| Research Article | ||
Open Vet. J.. 2025; 15(8): 3527-3540 Open Veterinary Journal, (2025), Vol. 15(8): 3527-3540 Research Article Hematological, biochemical, and antioxidant status in sheep with skin disorders suffering from zinc, copper, and vitamin A deficienciesAlshimaa Atef Mustafa Mohamed*, Aziza Mohamed Elsayed Eissa, Shimaa Mohamed Gouda and Ahmed Shehta Mohamed AliDepartment of Animal Medicine, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt *Corresponding Author: Alshimaa Atef Mustafa Mohamed, Department of Animal Medicine, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt. Email: alshimaaat896 [at] gmail.com Submitted: 03/06/2025 Revised: 13/07/2025 Accepted: 20/07/2025 Published: 31/08/2025 © 2025 Open Veterinary Journal
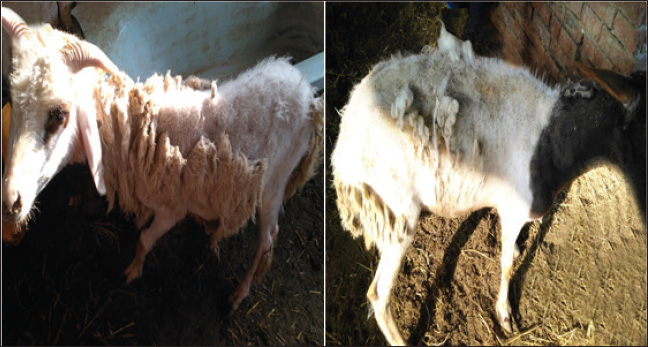
ABSTRACTBackground: A number of elements are essential for the body, including zinc (Zn), copper (Cu), vitamin A, and vitamin E. Nutritional deficiency diseases (ND diseases) are ailments that reduce the amount of animals produced, restrict commerce, and can result in severe economic losses. Mineral deficiencies, whether primary or secondary, create significant obstacles in livestock production because they are associated with decreased productivity, reproductive issues, and various health complications. Aim: This study aimed to determine the levels of Zn, Cu, and vitamin A, as well as the clinical signs, hematological, biochemical, and antioxidant changes that may occur due to the nutritional deficiencies of such trace elements and vitamin A in sheep. Methods: Several blood parameters were measured in normal or nutritionally deficient sheep. Such parameters included hematological levels, glucose, total protein, albumin, globulin, and oxidative stress GSH-Px, SOD, and CAT. This study was conducted on 100 sheep (male and female). Eighty sheep suffered from Zn, Cu, and vitamin A deficiency, and 20 were clinically healthy and served as the control group. Results: The diseased animals had the following clinical signs: depression, pica, pale mucous membrane, loss of hair around the eye, ear, and nose, erected wool, easily detached wool, depigmentation of the coat with apparent alopecia, decreased body weight gain with normal or decreased appetite. Some sheep were suffering from gait stiffness and parakeratosis with a marked increase in heart and respiratory rates, with normal body temperature. The obtained results showed significant decreases (p < 0.05) in Hb, PCV, RBCs, and WBCs in addition to significant decreases (p < 0.05) in glucose and total protein. Significant decreases in the levels of antioxidant enzymes (GSH-Px, CAT, and SOD) were also recorded. Conclusion: These results indicate that nutritional deficiencies of the trace elements Zn, Cu, and vitamin A make sheep liable to several skin disorders. Therefore, dietary supplementation of such macro and micro-elements is highly recommended to avoid such adverse effects. Keywords: Nutritional deficiencies, Zinc, Copper, Vitamin A, Skin disorders, Sheep. IntroductionSheep are responsible for 40% of cash income and 19% of household meat consumption. The most common sheep breeds in Egypt are the Rahmani, Ossimi, and Barki varieties, which account for approximately 65% of the livestock population in Egypt. According to Tefera (2004), they play a significant role in the production of wool, which is used in the leather industry and the production of meat. The skin performs several functions, including acting as an enclosing barrier, providing protection from the environment, regulating temperature, producing pigment and vitamin D, and providing sensory perception. The skin is the largest and heaviest single organ in the body, accounting for 12%–24% of an animal’s total body weight. The skin is much more than just an outer covering. According to van Beeck et al. (2015), its purpose is to keep the body in a state of homeostasis despite the daily assaults that come from the outside. Protein, vitamin A, vitamin E, essential fatty acids (EFAs), zinc (Zn), and copper (Cu) are some of the nutrients that can affect the skin and hair coat health of an animal. The coat of an animal is a reflection of its overall health and nutrition (Postnov et al., 2022). Any factor that affects the body has a direct effect on wool production, such as any systemic disease, stress, or nutritional deficiency that leads to wool weakening and fiber thinning, which causes wool breakage at any weak point. Skin diseases have a significant impact on the efficiency and quality of sheep wool production, which is an essential product for the sheep industry (Scott, 2015). Skin diseases resulting from different causes mostly present with a similar clinical appearance. The veterinarian must use a systemic approach to successfully detect skin problems and arrive at an accurate diagnosis by obtaining a complete history, performing a thorough physical examination, and using one or more simple diagnostic techniques (Carmalt et al., 2004). Infectious causes include parasitic causes (lice and mange), fungal infestation (ringworm), and viral infection (papillomatosis, lumpy skin disease, and contagious pustular dermatitis) (Prohic et al., 2022). According to Kusiluka et al. (1996), skin destruction caused by all infectious agents, including external parasites, fungi, bacteria, and viruses, results in a loss of mechanical protection between the animal and its