cultured at 37°C for 18–24 hours. The colony number was determined and calculated per volume of sample (1 mg). Application of C. sappan for treating E. coli infectionFeed treatment; supplemented feed was prepared by using C. sappan wood extract 1% and 2% with pig feed. Then, 1% and 2% mixed feed were fed diarrheal pigs group 1 and 2 of suckling and weaning pigs compared to the normal feed group. Fecal score and body weight were recorded daily (7 days). Diarrheal pigs were treated with C. sappan wood extract 500 mg/kg BW compared with those treated with enrofloxacin 5 mg/kg BW for 10 days. Fecal scoringThe fecal score was evaluated in the early morning by visualization of the fecal consistency, shape, and color with the following scores: 1=dark, firm, and bushy, 2=semi-solid with black and green, 3=relatively soft with greenish–yellow, 4=rather liquid (either loose or formed) with greenish yellow and grey, 5=watery diarrhea with yellow. Statistical analysisData were collected from individual pigs, and the differences in the mean values between the group, standard deviation, and the p-value for the statistical difference were analyzed by using Microsoft Excel. Qualitative analysis of E. coli shedding and body weight were performed using compute paired samples Wilcoxon test in R. Ordinal data of fecal shape and fecal color were analyzed by using nonparametric statistical methods “Kruskal Wallis test” (Statistical Program R; Free Software Foundation, Boston, MA). Ethical approvalThis study was approved by the ethics committee of Maejo University (Approval no . MACUC 035A /2560). ResultsThe MIC of both water and ethanol C. sappan wood extract for inhibiting E. coli growth was 16–32 mg/ml (Table 1). The average colony forming unit (CFU) of E. coli at 5 days before treatment with enrofloxacin and sappan wood extract were 5.70 × 108 and 6.10 × 108 CFU/ml, respectively. After treatment for 5 days, E. coli of the antibiotic treatment group was 2.8 × 107 CFU/ml, whereas in the C. sappan wood extract treatment group was 2.6 × 107 CFU/ml. After 10 days of treatment, 1.69 × 106 and 1.59 × 106 CFU/ml in enrofloxacin and Sappan wood extract treatment groups, respectively. The average number of E. coli (CFU/ml) before and after treatment for 5 and 10 days did not significantly differ between the enrofloxacin and C. sappan wood treatment groups (p > 0.05) (Table 2). Table 1. The MIC of C. sappan wood extract against E. coli.
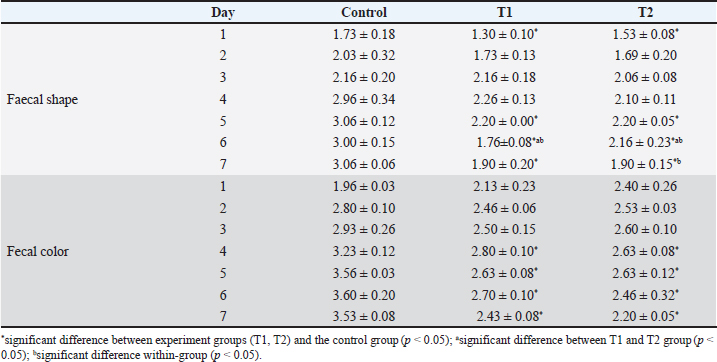
Treating with sappan wood extract and enrofloxacin showed significantly improved fecal shape and fecal color (p < 0.005). The Sappan wood extract treatment group showed better improvement than the enrofloxacin treatment group (p < 0.005). Comparison within the sappan wood extract and enrofloxacin treating groups determined that there was no significant difference in the improvement of fecal score and fecal color (p > 0.005) (data not showed). The average body weights of piglets in the enrofloxacin treatment group at 5 days before treatment and 5 and 10 days after treatment were 3.67, 4.23, and 4.67 kg. In the sappan wood extract treatment group, at 5 days before treatment and 5 and 10 days after treatment, the average body weights were 3.72, 4.25, and 4.93 kg. However, there were no statistically significant differences (p > 0.05) between the two groups (Table 3). Diarrheal-sucking pigs supplemented with 1% and 2% C. sappan wood extract feed, fecal shape differed significantly from the control group at days 1, 5, 6, and 7, whereas there were significant differences