| Research Article | ||
Open Vet J. 2024; 14(2): 683-691 Open Veterinary Journal, (2024), Vol. 14(2): 683-691 Original Research Suppressive effect of Yokukansan on glutamate released from canine keratinocytesYoichiro Kasuga1*, Ailing Hu2, Zenji Kawakami2, Masahiro Tabuchi2, Takuji Yamaguchi2, Hiroyuki Kobayashi2 and Shigaku Ikeda11Department of Dermatology and Allergology, Juntendo University Graduate School of Medicine, Tokyo, Japan 2Center for Advanced Kampo Medicine and Clinical Research, Juntendo University Graduate School of Medicine, Tokyo, Japan *Corresponding Author: Yoichiro Kasuga. Department of Dermatology and Allergology, Juntendo University Graduate School of Medicine, Tokyo, Japan. Email: y.kasuga.bl [at] juntendo.ac.jp Submitted: 03/11/2023 Accepted: 27/01/2024 Published: 29/02/2024 © 2024 Open Veterinary Journal
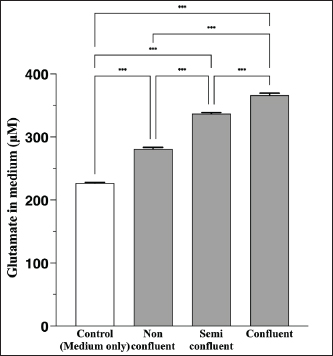
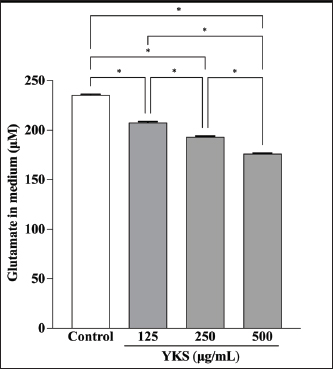
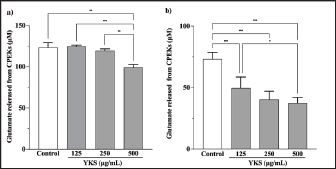
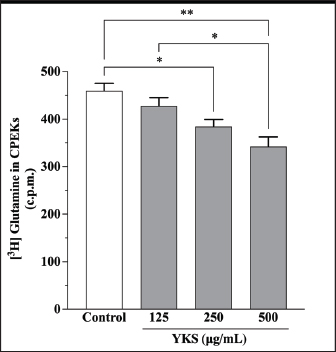
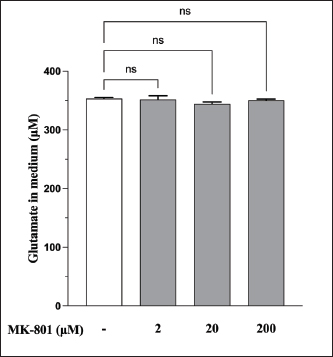
AbstractBackground: Canine atopic dermatitis (CAD) is caused by skin barrier dysfunction due to allergen exposure. Excessive glutamate release in the skin is associated with delayed skin barrier function recovery and epidermal thickening and lichenification. Treatment with Yokukansan (YKS), a traditional Japanese medicine, reduces dermatitis severity and scratching behavior in NC/Nga mice by decreasing epidermal glutamate levels. However, the association between canine keratinocytes and glutamate and the mechanism by which YKS inhibits glutamate release from keratinocytes remains unknown. Aim: We aimed to investigate glutamate release from canine progenitor epidermal keratinocytes (CPEKs) and the inhibitory effect of YKS on this release. We also explored the underlying mechanism of YKS to enable its application in CAD treatment. Methods: Glutamate produced from CPEKs in the medium at 24 hours was measured. The measurement conditions varied in terms of cell density and YKS concentration. CPEKs were treated with a glutamate receptor antagonist (MK-801), a glutamate transporter antagonist (THA), and a glutamate dehydrogenase inhibitor (epigallocatechin gallate; EGCG), and the inhibitory effect of YKS, YKS + THA, MK-801, and EGCG on this release was determined. MK-801 and glutamate dehydrogenase inhibitor were tested alone, and THA was tested in combination with YKS. Finally, glutamine incorporated into CPEKs at 24 hours was measured using radioisotope labeling. Results: CPEKs released glutamate in a cell density-dependent manner, inhibited by YKS in a concentration-dependent manner. Moreover, YKS reduced the intracellular uptake of radioisotope-labeled glutamine in a concentration-dependent manner. No involvement of glutamate receptor antagonism or activation of glutamate transporters was found, as suggested by previous studies. In addition, EGCG could inhibit glutamate release from CPEKs. Conclusion: Our findings indicated that glutamate release from CPEKs could be effectively inhibited by YKS, suggesting the utility of YKS in maintaining skin barrier function during CAD. In addition, CPEKs are appropriate for analyzing the mechanism of YKS. However, we found that the mechanism of action of YKS differs from that reported in previous studies, suggesting that it may have had a similar effect to EGCG in this study. Further research is warranted to understand the exact mechanism and clinical efficacy in treating CAD. Keywords: Canine atopic dermatitis, Canine progenitor epidermal keratinocyte, Glutamate, Glutamine, Yokukansan. IntroductionCanine atopic dermatitis (CAD) is an inflammatory and pruritic allergic disease of genetic predisposition with a reported prevalence of 10%–15% (Hillier and Griffin, 2001; Olivry et al., 2015; Hensel et al., 2015). Although its etiology remains largely unknown, studies suggest that skin barrier function and immune system abnormalities contribute to its development. Moreover, the pathogenesis of CAD is similar to that of human atopic dermatitis (Marsella and De Benedetto, 2017), which has also been shown to involve skin barrier dysfunction (Marenholz et al., 2006; Han et al., 2017). Therefore, maintaining skin barrier function is integral for treating CAD (Yoon et al., 2011; Marsella and De Benedetto, 2017). Glutamate is known to maintain skin barrier function, along with filaggrin and ceramide (Fuziwara et al., 2003; Davidson et al., 1997). However, research suggests that excessive glutamate in the skin impedes the recovery of skin barrier function, leading to epidermal thickening and lichenification in mouse models (Fuziwara et al., 2003). Kampo, a traditional herbal medicine used in Japan and China, has been shown to be effective against atopic dermatitis. However, its exact mechanism of action remains unknown (Saeki et al., 2022; Sheehan and Atherton, 1992; Sheehan et al., 1992). Studies have demonstrated that Yokukansan (YKS), a type of Kampo, could reduce dermatitis severity and scratching behavior in NC/Nga mice, a human atopic dermatitis model (Jiang et al., 2009). Japan’s Ministry of Health, Labour, and Welfare has approved the use of YKS, which comprises seven crude