environment. In turn, this makes it easier for other diseases to infiltrate the animal, which can have a negative impact on the animal’s overall health. Additionally, nutritional deficiencies, such as Cu, Zn, and vitamin A, can affect the skin and wool of animals. The proportions of various components, including proteins, lipids, minerals, and vitamins, affect the structure of wool. According to Ambilo and Melaku (2013), wool quality is indicated by its fiber diameter, yield, crimp, color, and strength. Any disruption in these nutrients can affect the skin’s barrier function and immune protection. Clinical signs of nutritional deficiencies in animals include diarrhea, anemia, alopecia, depigmentation, seborrhea, hyperkeratosis, parakeratosis, anorexia, fertility problems, development problems in young animals, decreased production, tetany, decreased protein synthesis, immune system insufficiencies, and pica (Antoniou et al., 2022). The term “Cu deficiency” refers to a condition in which the body is unable to absorb Cu in the digestive tract for a variety of reasons, the most important of which is the interplay between Cu, molybdenum, and sulfur. Copper thiomolybdate (CuMoS4) is formed when these three elements bind to each other, which prevents Cu from being absorbed and leads to the subsequent problem. In addition, its deficiency in soil induces swayback or enzootic ataxia, and the consequence of its deficiency is keratinization in the wool follicles. Depigmentation takes the form of a grayish-brown discoloration of the coat, particularly around the ear margins and eyes, giving the impression of a “spectacle eye” (Sándor et al., 2021). The availability of Zn and its capacity to be absorbed are all affected by calcium, phosphorus, iron, and molybdenum. The signs of Zn deficiency-induced parakeratosis of the skin in lambs suffering from wool eating sickness include growth retardation, diarrhea, poor appetite, salivation, malformed hooves, swollen joints, stiff gait, and loss of wool (Mayorga, 2021). A lack of vitamin A can result in a number of different cutaneous symptoms, one of which is phrynoderma, which is characterized by rough, hyperkeratotic, and follicular papules on the skin of the elbows and knees (Bondan et al., 2021). The primary objective of this research was to investigate the status of several biochemical, hematological, and antioxidant parameters in sheep suffering from nutritional deficiencies, including Zn, Cu, and vitamin A, in relation to certain skin diseases. Materials and MethodsAnimalsA survey was carried out on 100 sheep, and the results showed that both sexes of sheep aged 5 months to 3 years had skin problems. The sheep were collected from a variety of locations, including the Faculty of Veterinary Medicine at Zagazig University, private clinics, and private farms, and their weight ranged from 20 to 40 kg. The clinical examinations that were performed on each of the 100 cases included taking the body temperature, pulse rate, respiration rate, ruminal motions, mucous membrane health, and wool condition. Twenty sheep that appeared to be in good health were employed as a quality control. All samples were collected from sheep herds located in the vicinity of Sharkia Governorate from May to September 2023. Clinical examinationAll animals, including healthy and diseased animals, were subjected to general physical examination, including temperature, pulse, respiration, ruminal movement, and mucous membrane examination, as described by Radadostits et al. (2007). A thorough examination of the complete hair coat and skin is required for a good dermatological examination under high lighting. To examine the skin of large animals, it may be necessary to use flashlights or an ultraviolet lamp. An examination of the animal’s ventrum is essential because this is where most initial lesions and cutaneous parasites are discovered. A description of the affected location (such as mucocutaneous or truncal) is followed by a description of the gross lesions, which can be described as focal, multifocal, or diffuse in distribution. Lesions can be further classified as primary (macules or patches, papules, pustules, vesicles, wheals, and nodules) or secondary (epidermal collarettes, scars, excoriation, erosions or ulcers, and fissures) when examined more closely. Primary lesions are lesions found on the skin. Other potential lesions that may be present include alopecia, scale, follicular casts, blackheads, pigment alterations, and erythema (Lavari et al., 2022). According to Boelsma et al. (2003), for the best results, a healthy coat should be lustrous and smooth, rather than brittle or harsh, and healthy skin should be elastic and transparent, rather than oily, flaky, or bumpy. Blood collectionBlood samples were collected from the jugular vein using a disposable syringe with a capacity of 10 ml. The serum was separated by centrifugation at a speed of 3,000 revolutions per minute and stored at a temperature of −20°C. Whole blood examinationUsing an automatic cell counter, we were able to determine the CBC, which included the total erythrocyte count (RBCs), total leukocyte count (WBCs), differentiated leucocytic count, hemoglobin (Hb) level, and packed cell volume (PCV). To obtain a comprehensive blood picture, a clean, dry, and labeled EDTA tube was used to collect the initial blood sample, which was approximately 2 ml in volume (Jiwuba et al., 