between treatments with 1% and 2% C. sappan wood extract (p < 0.05) at day 6. Comparison within each group from day 1 to 7, feeding with 1% sappan wood showed significantly better improvement of fecal shape at day 6 than on day 5. Feeding with 2% sappan wood showed significantly better improvement of fecal shape on days 6 and 7 than on day 5. The fecal color of the 1% and 2% C. sappan wood extract treatment groups differed significantly from that of the control group on days 4, 5, 6, and 7, but there was no significant difference within the group (Table 4). The average daily gain (ADG) values of suckling pigs of the control group, the 1% feeding group, and the 2% feeding group did not significantly differ (data not showed). However, the feed intake of suckling pigs fed 1% (69.4 ± 25.1) and 2% (68.9 ± 24.1) was lower than that of the control group (92.2 ± 53.9). In weaning pigs fed with 1% and 2% mixed feed, there were no differences in fecal shape, fecal score, and ADG when compared to the control group. Fecal color and score did not significantly differ between the enrofloxacin treatment group and the sappan wood extract treatment group from day 1 to day 10 (p > 0.05) (data not showed). DiscussionEnteric colibacillosis in swine is mostly caused by enterotoxigenic E. coli. This bacterium produces one or more enterotoxins that can have local and systemic effects, causing the secretion of fluid and electrolytes into the intestinal lumen, which results in diarrhea, dehydration, and acidosis (Castro et al., 2022). One of the antibiotics used for the treatment of swine colibacillosis is enrofloxacin, which is generally used for the treatment of gram-negative bacterial infections of the urinary and gastrointestinal tract. Generally, E. coli isolated from pigs is most frequently resistant to many antibiotics. In northern Thailand, E.coli shows resistance to tetracycline (91.5%), nalidixic acid (67.4%), ampicillin (61.6%), florfenicol (51.8%), enrofloxacin (28.7%), ciprofloxacin (12.5%), ceftiofur (4.9%), and ceftriaxone (1.5%) (Hanson et al., 2002). Pathogenic E. coli isolated from diarrheal pigs in Thailand resisted multidrugs such as novobiocin, streptomycin, sulfamethoxazole, tetracyclin, and tiamulin (100%), followed by amoxicillin (98%), oxytetracycline (96%), nalidixic acid (82%), gentamicin (56%), enrofloxacin (54%), and Colistin sulfate (46%) but susceptible to (Chukiatsiri et al, 2021). While another study indicated that, E. coli isolates from diarrheal piglets showed highly resistant to amoxicillin (100%), followed by oxytetracycline (91.9%), enrofloxacin (89.2%), trimethoprim/sulfamethoxazole (86.5%), amoxicillin: clavulanic acid (81.1%), colistin and gentamicin (75.7%), ceftriaxone and ceftiofur (64.9%), and ceftazidime (35.1%); 97.3% showed multidrug resistance (Nguyet et al., 2022). The use of enrofloxacin in piglets become increases the odds for enrofloxacin resistance in piglets (OR=26.78; p ≤ 0.0001) and sows at weaning (OR=4.04; p ≤ 0.05) (Callens et al., 2015). Resistance of E. coli to enrofloxacin has been reported in many species of livestock such as cattle, poultry included swine (Lin et al., 2017; Astorga et al., 2019; Li et al., 2019). Table 2. Average numbers of E. coli at 5 days before treatment and 5 and 10 days after treatment.
Table 3. Average body weights of piglets before treatment and 5 and 10 days after treatment.
Table 4. Scoring of fecal shape and color in suckling pigs fed with 1% (T1) and 2% (T2) C. sappan wood extract feed compared with the control group (normal feed).
