| Research Article | ||
Open Vet J. 2023; 13(5): 510-514 Open Veterinary Journal, (2023), Vol. 13(5): 510–514 Original Research A prospective study on clinical signs, management, outcomes, and delayed neurologic sequelae due to metaldehyde poisoning in 26 dogsGuillaume Fabien Dutil1,2*, and Philippe Berny31Division of Neurology and Neurosurgery, CHV Atlantia, Nantes, France 2Division of Clinical Neurology, Department of Clinical Vetrerinary Medicine, VetSuisse Faculty, University of Bern, Bern, Switzerland 3Division of Pharmacology and Toxicology, VetAgro Sup, Marcy l'Etoile, France *Corresponding Author: Guillaume Fabien Dutil. Division of Neurology and Neurosurgery, CHV Atlantia, Nantes, France. Email: gdutil [at] live.fr. Submitted: 22/06/2022 Accepted: 03/04/2023 Published: 02/05/2023 © 2023 Open Veterinary Journal
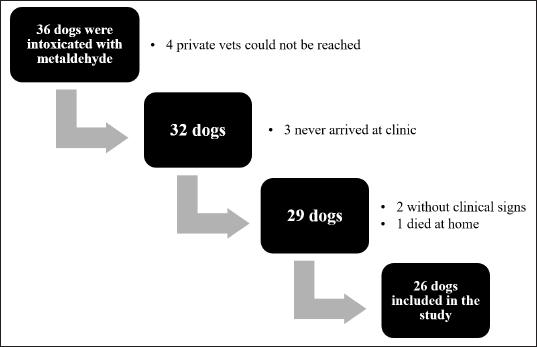
AbstractBackground: Metaldehyde poisoning in dogs is well known and described issue. Several studies focused on the incidence, epidemiological features, and clinical and pathological findings associated with this intoxication. However, there are no prospective studies of metaldehyde poisoning and late-onset seizures. Aims: To prospectively describe clinical signs, therapeutic management, outcomes, and delayed-onset seizures due to metaldehyde poisoning in dogs. Methods: A 15-month prospective study on dogs with a diagnosis of metaldehyde poisoning, either via phone call to the animal poison control center or analysis at the toxicology laboratory in Lyon, France. Clinical signs, therapeutic management and outcomes, and the late onset of seizures were assessed for at least 3 years. Results: Twenty-six dogs were enrolled in the study. The most prevalent clinical signs were ataxia (18 dogs), convulsions (17), hypersalivation (15), and tremors (15). Treatment was symptomatic (e.g., activated charcoal, emetic therapy, and intravenous fluids) with anticonvulsant therapy (mainly diazepam). The overall survival rate was 81% (21/26 dogs). All dogs that received active charcoal (11/11) or emetic therapy (4/4) survived. Twelve of 17 dogs had convulsions and survived; 9 were followed up for at least 3 years after poisoning, and none had any other seizure episode or neurological sequelae. Conclusion: This prospective study describes clinical signs, therapeutic management and outcome of metaldehyde poisoning in dogs, and late-onset neurologic sequelae. None of the nine cases that were followed for 3 years developed neurological signs after metaldehyde poisoning. Therefore, long-term antiepileptic therapy is not indicated. Keywords: Metaldehyde poisoning, Neurologic sequelae, Delayed-onset seizures, Reactive seizures, Canine. IntroductionMetaldehyde is a cyclic tetramer of acetaldehyde generally used in a dry granule form as snail and slug baits (Richardson et al., 2003). Worldwide, including France (Ephy Website, 2022), its concentration in most molluscicides is 2%–5%. Poisoning can occur due to direct exposure to the toxic agent in the environment or voluntary distribution by humans (Berny et al., 2010; Guitart et al., 2010; De Roma et al., 2017). The acute median lethal dose of metaldehyde is ~100–1,000 mg/kg of body weight in dogs (Booze and Oehme, 1986; Richardson et al., 2003; Bates et al., 2012). However, the mechanism by which metaldehyde is toxic remains unclear. Metaldehyde’s pharmacokinetics have been described in rodents but not dogs (Homeida and Cooke, 1982a). It can be either absorbed intact or transformed in the stomach by acid hydrolysis to acetaldehyde. The latter compound was previously thought to be the primary toxic molecule; however, several studies highlighted that this hypothesis was debatable and that metaldehyde was also, at least partially, responsible for the clinical signs observed (Booze and Oehme, 1986; Sparks et al., 1996; Shintani et al., 1999). Metaldehyde reaches the blood, brain, and liver (Tardieu et al., 1996; Richardson et al., 2003). In mice, metaldehyde is associated with an increased monoamine