2022). Serum biochemical analysisAfter allowing approximately 10 ml of blood to flow freely and gently over the inner surface of a clean and dry centrifuge tube, a second blood sample was obtained for serum biochemical analysis. This analysis included the determination of serum glucose, total protein, albumin, and globulin levels. The sample was allowed to coagulate at room temperature for approximately 2 h in a slanting position. Subsequently, the sample was centrifuged at a speed of three thousand revolutions per minute for 10 min. The clear sera were carefully aspirated using an automatic pipette. The clear sera were then transferred into clear dry Eppendorf tubes and stored at 20°C until examination (An et al., 2022). Determination of serum trace elements and vitaminsIn accordance with the methodology outlined in Anderson and Rings (2008), the trace elements “Cu, Zn, and Vit A” were identified by the utilization of an atomic absorption spectrophotometer “Model 210 VGP” and the utilization of specialized kits that were provided by Buck Scientific Company in the United States. The samples were examined in the central laboratory of the Faculty of Veterinary Medicine, Zagazig University. Determination of some serum antioxidant enzymesMeasurements were used to determine the levels of several antioxidant enzymes in the serum. These enzymes included glutathione peroxidase (GSH-Px), catalase (CAT), and superoxide dismutase (SOD). The serum glutathione content was evaluated using the method described by Haddad et al. (2022). The amount of CAT was determined using the colorimetric approach in accordance with (Aebi, 1984). Skin biopsyThe procedure for the skin biopsy involves cutting the hair shorter with scissors and injecting 2% lidocaine into the subcutaneous tissue (Abramo et al., 2001). A sufficient amount of lidocaine is injected to create a slight bump, often between 0.5 and 1 ml per site. Using forceps or a needle with a 25-g capacity, hold the edge of the sample and pull it up and out. The biopsy punch was then placed in the middle of the area. Place the sample immediately in formalin at a concentration of 10% (Colombo et al., 2012). An inspection using skin scraping involves scraping the afflicted area’s border until blood begins to seep out (Nardoni et al., 2013). To maximize the possibility of ectoparasite detection, several sites were eliminated from consideration. Direct microscopical inspection was utilized to identify an ectoparasite infestation (Mueller et al., 2022). A few drops of a 10% NaOH solution were added to the sample, a cover slip was applied, and debris removal was allowed to continue for 15–30 minutes prior to microscopic analysis to identify fungal infection in the form of spores or hyphae (Muller et al., 2011). Wool and hair samples: A tiny bundle of hair or wool was taken and thoroughly cleaned using a detergent that did not include any minerals. Subsequently, the bundle was rinsed multiple times in distilled water until the filtrate appeared clean. Finally, the bundle was rinsed with double-distilled water. To remove any oily substances, the hair and wool samples were washed twice using a mixture of acetone and alcohol composed of 50% each. The samples were placed in Petri dishes and dried in a hot air oven at a temperature of 100°C for approximately 3 hours. After drying, the samples were placed in clean, dry plastic bags and tagged with the first use date until they were ashed (Deb-Choudhury et al., 2016). Statistical analysisThe mean values and standard errors were calculated for the statistical analysis. JMP software from the United States was used for the statistical analysis, which consisted of a one-way analysis of variance followed by a Tukey’s HSD post-hoc test (Cox, 1972). Ethical approvalExperiments were performed according to the ethical guidelines of Zagazig University, Egypt. The ethical approval number is ZU-IACU/2/F/134/2023. ResultsAfter clinical and laboratory examination, the examined sheep in this survey were classified into two groups: control and diseased groups, as described in Table 1. Clinical examinationSheep appeared healthy “group 1”This group included 20 sheep that were used as a control group. The clinical examination of this group revealed good healthy condition which represented in “good appetite, regular increase in body weight gain, shiny coat, shiny eyes, and normal defecation in the form of small hard pellets” the systemic states included body temperature, heart rate, and respiratory rates were within the normal range, as shown in Table 2. Sheep suffering from Cu, Zn, and vit A “group 2”This group consists of 80 sheep of different ages ranging from 5 months to 3 years, in which there was no increase in body weight despite normal appetite, and in some cases, there was a decrease in feed intake causing unthriftiness (Fig. 1). Other sheep were suffering from depigmentation of wool either all over the body as brown wool converted to blushed red or around the eyes (Fig. 2). Easily detached wool (Fig. 3), diffused alopecia (Fig. 4). In some cases, there was a straightness and stringiness of wool “steely wool” (Fig. 5). Some sheep revealed staggering in gait. Some animals in this group had soft to watery feces. Table 1. Animal groups under experimentation.
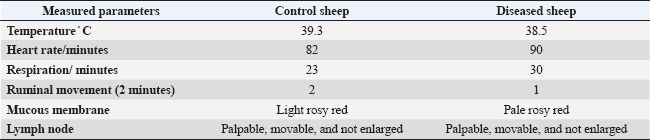
Table 2. Vital signs in apparently healthy sheep and sheep suffering from trace element deficiency.
