| Research Article | ||
Open Vet J. 2023; 13(5): 613-619 Open Veterinary Journal, (2023), Vol. 13(5): 613–619 Original Research Effects of glutamine and arginine combination on pro- and anti-inflammatory cytokinesMaulydia Maulydia1*, Nancy Margarita Rehatta2 and Subijanto Marto Soedarmo31Doctoral Program of Medical Science, Universitas Airlangga, Surabaya, Indonesia 2Department of Anesthesiology and Reanimation, Universitas Airlangga/Dr. Soetomo General Hospital, Surabaya, Indonesia 3Department of Child Health, Universitas Airlangga/Dr. Soetomo General Hospital, Surabaya, Indonesia *Corresponding Author: Maulydia Maulydia. Doctoral Program of Medical Science, Universitas Airlangga, Surabaya, Indonesia. Email: maulydia [at] fk.unair.ac.id. Submitted: 15/02/2023 Accepted: 12/04/2023 Published: 15/05/2023 © 2023 Open Veterinary Journal
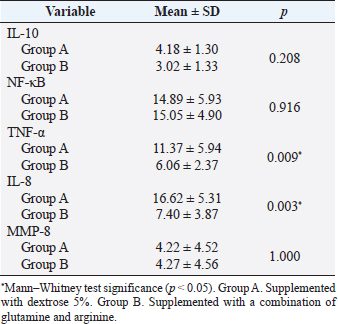
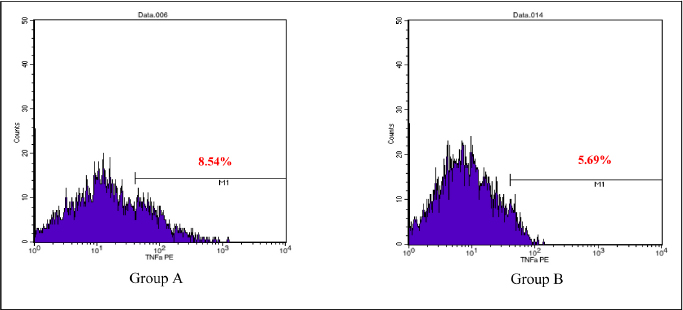
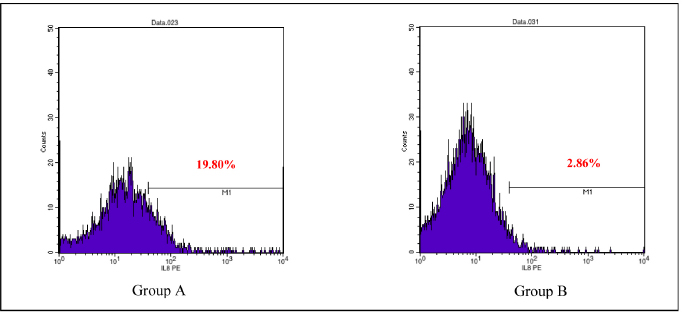
AbstractBackground: Cytokines were beneficial for diagnosis and treatment, which in clinical situations introduced from the perspective of pro and anti-inflammatory effects. An inflammatory response is commonly associated with various severe traumatic insults that consequently recruit the immune cells into the target organs and causing systemic inflammatory response that can lead to sepsis. Immune-modulating nutrients, such as glutamine and arginine, are known as pathophysiological modulate in inflammation. Aim: The aim of this study was to evaluate the effect of oral gavage supplementation with a combination of glutamine and arginine on inflammatory cytokines in intestinal mucosa, specifically jejunum. Methods: Sixteen Rattus norvegicus rats (average weight 150–200 g) were randomly divided into two groups: groups A and B, both intraperitoneal injected by 2 ml NaCl 0.9%. Group A orally supplemented with 1 ml dextrose 5% daily, meanwhile, group B orally supplemented with 1 ml combination of glutamine and arginine (contains 250 mg/kg glutamine and 250 mg/kg arginine) daily. The experiment lasted for 3 days. We compared the pro and anti-inflammatory cytokines (IL-10, NF-κB, TNF-α, IL-8, and MMP-8) between the two groups by the Mann–Whitney test. Results: More IL-10, TNF-α, and IL-8 cytokine-produced cells found in group A. Group B produced significantly lower TNF-α (p=0.009) and IL-8 (p=0.003). The number of NF-κB and MMP-8 were slightly higher in group B. Conclusion: Giving a combination of glutamine and arginine as nutrition supplementation has beneficial effects in decreasing almost half of the cells that produce TNF-α and IL-8. Further studies must be carried out to support a standard guideline for this recommendation. Keywords: Immunonutrient, Glutamine, Arginine, Inflammatory, Cytokine. IntroductionCytokines are defined as low-weight molecules of non-structural proteins that have critical roles in regulating inflammation and the immune system through complex interactions (Gulati et al., 2016). Cytokines are known as biomarkers for various diseases and are beneficial for diagnosis and treatment, which in clinical is introduced from the perspective of pro and anti-inflammatory effects. Pro-inflammatory cytokines (such as TNF-α, IL-8, IL-1β, and others) facilitate the reaction of inflammation and stimulate immunocompetent cells. In contrary, anti-inflammatory (such as IL-10, IL-6, IL-4, and others) major role was inhibiting the inflammation (Liu et al., 2021). In the biomolecular model, the T regulator plays a vital role together with TGF-β in suppressing the immune response, which has both positive and negative impacts on the immune system (Wan and Flavell, 2007). T regulator involves making a balance condition between Th1 and Th2 (Lin et al., 2019). Th1 affects the secretion of pro-inflammation cytokine such as TNF-α, IL-8, IL-6, and IL-1. Meanwhile, Th2 affecting to the IL-10 production (Dembic, 2015). Th1 and Th2 were