drugs (Wakabayashi et al., 2014), for treating neurosis, insomnia, and irritability in children. Notably, a similar study by Wakabayashi et al. (2014) showed that YKS decreased glutamate concentrations in the skin of NC/Nga mice. This study also explored the underlying mechanism using normal human epidermal keratinocytes (NHEKs). It showed that NHEKs release glutamate into the medium in a cell density-dependent manner and that this release was suppressed by YKS administration. Furthermore, upon exploring the mRNA expression in NHEKs treated with YKS, the study found that the expression of N-methyl-D-aspartate (NMDA) receptor 2D (NMDAR-2D) and glutamate aspartate transporter (GLAST), which are types of glutamate receptor and transporter, respectively, decreased (Wakabayashi et al., 2014). The study shows that YKS regulates extracellular glutamate levels by inhibiting NMDAR and stimulating glutamate transportation in NHEKs. Based on the successful experiments on NC/Nga mice, YKS can be potentially used to treat CAD. Nevertheless, the correlation between canine keratinocytes and glutamate has not yet been examined. Furthermore, previous studies that used NHEKs did not fully elucidate the inhibitory mechanism of YKS on glutamate release from keratinocytes. In the present study, we determined whether YKS can maintain skin barrier function by suppressing excess glutamate in canine keratinocytes and investigated its mechanism of action. Materials and MethodsCell cultureWe used 4th–5th-generation canine progenitor epidermal keratinocytes (CPEKs) derived from adult canine beagle epidermis and obtained from CELLnTEC Advanced Cell Systems (Bern, Switzerland). CPEKs were cultured in a specific medium, CnT-09 (CELLnTEC Advanced Cell System), prepared by supplementing CnT-BM2 with fetal bovine serum (supplement A) and glutamine (supplement B). CnT-09 was used to culture CPEKs, whereas CnT-BM2 served as the basal medium for mixing YKS and other reagents. CPEKs were cultured in 75-cm2 flasks (Corning, Corning, NY) and seeded at 4 × 103 cells/cm2 in 96- or 48-well plates after appropriate cell growth. YKSYKS contains seven crude drugs: Atractylodes lancea rhizome (4-g Atractylodes lancea De Candolle rhizome), Poria sclerotium (4-g Poria cocos Wolf sclerotium), Cnidium rhizome (3-g Cnidium officinale Makino rhizome), Japanese angelica root (3-g Angelica acutiloba Kitagawa root), Bupleurum root (2-g Bupleurum falcatum Linne root), Glycyrrhiza root (1.5-g root and stolon of Glycyrrhiza uralensis Fisher), and Uncaria thorn (3-g Uncaria rhynchophylla Miquel thorn). Dry powder extracts of YKS were provided by Tsumura & Co. (Tokyo, Japan). The YKS concentration was determined to be 125, 250, and 500 µg/ml based on previous research and reports (Wakabayashi et al., 2014; Kawakami et al., 2009; Kawakami et al., 2011b). Measurement of glutamate released from CPEKsWe investigated the correlation between the cell density of CPEKs and the increase in glutamate levels in the culture medium. CPEKs were cultured in 48-well plates (Corning) until reaching nonconfluency, subconfluency, and confluency (approximately 30%, 60%–80%, and 90%–100% of the area covered by cells, respectively). Once CPEKs reached a specific cell density, the cells were washed twice with phosphate-buffered saline (PBS) (Thermo Fisher Scientific Inc., Waltham, MA) and replaced with basal medium. They were placed in a CO2 incubator at 37°C. After a 24-hour incubation, glutamate in the medium was measured using a glutamate assay kit (Fluorometric, ab138883; Abcam, Cambridge, United Kingdom) based on the principle of a coupled enzyme system. In this system, L-glutamic acid reacts with NADP+ to produce NADPH, which is recognized by a specific NADPH sensor, before being converted to NADP+. The reaction produces a red fluorescence product, which is detected using a microplate reader (FLEX station3, Molecular Devices, San Jose, CA) at an excitation/emission wavelength of 540/590 nm. Effect of YKS on the glutamate assaySome of the crude drugs in Kampo can inhibit enzyme activity (Zhao et al., 2020). Therefore, to ensure that YKS does not interfere with glutamate assay measurements, we measured the glutamate in the basal medium at different YKS concentrations (0, 125, 250, and 500 µg/ml) before investigating its effect on CPEKs. Effects of YKS on glutamate release from CPEKsUsing CPEKs, we determined whether the decrease in glutamate levels in the medium depended on the YKS concentration. Once CPEKs reached a subconfluency, the cells were washed twice with PBS, replaced with basal medium supplemented with different YKS concentrations (0, 125, 250, and 500 µg/ml), and incubated for 24 hours in a CO2 incubator at 37°C. After incubation, the glutamate levels in the medium were measured via glutamate assay. The effect of YKS on the glutamate assay was eliminated by establishing test and control groups with and without cells, respectively. The amount of glutamate released by the cells over 24 hours was determined by subtracting the glutamate value of the control group at the same YKS concentration from that of the test group. Effect of YKS on the intracellular uptake of radioisotope-labeled glutamineWe