This study used the optimal conditions that provide the highest active ingredient (brazilin) content by extraction at a temperature of 95°C for 30 minutes, as suggested by Settharaksa (2019). These results indicate that C. sappan wood extract can inhibit the growth of E. coli, (MIC: 16–32 mg/ml). The MIC obtained in the present study was lower than that reported in a study that determined the antibacterial activity of C. sappan wood extract against foodborne pathogens, including S. aureus, E. coli, S. enteritidis, and V. parahaemolyticus, with a MIC of 200 mg/ml (Pattananandecha et al., 2022). But it showed higher than a previous which indicated that ethanolic extracts of C. sappan wood extract can be used against bacteria that cause chronic wound infection, such as S. aureus, with a MIC value of 0.625 mg/ml (Temrangsee, 2011) and MIC/MBC 2/2 mg/ml (Kawari et al, 2016). The Brasilin which used a bioassay-directed method for isolating showed a potential activity against antibiotic-resistant bacteria at the MIC 4–32 mg/ml (Xu and Lee, 2004). For treating E. coli-caused diarrheal, this study was designed to use C. sappan wood extract 500 mg/kg BW because of the unestimated number of bacteria in the gut of diarrheal pigs. The results determined that the efficiency of C. sappan wood extract by oral route treatment of E. coli did not significantly differ from that of enrofloxacin. Moreover, the average body weights of piglets treated with enrofloxacin and C. sappan wood extract showed similar. Indicated that C. sappan wood extract did not improve the growth rate of piglets. Feeding with 1% sappan wood extract mixed feed showed a higher improvement of fecal shape at day 6 after treatment than that of the 2% treatment and the control group. However, feed intake and ADG were lower than in the control group, most likely because of the bitter taste of sappan wood extract. Using sappan wood extract (1% and 2%) mixed with feed did not improve diarrheal signs in weaned pigs. Similar to the results of a previous study which indicated that the flavonoids in C. sappan extract can decrease the number of Salmonella, but not of E. coli, in quail intestines (Widigdyo et al., 2017). The results of the present study suggest that C. sappan wood extract can be used as an alternative in the treatment of E. coli infections in sucking pigs at a concentration of 1%, C. sappan wood extract supplemented in feed. These results showed that although treatment with C. sappan decreases the CFU/ml of E. coli; it does not show better efficiency with respect to enrofloxacin treatment. The potential biases or limitations inherent in the study design were a bitter test of sappan wood extract and animal death during the experiment. AcknowledgmentThe research team would like to thank the staff at the Faculty of Animal Science and Technology, Maejo University, for their support. Thanks are due to Mr. Polkrit Vijitpongsa, Mr. Jatupohn Tongkumheo, Mr. Nattapong Tonksak, and Miss Natkritta Kongkart for providing assistance in the experiments for this research. This project was not supported by any grants. Authors’ contributionsKridda Chukiatsiri: Study design, sampling, diagnosis, and analysis of the raw data. Kittiphong Tippaya: Sampling, diagnosis, and analysis of the raw data. Ruttayaporn Ngasaman: Analyzed the data and writing the manuscript. Conflict of interestThe authors declare that there is no conflict of interest. FundingMaejo University funding. Data availabilityAll data are provided in the manuscript. ReferencesAstorga, F., Navarrete-Talloni, M.J., Miró, M.P., Bravo, V., Toro, M., Blondel, C.J. and Hervé-Claude, L.P. 2019. Antimicrobial resistance in E. coli isolated from dairy calves and bedding material. Heliyon 5(11), e02773. British Society for Antimicrobial Chemotherapy (BSAC). 2011. BSAC methods for antimicrobial susceptibility testing. Version 7.1. London: BSAC, pp: 26–30. Callens, B., Faes, C., Maes, D., Catry, B., Boyen, F., Francoys, D., de Jong, E., Haesebrouck, F. and Dewulf, J. 2015. Presence of antimicrobial resistance and antimicrobial use in sows are risk factors for antimicrobial resistance in their offspring. Microb. Drug. Resist. (Larchmont, N.Y.) 21, 50–58. Castro, J., Barros, M.M., Araújo, D., Campos, A.M., Oliveira, R., Silva, S. and Almeida, C. 2022. Swine enteric colibacillosis: current treatment avenues and future directions. Front. Vet. Sci. 9, 981207. Clinical and Laboratory Standards Institute. 