affected by macrophage type 1 (M1) and type 2 (M2). Macrophage has two side effects, which can attenuate nitric oxide (NO) or promote ornithine. During inflammation, macrophage improves ornithine which leads to the wounds healing. Macrophage also has suppressor activity that inhibits specific T-cell responses during several chronic infections. In addition, the suppressor activity was correlated with NO production. Excessive production of NO could inhibit beneficial immune response (Mills, 2015). Response of inflammation is commonly associated with various severe traumatic insults. Traumatic injury increases pro-inflammatory mediators that consequently recruit the immune cells into the target organs and cause systemic inflammatory response (Yu et al., 2015). During inflammatory conditions, NF-κB is activated by local cytokines to promote macrophage activation at the site of infection (Baker et al., 2011). Elevation of NF-κB enhances the MMP-8 production, which is suspected as the main collagen in the process of mucosal damage (O’Sullivan et al., 2015; Lee, 2019). Recently, inflammation was correlated with the alteration of the gut microbiome, which is known as intestinal dysbiosis (Levy et al., 2017). This intestinal dysbiosis condition can lead to increase gut permeability and induce mucosal immune dysfunction (Wang et al., 2019). Immune-modulating nutrients, such as glutamine, arginine, and others, could modulate pathophysiological processes in critical illness, such as inflammatory and oxidative stress responses (van Zanten et al., 2014). In critically ill patients, glutamine supplementation was beneficial to reduce complication infection and shorter length of stay, which average, patients were to stay on 3 days only (Tao et al., 2018). Glutamine also showed a significant reduction of neutrophil count in infection of burn injury during 3 days of administration (Sudarsa et al., 2021). Glutamine and arginine independently have a role in growth, tissue recovery, regeneration of cell, and reducing bacteria translocation in sepsis patients (Cohen and Chin, 2012). Lack of glutamine level was affected by the worsening in critically ill (Casaer and Van den Berghe, 2014). Arginine–proline metabolism change was found in bacteria induced-jejunum (Ilaiwy et al., 2019). The use of immunonutrition combination to improve ICU patient outcomes has been challenged (Arabi et al., 2017). However, the recent studies showed that glutamine and arginine combination (GAC) supplementation significantly decreased pro-inflammatory cytokines (such as CRP, TNF-α, IL-1β, and IL-6), improve tissue repair, cell renewal, and collagen synthesis (Bakir et al., 2019). Several studies found that supplementation of GAC had beneficial effects on the critically ill, but the specific benefit remained unclear (Bakir et al., 2019; Zhou et al., 2012). Here, we aim to present the effects of giving glutamine and arginine as a nutritional support for preventing immune system deterioration. Materials and MethodsAnimal and treatmentSixteen adult male Rattus norvegicus strain Wistar (12–14 weeks) with average body weight between 150 and 200 g were chosen in this study. The animals were maintained in group cages of size 17.5 × 23.75 × 17.5 cm made from plastic with wire caps, each cage including eight rats, placed in a controlled environment at room temperature. They were given the same ratio of clean water and sawdust as food. Trauma modeling in this study uses an injection of NaCl 0.9% intraperitoneally and an orogastric route of nutrition, which is commonly used and known, can cause high stress in animal models (Turner et al., 2011; Sotocinal et al., 2011). The injection was in the lower left side abdomen, which sign of inflammation in the injection area was observed. All animals could adapt to the experimental conditions for 1 week. After the adaption period, the animals were weighed and randomly allocated into two groups: group A and group B. Each rat was injected with 2 ml of NaCl 0.9% intraperitoneally in the lower left side abdomen. One hour after injection, each rat started to have oral gavage supplemented, group A with 1 ml of dextrose 5% while group B with 1 ml of glutamine and arginine (containing 250 mg/kg glutamine and 250 mg/kg arginine). All animals were supplemented once a day for 3 days, at the same time every day. On the third day, 2 hours after supplementation, all animals were sacrificed. Injection and gavage were given by an expert veterinarian. Food intake, body weight, and body temperature were measured and noted by the team during the experiment. Sample collectionAfter the rats were killed, the small intestine was removed. Jejunum was taken and washed in aquadest, then wrecked into small pieces and soaked in the plastic bottle containing 5 ml of phosphate buffer saline. The jejunum samples were processed by FACS Calibur flow cytometer. Data analysisThis was a quasy experimental design. Inferential statistic test was used to analyze the number of cells producing anti-inflammatory (IL-10) and pro-inflammatory cytokines (NF-κB, TNF-α, IL-8, and MMP-8). Data were analyzed using Statistical Package for the Social Sciences with comparative non-parametric inferential method (Mann–Whitney test). Statistical significance was determined if p value < 0.05. Significant results then analyze continuance by using a histogram of flowcytometry (Bioscience, 2002). Ethical approvalThis study is ethically approved by the committee of ethics from the Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia (2.KE.027.03.2021). ResultsWe compared the number of cells producing pro and anti-inflammatory cytokine between group A and B. Results showed that there was no difference in the number of anti-inflammatory cells producing IL-10 between the two groups, nor there was any significant difference in the number of pro-inflammatory cells producing NF-κB and MMP-8. Meanwhile, there was a significant difference in the number of pro-inflammatory cells producing TNF-α and IL-8 as shown in the Table 1. Table 1 showed that GAC effect on the cell that produces IL-10, NF-κB, and MMP-8 are not significant with p=0.208, p=0.916, and p=1.000, consecutively. The combination of glutamine and arginine significantly decreases the cell that produces TNF-α (p=0.009) and IL-8 (p=0.003). This significancy also showed in the flowcytometry histogram of macrophage proliferation into M1 type on TNF-α examination for both groups (Fig. 1). Group A, which received 5% dextrose, showed a higher peak of M1 proliferation, with 8.54% cells producing TNF-α in macrophage. In group B, only 5.69% of cells have been found to produce TNF-α in macrophages that proliferate into M1. Figure 2 showed macrophage proliferation of M1 type on IL-8 examination of both groups. Group A showed a higher peak of M1 proliferation with 19.80% of cells producing IL-8, meanwhile in group B only 2.86% of cells have been found to produce IL-8. DiscussionInjection of 0.9% NaCl intraperitoneally can lead to inflammation and causing pain in rats (Coria-Avila et al., 2007). Our study used the injection of 2 ml 0.9% NaCl intraperitoneally in the lower left abdomen. The location for peritoneal injection refers to the study of Coria-Avila et al. (2007) that found the lower left injection showed more lesions in the cecum than the lower right injection. Hopefully, this treatment can stimulate the inflammation process in this study. We found that TNF-α significantly decreased in the group supplemented by a combination of glutamine and arginine. This result is in line with a study by Bakir et al. (2019) that showed GAC led to lower serum levels of TNF-α in the liver of rats with sepsis, and also a study by Zhou et al. (2012) that found decrease TNF-α mRNA in the intestinal mucosa of endotoxemic rats. Table 1. Comparison of the number of cells that produce cytokines.
