| Research Article | ||
Open Vet J. 2024; 14(2): 664-673 Open Veterinary Journal, (2024), Vol. 14(2): 664-673 Original Research Inflammatory responses to Opisthorchis viverrini infection in animal models: A comparison between susceptible and nonsusceptible hosts in different anatomical locationsSirikachorn Tangkawattana1,2*, Watcharapol Suyapoh4, Theerayut Thongrin5,6, Woro Danur Wendo5,7, Kanin Salao3, Sutas Suttiprapa2,3, Prasert Saichua2,3 and Prasarn Tangkawattana11Faculty of Veterinary Medicine, Khon Kaen University, Khon Kaen, Thailand 2WHO Collaborating Centre for Research and Control of Opisthorchiasis (Southeast Asian Liver Fluke Disease), Tropical Disease Research Center, Khon Kaen University, Khon Kaen, Thailand 3Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand 4Faculty of Veterinary Science, Prince of Songkla University, Songkhla, Thailand 5Graduate Program, Faculty of Veterinary Medicine, KhonKaen University, Khon Kaen, Thailand 6Faculty of Veterinary Medicine, Western University, Kanchanaburi, Thailand 7Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia *Corresponding Author: Sirikachorn Tangkawatana. Faculty of Veterinary Medicine, Khon Kaen University, Khon Kaen, Thailand. Email: sirikach [at] kku.ac.th Submitted: 21/10/2023 Accepted: 16/01/2024 Published: 29/02/2024 © 2024 Open Veterinary Journal
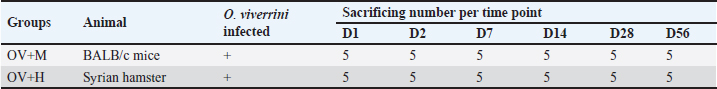
AbstractBackground: Inflammation caused by Opisthorchis viverrini infection increases the risk of cholangitis, cholecystitis, and leads to bile duct cancer (cholangiocarcinoma or CCA). However, only certain infected individuals are susceptible to CCA, suggesting the involvement of host factors in cancer development. In addition, there are reports indicating differences in the locations of CCA. Aim: This study aims to investigate cellular inflammatory responses in the common bile duct (CB), intrahepatic bile duct (IHB), and gallbladder (GB) in susceptible and non-susceptible hosts following O. viverrini infection. Methods: Thirty Syrian golden hamsters (a susceptible host) and 30 BALB/c mice (a non-susceptible host) infected with O. viverrini were studied at six time points (five animals per group). Histopathological evaluations were conducted on samples from the IHB, CB, and GB. Inflammatory cell infiltration was quantitatively assessed and compared between groups and time points. Statistical analysis was performed using one-way ANOVA, with a significance level of p < 0.05. Results: Inflammation was significantly more pronounced in the IHB compared to the other two biliary locations. In comparison between susceptible and non-susceptible hosts, the intensity of inflammation was higher in the OV+H group than in the OV+M group (p < 0.05). Conclusion: This study highlights the association between host response to inflammation, tissue location, and host susceptibility, with the IHB showing particular susceptibility to inflammation and pathological changes. These findings contribute to our understanding of the increased risk of CCA in susceptible hosts. Keywords: Opisthorchis viverrini, Susceptible host, Non-susceptible host, Locations, Intrahepatic bile duct. IntroductionOpisthorchis viverrini (O. viverrini), a foodborne trematode, remains a significant public health issue in the Greater Mekong Subregion, affecting approximately 12 million individuals (Sripa et al., 2021). Infection occurs through the consumption of raw or undercooked cyprinid fish containing the infective stage of the fluke, known as the metacercaria (Nair et al., 2011). Upon ingestion, the flukes migrate through the common bile duct (CB), primarily inhabiting the biliary tree, which includes both intra- and extrahepatic ducts as well as the gall bladder and pancreatic duct (PD) (Kaewkes and Sripa, 2004; Sithithaworn et al., 2014). Inflammation resulting from O. viverrini infection can lead to various biliary tract abnormalities, with the severity often associated with the specific location. Chronic inflammation of the gallbladder (GB) typically presents as cholecystitis, GB abscess, and cholelithiasis (Bhamarapravati et al., 1978; Harinasuta et al., 1984; Riganti et al., 1989), while lesions in the biliary tree can cause cholangitis, periductal fibrosis, and potentially progress to bile duct cancer (Sripa et al., 2018). Notably, the development of pathological lesions appears to be influenced by the location of the biliary tract. Chronic O. viverrini infection is believed to contribute to the development of bile duct cancer, specifically cholangiocarcinoma (Campbell et al., 2017), through a «perfect storm» of carcinogenic stimuli (Sripa and Pairojkul, 2008; Sripa et al., 2012). However, less than 5% of individuals with opisthorchiasis develop malignancy (Sripa and Pairojkul, 2008; Parkin et al., 2010; Ghouri et al., 2015), indicating that susceptible individuals with a specific phenotype are more likely to develop cancer in response to persistent inflammation caused by the infection (Sripa et al., 2012). The Syrian golden hamster has been identified as a susceptible host for liver fluke infection in the liver fluke model, while mice naturally reject and expel the worms from