investigated the effect of YKS on cellular glutamine uptake using radioisotope (RI)–labeled glutamine. CPEKs were cultured in a 96-well plate (Cytostar-T scintillating microplate, RPNQ0162; PerkinElmer, Waltham, MA) until subconfluency was reached. Once CPEKs reached a certain density, the cells were washed twice with PBS. The basal medium was replaced with medium supplemented with 1-mM glutamine, L-[3,4-3H(N)]-glutamine (PerkinElmer), and various concentrations of YKS (0, 125, 250, and 500 µg/ml). The amount of the added L-[3,4-3H(N)]-glutamine was equivalent to 0.012 MBq per well, and incorporation of RI was evaluated after 24 hours of incubation in a CO2 incubator at 37°C. Effect of NMDA receptor antagonist on glutamate releaseWe investigated whether MK-801 alone could inhibit the release of glutamate from CPEKs. CPEKs were cultured in a 48-well plate until reaching subconfluency. Once the CPEKs reached a certain density, the cells were washed twice with PBS, replaced with basal medium containing different concentrations of MK-801 (Abcam) (2, 20, and 200 µM) or basal medium alone, and incubated for 24 hours at 37°C in a CO2 incubator. After incubation, the glutamate in the medium was measured using the glutamate assay kit. Effects of glutamate uptake inhibitors (THA) on glutamate release by YKSWe investigated whether THA could be used simultaneously with YKS to inhibit the release of glutamate from CPEKs. CPEKs were cultured in 48-well plates until reaching subconfluency. Once CPEKs reached a certain density, the cells were washed twice with PBS, replaced with basal medium containing 500-µg/ml YKS and various concentrations of THA (Sigma-Aldrich, St. Louis, MO) (0, 10, 100, and 1000 µM), and incubated at 37°C in a CO2 incubator. After 24 hours of incubation, the glutamate in the medium was measured using a glutamate assay kit. Effect of epigallocatechin gallate (EGCG) on glutamate release by CPEKsEGCG is a catechin found in green tea that has also been reported to inhibit glutamate dehydrogenase (GDH). To confirm the effect of YKS, we determined whether EGCG with GDH inhibitory effect exhibits a similar effect (Li et al., 2006; Moon et al., 2007; Li et al., 2011; Li et al., 2012) by replicating the experiments described in the subsection titled “Effect of YKS on glutamate release from CPEK.” CPEKs were cultured in a 48-well plate until reaching subconfluency. Once the CPEKs reached a certain density, the cells were washed twice with PBS, replaced with basal medium supplemented with different concentrations of EGCG (0, 10, 50, and 100 µM), and then incubated in a CO2 incubator at 37°C. After 24 hours of incubation, glutamate in the medium was measured using the glutamate assay kit and determined as the amount released from the cells over 24 hours. Statistical analysisStatistical analysis was conducted via analysis of variance (ANOVA), followed by the appropriate post hoc test or Student’s t-test. Statistical analyses were conducted using GraphPad Prism for Mac (Prism 9, GraphPad Software, San Diego, CA). A p-value < 0.05 was considered statistically significant. The results were expressed as mean ± SEM. Ethical approvalNot required for this type of study. ResultsAssociation between the cell density of CPEKs and glutamate in the mediumWe observed that the glutamate in the medium increased with the cell density of CPEKs (Fig. 1). Effect of YKS on glutamate assay measurementsThe glutamate in the medium decreased in a YKS concentration-dependent manner, whereas that in the basal medium remained the same (Fig. 2). Effect of YKS on glutamate released from CPEKsYKS decreased the glutamate released from the cells in a concentration-dependent manner in CPEKs, similar to that observed in NHEKs (Wakabayashi et al., 2014). At confluence (Fig. 3a), we observed a significant decrease in glutamate levels at 500 µg/ml YKS compared with the control. Contrarily, in the subconfluent cells (Fig. 3b), we observed significant differences across all YKS concentrations compared with the control. Effect of YKS on glutamine uptake into CPEKsYKS suppressed the uptake of RI-labeled glutamine into CPEKs in a concentration-dependent manner (Fig. 4). Investigating the mechanism underlying glutamate inhibition by YKS using an NMDAR antagonistWakabayashi et al. showed that the mRNA expression of NMDAR-D2, a glutamate receptor, decreased in NHEKs in a YKS concentration-dependent manner. Based on this finding, they hypothesized that YKS might act as an NMDAR antagonist in suppressing glutamate in the culture medium (Wakabayashi et al., 2014). A previous study showed that YKS might act as an NMDA receptor antagonist, as it could inhibit glutamate cytotoxicity in neurons similar to MK-801, an NMDA receptor antagonist (Kanno et al., 2014; Kawakami et al., 2011a). Thus, this study hypothesized that if NMDA receptor antagonists can indeed reduce glutamate levels in the medium, administration of MK-801 alone instead of YKS might yield identical results. We observed that the glutamate levels in the medium were not reduced by the NMDAR antagonist MK-801 alone (Fig. S1), suggesting that NMDAR antagonism is not the underlying mechanism behind the inhibition of glutamate by YKS.