We chose jejunum as the sample and found that TNF-α was significantly low for the combination of glutamine and arginine treatment. Another study found significantly low TNF-α in the combination of glutamine and arginine treatment in the ileum segment of endotoxemic rats, but in the jejunum segment even though decreasing TNF-α was found but not significantly. Another study also found decreased TNF-α in the enterally supplementation of GAC, and also for glutamine or arginine only (Zhou et al., 2012). Our study found a significant decrease in IL-8. We could not find the same studies that used a combination of glutamine and arginine to decrease IL-8 expression. However, Huang et al. (2021) used glutamine gavage supplemented only for mice with acute lung injury induced by lipopolysaccharide (LPS) and showed that glutamine adequately depressed IL8 production. Several studies also found low levels of IL-8 in arginine treatment. Arginine with a dose of 8 mM effectively decreased IL-8 in the IPEC-J2 cells induced by LPS (Lan et al., 2020). Another study also found that the administration of arginine topical showed low levels of IL-8 in laparotomy mice (Jeronimo et al., 2016). Low levels of TNF-α and IL-8 might be caused by the inhibition of M1 macrophage proliferation pathways. Our study found short peaks of M1 macrophage proliferation in cells that produce TNF-α and IL-8 in a group with a combination of glutamine and arginine. M1 macrophage was activated by IFN-γ which was secreted by CD4+ helper T cells, NK cells, and NKT cells (Wu et al., 2014; Kanno et al., 2019). Unfortunately, we did not measure those parameters in this study. M1 macrophage plays a role in the secretion of pro-inflammatory cytokines such as TNF-α and IL-8 (Cruzat et al., 2018; Gomarasca et al., 2020). Glutamine availability was regulating the activation of M1 macrophages (Cruzat et al., 2018). In addition, glutamine, known as essential amino acid in several conditions because of the widely used glutamine in the intestinal cell, renal, and immune cells, exceeds endogen production (Kim and Kim, 2017). During inflammation, glutamine was beneficial in modulating the imbalance production of cytokine (Cruzat et al., 2018). Meanwhile, arginine was involved in any of the various processes one of which is related to macrophage polarization into M1 and M2 type (Ley, 2017). M1 macrophages release NO synthesize that metabolized arginine into NO and cytruline, which is NO will metabolize into reactive nitrogen species which is commonly followed by tissue damage, hence M2 macrophage were needed for tissue purify and renewal. M2 macrophage expressed arginase that hydrolyze arginine into ornithine and urea, which ornithine changes into polyamine, glutamate, and proline for cell proliferation and tissue repair (Rath et al., 2014).
Fig. 1. Flowcytometry of M1 proliferation in TNF-α. Group A: Supplemented with dextrose 5%. Group B: Supplemented with a combination of glutamine and arginine.