their bodies (Choi et al., 2003; Chung et al., 2004; Boonmars et al., 2009). Furthermore, the Syrian golden hamster is a preferred model for cancer research, particularly in the study of CCA (Bhamarapravati et al., 1978; Sudsarn et al., 2014; Hanpanich et al., 2017; Loeuillard et al., 2019). These findings suggest that the host’s susceptibility plays a crucial role in the development of CCA. Bile duct cancer manifests along the biliary system, and has been categorized into three types based on their anatomical site of origin: intrahepatic (iCCA), perihilar (pCCA), and distal CCA (dCCA) types (Sarcognato et al., 2021). However, not all biliary locations have an equal propensity for CCA development; certain areas, particularly the intrahepatic bile duct (IHB), are major sites for cancer establishment (Rizvi et al., 2018). A strong association has been established between O. viverrini infestation and the development of CCA (Cardinale et al., 2018). The inflammatory response is believed to be involved in cancer susceptibility, as individuals with a severe inflammatory response are considered more prone to developing CCA based on the concept of «helminth infection-induced malignancy» (Brindley and Loukas, 2017; Fried et al., 2011). Consequently, we formulate a hypothesis that the establishment phase of infection may be linked to the host’s inflammatory response. However, the association between inflammatory responses at usual locations of the biliary system, including IHB, CB, and GB, and host susceptibility has not yet been established. To investigate the pattern and response of cellular inflammation in three different locations among both susceptible and non-susceptible hosts, a study was designed using hamster and BALB/c mouse models. The objective of this study was to confirm the role of the inflammatory cell response in each location during the early phase of O. viverrini infection. Materials and MethodsFish collection, O. viverrini metacercariae isolation, and identificationTo isolate and select the infective stage (metacercariae) of O. viverrini, freshwater cyprinoid fish were procured from water reservoirs located within the endemic region of Northeast Thailand. The fish underwent homogenization using an electronic blender, along with a solution comprising 0.25% pepsin and 1.5% hydrochloric acid (HCl) sourced from Wako Pure Chemical Industries, Osaka, Japan. This concoction was subsequently subjected to an incubation process within a water bath at 37°C, lasting for 1 hour. Upon completion of digestion, the resultant solutions underwent filtration through sieves of varying mesh sizes (1,000, 300, 106, and 250 µm) to meticulously isolate the metacercariae. Following this, the metacercariae were precipitated within a 0.85% NaCl solution, following a previously documented procedure (Bhamarapravati et al., 1978), and were subsequently identified based on morphological characteristics observed under a stereomicroscope (Kaewkes and Sripa, 2004). Animal models and sample collectionSix-week-old male Syrian golden hamsters (Mesocricetus auratus) obtained from the Laboratory Animal Unit, Faculty of Medicine, Khon Kaen University, and 6-week-old male BALB/c mice from Nomura Siam International were used as animal models. A total of 30 animals were allocated to each group, with Group I (OV+M) consisting of BALB/c mice infected with O. viverrini and Group II (OV+H) consisting of Syrian golden hamsters infected with O. viverrini (Table 1). The animals were given 50 metacercariae by orogastric intubation. At specific time points Day 1, 2, 3, 7, 14, 28, and 56 (Table 1), five animals from each group were sacrificed using an overdose of isoflurane inhalation. Their livers including the IHB, CB, and gall bladder (GB) were dissected and collected for subsequent histopathological studies (Fig. 1). Quantitative study of inflammatory pattern on various O. viverrini-residing locationsTo quantitatively study, the inflammatory cell infiltration of different locations, namely the IHB, CB, and GB, the tissues from the biliary system were processed using routine histology techniques. The tissues were embedded in paraffin blocks, and 4 μm-thick sections were cut using a microtome and placed on coated glass slides. Subsequently, the tissue sections were deparaffinized and stained with routine hematoxylin and eosin. Inflammatory cell infiltration was identified following the method described by Dulaimi et al. (2018). Infiltrating leukocytes were then quantitatively evaluated by counting the cells from ten non-overlapping high-power fields under a light microscope (Suyapoh et al., 2021a). Table 1. The experimental groups consisted of Syrian golden hamsters and BALB/c mice that were infected with O. viverrini (Ov). At each designated time point, five mice and five hamsters from each group were euthanized for subsequent analysis.
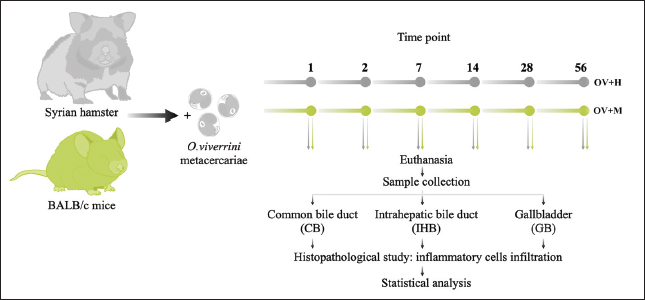
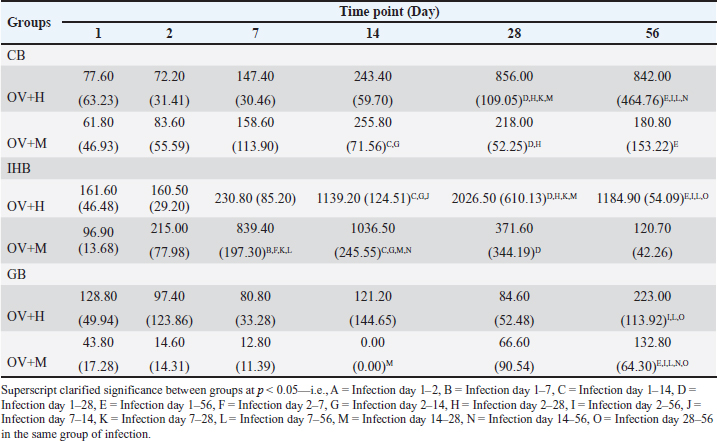