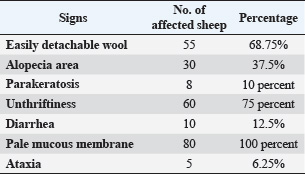
Diseased sheep suffering from trace element deficiency exhibited different signs, with some animals showing only one sign and others showing multiple signs. The most common sign among herds suffering from trace element deficiency was unthriftiness. All diseased sheep showed a pale mucous membrane. The lowest signs were ataxia, tip-toe, and parakeratosis (Table 3). When compared with the control group, the hematological parameters exhibited a significant decrease (p < 0.05) in the erythrocyte count (RBCs count), hemoglobin concentration (Hb), and packed cell volume. On the other hand, the results indicated a significant difference in the mean corpuscular volume (MCV) of serum in sheep with Zn, Cu, and vitamin A deficiency. The mean type of anemia was normocytic normochromic (Table 4). Animals suffering from nutritional deficiencies showed a considerable decrease in their serum glucose levels, and there was a minor decrease in the serum albumin levels in animals lacking Zn (Table 5). The results of the study indicated a noteworthy decrease, with a p-value of 0.05, in the concentration of Zn, Cu, and vitamin A in the serum of sheep that were exhibiting symptoms of Zn, Cu, and vitamin A insufficiency when compared with the control group (Table 6). The result showed a significant decrease (p < 0.05) of glutathione and CAT levels in animals with Zn, Cu, and vitamin A deficiency compared with the control group (Table 7). Blood smear examination was negative in the two groups, indicating that all sheep were free from blood parasites. Fecal examination was negative in all the control groups. Some of the sheep suffering from trace element deficiency were positive for gastrointestinal nematodes “Trichostrongylus” egg, which was oval in shape and double-walled with a small-sized embryo, and there was a distance between the embryo and the egg wall. Scraps taken from the animals showed alopecia, revealing that it was free from external parasites. DiscussionRecent advances in veterinary medicine have focused on ways to increase the quality and quantity of meat, milk, and wool while lowering food costs. This can only be achieved by improving the growth rate, food utilization efficiency, and productivity. In addition, minerals and vitamins play a crucial role in metabolism as cofactors, coenzymes, or as constituents of other enzymes, activating certain enzymes, and stabilizing secondary molecular structures (Anderson and Rings, 2008). In our study, the diseased animals (Zn, Cu, and vitamin A deficiency) suffered from depression, pica, pale mucous membrane, loss of hair around the eye, ear, and nose, erected wool, easily detached wool, depigmentation of the coat with apparent alopecia, and decreased body weight gain with normal or decreased appetite. Some sheep are suffering from gait stiffness, unthriftiness, diarrhea, and parakeratosis. Cu deficiency is linked to anemia, diarrhea, growth retardation, wool color changes, long bone fragility, and possibly death (Constable et al., 2016). Zn and Cu deficiencies adversely affect the endocrine system (Underwood and Suttle, 2001). Additionally, variations in the amounts of numerous trace elements in blood, urine, and other tissues are connected to or impacted by variations in the blood concentrations of different hormones. Zn is essential for hormone synthesis and is a crucial component of animal nutrition (Constable et al., 2016). Zn deficiency results in reduced feed intake, decreased appetite, and problems in synthesizing proteins with sulfur-containing amino acids (Yousef et al., 2001). Anorexia is one of the primary clinical characteristics of Zn-deficient lambs. A lack of trace elements may cause lambs, sheep, and goats to eat wool. Moreover, sheep with Zn deficiency exhibit severe alopecia and wool-eating syndrome (Bida and Garba, 2012). In lambs that ate wool, Suliman et al. (1988) observed a drop in serum Zn levels but no change in Cu levels. According to some studies, sheep, lambs, and goats that are fed specific rations that are low in Zn, Cu, sulfur, and trace elements over an extended period of time may suffer from deficient symptoms, such as alopecia and wool chewing (Youde and Huaitao, 2001).
Fig. 1. Sheep suffering from unthriftiness. In the current study, the sheep had alopecia, which was dispersed across the body, including the lumbar area, axillae, fatty tail, and both sides of the neck. In most cases, the coat was rough, the black or brown wool was steely, and the wool was easily removed and displayed depigmentation or discoloration. Alopecia and wool eating have been observed in Zn-deficient animals (Nelson et al., 1985), whereas White (2004) reported poor wool growth and improper keratinization of wool fibers in Zn-deficient sheep. Additionally, our serum Zn results were consistent with those of Bires et al. (1991), El-Sangary (1999), and Nelson et al. (1985). Zn deficiency is linked to decreased growth rate, decreased reproductive performance, poor immune responses, and skin infections (El-Attar, 1979; Chan et al., 1998; Gooneratne et al., 1989; Mozaffari and Derakhshanfar, 2007). Harold et al. (2011) also reported that Zn, as a trace element, plays an important role in numerous enzymatic reactions. A correlation exists between Zn deficiency and alopecia. Hefnawy et al. (2018) found that the skin contains a higher amount of Zn, particularly in the epidermis, than in the dermis, which is necessary for epidermal cell proliferation and differentiation. Watson (1998) noted that Zn plays a role in the immune system and vitamin A metabolism, which may result in a decreased serum vitamin A level in cases of Zn insufficiency. In addition, we found a significant decrease in serum Zn and Cu levels as well as a significant decrease in serum vitamin A levels in patients with noninfectious skin infections. The link between vit A metabolism and Zn metalloenzymes are necessary for the conversion of retinol to vit. Aldehyde (Underwood and Shuttle, 2000). According to Berger (2002), Zn is present in approximately 300 enzyme components. Alopecia, crusting, and scaling are symptoms of Zn insufficiency. Zn promotes wound healing, keratin synthesis, epidermal cell proliferation, and cellular integrity. Epidermal keratinocytes proliferate and differentiate (Ogawa et al., 2016). The symptoms we noticed in our survey were matched with those recorded by Kendall et al. (2000) as parakeratosis, wrinkled skin, wool loss, and alopecia.