Fig. 2. Flowcytometry of M1 proliferation in IL-8. Group A: Supplemented with dextrose 5%. Group B: Supplemented with a combination of glutamine and arginine. The immune system showed that TNF-α and IL-8 were correlated. TNF-α could activate the secretion of IL-8 (Baratawidjaja, 2012). Both TNF-α and IL-8 were affected by NF-κB activation (Sudiana, 2017; Kany et al., 2019). Glutamine could suppress NF-κB production by stimulating Ikβ (Kim and Kim, 2017). Meanwhile, arginine attenuates NF-κB by increasing IL-10 production (Rath et al., 2014; Lee, 2019; Bergmann et al., 2021). Our study found that GAC had higher NF-κB production and lower IL-10. This result is in line with a study by Zhou et al. (2012) in endotoxemic rats, which showed a decrease in IL-10. Arginine supplementation only showed that topical administration did not affect IL-10 production in mice with laparotomy (Jeronimo et al., 2016). In Contrary, several studies showed that glutamine only affects the high production of IL-10 in lobectomy patients (Wang et al., 2018) and in bone marrow-derived macrophages induced by LPS (de Oliveira et al., 2018). Our study did not find a significant difference in cells that produce NF-κB. Until now, we could not find another study that uses a combination of glutamine and arginine and its effects on NF-κB expression. We found several studies used glutamine only or arginine only with a different result for the expression of NF-κB. Glutamine administration could inhibit the expression of NF-κB and showed advantages effect to protecting the intestinal sepsis rats (Wu and Zhang, 2019). As well as glutamine, 500 µM arginine significantly inhibits NF-κB in IPEC-J2 cells injected with LPS (Qiu et al., 2019). MMP-8 is suspected as the main collagen in the process of mucosal damage (Lee, 2019). MMP-8 production was affected by NF-κB presence (O’Sullivan et al., 2015). In our study, there is no difference in cells producing MMP-8 between the two groups. It might be caused by no differences in NF-κB cell production for both groups. No differences in cells that produce IL-10, NF-κB, and MMP-8 in our study may be caused by the dose and the time of administration. A study by de Oliveira et al. (2018) found that glutamine modulation in cytokine expression depended on the time of administration, in which high dosages could increase IL-10 affecting the inflammation response. A review by Sepandi et al. (2019) mentioned that a previous study reported contradictory evidence of arginine effects on indices of lipid profiles and inflammatory markers. Several studies showed the beneficials of arginine for lipid disorder and on inflammatory markers, but some studies have been failed to prove it. ConclusionStudies about giving a combination of glutamine and arginine are limited. In our study, nutritional supplementation with GAC has beneficial effects in decreasing almost half of the cells that produce TNF-α and IL-8. Further and larger studies are required to make a more established and standard guideline. Conflict of interestThe authors declare that there is no conflict of interest in this study. Author contributionsM, NMR, and SMS have made substantial contributions to the conception; design of the work; the acquisition, analysis, and interpretation of data; the creation of new software used in the work; have drafted the work substantively revised it. ReferencesArabi, Y.M., Casaer, M.P., Chapman, M., Heyland, D.K., Ichai, C., Marik, P.E., Martindale, R.G., McClave, S.A., Preiser, J.C., Reignier, J., Rice, T.W., Van den Berghe, G., van Zanten, A.R.H. and Weijs, P.J.M. 2017. The intensive care medicine research agenda in nutrition and metabolism. Inten. Care Med. 43, 1239–1256. Baker, R.G., Hayden, M.S. and Ghosh, S. 2011. NF-κB, inflammation, and metabolic disease. Cell Metabolism. 