oxidase activity and decreases in gamma-aminobutyric acid, norepinephrine, and 5-hydroxytryptamine concentrations. These modifications cause neuronal excitation and decrease the seizure threshold, both of which can result in convulsions (Homeida and Cooke, 1982a, 1982b; Sparks et al., 1996). Metaldehyde poisoning can also lead to (severe) metabolic acidosis (Yas-Natan et al., 2007). In humans, other poisoning cases, such as carbon monoxide or diethylene glycol poisoning, are responsible for neurologic sequelae (Alfred et al., 2005; Jeon et al., 2018). In one retrospective study in dogs, metaldehyde intoxication did not seem to cause neurological sequelae or structural epilepsy (Jull et al., 2011). Previous retrospective studies on metaldehyde poisoning in dogs focused on the incidence, epidemiological features, and clinical and pathological findings associated with this intoxication. However, there are no prospective studies of metaldehyde poisoning and late-onset seizures. The objective was to prospectively describe the clinical signs, management, and outcome of metaldehyde poisoning in dogs. Furthermore, this was apparently the first study to prospectively investigate delayed onset seizures or neurological abnormalities in dogs several years after poisoning. Materials and MethodsSelection of casesThe study took place from October 2014 to December 2015. First, all calls by a veterinarian for dogs with confirmed and unique witnessed metaldehyde ingestion during the opening hours (800–1,830) of the animal poison control center in Lyon (Centre National d'Informations Toxicologiques Vétérinaires—CNITV) were recorded. Second, all dogs with the presence of metaldehyde in blood, gastric content, or organs tested at the toxicology laboratory were also included. Veterinary practitioners were informed during the call that they would be called back within ~1 week for further information about therapy and outcome. Dogs that had died before arrival at the veterinarian (one dog) or that were never presented to a veterinarian (three dogs) were excluded from the study. The data collected included as much information as possible about symptoms, physical and neurological examination findings, the interval from ingestion to clinical signs, or to the start of therapy, duration of hospitalization, and short- and long-term outcomes. Specific endpoints collected included: the interval from poisoning to the onset of clinical signs, the interval from poisoning to presentation to a veterinary practitioner, the duration of clinical signs, and the duration of hospitalization. Laboratory testingA screening step was performed on the gastric content using a colorimetric method (Braselton and Johnson, 2003). Metaldehyde was measured using gas chromatography-mass spectrometry (GC-MS), based on a previous report (Braselton and Johnson, 2003). In brief, after chloroform extraction, analysis was performed on a GC-MS 6973 apparatus with a 30-m Restek 5 column. Metaldehyde was detected at 5.2 minutes (molecular fragment 89 was used for quantification and fragments 117 and 131 were used for identification) and quantified with a linear calibration curve from 10 to 100 mg/kg. Recovery (determined on spiked samples) was >80%. The detection threshold was 1 µg/g or 1 µg/ml. Ethical approvalNo ethical approval was needed for this study. ResultsIn total, 26 dogs (Fig. 1) were involved in our study (22 from CNITV and 4 from the toxicology laboratory). The breeds most represented were Breton Spaniel (four) and Border Collie (three). Age was available for 24 dogs (other dogs were adults), with a mean of 4.4 years (median 4 years, range 0.2–10). Bodyweight was available for 24 dogs (other dogs were Labrador and Beagle); mean body weight was 18.7 kg (median 20, range 3–42). There were 17 female dogs (65%) and 9 male dogs (35%). Eleven dogs were intoxicated in autumn, seven during spring, five in winter, and three in summer. Four dogs underwent gastric content analysis [1,986, 443 (for two dogs from the same owner), and 137 µg/g].