Fig. 2. Sheep suffering from coat depigmentation and wool depigmentation around the eyes.
Fig. 3. Sheep suffering from easily detached wool.
Fig. 4. Sheep suffering from diffused alopecia. Our study revealed that the mean body temperature, heart rate, respiration rate, and ruminal movement were 38.5ºC, 90/minutes, 30/minutes, and 1/2 minutes, respectively. Our results were in conformity with the data acquired by Sobiech and Kuleta (2002), who indicated that sheep showed normal appetite, whereas in our experiment, there was a decrease in sheep appetite. Because of anemia caused by a lack of Cu and iron, the mucous membrane was paler than usual. Although our investigation found negative interior and external parasites as well as a lack of Cu and iron, pale mucous membranes, and anemia severity are linked to gastrointestinal nematodes. Alopecia, parakeratosis, alterations in the coat (steely wool), a minor decrease in appetite, a drop in body weight gain, locomotor disruption, and anxious symptoms were among the other clinical indicators displayed by the afflicted sheep. Similar findings have been documented in other related studies (Abd El-Raof and Ghanem, 2006; Aitken, 2008).
Fig. 5. Sheep suffering from steely wool. Table 3. Incidence of different clinical signs in sheep under investigation.
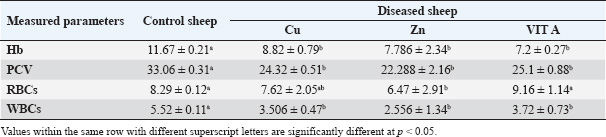
Our study revealed a significant decrease in hematological parameters (Hb, RBCs, WBCs, and PCV). This decrease might be due to a disturbance in the regular metabolism of iron as Cu deficiency decreases the absorption of iron, releasing of iron from body stores, and utilization in Hb synthesis (Abd El-Raof and Ghanem, 2006; Pond et al., 2004). However, this could be linked to a decrease in the level of serum ceruloplasmin, which moves iron from intestinal and liver storage cells to transferase in the plasma. Enzyme transferase reduces the release of iron from typically damaged erythrocytes and delivers iron to the bone marrow for Hb synthesis (Sharma et al., 2005; Kaneko et al., 2008). In the current study, normochromic normocytic anemia was detected in the blood of the affected sheep. The low serum Cu levels suggest that the ewes were fed a low-concentration diet (Pugh and Baird, 2012; Smith, 2014). Furthermore, increasing calcium and phosphorus consumption is the primary cause of Zn deficiency in sheep because it reduces Zn absorption. According to the current clinical assessment, most farmers only feed sheep large concentrations of grains, especially barley, which may cause a Zn shortage. The mean mineral value results of the current study are consistent with those published by Pugh and Baird (2012). The role of trace elements in hematopoiesis and immunity is responsible for this hematological change. Hypochromic microcytic anemia develops in the advanced stages of hypocuprosis due to poor iron absorption and Hb synthesis failure. In addition, Gehrke et al. (1998) noted that Fe is not used in Hb formation and stays in the liver until Cu is given, when anemia results from either a Cu or Fe deficit. Anemia results from hypocuprosis because less iron is released, which reduces the amount of iron available for erythropoiesis and many other processes. Cu is required for iron to be properly distributed, absorbed, and released throughout the body. Therefore, for erythropoiesis, there is less Fe available. Cu status also affects the Heinz body count and Hb concentration (Lukaski, 2004; Murray et al., 1999). In conclusion, the substantial reduction in the hematological picture highlights the critical role of trace elements in hematopoiesis, as demonstrated subsequently by serum analysis. Cu-dependent enzymes help to maintain the red cell membrane and allow iron absorption and hemosynthesis. This outcome was also reported by Mohammed et al. (2013) and Al-Nakeeb et al. (2016). Table 4. Hematological parameters in apparently healthy sheep and sheep with trace element deficiency.
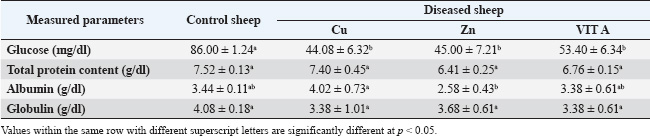
Table 5. Serum glucose, total protein, albumin, and globulin in apparently healthy sheep and sheep with trace element deficiency.