13, 11–22. Bakir, B.O., Oztezcan, S., Saka, M., Karalti, I., Ozkan, F. and Ok, M.A. 2019. The effects of enteral supplementation of glutamine and arginine in lipopolysaccharide (LPS) induced sepsis. Progr. Nutr. 21, 244–250. Baratawidjaja, K.G. 2012. Imunologi Dasar, 10th ed. Jakarta, Indonesia: Badan Penerbit Fakultas Kedokteran Universitas Indonesia. Bergmann, C.B., Beckmann, N., Salyer, C.E., Hanschen, M., Crisologo, P.A. and Caldwell, C.C. 2021. Potential targets to mitigate trauma- or sepsis induced immune suppression. Front. Immunol. 12, 1–19. Bioscience, BD. 2002. Introduction to flow cytometry: a learning guide. Franklin Lakes, NJ: Becton, Dickinson and Company. Casaer, M.P. and Van den Berghe, G. 2014. Nutrition in the acute phase of critical illness. N. Engl. J. Med. 370, 1227–1236. Cohen, J. and Chin, W.D.N. 2012. Nutrition and sepsis. Nutr. Inten. Care Med. Beyond Physiol. 105, 116–125. Coria-Avila, G.A., Gavrila, A.M., Menard, S., Ismail, N. and Pfaus, J.G. 2007. Cecum location in rats and the implications for intraperitoneal injections. Lab Anim. 36, 25–30. Cruzat, V., Rogero, M.M., Keane, K.N., Curi, R. and Newsholme, P. 2018. Glutamine: metabolism and immune function, supplementation and clinical translation. Nutrients. 10, 1–31. Dembic, Z. 2015. The cytokines of the immune system. San Diego, CA: Elsevier. de Oliveira D.C., Horwitz, B.H. and Fock, R.A. 2018. Glutamine modulates cytokines gene expression in a dose and time dependent manner. FASEB J. 31, 964. Gomarasca, M., Banfi, G. and Lombardi, G. 2020. Myokines: the endocrine coupling of skeletal muscle and bone. Adv. Clin. Chem. 94, 155–218. Gulati, K., Guhathakurta, S., Joshi, J., Rai, N. and Ray, A. 2016. Cytokines and their role in health and disease: a brief overview. MOJ Immunol. 4, 121–128. Huang, J., Liu, J., Chang, G., Wang, Y., Ma, N., Roy, A.C. and Shen, X. 2021. Glutamine supplementation attenuates the inflammation caused by LPS-induced acute lung injury in mice by regulating the tlr4/mapk signaling pathway. Inflammation 44, 2180–2192. Ilaiwy, A., Ten Have, G.A.M., Bain, J.R., Muehlbauer, M.J., O’Neal, S.K., Berthiaume, J.M., Parry, T.L., Deutz, N.E. and Willis, M.S. 2019. Identification of metabolic changes in ileum, jejunum, skeletal muscle, liver, and lung in a continuous i.v. psudomonas aeruginosa model of sepsis using nontrageted metabolomics analysis. Am. J. Pathol. 189, 1797–1813. Jeronimo, M.S., do Prado Barros A., Morital, V.E.Z., Alves, E.O., de Souza N.L.B., de Almeida, R.M., Nobrega, Y.K.M., Neto, F.F.C., Amorin, R., de Fatima Borin, M. and Bocca, A.L. 2016. Oral or topical administration of L-arginine changes the expression of TGF and iNOS and results in early wounds healing. Avta Cir. Bras. 31, 586–596. Kanno, E., Tanno, H., Masaki, A., Sasaki, A., Sato, N., Goto, M., Shisai, M.,Yamaguchi, K., Takagi, N., Shoji, M., Kitai, Y., Sato, K., Kasamatsu, J., Ishii, K., Miyasaka, T., Kawakami, K., Imai, Y., Iwakura, Y., Maruyama, R., Tachi, M. and Kawakami, K. 2019. Defect of interferon γ leads to impaired wound healing through prolonged neutrophilic inflammatory response and enhanced mmp-2 activation. Int. J. Mol. Sci. 20, 5657–5669. Kany, S., Volrath, J.T. and