Fig. 1. In the location study, the experimental design encompassed the infection of two different animal species. Subsequently, these animals underwent sacrifice, and sample collection was conducted at specific time intervals: 1, 2, 7, 14, 28, and 56 days. The experimental groups were designated as follows: OV+M (BALB/c mice with O. viverrini infection), and OV+H (Syrian golden hamsters subjected to O. viverrini infection). Statistical analysisStatistical analysis in this study was conducted using SPSS version 23.0. To compare the mean values between different groups, a One-way ANOVA with Fisher’s Least significant difference post hoc test was employed. A p-value of less than 0.05 was considered statistically significant. Ethical approvalThe study protocol was subjected to review and approved by the Animal Ethics Committee of Khon Kaen University approved the study protocol (IACUC-KKU 78/2561). ResultsInfiltration pattern of inflammatory cells across distinct biliary in susceptible and non-susceptible hostsTo investigate the pattern of inflammatory responses in the biliary tract system of hamsters and mice infected with O. viverrini, we assessed the infiltration of inflammatory cells in three relevant tissues: the IHB, CB, and GB. Quantitative cell counts were performed through histopathology at six different time points after infection (Table 2). Overall, the pattern of inflammatory cell infiltration differed between the early and chronic phases but remained similar across all biliary and GB tissues. Initially, a small number of neutrophils, eosinophils, and mononuclear cells were observed in the lamina propria and submucosa of IHB, CB, and GB. These cells were particularly detected at the portal area where O. viverrini resided. As the infection progressed beyond the acute phase, a greater infiltration of mononuclear cells was observed, with some of these cells penetrating the intra-epithelium of the mucosa, particularly in the IHB and GB tissues. In addition, a high number of eosinophils were detected during this period, primarily located beyond the mucosal area infected by the worm. The extent of inflammatory cell infiltration varied but was most extensive in IHB, followed by CB and GB, respectively (Fig. 2). In the hamster model, the pattern of inflammatory cell infiltration in the CB tissue exhibited low levels during the acute phase, specifically at 1–2 days post-infection (d.p.i.). Subsequently, the inflammation increased significantly during 7–14 d.p.i. and reached its maximum level at 28 d.p.i. The inflammation remained stable thereafter until 56 d.p.i. On the other hand, the IHB tissue showed a similar trend, with inflammation increasing during the early phase but declining at 56 d.p.i. On the contrary, the levels of leukocyte infiltration in the GB tissue remained unchanged throughout the infection period. In the mice model, the inflammation levels at CB and IHB slightly increased during the 1–7 d.p.i., reached a significant peak at the 14 d.p.i., and then declined at 28–56 d.p.i. Meanwhile, the infiltration pattern at the GB tissue showed slight detection at the early period of infection, dropped during 7–14 d.p.i, and then rose toward the end of the experiment. For more detailed information on the quantitative cell count of overall inflammation (Table 2). Table 2. Quantitative inflammatory cell infiltration in the CB, IHB, and GB in hamsters and mice at different time periods is presented below. O. viverrini infected hamster (OV+H), O. viverrini infected BALB/c mice (OV+M). The data is reported as mean with standard deviation (SD).
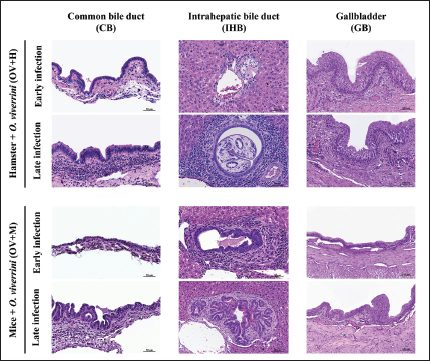
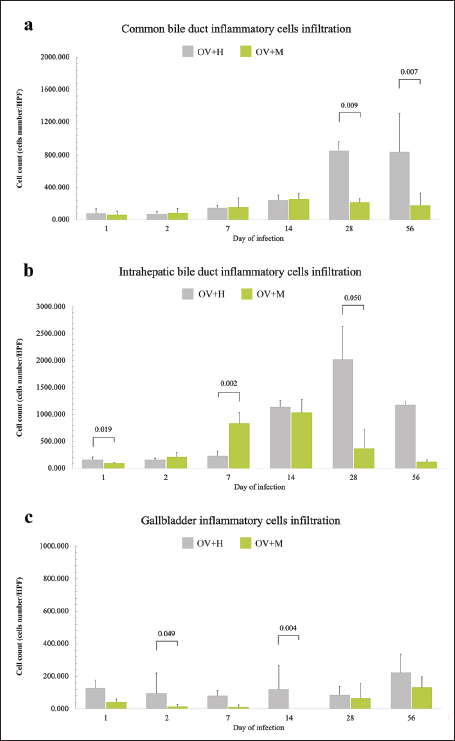
Fig. 2. Histopathological features of inflammatory cell infiltration in the biliary tract of O. viverrini infected hamsters and mice. Notably, there are distinct patterns observed during the early and late infection periods. In both the IHB and CB, the inflammation is more pronounced in the early stage of infection when compared to the later stages in both groups (left and middle column). Conversely, the GB exhibits a moderate and consistent level of leukocyte infiltration in the OV+H group, which contrasts with the OV+M group (right column) [H&E, original magnification=×40, the scale bar represents 50 μm]. Comparison of inflammatory pattern between susceptible and non-susceptible hostsThe quantitative comparison of biliary tract inflammation between OV+H and OV+M was assessed based on histopathological sections during the early and late phases (Fig. 3). In general, the inflammation observed in the OV+H was more intense compared to the OV+M across all locations. Specifically, in the CB, the OV+M group exhibited slightly higher inflammatory cell infiltration during the early phase (2–7 d.p.i.) than in the late phase, and there was no significant difference between the 2 groups (Fig. 3a). However, in the OV+H group, O. viverrini infection significantly enhanced leukocyte infiltration during the late period, particularly at 28 and 56 d.p.i. with p=0.009 and 0.007, respectively (Fig. 3a). These findings suggest that the inflammatory response in the biliary tract, specifically in the CB, differs between the two animal models. While the OV+M group shows a relatively stable level of inflammation, the OV+H group exhibits a significant increase in leukocyte infiltration during the late phase of infection.