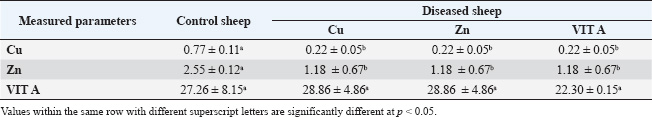
Table 6. Trace element content (ppm) in apparently healthy sheep and sheep with trace element deficiency.
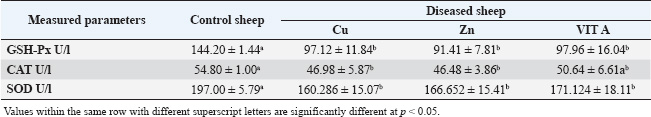
Table 7. Antioxidant enzyme levels in apparently healthy sheep and sheep suffering from trace element deficiency
Our study showed a significant decrease in serum glucose, total protein, albumin, and globulin levels. Our findings concurred with those of Rosolova et al. (1997) regarding serum glucose levels. This is explained by the disease, which causes anorexia and impaired metabolic and absorption processes, which in turn affect glucose metabolism and subsequently its level (Coles, 1986). Glucose is the energy source of the organism, and its level in the blood varies among species. The blood glucose level in sheep is between 50 and 80 mg/dL (Mert et al., 2003). To support this association between glucose and lower body weight, the present study provided further evidence as the serum glucose level in yearling rams with retarded growth was lower than that of the control. Hypoproteinemia may be caused by intestinal tract malabsorption of dietary content (Coles, 1986). The condition may be linked to a reduction in feed intake and general slackness, which worsen the condition by affecting the hepatic parenchyma and preventing protein synthesis. Albumin may be the main extracellular source of thiols, which scavenge free radicals and enable albumin to act as an antioxidant (Ganaie et al., 2013). Inadequate protein synthesis may be the cause of hypoalbuminemia in animals (El-Sangary, 1999; Nasser et al., 2000). Conversely, hypoproteinemia was linked to parasitism and a reduction in protein intake. Our findings disagreed with those of Akgul et al. (2000), who found that total serum protein had significantly decreased while albumin, globulin, and glucose levels were within the reference range. Tag El Din et al. (2022) also found that total protein and globulin levels were significantly increased, whereas albumin levels were significantly decreased. The kind of diet the animals were fed may be responsible for this. These studies supported the notion that hypoalbuminemia, a hallmark of parasite infestation in ruminants, primarily reduces the levels of most trace elements. The impairment of food digestion, absorption, and use is reflected in this decrease in serum albumin in the blood. While Amina (2002) found no significant change in serum globulin in dystrophic sheep, Rock et al. (2001) found a decrease in serum globulin in diseased sheep and attributed this decrease to the effect of selenium (Se), which increases the absorption of globulin and essential protein-digested products. Our results regarding serum globulin level coincided with their findings. In this study, there was a significant decrease in the serum Cu level compared with that of apparently healthy sheep. Our data were consistent with those of El-Sangary (1999) and Draksler et al. (2002). A greater level was observed by Ali (2000). The observed clinical indications that raised suspicions of a Cu shortage in the diseased sheep were consistent with the declining serum Cu level in this study. Reduced Hb content, PCV, and total red blood cell count were among the hematological data observed in this study; these results also suggested a Cu shortage in sick sheep (Nasser et al., 2000). Cu is necessary for immunity through energy production, neutrophil production and activity, antioxidant enzyme production, antibody development, and lymphocyte replication. Therefore, a deficiency in ruminants interferes with tissue oxidation, leading to a variety of clinical manifestations, including decreased body weight gain (Niederman et al., 1994; Nocklels, 1994). The primary causes of Cu insufficiency in infants are low maternal Cu status and inadequate milk secretion. A significant decrease in serum Zn was observed in this group compared with the apparently healthy sheep. Our results were in agreement with those obtained by Tag El Din et al. (2022), while Ali (2000) registered a higher value. Zn is an essential nutrient for animals, largely or entirely involved in enzymatic systems and protein synthesis, carbohydrate metabolism, and many other biochemical reactions. According to Attia et al. (1987), the addition of Zn oxide to the ration improves the growth rate, feed efficiency, and rumen functions of animals. Zn is necessary to increase body weight because a decrease in gain and conversion is one of the first signs of a marginal Zn deficiency and frequently appears before any change in blood or liver levels (Engle et al., 1989). Zn also affects the immune system by producing energy and proteins, stabilizing membranes against bacterial endotoxins, producing antioxidant enzymes, maintaining lymphocyte replication, and producing antibodies (Kidd et al., 