Relja, B. 2019. Cytokines in inflammatory disease. Int. J. Mol. Sci. 20, 6008–6038. Kim, M.H. and Kim, H. 2017. The roles of glutamine in the intestine and its implication in intestinal diseases. Int. J. Mol. Sci. 18, 1051–1065. Lan, J., Dou, X., Li, J., Yang, Y., Xue, C., Wang, C., Gao, N. and Shan, A. 2020. L-arginine ameliorates lipopolysaccharide-induced intestinal inflammation through inhibiting the TLR4/NF-κB and mapk pathways and stimulating β-defensin expression in vivo and in vitro. J. Agric. Food Chem. 68, 2648–2663. Lee, K.Y. 2019. M1 and M2 polarization of macrophages: a mini-review. Med. Bio. Sci. Eng. 2, 1–5. Levy, M., Kolodziejczyk, A.A., Thaiss, C.A. and Elinav, E. 2017. Dysbiosis and the immune system. Immunology. 17, 219–232. Ley, K. 2017. M1 means kill; m2 means heal. J. Immunol. 199, 2191–2193. Lin, W., Niu, Z., Zhang, H., Kong, Y., Wang, Z., Yang, X. and Yuan, F. 2019. Imbalance of Th1/Th2 and Th17/Treg during the development of uterine cervical cancer. Int. J. Clin. Exp. Pathol. 12, 3604–3612. Liu, C., Chu, D., Zadeh, K.K., George, J., Young, H.A. and Liu, G. 2021. Cytokines: from clinical significance to quantification. Adv. Sci. 8, 2004433. Mills, C.D. 2015. Anatomy of a discovery: m1 and m2 macrophages. Front. Immunol. 6, 1–12. O’Sullivan, S., Gilmer, J.F. and Medina, C. 2015. Matrix metalloproteinases in inflammatory bowel disease: an update. Mediat. Inflam. 2015, 1–19. Qiu, Y., Yang, X., Wang, L., Gao, K. and Jiang, Z. 2019. L-arginine inhibited inflammatory response and oxidative stress induced by lipopolysaccharide via arginase-1 signaling in IPEC-J2 cells. Int. J. Mol. Sci. 20, 1–14. Rath, M., Muller, I., Kropf, P., Closs, E.I. and Munder, M. 2014. Metabolism via arginase or nitric oxide synthase: two competing arginine pathways in macrophages. Front. Immunol. 5, 1–10. Sepandi, M., Abbaszadeh, S., Qobady, S., Taghdir, M. 2019. Effect of l-arginine supplementation on lipid profiles and inflammatory markers: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 148, 1–8. Sotocinal, S.G., Sorge, R.E., Zaloum, A., Tuttle, A.H., Martin, L.J., Wieskopf, J.S., Mapplebeck, J.C.S., Wei, P., Zhan, S., Zhang, S., McDougall, J.J., King, O.D. and Mogil, J.S. 2011. The rat grimace scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol. Pain 7, 1–10. Sudarsa, S.D., Hamid, A.R.R.H., Harimawan, A.I.W. and Budayanti, N.N.S. 2021. Correlation of glutamine and serial absolute neutrophil count as a parameter of infection in major burn trauma patients at Sanglah General Hospital, Bali, Indonesia. Intisari Sains Med. 12, 187–191. Sudiana, I.K. 2017. Hantaran sinyal pada proses inflamasi. Surabaya, Indonesia: Airlangga University Press. Tao, K.M., Li, X.Q., Yang, L.Q., Yu, W.F., Lu, Z.J., Sun, Y.M. and Wu, F.X. 2018. Glutamine supplementation for critically ill adults (review). Cochrane Database Syst. Rev. 2014, 1–99. Turner, P.V., Brabb, T., Pekow, C. and Vasbinder, M.A. 2011. Administration of substances to laboratory animals: routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 50, 600–613. van Zanten, A.R.H., Sztark, F., Kaisers, U.X., Zielmann, S., Felbinger, T.W., Sablotzki, A.R., De Waele, J.J., Timsit J.F., Honing, M.L.H., Keh, D., Vincent J.L., Zazzo, J.F., Fijn, H.B.M., Petit, L., Preiser, J.C., van Horssen, P.J. and Hofman, Z. 2014. High-protein enteral nutrition enriched with immune-modulating nutrients vs standard high-protein enteral nutrition and nosocomial infections in the ICU: a randomized clinical trial. J. Am. Med. Assoc. 