Fig. 3. A comparison of inflammatory cell infiltration at the biliary and GB area of the hamsters and mice infected with O. viverrini from 1 to 56 days. (a) CB. (b) IHB. (c) GB. The comparison data was illustrated with a significant difference, p < 0.05. In the IHB, the inflammatory pattern in both the short and long term exhibited a similar trend to that observed in the CB. On the first day of infection, a significantly high number of infiltrating leukocytes was detected in the OV+H group (p=0.019), but this number declined by day 2 post-infection. At day 7 post-infection, the average number of infiltrating leukocytes in the OV+M group was significantly higher than in the OV+H group (p=0.022). However, at later infection periods (14, 28, and 56 d.p.i.), a higher number of inflammatory cells were detected in the OV+H group. The maximum number of leukocyte infiltrations was significantly found at day 28 (p=0.050) (Fig. 3b). Regarding the GB, minimal levels of inflammation were observed in both the OV+H and OV+M groups. However, a higher infiltration of inflammatory cells was observed in infected hamsters compared to mice. The significantly highest inflammation was found on days 2 and 14 in the OV+H group (p=0.049 and 0.004, respectively) (Fig. 3c). These results indicate that the inflammatory response in the IHB and GB differs between the OV+H and OV+M groups. While the OV+H group exhibits a higher number of infiltrating leukocytes in the IHB and GB during the later stages of infection, the OV+M group shows a relatively higher number of inflammatory cells in the IHB during the early phase of infection. DiscussionOpisthorchis viverrini is classified by the International Agency for Research on Cancer [28] as a Group I biological carcinogen to humans, specifically causing bile duct lesions and cholangiocarcinoma (Group 1) (IARC, 1994). Chronic inflammation resulting from O. viverrini infection is believed to be a risk factor for severe biliary abnormalities (Aksorn et al., 2018; Sripa et al., 2009). This phenomenon typically appears in different locations along the biliary tree (Pungpak et al., 1985; Riganti et al., 1989; Sripa and Kaewkes, 2002). Unfortunately, some individuals affected by O. viverrini infection may ultimately develop cholangiocarcinoma (Ghouri et al., 2015; Parkin et al., 2010; Sripa and Pairojkul, 2008). This bile duct cancer can be classified into 3 subtypes based on anatomical locations: intrahepatic type (iCCA) which develops from either large or small IHBs; perihilar (pCCA), located at the hilar region, and distal CCA (dCCA) arising from the extrahepatic bile duct (EHB) (Bragazzi et al., 2018; Sarcognato et al., 2021). The severity of lesions and symptoms appears to be associated with individual susceptibility, both in human and animal models (Boonmars et al., 2009; Choi et al., 2003; Chung et al., 2004; Khuntikeo et al., 2018). In this study, a systematic investigation of the inflammatory response in different biliary locations and susceptible/non-susceptible animal models was conducted, revealing that O. viverrini infection leads to more severe inflammation in the susceptible hamster model compared to the non-susceptible mouse model. Among the different biliary locations, the inflammation in the IHB was found to be particularly intense. These results suggest that hosts susceptible to O. viverrini infection experience more severe inflammation, especially in the IHB area. Inflammatory cell infiltration including neutrophils, eosinophils, macrophages, lymphocytes, and mast cells, is commonly observed in areas infected by O. viverrini (Bhamarapravati et al., 1978; Lvova et al., 2012; Sripa et al., 2018; Suyapoh et al., 2021a). Studies have reported minimum reactions with neutrophils, macrophages, eosinophils, and lymphocytes in hamsters, occurring in the EHB including CB and GB on days 1–7 of infection (Sripa and Kaewkes, 2002). This suggests that the inflammation may be a response to the migration of juvenile liver fluke [3, 37]; and the secretory antigens by immature parasites [38], the first-line defense mechanisms such as mucus hypersecretion (Kaewkes and Sripa, 2004; Nithikathkul et al., 2007), and the mucosal immune response in bile fluid, specifically involving IgA and IgE (Wongratanacheewin et al., 1988). During migration, immature fluke reaches the IHB and induces a slightly inflammatory response with neutrophils and eosinophils (Bhamarapravati et al., 1978; Sripa and Kaewkes, 2000). As the flukes develop into adult worms, they are commonly detected in the larger ducts of the intrahepatic biliary tree (Pungpak et al., 1985; Pungpak et al., 1989). Mature O. viverrini worms induce strongly cell-mediated immune responses in the periductal area (Bhamarapravati et al., 1978; Sripa et al., 2018). Various animal studies have described patterns of leukocyte infiltration, where chronic inflammation with intense mononuclear cells, a moderate number of eosinophils, and mast cells are detected after a week post-infection (Bhamarapravati et al., 1978; Sripa and Haswell, 2021; Sripa and Kaewkes, 2000). In heavy infection with O. viverrini, the worms can be found in other locations, including EHB, PD, GB, and CB (Pungpak et al., 1985; Pungpak et al., 1989). The adult helminths are more frequently detected in GB than in the CB, likely due to the larger space available in the GB. Gross findings may reveal thickening, opacity, and dilatation of the GB (Boonmars et al., 2009; Wonkchalee et al., 2011). Microscopically, mild-to-moderate infiltrations of eosinophils and mononuclear cell infiltration can be seen 7–14 d.p.i. (Sripa and Kaewkes, 2002). The severity of inflammation depends on the number of flukes in close contact with the mucosal surface of those organs (Sripa and Kaewkes, 2002). The mechanisms of hepatobiliary inflammation initiated by adult flukes of O viverrini are multi-factorial and involve various factors, including mechanical damages, bacterial colonization, and