1996). Antioxidants are a class of naturally occurring chemicals that can either prevent or lessen oxidative stress. Because oxygen is regularly used by the body, free radicals are continuously produced. Cell damage in the body is caused by reactive oxygen species (ROS), which are free radicals. Antioxidants are essential for shielding cells from pro-oxidant reactants produced during normal metabolic processes. The antioxidant system includes antioxidant enzymes, such as superoxide dismutase, GSH-Px, glutathione reductase, CAT, and various peroxidases; trace elements, such as Cu, iron, Zn, and Se; and vitamins, such as vitamin E and vitamin C (Singh et al., 2009; McDowell et al., 2007; Donia et al., 2014). In our study, there was a significant decrease in the serum level of GSH-PX compared with the control. The result concerning serum GSH-PX activity coincided with those obtained by Scott (2006), while Amina (2002) reported a higher level. According to Zago and Oteiza (2001), Zn and Cu are involved in antioxidant mechanisms, such as Cu-Zn SOD, and protect against iron-induced lipid peroxidation. This explains the correlation between Zn and Cu deficiency and lower SOD serum levels. Our results showed a significant decrease in both GSH-PX, CAT, and SOD levels. According to Jenkins et al. (1982), the sheep’s age, sex, and breed all contributed to the differences in serum enzymatic activity. Fecal examination was negative in all the control groups. Some sheep suffering from trace element deficiency were positive for gastrointestinal nematodes “Trichostrongylus”. Due to malabsorption, impaired food assimilation, and interference with the delivery of micronutrients by parasite toxins, parasitic infestations, particularly those involving gastrointestinal nematodes, coccidiosis, and lice, decrease the concentration of Fe, Cu, and Zn. Internal parasites can decrease the solubility of Cu in the abomasum by up to 70%, decrease the subsequent uptake of dissolved Cu by the liver by up to 50%, and increase Cu losses in the animal. Heavy worm burdens can also impair Cu uptake by changing the PH in the gut, which makes Cu less soluble (Lazzaro, 2005). According to Saleh (2007), animals affected with internal parasites had lower mean values of minerals, particularly trace elements. Our results showed a significant decrease in Cu, Zn, and vitamin A levels. According to the data, the overall ratio content of Cu, Zn, and vit A is significantly lower than the NRC’s 2007 assessment of the sheep’s appropriate requirement. Compared with the results obtained by Amina (2002), the present values were significantly lower. According to Suttle (2010), the “primary deficiency” is caused by a diet lacking certain components. Our study revealed decreased mean values of Cu, Zn, and vit A in the sera. Serum Cu levels in the study’s obviously healthy sheep were in line with those found by Ali (2000) and Amina (2002), although Green et al. (1995) found higher levels (108 ± 8.4 ug/ml and 12 ug/ml, respectively). The serum Zn level results from Barge and Mazzaco (1982) were consistent with the results from the sheep in this investigation that appeared to be in good condition; however, Eassa (1987) recorded a lower value for serum Zn (0.96 ± 0.06 ug/ml). The aforementioned variances may be related to the sheep’s breed, age, live body weight, or performance, even when they fall within the typical range. ConclusionThis study indicated a significant reduction in hematological parameters, glucose, and antioxidant levels in sheep suffering from trace element deficiency, which makes them highly susceptible to diseases. Therefore, regular Cu supplementation, Zn, and vitamins are highly recommended to reduce the incidence of skin diseases in sheep. FundingNot available Authors’ contributionsAll authors contributed equally. Conflict of interestThe authors declare that there is no conflict of interest. Data availabilityAll related data are included in the manuscript. ReferencesAbd El-Raof, Y.M. and Ghanem, M.M. 2006. Clinical and haemato-biochemical studies on cases of alopecia in sheep due to deficiency of some trace elements. Suez Canal Vet. Med. J. 10(1), 17–25. Abramo, F., Vercelli, A. and Mancianti, F. 2001. Two cases of dermatophytic pseudomycetoma in the dog: an immunohistochemical study. Vet. Dermatol. 12(4), 203–207. Aebi, H. 1984. Catalase in vitro. In Methods in enzymology. Ed., Packer, L. Philadelphia, PA: Academic Press, vol. 105, pp: 121–126. Aitken, A. 2008. Diseases of sheep. Edinburgh, UK: John Wiley & Sons. Akgul, Y., Agaoglu, Z.T., Kaya, A. and Sahin, T. 2000. The relationship between the syndromes of wool eating and alopecia in Akkaraman and Morkaraman sheep fed corn silage and blood changes (Haematological, Biochemical and Trace elements). Isr. Vet. Med. Assoc. 