312, 514–524. Wan, Y.Y. and Flavell, R.A. 2007. Yin-Yang functions of TGF-β and Tregs in immune regulation. Immunol. Rev. 220, 199–213. Wang, C., Li, Q. and Ren, J. 2019. Microbiota-immune interaction in the pathogenesis of gut-derived infection. Front. Immunol. 10, 1–14. Wang, X., Huang, L., Qu, Y., Hongmei, L.V. and He X. 2018. Effects of glutamine on cytokines 1L-1 and TNF-α in rehabilitation and prognosis of patients with lobectomy. Exp. Therap. Med. 16, 2303–2308. Wu, C., Xue, Y., Wang, P., Lin, L., Liu, Q., Li, N, Xu, J and Cao, X. 2014. IFN-γ primes macrophage activation by increasing phosphatase and tensin homolog via downregulation miR-3473b. J. Immunol. 193, 3036–3044. Wu, T.Y. and Zhang, H.B. 2019. Glutamine has a protective role on intestinal tissues via targeting NF-κB pathway in rats with sepsis. Eur. Rev. Med. Pharmacol. Sci. 23, 184–191. Yu, H.P., Chaudry, I.H., Choudhry, M.A., Hsing, C.H., Liu, F.C. and Xia, Z. 2015. Inflammatory response to traumatic injury: clinical and animal researches in inflammation. Mediat. Inflamm. 2015, 1–2. Zhou, X., Wu, X., Yin, Y., Zhang, C. and He, L. 2012. Preventive oral supplementation with glutamine and arginine has beneficial effects on the intestinal mucosa and inflammatory cytokines in endotoxemic rats. Amino Acids 4, 813–821. | ||
| How to Cite this Article |
| Pubmed Style Maulydia M, Rehatta NM, Sudarmo SM. Effects of glutamine and arginine combination on pro- and anti-inflammatory cytokines. Open Vet J. 2023; 13(5): 613-619. doi:10.5455/OVJ.2023.v13.i5.14 Web Style Maulydia M, Rehatta NM, Sudarmo SM. Effects of glutamine and arginine combination on pro- and anti-inflammatory cytokines. https://www.openveterinaryjournal.com/?mno=143623 [Access: May 01, 2024]. doi:10.5455/OVJ.2023.v13.i5.14 AMA (American Medical Association) Style Maulydia M, Rehatta NM, Sudarmo SM. Effects of glutamine and arginine combination on pro- and anti-inflammatory cytokines. Open Vet J. 2023; 13(5): 613-619. doi:10.5455/OVJ.2023.v13.i5.14 Vancouver/ICMJE Style Maulydia M, Rehatta NM, Sudarmo SM. Effects of glutamine and arginine combination on pro- and anti-inflammatory cytokines. Open Vet J. (2023), [cited May 01, 2024]; 13(5): 613-619. doi:10.5455/OVJ.2023.v13.i5.14 Harvard Style Maulydia, M., Rehatta, . N. M. & Sudarmo, . S. M. (2023) Effects of glutamine and arginine combination on pro- and anti-inflammatory cytokines. Open Vet J, 13 (5), 613-619. doi:10.5455/OVJ.2023.v13.i5.14 Turabian Style Maulydia, Maulydia, Nancy Margarita Rehatta, and Subijanto Marto Sudarmo. 2023. Effects of glutamine and arginine combination on pro- and anti-inflammatory cytokines. Open Veterinary Journal, 13 (5), 613-619. doi:10.5455/OVJ.2023.v13.i5.14 Chicago Style Maulydia, Maulydia, Nancy Margarita Rehatta, and Subijanto Marto Sudarmo. "Effects of glutamine and arginine combination on pro- and anti-inflammatory cytokines." Open Veterinary Journal 13 (2023), 613-619. doi:10.5455/OVJ.2023.v13.i5.14 MLA (The Modern Language Association) Style Maulydia, Maulydia, Nancy Margarita Rehatta, and Subijanto Marto Sudarmo. "Effects of glutamine and arginine combination on pro- and anti-inflammatory cytokines." Open Veterinary Journal 13.5 (2023), 613-619. Print. doi:10.5455/OVJ.2023.v13.i5.14 APA (American Psychological Association) Style Maulydia, M., Rehatta, . N. M. & Sudarmo, . S. M. (2023) Effects of glutamine and arginine combination on pro- and anti-inflammatory cytokines. Open Veterinary Journal, 13 (5), 613-619. doi:10.5455/OVJ.2023.v13.i5.14 |