secreted proteins (Brindley and Loukas, 2017; Gouveia et al., 2017; Sripa et al., 2012; Sripa et al., 2017). The mechanical action of sucking, facilitated by the oral and ventral suckers of the fluke, contributes to O. viverrini-mediated inflammation (Sripa et al., 2012). In addition, the liver fluke plays a significant role as a carrier of carcinogenic bacteria, Helicobacter pylori (Deenonpoe et al., 2015). The presence of O. viverrini enhances the migration and colonization of H. pylori, leading to severe inflammation and pathological changes in the biliary system (Suyapoh et al., 2021a; Suyapoh et al., 2021b). Furthermore, there is evidence that O. viverrini can modify the intestinal microbiome. Plieskatt et al. (2013) reported that O. viverrini infection results in alterations in the composition of the intestinal flora the evidence of liver fluke-modification of intestinal microbiome. This dysbiosis of the intestinal microbiota can promote the translocation of microorganisms toward the liver, leading to an immunological response known as the «Leaky-Gut Hypothesis» (Giordano et al., 2018). The «leaky-gut hypothesis» suggests that impaired intestinal barrier function can result in increased permeability, allowing the translocation of gut bacteria and their products into the liver. This translocation can trigger an inflammatory response (Giordano et al., 2018). In the case of O. viverrini infection, it has been proposed that the parasite may disrupt the intestinal microbiome, leading to dysbiosis and increased intestinal permeability (Harris and Loke, 2017; Plieskatt et al., 2013). This, in turn, may facilitate the translocation of bacteria and their products to the liver and biliary system. The chronic inflammation caused by O. viverrini infection is believed to be a key factor in the development of cholangiocarcinoma (Oh et al., 2014). O. viverrini secretes excretory/secretory products (OvES) that have been implicated in the pathogenesis of liver fluke-induced bile duct cancer (Jittimanee et al., 2007; Nair et al., 2011). OvES may promote the proliferation of bile duct epithelial cells and contribute to the development of IHB cancer (Nair et al., 2011). Liver fluke infection is also associated with alterations in the host’s immune response. It can modulate cytokine production and antigen presentation, which may contribute to the chronic inflammation and carcinogenesis observed in infected individuals (Chen et al., 2012). Indeed, O. viverrini infection can have multiple effects on the liver and biliary system that contribute to chronic inflammation and the development of cholangiocarcinoma. The alteration of the liver microbiome and the promotion of H. pylori growth by the parasitic infection can enhance inflammation and potentially increase the risk of malignancy [50, 53]. Various studies have reported the production of excretory/secretory products (OvES) by O. viverrini (Oh et al., 2014; Rim, 2005). The production of OVES by the fluke, which increases as the parasite matures, has been associated with heavy leukocyte infiltration and proliferation of bile duct epithelium (Nair et al., 2011; Sripa and Kaewkes, 2000). This proliferation of the bile duct epithelium, triggered by the endocytosis of OvES by cholangiocytes, is thought to contribute to the higher incidence of IHB cancer compared to other types of cholangiocarcinoma. The increased production of OvES with the maturation of the parasite corresponds to the heavy infiltration of leukocytes, indicating a possible role in the inflammatory response. Studies have demonstrated that OvES can promote the growth of cholangiocarcinoma cells both in vitro and in vivo, indicating a potential role in the development of cholangiocarcinoma (Chen et al., 2012; Harris and Loke, 2017). Epidemiological studies have extensively documented the association between O. viverrini infection and CCA, further supporting the idea that chronic inflammation induced by the parasite plays a crucial role in the development of this cancer (Chen et al., 2012; Sripa et al., 2018). The variability in the inflammatory response to O. viverrini infection between different host types is indeed interesting. In BALB/c mice, the inflammatory response in the CB and GB was generally low, with mild to moderate inflammation observed at 1–2 weeks post-infection, consistent with previous studies (Boonmars et al., 2009). Gross examination of the livers of infected mice did not reveal any notable abnormalities. The complexity of the inflammatory response to trematode infections, such as Clonorchis sinensis (C. sinensis), is influenced by various factors, including the parasite type, the host immune response, and the genetic background of the host. Notably, C. sinensis, similar to O. viverrini, is commonly found in the IHB (Rim, 2005; Oh et al., 2014). In C. sinensis infection, a peak of leukocyte infiltration was reported at 2–3 weeks post-infection, followed by a gradual decrease (Choi et al., 2003). This non-susceptibility phenomenon may be associated with an early significant increase in Th1 cytokines, such as IFN-γ, IL-2, and IL-10, at this time point (Choi et al., 2003; Wang et al., 2021). In addition, similar results were recently reported in another liver fluke, Opisthorchis felineus (Avgustinovich et al., 2021). In non-susceptible hosts, it is possible that the immune system effectively eliminates the parasite with the assistance of immune cell infiltration. Furthermore, in other opisthorchiasis models, the infiltration of immune cells appears to depend on the host’s master coregulatory or MTA1, which plays a role in host-parasite interactions (Nair et al., 2011). One study found that intense bile duct inflammation was only detected in wild-type Mta1+/+ mice, whereas inflammation was less severe in Mta1-/- mice (Nair et al., 2011). This suggests that host factors, including coregulators and genetic factors, can influence the inflammatory response to opisthorchiasis. The