Fig. 1. Correlation between the cell density of CPEKs and glutamate release. Mean + SEM (n=6), one-way ANOVA + Tukey’s test, *p < 0.001.
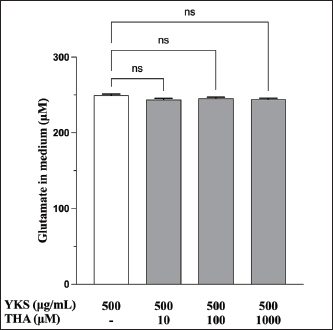
Fig. 2. YKS inhibits measurements by the glutamate assay kit in a concentration-dependent manner. Mean + SEM (n=6), one-way ANOVA + Tukey’s test, *p < 0.001. Role of glutamate transporter activation in glutamate reduction by YKS in the mediumWakabayashi et al. showed that YKS enhanced the mRNA expression of the GLAST glutamate transporter in a concentration-dependent manner. They suggested that YKS might activate glutamate transporters, triggering glutamate uptake by the cells, which subsequently decreases the glutamate level in the medium (Wakabayashi et al., 2014). Similarly, another study showed that the addition of DL-three-β-hydroxyaspartic acid (THA), a glutamate transporter inhibitor, to YKS results in a decrease in glutamate uptake by the cells (Kawakami et al., 2009; Kawakami et al., 2019). Hence, we hypothesized that if YKS decreases glutamate in the medium, enhancing cellular uptake by activating glutamate transporters, administration of THA, a glutamate uptake inhibitor, would not lead to a decrease in the glutamate levels in the medium. Therefore, in this experiment, we focused on the glutamate released from CPEKs by administering YKS and THA simultaneously.
Fig. 3. Effect of YKS on glutamate released from CPEKs under (a) confluent and (b) subconfluent conditions. Glutamate was calculated as the amount released from the cells over 24 hours. Mean + SEM (n=6), one-way ANOVA + Tukey’s test, *p < 0.05, **p < 0.01, ***p < 0.001.
Fig. 4. Glutamine uptake by CPEKs decreased in a YKS concentration-dependent manner. No significant difference was observed at 125 μg/ml as compared with control; however, there was a significant decrease at 250 and 500 μg/ml. Mean + SEM (n=6), one-way ANOVA + Tukey’s test, *p < 0.05, **p < 0.001.
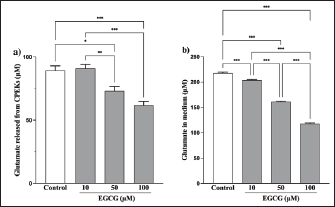
Fig. 5. EGCG affects glutamate release. Glutamate levels in the (a) cells and (b) medium were measured via a glutamate assay. No significant difference was observed at 125 μg/ml compared with controls, but there were significant decreases at 250 and 500 μg/ml (a). Mean + SEM (n=6), one-way ANOVA + Tukey’s test, *p < 0.05, **p < 0.01, ***p < 0.001. Although the glutamate in the medium was reduced by 500 µg/ml of YKS, this effect was not inhibited by the administration of THA, a glutamate transporter inhibitor (Fig. S2). This indicates that the glutamate-reducing effect of YKS is not due to the activation of glutamate transporters. Effect of EGCG on glutamate release from CPEKsEGCG, a GDH inhibitor, decreased glutamate release from CPEKs (Fig. 5a). Interestingly, glutamate in the control group (without cells) also decreased in an EGCG concentration-dependent manner (Fig. 5b). Similar to YKS (Fig. 2), EGCG was shown to affect the measurement of glutamate in the glutamate assay. DiscussionMaintaining skin barrier function is important in canines with CAD, similar to humans (Marsella and De Benedetto, 2017; Santoro et al., 2015; Yoon et al., 2011). Studies in mice have demonstrated that elevated levels of skin glutamate delayed the recovery of skin barrier function and caused epidermal thickening and lichenification (Fuziwara et al., 2003). Experiments on NC/Nga mice have shown a positive correlation between dermatitis score, scratching behavior, and glutamate in the skin (Wakabayashi et al., 2014). Although YKS and Kampo are occasionally used to treat atopic dermatitis in humans, their mechanism of action remains poorly understood (Saeki et al., 2022; Sheehan and Atherton, 1992; Sheehan et al., 1992). Wakabayashi et al. (2014) demonstrated that YKS decreases excess glutamate in the skin. However, glutamate release in canine keratinocytes has not yet been shown. Thus, our objective was to demonstrate glutamate release by canine keratinocytes and its inhibition by YKS. Our results indicated that CPEKs can release glutamate into the extracellular space. Consequently, this excessive glutamate may be implicated in CAD, indicating that it can be a potential therapeutic target. We also demonstrated concentration-dependent inhibition of glutamate released from CPEKs by YKS. Furthermore, we observed that YKS suppressed glutamate release more effectively under subconfluent conditions than under confluent ones. This result indicates that YKS can effectively inhibit glutamate release in cell proliferation. Although in vivo studies on dog and mouse skin to elucidate the mechanism of action of YKS are informative, the use of specific cell lines is more beneficial from the perspective of animal welfare. To this end, our findings showing the inhibition of glutamate release in CPEKs by YKS suggest that CPEKs are ideal for studying the mechanism of YKS. One of the challenges in