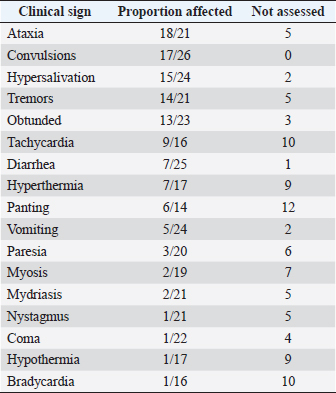
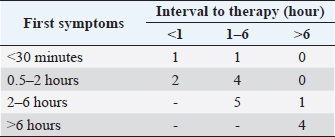
Fig. 1. Number of cases collected to be included in the study. The overall survival rate was 81% (21/26). Ataxia, convulsion, and hypersalivation were the most frequent clinical signs (Table 1). The interval from poisoning to the onset of clinical signs was available for 19 dogs (Table 2). The majority of cases (15/19) had their first clinical signs within 6 hours and there was no significant association between the time of first clinical signs and survival. Twenty-five dogs received therapy, and the interval from the first clinical sign to initiation of therapy was assessed for 20 of them (Table 3). There was no significant association between survival rate and either time of poisoning or initiation of therapy. It was possible to administrate orally (patients able to swallow normally) activated charcoal to 11 dogs and all survived, whereas 9 of the 15 dogs that did not receive activated charcoal (due to severe clinical signs with an inability to swallow correctly) survived (higher survival with activated charcoal, p < 0.05). Intravenous fluid therapy was used for 22 dogs. Emetic therapy (apomorphine SQ) was used for four dogs (all survived) prior onset of severe clinical signs (e.g., seizures). Emesis confirmed ingestion of metaldehyde slug bait. None of the dogs had gastric lavage. The duration of clinical signs was available for 15 dogs [mean of 15.7 hours (1.5–72)], with 4, 4, 2, and 5 dogs having clinical signs for 0–2, 2–4, 4–12, and >12 hours, respectively. Overall, 23 dogs were hospitalized; despite therapy, 4 seizuring dogs died after 3, 5, 12, and 24 hours of hospitalization. The duration of hospitalization was available for 19 dogs, with 2, 2, 9, and 6 dogs being hospitalized for 0–6, 6–12, 12–24, and >24 hours, respectively [mean of 24.8 hours (3.5–72)]. Sixteen out of 17 dogs with reactive seizures received diazepam (1 died upon arrival and did not receive any drug). In addition to diazepam, one received pentobarbital and ketamine, one received pentobarbital only, one received phenobarbital, and one received tiletamine and zolazepam. Two dogs received general anesthesia due to seizures unresponsive to diazepam (one died and the other survived). Table 1. Clinical signs of dogs intoxicated with metaldehyde.
Of the 12 dogs that presented reactive seizures and survived, 9 had a long-term follow-up. One was euthanized 2 years after poisoning for a reason unrelated to neurological disease and did not have seizures in the 2 years. The other eight had a minimum of 3 years of long-term follow-up, and none had late-onset neurological sequelae or seizures. None of the dogs with reactive seizures that survived received anti-epileptic drugs after discharge. DiscussionMetaldehyde poisoning in dogs is described worldwide in several retrospective studies (Studdert, 1985; Robertson et al., 1992; Yas-Natan et al., 2007; Bates et al., 2012; De Roma et al., 2017; Teichmann-Knorrn et al., 2020) as well as for numerous other domestic or wildlife species, such as cats, cattle, red fox, or birds (Firth, 1992; Berny et al., 2010; Guitart et al., 2010; Wang et al., 2011; Sabater et al., 2014; Bille et al., 2016). These have raised questions about the consequences of using or storing metaldehyde close to dogs. This study presents prospective data about the clinical presentation of metaldehyde poisoning and discusses, for the first time, long-term outcomes such as structural epilepsy or neurological sequelae. We registered 26 cases of metaldehyde poisoning during the 15 months of our study. These findings were in the same range as other studies and relied mostly on the area investigated and data collected (Yas-Natan et al., 2007; Bates et al., 2012; De Roma et al., 2017). The season of intoxication in our study cannot confirm observations from Italy and the UK, where poisoning was higher during summer and winter (Bates et al., 2012; De Roma et al., 2017). Among our limitations, collecting data only during opening hours may have limited the scope of the collection. Both age (1 month to 17 years with a mean of 4 years) and weight (5–40 kg with a mean of 17 kg), data were as wide as in other studies and confirmed that all dogs at all ages can be affected (Yas-Natan et al., 2007; Bates et al., 2012). Half of our dogs were presented before 2 hours, comparable to a previous study (13) in which the mean interval before therapy was 135 minutes (40–600 minutes) in 8 dogs. In terms of the interval between poisoning and first clinical signs, it was previously reported as 2.9 hours (range 2 minutes–24 hours) in 290 cases, with 50.3% developing effects within 1 hour (Bates et al., 2012). With 81% survival, our study was similar to others, which ranged from 83% to 92% (Studdert, 1985; Robertson et al., 1992; Yas-Natan et al., 2007; Bates et al., 2012). Table 2. Interval from metaldehyde poisoning to first clinical signs in all dogs and survivors only.