extensive cellular infiltration observed in the biliary system of Syrian golden hamsters in our study is consistent with previous findings reported by Bhamarapravati et al. (1978) and Lvova et al. (2012). It has been suggested that the higher inflammation observed in hamsters is associated with the early expression of IL-12 in the liver in response to the parasite antigens, followed by a shift toward Th2 cytokine, particularly IL-10 (Jittimanee et al., 2007). The predominance of a Th2-type immune response and reduced Th1 response may contribute to immune homeostasis and allow the survival of the parasite (Chen et al., 2012; Harris and Loke, 2017). Our results further support the notion that hamsters exhibit a more intense cellular immune response compared to mice, especially during the late phase of infection (4 weeks or one month). This heightened immune response in susceptible hosts like those hamsters may contribute to the long-term survival of O. viverrini. However, it is important to acknowledge the differences in immune responses between species. The immune system of humans is more complex and may respond differently to O. viverrini infection compared to animal models. Further studies are required to fully understand the mechanisms underlying the pathogenesis of opisthorchiasis and the immune response to O. viverrini infection in humans. Nonetheless, the data obtained from animal models such as hamsters and mice are valuable in elucidating the fundamental mechanisms involved in host-parasite interaction and can provide insights into potential therapeutic targets for further exploration. ConclusionIndeed, the inflammatory responses to O. viverrini infection are multifaceted and influenced by a range of factors. The interplay between the host’s immune system, the parasite’s virulence factors, and the specific location of the infection all contribute to the observed inflammatory patterns. The intense inflammatory response observed in the IHB tissue of susceptible hosts suggests a potential link to the development of intraductal cholangiocarcinoma. On the other hand, the lower inflammatory reaction seen in non-susceptible hosts may indicate a reduced risk of developing this particular type of cancer. Gaining a comprehensive understanding of the mechanisms underlying these differences in susceptibility and immune response is crucial for developing effective treatments and prevention strategies for parasitic infections. Further research is needed to elucidate the specific immune pathways and molecular mechanisms involved in the host-parasite interaction during O. viverrini infection. AcknowledgementsThe authors would like to thank Mr. Suwit Balthaisong for sample collection assistance. Conflict of interestThe authors declare that there is no conflict of interest. Author contributionConceptualization, ST; Methodology, TT, WDW, and ST; Validation, WS, ST; Formal Analysis, WS; Investigation, ST; Resources, TT, ST; KS, PS Writing—Original Draft Preparation, WS, ST; Writing—Review and Editing, WS, ST; Supervision and Editing, SS, ST, PT. All authors have read and agreed to the submitted version of the manuscript. FundingThe research was supported by the Fundamental Fund of Khon Kaen University; Veterinary Medicine Annual Research Fund 2021. Data availabilityAll data are provided in the manuscript. ReferencesAksorn, N., Roytrakul, S., Kittisenachai, S., Leelawat, K., Chanvorachote, P., Topanurak, S., Hamano, S. and Lek-Uthai, U. 2018. Novel potential biomarkers for Opisthorchis viverrini infection and associated cholangiocarcinoma. In Vivo 32, 871–878. Avgustinovich, D., Kovner, A., Kashina, E., Shatskaya, N., Vishnivetskaya, G., Bondar, N. and Lvova, M. 2021. The pathogenic potential of the combined action of chronic Opisthorchis felineus infection and repeated social defeat stress in C57BL/6 mice. Int. J. Parasitol. 51(5), 353–363. Bhamarapravati, N., Thammavit, W. and Vajrasthira, S. 1978. Liver changes in hamsters infected with a liver fluke of man, Opisthorchis viverrini. Am. J. Trop. Med. Hyg. 27, 787–794. Boonmars, T., Boonjaraspinyo, S. and Kaewsamut, B. 2009. Animal models for Opisthorchis viverrini infection. Parasitol. Res. 104, 701–703. Bragazzi, M.C., Ridola, L., Safarikia, S., Matteo, S.D., Costantini, D., Nevi, L. and Cardinale, V. 2018. New insights into cholangiocarcinoma: multiple stems and related cell lineages of origin. Ann. Gastroenterol. 31, 42–55. Brindley, P.J. and Loukas, A. 2017. Helminth infection-induced malignancy. PLoS Pathog. 13, e1006393. Campbell, S.J., Nery, S.V., Wardell, R., D’Este, C.A., Gray, D.J., McCarthy, J.S., Traub, R.J., Andrews, R.M., Llewellyn, S., Vallely, A.J., Williams, G.M. and Clements, A.C. 2017. Water, sanitation and hygiene (WASH) and environmental risk factors for soil-transmitted helminth intensity of infection in Timor-Leste, using real time PCR. PLoS Negl. Trop. Dis. 11, e0005393. Cardinale, V., Bragazzi, M.C., Carpino, G., Matteo, S.D., Overi, D., Nevi, L., Gaudio, E. and Alvaro, D. 2018. Intrahepatic cholangiocarcinoma: review and update. Hepatoma Res. 4, 20. Chen, F., Liu, Z., Wu, W., Rozo, C., Bowdridge, S., Millman, A., Van Rooijen, N., Urban, J.F., Jr., Wynn, T.A. and Gause, W.C. 2012. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat. Med. 18, 260–266. Choi, Y.K., Yoon, B.I., Won, Y.S., Lee, C.H., Hyun, B.H., Kim, H.C., Oh, G.T. and Kim, D.Y. 2003. Cytokine responses in mice infected with Clonorchis sinensis. Parasitol. Res. 91, 87–93. Chung, B.-S., Zhang, H., Choi, M.-H., Jeon, D., Li, S., Lee, M. and Hong, S.-T. 2004. Development of resistance to reinfection by Clonorchis sinensis in rats. Korean J. Parasitol. 