this study was the inconsistencies in the glutamate assay measurements. The addition of YKS resulted in lower readings than the actual glutamate levels. This resulted in a difficult direct comparison of results with and without YKS and with different YKS concentrations. To address this problem, we established test and control groups with and without cells, respectively, and treated them with different YKS concentrations. The amount of glutamate produced by the cells was then calculated by subtracting the control group from the test group at the same YKS concentration to eliminate the effect of YKS. In this study, we examined the impact of YKS on the inhibition of glutamate release from CPEKs. A previous study used NHEKs to elucidate the mechanism underlying the inhibitory effect of YKS on glutamate release via RT-PCR analysis. This study suggested that YKS regulates extracellular glutamate by suppressing NMDA receptors and activating glutamate transport in NHEKs (Wakabayashi et al., 2014). Thus, we prioritized investigating these two mechanisms. Experiments using MK-801 showed that NMDAR antagonism might not be involved in the inhibition of glutamate by YKS. Similarly, experiments using THA showed that glutamate transporter activation might not be implicated in the reduction of glutamate levels. Hence, a novel hypothesis should be considered regarding the inhibitory effect of YKS on glutamate released from cells. We investigated the involvement of GDH in the inhibition of glutamate release from CPEKs by YKS for two reasons. First, some crude drugs in Kampo have been reported to inhibit enzyme activity (Zhao et al., 2020). Second, GDH is involved in cell metabolism. It was also used in the glutamate assay in this study, which measures NADPH produced after GDH reacts with glutamate along with alpha-ketoglutarate (a-KG) (Han et al., 2019). If YKS inhibits GDH, the same effect can be expected when EGCG, a GDH inhibitor, is used (Moon et al., 2007; Li et al., 2006; Li et al., 2011; Li et al., 2012). We observed that glutamate released from the cells treated with EGCG was suppressed in a concentration-dependent manner. Interestingly, this decrease was also observed in the control group (without cells), which was also shown to affect glutamate assay measurements. This implies that the mechanisms of action of YKG and EGCG are similar. The findings support the hypothesis that YKS can inhibit GDH. GDH inhibitors decrease cellular activity through glutaminolysis (Jin et al., 2015), a mitochondrial pathway that involves the initial deamination of glutamine by glutaminase, which yields glutamate and ammonia. Glutamate is converted into a-KG, a TCA cycle intermediate, producing ATP and anabolic carbons required for synthesizing amino acids, nucleotides, and lipids (DeBerardinis et al., 2007; Wise and Thompson, 2010). The conversion of glutamate into a-KG is catalyzed by glutamate dehydrogenase 1 (GDH1, also known as GLUD1, GLUD, GDH) or other transaminases, such as glutamate pyruvate transaminase 2 (GPT2, also known as alanine aminotransferase) and glutamate oxaloacetate transaminase 2 (GOT2, also known as aspartate aminotransferase). These enzymes convert alpha-keto acids to the corresponding amino acids in the mitochondria (Quagliariello et al., 1965; Kovacevic, 1971). Thus, the reduction in glutamate release from cells by YKS might be caused by reduced cellular activity, as shown above. This is consistent with the finding that YKS suppressed glutamate release from cells under the subconfluent condition, in which cells proliferate and consume more energy, compared with that under the confluent condition, in which they consume less energy. Studies using RI-labeled glutamine have demonstrated that GDH inhibitors can decrease cellular glutamine uptake (Jin et al., 2015). We determined whether YKS reduces cellular glutamine uptake by inhibiting GDH using tritium-labeled glutamine. We demonstrated that YKS decreased cellular glutamine uptake in a concentration-dependent manner while inhibiting glutamate release from the cells. To the best of our knowledge, this is the first study to investigate the relationship between canine keratinocytes and glutamate. We observed similarities between glutamate release by canine and human keratinocytes. Our findings indicated that excessive glutamate release in the skin during CAD can be a potential therapeutic target. Although reports have demonstrated the efficacy of Kampo in treating atopic dermatitis (Zhao et al., 2020), the detailed mechanism is poorly understood (Jiang et al., 2009; Wakabayashi et al., 2014). In this study, we explored the mechanism by which YKS inhibits glutamate release from cells. However, unlike previous studies, we found that YKS acts neither as an NMDA-R antagonist nor as an activator of glutamate transporters, indicating that other mechanisms might