2008. Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals; approved standard. 3rd ed. Wayne, Pennsylvania, pp: 13–23. Chukiatsiri, K., Tippaya, K. and Ngasaman, R. 2021. Effects of pomegranate rind (Punica granatum Linn.) and guava leaf extract (Psidium guajava Linn.) for inhibition of multidrug resistant Escherichia coli recovered from diarrhoeal piglets. SJST 43(1), 45–49. Hanson, R., Kaneene, J.B., Padungtod, P., Hirokawa, K. and Zeno, C. 2002. Prevalence of Salmonella and E. coli, and their resistance to antimicrobial agents, in farming communities in northern Thailand. Southeast Asian J. Trop. Med. Public Health. 33(3), 120–126. Kaur, H., Amini, M.H., Prabhakar, D.P., Singh, A. and Suttee, A. 2016. Phytochemical screening and antimicrobial activity of Caesalpinia sappan L. leaves. Int. J. Pharmacogn. Phytochem. Res. 8, 1064–1069. Kawari, R., Tawkanchan, N. and Tharachay Y. 2016. Inhibitory effect on pathogenic bacteria of crude ethanol extract from sappan wood extract. ChiangMai, Thailand: Faculty of Science, Maejo University, pp: 522–527. Kim, K.-J., Yu, H.-H., Jeong, S.-I., Cha, J.-D., Kim, S.-M. and You, Y.-O. 2004. Inhibitory effects of Caesalpinia sappan on growth and invasion of methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol. 91, 81–87. Li, J., Hao, H., Dai, M., Zhang, H., Ning, J., Cheng, G., Shabbir, M.A.B., Sajid, A. and Yuan, Z. 2019. Resistance and virulence mechanisms of Escherichia coli selected by enrofloxacin in chicken. Antimicrob. Agents Chemother. 63(5), e01824–18. Li, X., Liu, H., Cao, S., Cheng, P., Li, F., Ishfaq, M., Sun, J. and Zhang, X. 2021. Resistance detection and transmission risk analysis of pig-derived pathogenic Escherichia coli in East China. Front. Vet. Sci. 8, 614–651. Lin, D., Chen, K., Xie, M., Ye, L., Chan, E.W. and Chen, S. 2017. Effect of ceftiofur and enrofloxacin on E. coli sub-population in pig gastrointestinal tract. J. Glob. Antimicrob. Resist. 10, 126–130. Liu, X., Liu, Q., Cheng, Y., Liu, R., Zhao, R., Wang, J., Wang, Y., Yang, S. and Chen, A. 2022. Effect of bacterial resistance of Escherichia coli from swine in large-scale pig farms in Beijing. Front. Microbiol. 13, 820–833. Lupindu, A.M. 2017. Isolation and characterization of Escherichia coli from animals, humans, and environment. E. coli recent advances on physiology, pathogenesis and biotechnological applications. London, UK: In Tech; doi:10.5772/67390. Mith, H., Duré, R., Delcenserie, V., Zhiri, A., Daube, G. and Clinquart, A. 2014. Antimicrobial activities of commercial essential oils and their components against food-borne pathogens and food spoilage bacteria. Food Sci. Nutr. 2(4), 403–416. Nguyet, L.T.Y., Keeratikunakorn, K., Kaeoket, K. and Ngamwongsatit, N. 2022. Antibiotic resistant Escherichia coli from diarrheic piglets from pig farms in Thailand that harbor colistin-resistant mcr genes. Sci. Rep. 12(1), 9083. Nirmal, N.P. and Panichayupakaranant, P. 2015. Antioxidant, antibacterial, and anti-inflammatory activities of standardized brazilin-rich Caesalpinia sappan extract. Pharmaceut. Biol. 53, 1339–1343. Österberg, J., Wingstrand, A., Nygaard Jensen, A., Kerouanton, A., Cibin, V., Barco, L., Denis, M., Aabo, S. and Bengtsson, B. 2016. Antibiotic resistance in Escherichia coli from pigs in organic and conventional farming in four European countries. PLoS One 11, e0157049. Pattananandecha, T., Apichai, S., Julsrigival, J., Ogata, F., Kawasaki, N. and Saenjum, C. 2022. Antibacterial activity against foodborne pathogens and inhibitory effect on anti-inflammatory mediators’ production of brazilin-enriched extract from Caesalpinia sappan Linn. Plants (Basel, Switzerland), 11(13), 1698. Settharaksa, S., Monton, C. and Charoenchai, L. 2019. Optimization of Caesalpinia sappan L. heartwood extraction procedure to obtain the highest content of brazilin and greatest antibacterial activity. J. Integr. Med. 17(5), 351–358. Sinha, R., Sahoo, N.R., Kumar, P., Qureshi, S., Kumar, A., Ravikumar, G.V.P.P.S. and Bhushan, B. 2018. Comparative jejunal expression of MUC 13 in Indian native pigs differentially adhesive to diarrhoeagenic E. coli. J. Appl. Anim. Res. 46, 107–111. Srinivasan, R., Selvam, G., Karthik, S., Krishnamurthy, M., Baskaran, R., Karthikeyan, M., Gopi, M. and Govindasamy, C. 2012. In vitro antimicrobial activity of Caesalpinia sappan L. Asian Pac. J. Trop. Biomed. 