Table 3. Interval from first clinical sign to initiation of therapy in dogs with metaldehyde poisoning.
The main clinical signs observed were ataxia, convulsions, and hypersalivation. Seizures appear to be common as suggested in the most recent as well as older studies (Firth, 1992; Richardson et al., 2003; Yas-Natan et al., 2007; Bates et al., 2012). No blindness was reported, but it was not actively assessed as compared to other studies (Bates et al., 2012). Metabolic acidosis was not reported due to the lack of facilities to measure blood gases in most of the private practices involved. Interval to full recovery or duration of hospitalization was shorter than previously reported (Yas-Natan et al., 2007; Bates et al., 2012). Medical management is a difficult metric to compare between studies due to the biased selection of cases in private vet clinics and therapy availability. Nevertheless, in our study, 100% of dogs (11/11) that were able to receive activated charcoal (recent ingestion and before severe clinical signs) survived. This finding confirmed a previous report of increased odds of survival in rats after activated charcoal administration (Shintani et al., 1999). However, in one study (Botelho et al., 2020), a dog with metaldehyde poisoning was given activated charcoal and died. In the absence of specific antidotes, symptomatic treatments, including activated charcoal, are not always effective. Many factors are involved in the toxicity and final outcome, including the dose of metaldehyde ingested, other comorbidities, the severity of clinical signs, and interval from onset of signs to initiation of treatment. Intravenous lipid emulsion therapy or hemodyalisis was not used in our cases but successful management of metaldehyde poisoning in dogs was already described (Lelescu et al., 2018; Teichmann-Knorrn et al., 2020). Interestingly, none of the dogs that responded to diazepam received phenobarbital treatment. Thus, the protective effect of phenobarbital in metaldehyde intoxication, as suggested in rats with possible accelerated degradation of metaldehyde, cannot be assessed (Tardieu et al., 1996). None of the dogs that presented seizures and survived received anti-epileptic drugs in our study; furthermore, only 4/17 were under therapy after status epilepticus (Jull et al., 2011). None of the dogs that presented seizures and survived presented neurological sequelae at least 3 years after poisoning. Our results were similar to those of a retrospective study (Jull et al., 2011) Furthermore, results differed from carbon monoxide, chlorpyrifos, and ethylene glycol poisoning in humans (Alfred et al., 2005; Jeon et al., 2018). One hypothesis is that metaldehyde does not cause the same histological changes as other convulsant drugs. It may also be linked to the lack of knowledge of the mechanism of action of metaldehyde poisoning or perhaps without electroencephalographies, severe tremors may be confused with seizures. Most importantly, our study confirmed that using long-term antiepileptic drugs in metaldehyde poisoning is not mandatory even a few hours after improvement. There were many limitations to our prospective study in the management of cases because it involved several clinicians. However, information concerning therapy and outcome was well reported because vets were informed at the beginning of the study that these dogs would be part of our investigation. In conclusion, metaldehyde intoxication can lead to severe neurological signs and death, but with immediate medical management, the prognosis is generally good. Moreover, even with seizures, antiepileptic drugs after hospitalization are not needed and long-term neurological sequelae are unlikely, which results in a good long-term prognosis. Conflict of interestThe Author declares that there is no conflict of interest. Author