be involved. Furthermore, we observed similarities between the inhibitory activity of EGCG and YKS on glutamate release from cells; however, this needs to be further validated as EGCG has been reported to exert various effects, such as antioxidant, antitumor, and anti-inflammatory effects in addition to its GDH inhibitory effects (Higdon and Frei 2003; Hsu et al., 2007; Yang et al., 2009). Although Kampo, composed of several crude drugs, is effective when acting as a whole, its action is very complex. Therefore, elucidating its mechanism of action is difficult. Our results suggest that YKS maintains skin barrier function by inhibiting glutamate released from keratinocytes. Although the underlying mechanism was not similar to that reported in previous studies, we were unable to elucidate the exact mechanism. We believe that the similarity in the results obtained with EGCG and the reduced cellular glutamine uptake might provide clues for future mechanistic elucidation. In the future, we plan to conduct further mechanistic analysis of YKS and clinical studies to determine whether it is effective in alleviating dermatitis and pruritus severity in CAD. AcknowledgmentsThe authors thank the Laboratory of Molecular and Biochemical Research, Laboratory of Proteomics and Biomolecular Science, and Laboratory of Radioisotope Research, Juntendo University Graduate School of Medicine, for technical assistance. Conflict of interestThe authors declare that there is no conflict of interest. Author contributionsY.K. and S.I. conceptualized the study; I.K. and T.Y. contributed to data analysis and interpretation; Z.K., M.T., and H.K. contributed to the manuscript drafting. All authors critically reviewed and revised the manuscript draft and approved the final version for submission. FundingThis research received no specific grant. Data availabilityAll data supporting the findings of this study are available within the manuscript. ReferencesDavidson, E.M., Coggeshall, R.E. and Carlton, S.M. 1997. Peripheral NMDA and non-NMDA glutamate receptors contribute to nociceptive behaviors in the rat formalin test. Neuro Report. 8, 941–946. De Berardinis, R.J., Mancuso, A., Daikhin, E., Nissim, I., Yudkoff, M., Wehrli, S. and Thompson, C.B. 2007. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA. 104, 19345–19350. Fuziwara, S., Inoue, K. and Denda, M. 2003. NMDA-type glutamate receptor is associated with cutaneous barrier homeostasis. J. Invest. Dermatol. 120, 1023–1029. Han, H., Roan, F. and Ziegler, S.F. 2017. The atopic march: current insights into skin barrier dysfunction and epithelial cell-derived cytokines. Immunol. Rev. 278, 116–130. Han, T.L., Cannon, R.D., Gallo, S.M. and Villas-Bôas, S.G. 2019. A metabolomic study of the effect of Candida albicans glutamate dehydrogenase deletion on growth and morphogenesis. NPJ Biofilms Microbiomes 5, 13. Hensel, P., Santoro, D., Favrot, C., Hill, P. and Griffin, C. 2015. Canine atopic dermatitis: detailed guidelines for diagnosis and allergen identification. BMC Vet. Res. 11, 196. Higdon, J.V. and Frei, B. 2003. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit. Rev. Food Sci. Nutr. 43, 89–143. Hillier, A. and Griffin, C.E. 2001. The ACVD task force on canine atopic dermatitis (I): incidence and prevalence. Vet. Immunol. Immunopathol. 81, 147–151. Hsu, S.D., Dickinson, D.P., Qin, H., Borke, J., Ogbureke, K.U., Winger, J.N., Camba, A.M., Bollag, W.B., Stöppler, H.J., Sharawy, M.M. and Schuster, G.S. 2007. Green tea polyphenols reduce autoimmune symptoms in a murine model for human Sjogren’s syndrome and protect human salivary acinar cells from TNF-alpha-induced cytotoxicity. Autoimmunity 40, 138–147. Jiang, J., Yamaguchi, T., Funakushi, N., Kuhara, T., Fan, P.-S., Ueki, R., Suto, H., Kase, Y., Ikeda, S. and Ogawa, H. 2009. Oral administration of yokukansan inhibits the development of atopic dermatitis-like lesions in isolated NC/Nga mice. J. Dermatol. Sci. 56, 37–42. Jin, L., Li, D., Alesi, G.N., Fan, J., Kang, H.B., Lu, Z., Boggon, T.J., Jin, P., Yi, H., Wright, E.R., Duong, D., Seyfried, N.T., Egnatchik, R., DeBerardinis, R.J., Magliocca, K.R., He, C., Arellano, M.L., Khoury, H.J., Shin, D.M., Khuri, F.R. and Kang, S. 2015. Glutamate dehydrogenase 1 signals through antioxidant glutathione peroxidase 1 to regulate redox homeostasis and tumor growth. Cancer Cell. 27, 257–270. Kanno, H., Kawakami, Z., Mizoguchi, K., Ikarashi, Y. and Kase, Y. 2014. Yokukansan, a kampo medicine, protects PC12 cells from glutamate-induced death by augmenting gene expression of cystine/glutamate antiporter system Xc-. PLOS One. 9, e116275. Kawakami, Z., Ikarashi, Y. and Kase, Y. 2011a. Isoliquiritigenin is a novel NMDA receptor antagonist in kampo medicine yokukansan. Cell. Mol. Neurobiol. 31, 1203–1212. Kawakami, Z., Kanno, H., Ikarashi, Y. and Kase, Y. 2011b. Yokukansan, a kampo medicine, protects against glutamate cytotoxicity due to oxidative stress in PC12 cells. J. Ethnopharmacol. 