42, 19. Deenonpoe, R., Chomvarin, C., Pairojkul, C., Chamgramol, Y., Loukas, A., Brindley, P.J. and Sripa, B. 2015. The carcinogenic liver fluke Opisthorchis viverrini is a reservoir for species of Helicobacter. Asian Pac. J. Cancer Prev. 16, 1751–1758. Dulaimi, K.A.L., Banks, J., Chandran, V., Tomeo-Reyes, I. and Nguyen, K., 2018. Classification of white blood cell types from microscope images: techniques and challenges. Formatex Research Center, Spain. Fried, B., Reddy, A. and Mayer, D. 2011. Helminths in human carcinogenesis. Cancer Lett. 305, 239–249. Ghouri, Y.A., Mian, I. and Blechacz, B. 2015. Cancer review: cholangiocarcinoma. J. Carcinog. 14, 1. Giordano, D.M., Pinto, C., Maroni, L., Benedetti, A. and Marzioni, M. 2018. Inflammation and the gut-liver axis in the pathophysiology of cholangiopathies. Int J Mol Sci 19(10), 3003. Gouveia, M.J., Pakharukova, M.Y., Laha, T., Sripa, B., Maksimova, G.A., Rinaldi, G., Brindley, P.J., Mordvinov, V.A., Amaro, T., Santos, L.L., Costa, J. and Vale, N. 2017. Infection with Opisthorchis felineus induces intraepithelial neoplasia of the biliary tract in a rodent model. Carcinogenesis 38, 929–937. Hanpanich, P., Laha, T., Sripa, B., Mairiang, E., Sereerak, P., Upontain, S., Tangkawattana, P., Brindley, P.J. and Tangkawattana, S. 2017. Decreased risk of cholangiocarcinogenesis following repeated cycles of Opisthorchis viverrini infection-praziquantel treatment: magnetic resonance imaging (MRI) and histopathological study in a hamster model. Parasitol. Int. 66, 464–470. Harinasuta, T., Riganti, M. and Bunnag, D. 1984. Opisthorchis viverrini infection: pathogenesis and clinical features. Arzneimittelforschung 34, 1167–1169. Harris, N.L. and Loke, P. 2017. Recent advances in type-2-cell-mediated immunity: insights from helminth infection. Immunity 47, 1024–1036. IARC 1994. Infection with liver flukes (Opisthorchis viverrini, Opisthorchis felineus and Clonorchis sinensis). IARC Monogr Eval Carcinog Risks Hum. 61, 121–175. Jittimanee, J., Sermswan, R.W., Puapairoj, A., Maleewong, W. and Wongratanacheewin, S. 2007. Cytokine expression in hamsters experimentally infected with Opisthorchis viverrini. Parasite Immunol. 29, 159–167. Kaewkes, S. and Sripa, B. 2004. Migratory pattern of Opisthorchis viverrini in hamsters. Southeast Asian J. Trop. Med. Public Health 35, 306–308. Khuntikeo, N., Titapun, A., Loilome, W., Yongvanit, P., Thinkhamrop, B., Chamadol, N., Boonmars, T., Nethanomsak, T., Andrews, R.H., Petney, T.N. and Sithithaworn, P. 2018. Current perspectives on opisthorchiasis control and cholangiocarcinoma detection in Southeast Asia. Front. Med. (Lausanne) 5, 117. Loeuillard, E., Fischbach, S.R., Gores, G.J. and Rizvi, S. 2019. Animal models of cholangiocarcinoma. Biochimica et Biophysica Acta (BBA) Mol. Basis Dis. 1865, 982–992. Lvova, M.N., Tangkawattana, S., Balthaisong, S., Katokhin, A.V., Mordvinov, V.A. and Sripa, B., 2012. Comparative histopathology of Opisthorchis felineus and Opisthorchis viverrini in a hamster model: an implication of high pathogenicity of the European liver fluke. Parasitol. Int. 61(1), 167–172. Nair, S.S., Bommana, A., Pakala, S.B., Ohshiro, K., Lyon, A.J., Suttiprapa, S., Periago, M.V., Laha, T., Hotez, P.J., Bethony, J.M., Sripa, B., Brindley and P.J., Kumar, R. 2011. Inflammatory response to liver fluke Opisthorchis viverrini in mice depends on host master coregulator MTA1, a marker for parasite-induced cholangiocarcinoma in humans. Hepatology 54, 1388–1397. Nithikathkul, C., Tesana, S., Sithithaworn, P. and Balakanich, S. 2007. Early stage biliary and intrahepatic migration of Opisthorchis viverrini in the golden hamster. J. Helminthol. 81, 39–41. Oh, J.T., Kang, D.B. and Jo, H.J., 2014. Acute cholecystitis associated with Clonorchis sinensis infection. Ann. Surg. Treat. Res. 87, 104–107. Parkin, D.M., Ferlay, J., Curado, M.P., Bray, F., Edwards, B., Shin, H.R. and Forman, D., 2010. Fifty years of cancer incidence: CI5 I-IX. Int. J. Cancer 127, 2918–2927. Plieskatt, J.L., Deenonpoe, R., Mulvenna, J.P., Krause, L., Sripa, B., Bethony, J.M. and Brindley, P.J., 2013. Infection with the carcinogenic liver fluke Opisthorchis viverrini modifies intestinal and biliary microbiome. Faseb. J. 27, 4572–4584. Pungpak, S., Riganti, M., Bunnag, D. and Harinasuta, T. 1985. Clinical features in severe Opisthorchiasis viverrini. Southeast Asian J. Trop. Med. Public Health 16, 405–409. Pungpak, S., Sornmani, S., Suntharasamai, P. and Vivatanasesth, P. 1989. Ultrasonographic study of the biliary system in opisthorchiasis patients after treatment with praziquantel. Southeast Asian J. Trop. Med. Public Health 20, 157–162. Riganti, M., Pungpak, S., Punpoowong, B., Bunnag, D. and Harinasuta, T. 1989. Human pathology of Opisthorchis viverrini infection: a comparison of adults and children. Southeast Asian J. Trop. Med. Public Health 20, 95–100. Rim, H.J., 2005. Clonorchiasis: an update. J. Helminthol. 79, 269–281. Rizvi, S., Khan, S.A., Hallemeier, C.L., Kelley, R.K. and Gores, G.J. 2018. Cholangiocarcinoma-evolving concepts and therapeutic strategies. Nature Rev. Clin. Oncol. 15, 95–111. Sarcognato, S., Sacchi, D., Fassan, M., Fabris, L., Cadamuro, M., Zanus, G., Cataldo, I., Capelli, P., Baciorri, F., Cacciatore, M. and Guido, M. 2021. Cholangiocarcinoma. Pathologica 113, 158–169. Sithithaworn, P., Yongvanit, P., Duenngai, K., Kiatsopit, N. and Pairojkul, C. 2014. Roles of liver fluke infection as risk factor for cholangiocarcinoma. J. Hepatobiliary Pancreat. Sci. 21, 301–308. Sripa, B., Brindley, P.J., Mulvenna, J., Laha, T., Smout, M.J., Mairiang, E., Bethony, J.M. and Loukas, A. 2012. The tumorigenic liver fluke Opisthorchis viverrini--multiple pathways to cancer. Trends Parasitol. 28, 395–407. Sripa, B., Deenonpoe, R. and Brindley, P.J. 2017. Co-infections with liver fluke and helicobacter species: a paradigm change in pathogenesis of opisthorchiasis and cholangiocarcinoma? Parasitol. Int. 66, 383–389. Sripa, B. and Haswell, M.R. 2021. Mast cell hyperplasia in Opisthorchis viverrini-associated cholecystitis. Parasitol. Res. 120, 373–376. Sripa, B. and Kaewkes, S. 2000. Localisation of parasite antigens and inflammatory responses in experimental opisthorchiasis. Int. J. Parasitol. 