contributionsAuthors participate equally in designing, writing, correcting, and submitting the manuscript. DGF was responsible for calling vets and collecting data by phone. AcknowledgmentsThe authors extend great thanks to Dr. Laurence Tavernier for her help collecting phone call cases at CNTIV. ReferencesAlfred, S., Coleman, P., Harris, D., Wigmore, T., Stachowski, E. and Graudins, A. 2005. Delayed neurologic sequelae resulting from epidemic diethylene glycol poisoning. Clin. Toxicol. (Phila) 43, 155–159. Bates, N.S., Sutton, N.M. and Campbell, A. 2012. Suspected metaldehyde slug bait poisoning in dogs: a retrospective analysis of cases reported to the Veterinary Poisons Information Service. Vet. Rec. 171, 324–324. Berny, P., Caloni, F., Croubels, S., Sachana, M., Vandenbroucke, V., Davanzo, F. and Guitart, R. 2010. Animal poisoning in Europe. Part 2: companion animals. Vet. J. 183, 255–259. Bille, L., Toson, M., Mulatti, P., Dalla Pozza, M., Capolongo, F., Casarotto, C., Ferrè, N., Angeletti, R., Gallocchio, F. and Binato, G. 2016. Epidemiology of animal poisoning: an overview on the features and spatio-temporal distribution of the phenomenon in the north-eastern Italian regions. Forensic Sci. Int. 266, 440–448. Booze, T.F. and Oehme, F.W. 1986. An investigation of metaldehyde and acetaldehyde toxicities in dogs. Fundam. Appl. Toxicol. 6, 440–446. Botelho, A.F.M., Machado, A.M.D., da Silva, R.H.S., Faria, A.C., Machado, L.S., Santos, H., Braga, S. de M., Torres, B.B.J., Miguel, M.P., Chaves, A.R. and Melo, M.M. 2020. Fatal metaldehyde poisoning in a dog confirmed by gas chromatography. BMC Vet. Res. 16, 139. Braselton, W.E. and Johnson, M. 2003. Thin layer chromatography convulsant screen extended by gas chromatography-mass spectrometry. J. Vet. Diagn. Invest. 15, 42–45. De Roma, A., Miletti, G., D’Alessio, N., Rossini, C., Vangone, L., Galiero, G. and Esposito, M. 2017. Metaldehyde poisoning of companion animals: a three-year retrospective study. J. Vet. Res. 61, 307–311. Ephy Website. Metaldehyde. 2022. Available via https://ephy.anses.fr/substance/metaldehyde (Accessed 2 June 2022). Firth, A.M. 1992. Treatment of snail bait toxicity in dogs: retrospective study of 56 cases. J. Vet. Emerg. Crit. Care 2, 31–36. Guitart, R., Croubels, S., Caloni, F., Sachana, M., Davanzo, F., Vandenbroucke, V. and Berny, P. 2010. Animal poisoning in Europe. Part 1: farm livestock and poultry. Vet. J. 183, 249–254. Homeida, A.M. and Cooke, R.G. 1982a. Pharmacological aspects of metaldehyde poisoning in mice. J. Vet. Pharmacol. Ther. 5, 77–81. Homeida, A.M. and Cooke, R.G. 1982b. Anti-convulsant activity of diazepam and clonidine on metaldehyde-induced seizures in mice: effects on brain γ-amino butyric acid concentrations and monoamine oxidase activity. J. Vet. Pharmacol. Ther. 5, 187–190. Jeon, S.-B., Sohn, C.H., Seo, D.-W., Oh, B.J., Lim, K.S., Kang, D.-W. and Kim, W.Y. 2018. Acute brain lesions on magnetic resonance imaging and delayed neurological sequelae in carbon monoxide poisoning. JAMA Neurol. 75, 436–443. Jull, P., Risio, L.D., Horton, C. and Volk, H.A. 2011. Effect of prolonged status epilepticus as a result of intoxication on epileptogenesis in a UK canine population. Vet. Rec. 169, 361–361. Lelescu, C.A., Mureșan, C., Muste, A., Taulescu, M.A., Neagu, A.M. and Nagy, A.L. 2018. Successful treatment of metaldehyde toxicosis with intravenous lipid emulsion in a dog. Acta Vet. Brno 86, 379–383. Richardson, J.A., Welch, S.L., Gwaltney-Brant, S.M., Huffman, J.D. and Rosendale, M.E. 2003. Metaldehyde toxicoses in dogs. Compen. Contin. Educ. Pract. Vet. 25, 376–80. Robertson, I.D., Leggoe, M., Dorling, P.R., Shaw, S.E. and Clark, W.T. 1992. A retrospective study of poisoning cases in dogs and cats: comparisons between a rural and an urban practice. Aust. Vet. J. 69(8):194–195. Sabater, M., Pérez, M. and Calvo Carrasco, D. 2014. Metaldehyde intoxication in a red fox (Vulpes vulpes). Vet. Rec. Case Rep. 2, e000062. Shintani, S., Goto, K., Endo, Y., Iwamoto, C. and Ohata, K. 1999. Adsorption effects of activated charcoal on metaldehyde toxicity in rats. Vet. Hum. Toxicol. 