134, 74–81. Kawakami, Z., Kanno, H., Ueki, T., Terawaki, K., Tabuchi, M., Ikarashi, Y. and Kase, Y. 2009. Neuroprotective effects of yokukansan, a traditional Japanese medicine, on glutamate-mediated excitotoxicity in cultured cells. Neuroscience 159, 1397–1407. Kawakami, Z., Omiya, Y. and Mizoguchi, K. 2019. Comparison of the effects of yokukansan and Yokukansankachimpihange on glutamate uptake by cultured astrocytes and glutamate-induced excitotoxicity in cultured PC12 cells. Evid. Based Complement. Alternat. Med. 2019, 9139536. Kovacevic, Z. 1971. The pathway of glutamine and glutamate oxidation in isolated mitochondria from mammalian cells. Biochem. J. 125, 757–763. Li, C., Allen, A., Kwagh, J., Doliba, N.M., Qin, W., Najafi, H., Collins, H.W., Matschinsky, F.M., Stanley, C.A. and Smith, T.J. 2006. Green tea polyphenols modulate insulin secretion by inhibiting glutamate dehydrogenase. J. Biol. Chem. 281, 10214–10221. Li, C., Li, M., Chen, P., Narayan, S., Matschinsky, F.M., Bennett, M.J., Stanley, C.A. and Smith, T.J. 2011. Green tea polyphenols control dysregulated glutamate dehydrogenase in transgenic mice by hijacking the ADP activation site. J. Biol. Chem. 286, 34164–34174. Li, M., Li, C., Allen, A., Stanley, C.A. and Smith, T.J. 2012. The structure and allosteric regulation of mammalian glutamate dehydrogenase. Arch. Biochem. Biophys. 519, 69–80. Marenholz, I., Nickel, R., Rüschendorf, F., Schulz, F., Esparza-Gordillo, J., Kerscher, T., Grüber, C., Lau, S., Worm, M., Keil, T., Kurek, M., Zaluga, E., Wahn, U. and Lee, Y.A. 2006. Filaggrin loss-of-function mutations predispose to phenotypes involved in the atopic march. J. Allergy Clin. Immunol. 118, 866–871. Marsella, R. and De Benedetto, A. 2017. Atopic dermatitis in animals and people: an update and comparative review. Vet. Sci. 4, 37. Moon, H.S., Lee, H.G., Choi, Y.J., Kim, T.G. and Cho, C.S. 2007. Proposed mechanisms of (-)-epigallocatechin-3-gallate for anti-obesity. Chem. Biol. Interact. 167, 85–98. Olivry, T., Deboer, D.J., Favrot, C., Jackson, H.A., Mueller, R.S., Nuttall, T., Prélaud, P. and International Committee on Allergic Diseases of Animals. 2015. Treatment of canine atopic dermatitis: 2015 updated guidelines from the International Committee on Allergic Diseases of Animals (ICADA). BMC Vet. Res. 11, 1–15. Quagliariello, E., Papa, S., Saccone, C., Palmieri, F. and Francavilla, A. 1965. The oxidation of glutamate by rat-liver mitochondria. Biochem. J. 95, 742–748. Saeki, H., Ohya, Y., Furuta, J., Arakawa, H., Ichiyama, S., Katsunuma, T., Katoh, N., Tanaka, A., Tsunemi, Y., Nakahara, T., Nagao, M., Narita, M., Hide, M., Fujisawa, T., Futamura, M., Masuda, K., Matsubara, T., Murota, H. and Yamamoto-Hanada, K. 2022. English version of clinical practice guidelines for the management of atopic dermatitis 2021. J. Dermatol. 49, e315–e375. Santoro, D., Marsella, R., Pucheu-Haston, C.M., Eisenschenk, M.N., Nuttall, T. and Bizikova, P. 2015. Review: pathogenesis of canine atopic dermatitis: skin barrier and host-micro-organism interaction. Vet. Dermatol. 26, 84-e25. Sheehan, M.P. and Atherton, D.J. 1992. A controlled trial of traditional Chinese medicinal plants in widespread non-exudative atopic eczema. Br. J. Dermatol. 126, 179–184. Sheehan, M.P., Rustin, M.H., Atherton, D.J., Buckley, C., Harris, D.W., Brostoff, J., Ostlere, L. and Dawson, A. 1992. Efficacy of traditional Chinese herbal therapy in adult atopic dermatitis. Lancet 340, 13–17. Wakabayashi, M., Hasegawa, T., Yamaguchi, T., Funakushi, N., Suto, H., Ueki, R., Kobayashi, H., Ogawa, H. and Ikeda, S. 2014. Yokukansan, a traditional Japanese medicine, adjusts glutamate signaling in cultured keratinocytes. BioMed Res. Int. 2014, 364092. Wise, D.R. and Thompson, C.B. 2010. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem. Sci. 35, 427–433. Yang, C.S., Wang, X., Lu, G. and Picinich, S.C. 2009. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat. Rev. Cancer 9, 429–439. Yoon, J.S., Nishifuji, K., Sasaki, A., Ide, K., Ishikawa, J., Yoshihara, T. and Iwasaki, T. 2011. Alteration of stratum corneum ceramide profiles in spontaneous canine model of atopic dermatitis. Exp. Dermatol. 20, 732–736. Zhao, H.H., Liu, Y.Q. and Chen, J. 2020. Screening acetylcholinesterase inhibitors from traditional Chinese medicines by paper-immobilized enzyme combined with capillary electrophoresis analysis. J. Pharm. Biomed. Anal. 190, 113547. Supplementary material
Fig. S1. Effect of MK-801 on YKS-induced reduction of glutamate in the medium. The effect of the NMDAR antagonist activity was investigated using only MK-801. No statistically significant differences were observed between no MK-801 and with MK-801 (2, 20, and 200 μM). Mean + SEM (n=6), one-way ANOVA + Dunnett’s test, NS: Not Significant.