2, S136–S139. Temrangsee, P., Kondo, S. and Itharat, A. 2011. Antibacterial activity of extracts from five medical plants and their formula against bacteria that cause chronic wound infection. J. Med. Assoc. Thail. 94(7), 166–171. Wang, H., Zhong, Z., Luo, Y., Cox, E. and Devriendt, B. 2019. Heat-stable enterotoxins of enterotoxigenic Escherichia coli and their impact on host immunity. Toxins. 11, 24. Widigdyo, A., Widodo, E. and Djunaidi, I. 2017. Extract of Caesalpinia sappan L. as antibacterial feed additive on intestinal microflora of laying quail. J. Exp. Life Sci. 7, 7–10. Xu, H.X. and Lee, S.F. 2004. The antibacterial principle of Caesalpina sappan. Phytother. Res. 18(8), 647–651. Zhao, M.B., Li, J., Shi, S.P., Cai, C.Q., Tu, P.F., Tang, L., Zeng, K.W. and Jiang, Y. 2013. Two new phenolic compounds from the heartwood of Caesalpinia sappan L. Molecules (Basel, Switzerland). 19, 1–8. Zuo, G.Y., Han, Z.Q., Han, J., Hao, X.Y., Tang, H.S. and Wang, G.C. 2015. Antimicrobial activity and synergy of antibiotics with two biphenyl compounds, protosappanins A and B from Sappan Lignum against methicillin-resistant Staphylococcus aureus strains. J. Pharm. Pharmacol. 67, 1439–1447. | ||
| How to Cite this Article |
| Pubmed Style Chukiatsiri K, Tippaya K, Ngasaman R. Pharmaceutical activity of sappan wood extract (Caesalpinia sappan L.) for treating Escherichia coli infection in piglets. Open Vet. J.. 2024; 14(4): 973-979. doi:10.5455/OVJ.2024.v14.i4.4 Web Style Chukiatsiri K, Tippaya K, Ngasaman R. Pharmaceutical activity of sappan wood extract (Caesalpinia sappan L.) for treating Escherichia coli infection in piglets. https://www.openveterinaryjournal.com/?mno=173362 [Access: January 25, 2026]. doi:10.5455/OVJ.2024.v14.i4.4 AMA (American Medical Association) Style Chukiatsiri K, Tippaya K, Ngasaman R. Pharmaceutical activity of sappan wood extract (Caesalpinia sappan L.) for treating Escherichia coli infection in piglets. Open Vet. J.. 2024; 14(4): 973-979. doi:10.5455/OVJ.2024.v14.i4.4 Vancouver/ICMJE Style Chukiatsiri K, Tippaya K, Ngasaman R. Pharmaceutical activity of sappan wood extract (Caesalpinia sappan L.) for treating Escherichia coli infection in piglets. Open Vet. J.. (2024), [cited January 25, 2026]; 14(4): 973-979. doi:10.5455/OVJ.2024.v14.i4.4 Harvard Style Chukiatsiri, K., Tippaya, . K. & Ngasaman, . R. (2024) Pharmaceutical activity of sappan wood extract (Caesalpinia sappan L.) for treating Escherichia coli infection in piglets. Open Vet. J., 14 (4), 973-979. doi:10.5455/OVJ.2024.v14.i4.4 Turabian Style Chukiatsiri, Kridda, Kittiphong Tippaya, and Ruttayaporn Ngasaman. 2024. Pharmaceutical activity of sappan wood extract (Caesalpinia sappan L.) for treating Escherichia coli infection in piglets. Open Veterinary Journal, 14 (4), 973-979. doi:10.5455/OVJ.2024.v14.i4.4 Chicago Style Chukiatsiri, Kridda, Kittiphong Tippaya, and Ruttayaporn Ngasaman. "Pharmaceutical activity of sappan wood extract (Caesalpinia sappan L.) for treating Escherichia coli infection in piglets." Open Veterinary Journal 14 (2024), 973-979. doi:10.5455/OVJ.2024.v14.i4.4 MLA (The Modern Language Association) Style Chukiatsiri, Kridda, Kittiphong Tippaya, and Ruttayaporn Ngasaman. "Pharmaceutical activity of sappan wood extract (Caesalpinia sappan L.) for treating Escherichia coli infection in piglets." Open Veterinary Journal 14.4 (2024), 973-979. Print. doi:10.5455/OVJ.2024.v14.i4.4 APA (American Psychological Association) Style Chukiatsiri, K., Tippaya, . K. & Ngasaman, . R. (2024) Pharmaceutical activity of sappan wood extract (Caesalpinia sappan L.) for treating Escherichia coli infection in piglets. Open Veterinary Journal, 14 (4), 973-979. doi:10.5455/OVJ.2024.v14.i4.4 |