41, 15–18. Sparks, S.E., Quistad, G.B., Cole, L.M. and Casida, J.E. 1996. Metaldehyde molluscicide action in mice: distribution, metabolism, and possible relation to GABAergic system. Pesticide Biochem. Physiol. 55, 226–236. Studdert, V.P. 1985. Incidence of poisonings in dogs and cats in Melbourne. Aust. Vet. J. 62, 133–135. Tardieu, D., Thouvenot, N., Fargier, C., de Saqui-Sannes, P. and Petit, C. 1996. Phenobarbital-type P-450 inducers protect rats against metaldehyde toxicity. Vet. Hum. Toxicol. 38, 454–456. Teichmann-Knorrn, S., Doerfelt, S. and Doerfelt, R. 2020. Retrospective evaluation of the use of hemodialysis in dogs with suspected metaldehyde poisoning (2012–2017): 11 cases. J. Vet. Emerg. Crit. Care 30, 194–201. Wang, M., Dai, Y., Han, Y., Haacke, E.M., Dai, J. and Shi, D. 2011. Susceptibility weighted imaging in detecting hemorrhage in acute cervical spinal cord injury. Magn. Reson. Imaging 29, 365–373. Yas-Natan, E., Segev, G. and Aroch, I. 2007. Clinical, neurological and clinicopathological signs, treatment and outcome of metaldehyde intoxication in 18 dogs. J. Small Anim. Pract. 48, 438–443. | ||
| How to Cite this Article |
| Pubmed Style Dutil GF, Berny P. A prospective study on clinical signs, management, outcomes, and delayed neurologic sequelae due to metaldehyde poisoning in 26 dogs. Open Vet J. 2023; 13(5): 510-514. doi:10.5455/OVJ.2023.v13.i5.2 Web Style Dutil GF, Berny P. A prospective study on clinical signs, management, outcomes, and delayed neurologic sequelae due to metaldehyde poisoning in 26 dogs. https://www.openveterinaryjournal.com/?mno=55612 [Access: April 28, 2024]. doi:10.5455/OVJ.2023.v13.i5.2 AMA (American Medical Association) Style Dutil GF, Berny P. A prospective study on clinical signs, management, outcomes, and delayed neurologic sequelae due to metaldehyde poisoning in 26 dogs. Open Vet J. 2023; 13(5): 510-514. doi:10.5455/OVJ.2023.v13.i5.2 Vancouver/ICMJE Style Dutil GF, Berny P. A prospective study on clinical signs, management, outcomes, and delayed neurologic sequelae due to metaldehyde poisoning in 26 dogs. Open Vet J. (2023), [cited April 28, 2024]; 13(5): 510-514. doi:10.5455/OVJ.2023.v13.i5.2 Harvard Style Dutil, G. F. & Berny, . P. (2023) A prospective study on clinical signs, management, outcomes, and delayed neurologic sequelae due to metaldehyde poisoning in 26 dogs. Open Vet J, 13 (5), 510-514. doi:10.5455/OVJ.2023.v13.i5.2 Turabian Style Dutil, Guillaume Fabien, and Philippe Berny. 2023. A prospective study on clinical signs, management, outcomes, and delayed neurologic sequelae due to metaldehyde poisoning in 26 dogs. Open Veterinary Journal, 13 (5), 510-514. doi:10.5455/OVJ.2023.v13.i5.2 Chicago Style Dutil, Guillaume Fabien, and Philippe Berny. "A prospective study on clinical signs, management, outcomes, and delayed neurologic sequelae due to metaldehyde poisoning in 26 dogs." Open Veterinary Journal 13 (2023), 510-514. doi:10.5455/OVJ.2023.v13.i5.2 MLA (The Modern Language Association) Style Dutil, Guillaume Fabien, and Philippe Berny. "A prospective study on clinical signs, management, outcomes, and delayed neurologic sequelae due to metaldehyde poisoning in 26 dogs." Open Veterinary Journal 13.5 (2023), 510-514. Print. doi:10.5455/OVJ.2023.v13.i5.2 APA (American Psychological Association) Style Dutil, G. F. & Berny, . P. (2023) A prospective study on clinical signs, management, outcomes, and delayed neurologic sequelae due to metaldehyde poisoning in 26 dogs. Open Veterinary Journal, 13 (5), 510-514. doi:10.5455/OVJ.2023.v13.i5.2 |