Fig. S2. Effect of THA on the YKS-induced reduction of glutamate in the medium. YKS and THA were coadministered to examine the involvement of glutamate transporter. No statistically significant differences were observed between no THA and with THA at 10, 100, and 1,000 μM. Mean + SEM (n=6), one-way ANOVA + Dunnett’s test, NS: Not Significant. | ||
| How to Cite this Article |
| Pubmed Style Kasuga Y, Hu A, Kawakami Z, Tabuchi M, Yamaguchi T, HK, Ikeda S. Suppressive effect of Yokukansan on glutamate released from canine keratinocytes. Open Vet J. 2024; 14(2): 683-691. doi:10.5455/OVJ.2024.v14.i2.8 Web Style Kasuga Y, Hu A, Kawakami Z, Tabuchi M, Yamaguchi T, HK, Ikeda S. Suppressive effect of Yokukansan on glutamate released from canine keratinocytes. https://www.openveterinaryjournal.com/?mno=174198 [Access: April 27, 2024]. doi:10.5455/OVJ.2024.v14.i2.8 AMA (American Medical Association) Style Kasuga Y, Hu A, Kawakami Z, Tabuchi M, Yamaguchi T, HK, Ikeda S. Suppressive effect of Yokukansan on glutamate released from canine keratinocytes. Open Vet J. 2024; 14(2): 683-691. doi:10.5455/OVJ.2024.v14.i2.8 Vancouver/ICMJE Style Kasuga Y, Hu A, Kawakami Z, Tabuchi M, Yamaguchi T, HK, Ikeda S. Suppressive effect of Yokukansan on glutamate released from canine keratinocytes. Open Vet J. (2024), [cited April 27, 2024]; 14(2): 683-691. doi:10.5455/OVJ.2024.v14.i2.8 Harvard Style Kasuga, Y., Hu, . A., Kawakami, . Z., Tabuchi, . M., Yamaguchi, . T., , . H. K. & Ikeda, . S. (2024) Suppressive effect of Yokukansan on glutamate released from canine keratinocytes. Open Vet J, 14 (2), 683-691. doi:10.5455/OVJ.2024.v14.i2.8 Turabian Style Kasuga, Yoichiro, Ailing Hu, Zenji Kawakami, Masahiro Tabuchi, Takuji Yamaguchi, Hiroyuki Kobayashi, and Shigaku Ikeda. 2024. Suppressive effect of Yokukansan on glutamate released from canine keratinocytes. Open Veterinary Journal, 14 (2), 683-691. doi:10.5455/OVJ.2024.v14.i2.8 Chicago Style Kasuga, Yoichiro, Ailing Hu, Zenji Kawakami, Masahiro Tabuchi, Takuji Yamaguchi, Hiroyuki Kobayashi, and Shigaku Ikeda. "Suppressive effect of Yokukansan on glutamate released from canine keratinocytes." Open Veterinary Journal 14 (2024), 683-691. doi:10.5455/OVJ.2024.v14.i2.8 MLA (The Modern Language Association) Style Kasuga, Yoichiro, Ailing Hu, Zenji Kawakami, Masahiro Tabuchi, Takuji Yamaguchi, Hiroyuki Kobayashi, and Shigaku Ikeda. "Suppressive effect of Yokukansan on glutamate released from canine keratinocytes." Open Veterinary Journal 14.2 (2024), 683-691. Print. doi:10.5455/OVJ.2024.v14.i2.8 APA (American Psychological Association) Style Kasuga, Y., Hu, . A., Kawakami, . Z., Tabuchi, . M., Yamaguchi, . T., , . H. K. & Ikeda, . S. (2024) Suppressive effect of Yokukansan on glutamate released from canine keratinocytes. Open Veterinary Journal, 14 (2), 683-691. doi:10.5455/OVJ.2024.v14.i2.8 |