30, 735–740. Sripa, B. and Kaewkes, S. 2002. Gall bladder and extrahepatic bile duct changes in Opisthorchis viverrini-infected hamsters. Acta Trop. 83, 29–36. Sripa, B., Mairiang, E., Thinkhamrop, B., Laha, T., Kaewkes, S., Sithithaworn, P., Tessana, S., Loukas, A., Brindley, P.J. and Bethony, J.M. 2009. Advanced periductal fibrosis from infection with the carcinogenic human liver fluke Opisthorchis viverrini correlates with elevated levels of interleukin-6. Hepatology 50, 1273–1281. Sripa, B. and Pairojkul, C. 2008. Cholangiocarcinoma: lessons from Thailand. Curr. Opin. Gastroenterol. 24, 349–356. Sripa, B., Suwannatrai, A.T., Sayasone, S., Do, D.T., Khieu, V. and Yang, Y. 2021. Current status of human liver fluke infections in the Greater Mekong Subregion. Acta Trop. 224, 106133. Sripa, B., Tangkawattana, S. and Brindley, P.J. 2018. Update on pathogenesis of opisthorchiasis and cholangiocarcinoma. Adv. Parasitol. 102, 97–113. Sudsarn, P., Wongchalee, N., Boonmars, T., Laummaunwai, P., Chamgramol, Y., Pairojkul, C., Juasook, A. and Boonjaraspinyo, S. 2014. Sex differences in opisthorchiosis and the development of cholangiocarcinoma in Syrian hamster model. Parasitol. Res. 113, 829–835. Suyapoh, W., Tangkawattana, S., Suttiprapa, S., Punyapornwithaya, V., Tangkawattana, P. and Sripa, B. 2021a. Synergistic effects of cagA+ Helicobacter pylori co-infected with Opisthorchis viverrini on hepatobiliary pathology in hamsters. Acta Trop. 213, 105740. Suyapoh, W., Tirnitz-Parker, J.E.E., Tangkawattana, S., Suttiprapa, S. and Sripa, B., 2021b. Biliary migration, colonization, and pathogenesis of O. viverrini co-infected with CagA+ H. pylori. Pathogens 10(9), 1089. Wang, N., Bai, X., Jin, X., Tang, B., Yang, Y., Sun, Q., Li, S., Wang, C., Chang, Q., Liu, M. and Liu, X. 2021. The dynamics of select cellular responses and cytokine expression profiles in mice infected with juvenile Clonorchis sinensis. Acta Trop. 217, 105852. Wongratanacheewin, S., Bunnag, D., Vaeusorn, N. and Sirisinha, S. 1988. Characterization of humoral immune response in the serum and bile of patients with opisthorchiasis and its application in immunodiagnosis. Am. J. Trop. Med. Hyg. 38, 356–362. Wonkchalee, O., Boonmars, T., Kaewkes, S., Chamgramol, Y., Pairojkul, C., Wu, Z., Juasook, A., Sudsarn, P. and Boonjaraspinyo, S. 2011. Opisthorchis viverrini infection causes liver and biliary cirrhosis in gerbils. Parasitol. Res. 109, 545–551. | ||
| How to Cite this Article |
| Pubmed Style Tangkawattana S, Suyapoh W, TT, Wendo WD, Salao K, Suttiprapa S, Saichua P, Tangkawattana P. Inflammatory Responses to Opisthorchis viverrini Infection in Animal Models: A comparison between susceptible and non-susceptible hosts in different anatomical locations. Open Vet J. 2024; 14(2): 664-673. doi:10.5455/OVJ.2024.v14.i2.6 Web Style Tangkawattana S, Suyapoh W, TT, Wendo WD, Salao K, Suttiprapa S, Saichua P, Tangkawattana P. Inflammatory Responses to Opisthorchis viverrini Infection in Animal Models: A comparison between susceptible and non-susceptible hosts in different anatomical locations. https://www.openveterinaryjournal.com/?mno=172885 [Access: April 27, 2024]. doi:10.5455/OVJ.2024.v14.i2.6 AMA (American Medical Association) Style Tangkawattana S, Suyapoh W, TT, Wendo WD, Salao K, Suttiprapa S, Saichua P, Tangkawattana P. Inflammatory Responses to Opisthorchis viverrini Infection in Animal Models: A comparison between susceptible and non-susceptible hosts in different anatomical locations. Open Vet J. 2024; 14(2): 664-673. doi:10.5455/OVJ.2024.v14.i2.6 Vancouver/ICMJE Style Tangkawattana S, Suyapoh W, TT, Wendo WD, Salao K, Suttiprapa S, Saichua P, Tangkawattana P. Inflammatory Responses to Opisthorchis viverrini Infection in Animal Models: A comparison between susceptible and non-susceptible hosts in different anatomical locations. Open Vet J. (2024), [cited April 27, 2024]; 14(2): 664-673. doi:10.5455/OVJ.2024.v14.i2.6 Harvard Style Tangkawattana, S., Suyapoh, . W., , . T. T., Wendo, . W. D., Salao, . K., Suttiprapa, . S., Saichua, . P. & Tangkawattana, . P. (2024) Inflammatory Responses to Opisthorchis viverrini Infection in Animal Models: A comparison between susceptible and non-susceptible hosts in different anatomical locations. Open Vet J, 14 (2), 664-673. doi:10.5455/OVJ.2024.v14.i2.6 Turabian Style Tangkawattana, Sirikachorn, Watcharapol Suyapoh, Theerayut Thongrin, Woro Danur Wendo, Kanin Salao, Sutas Suttiprapa, Prasert Saichua, and Prasarn Tangkawattana. 2024. Inflammatory Responses to Opisthorchis viverrini Infection in Animal Models: A comparison between susceptible and non-susceptible hosts in different anatomical locations. Open Veterinary Journal, 14 (2), 664-673. doi:10.5455/OVJ.2024.v14.i2.6 Chicago Style Tangkawattana, Sirikachorn, Watcharapol Suyapoh, Theerayut Thongrin, Woro Danur Wendo, Kanin Salao, Sutas Suttiprapa, Prasert Saichua, and Prasarn Tangkawattana. "Inflammatory Responses to Opisthorchis viverrini Infection in Animal Models: A comparison between susceptible and non-susceptible hosts in different anatomical locations." Open Veterinary Journal 14 (2024), 664-673. doi:10.5455/OVJ.2024.v14.i2.6 MLA (The Modern Language Association) Style Tangkawattana, Sirikachorn, Watcharapol Suyapoh, Theerayut Thongrin, Woro Danur Wendo, Kanin Salao, Sutas Suttiprapa, Prasert Saichua, and Prasarn Tangkawattana. "Inflammatory Responses to Opisthorchis viverrini Infection in Animal Models: A comparison between susceptible and non-susceptible hosts in different anatomical locations." Open Veterinary Journal 14.2 (2024), 664-673. Print. doi:10.5455/OVJ.2024.v14.i2.6 APA (American Psychological Association) Style Tangkawattana, S., Suyapoh, . W., , . T. T., Wendo, . W. D., Salao, . K., Suttiprapa, . S., Saichua, . P. & Tangkawattana, . P. (2024) Inflammatory Responses to Opisthorchis viverrini Infection in Animal Models: A comparison between susceptible and non-susceptible hosts in different anatomical locations. Open Veterinary Journal, 14 (2), 664-673. doi:10.5455/OVJ.2024.v14.i2.6 |