56(1), 102–110. Ali, A.A. 2000. Influence of some diseased conditions on blood serum levels of antioxidant vitamin and some trace elements of Egyptian balady sheep in Assuit Governorate. Assuit Vet. Med. J. 42(84), 120–133. Al-Nakeeb, N.K., Judi, A.M.H. and Jassim, A. 2016. Clinical and hematological study of experimentally induced secondary copper deficiency in sheep. AL-Qadisiya J. Vet. Med. Sci. 15(1), 45–50. Ambilo, A. and Melaku, A. 2013. Major skin diseases of cattle: prevalence and risk factors in and around Hawassa, Southern Ethiopia. J. Adv. Vet. Res. 3(4), 147–153. Amina, E.M.F. 2002. Clinico-biochemical and diagnostic studies on some nutritional deficiency problems in sheep and its treatment. Ph.D. Thesis Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt. An, J., Yang, M., Kang, J.H., Ku, J.Y., Cha, S.E., Seo, M., Park, K.M. and Park, J. 2022. Age-related changes in the electrophoresis pattern of serum proteins in Korean indigenous calves. Can. J. Vet. Res. 86(3), 233–237. Anderson, D.E. and Rings, M. 2008. Current veterinary therapy: food animal practice. St Louise, MO: Elsevier Health Sciences. Antoniou, E., Dagla, M., Iliadou, M., Palaska, E. and Orovou, E. 2022. Early Introduction of solid foods in infant’s nutrition and long-term effects on childhood: a systematic. Int. J. Innov. Res. Med. Sci. 7, 12. Attia, E.A. 2007. Studies on nutritional deficiencies in sheep and goats in Sharkia Governorate. M.V.Sc. Thesis, Faculty of Veterinary Medicine, Zagazig University. Barge, M.T. and Mazzocco, P. 1982. The functional activity of zinc in the feeding of ruminants of economic importance – 2nd Part – experimental deficiency by full‐grown ewes. Z. Tierphysiol. Tierernahr. Futtermittelkd. 48(1–5), 36–46. Berger, L.L. 2002. Zinc Nutritional and pharmacological roles. In: Salt and Trace Minerals, Salt Institute for the Animal Nutrition Professional, Alexandria, VA, 34(3), 1– 3. Bida, A. and Garba, M. 2012. Zinc deficiency (hypozincemia) in a lamb: clinical field case. Int. J. Agro Vet. Med. Sci. 6, 349–352. Bires, J., Kovac, G. and Vrzgula, L. 1991. Mineral profile of serum in experimental copper intoxication of sheep from industrial emissions. Vet. Hum. Toxicol. 33(5), 431–435. Boelsma, E., Van De Vijver, L.P., Goldbohm, R.A., Klöpping-Ketelaars, I.A., Hendriks, H.F. and Roza, L. 2003. Human skin condition and its associations with nutrient concentrations in serum and diet. Am. J. Clin. Nutr. 77(2), 348–355. Bondan, C., Folchini, J.A., Guimarães, L., Noro, M., Zanella, R., Alves, L.P., Fontaneli, R.S. and Gonzalez, F. 2021. Milk yield and composition in dairy cows with post-partum disorders. Arq. Bras. Med. Vet. Zootec. 73(3), 639–646. Carmalt, J.L., Ashburner, S.J. and Clark, T. 2004. Equine dermatology. Large Anim. Vet. Rounds 4(8), 1–5. Chan, S., Gerson, B. and Subramaniam, S. 1998. The role of copper, molybdenum, selenium, and zinc in nutrition and health. Clin. Lab. Med. 18, 673–685. Coles, E.H. 1986. Veterinary clinical pathology. 4th ed. London, UK: WB Saunders Company, pp: 136–70. Colombo, S., Scarampella, F., Ordeix, L. and Roccabianca, P. 2012. Dermatophytosis and papular eosinophilic/mastocytic dermatitis (urticaria pigmentosa-like dermatitis) in three Devon Rex cats. J. Feline Med. Surg. 14(7), 498–502. Constable, P.D., Hinchcliff, K.W., Done, S.H. and Grünberg, W. 2016. Veterinary medicine: a textbook of the diseases of cattle, horses, sheep, pigs and goats. St Louise, MO: Elsevier Health Sciences. Cox, D.R. 1972. Regression models and life-tables. J. Roy. Stat. Soc. Ser. B Stat. Methodol. 34, 187–202. Deb-Choudhury, S., Plowman, J.E. and Harland, D.P. 2016. Isolation and analysis of keratins and keratin associated proteins from hair and wool. In Methods in enzymology. Eds., Bishr Omary, M. and Liem, RKH. Philadelphia, PA: Academic Press, vol. 568, pp: 279–301. Donia, G.R.; Wassif, I.M.; El Ebissy, I.A. 2014. Impact of some environmental factors and microbes causing respiratory diseases on antioxidants levels in small ruminants. Glob. Vet. J. 12(3), 299–306. Draksler, D., Núñez, M., Apella, M.C., Agüero, G. and González, S. 2002. Copper deficiency in Creole goat kids. Reprod. Nutr. Develop. 42, 243–249. Eassa, A. 1987. Wool and hair as an effective tool for diagnosis of some deficiency diseases and environmental pollution. Ph.D. Thesis Faculty of Veterinary Medicine, Zagazig University. El- Sangary, F.H.M. 1999. Studies on causes, diagnosis, biochemical changes and treatment of unthriftiness in sheep. Ph.D. Thesis, Faculty of Veterinary Medicine, Zagazig University. El-Attar, H.M. 1979. Zinc deficiency and nervous manifestation in lambs, 4th ed. Zagazig, Egypt: Zagazig University. El-Sangary, F.H.M. 1999. Studies on causes, diagnosis, biochemical changes and treatment of unthrifitness in sheep. Ph.D. thesis Zagazig University, Zagazig, Egypt. Engle, T.E., Nockels, C.F., Kimberling, C.V., Weaber, D.L. and Johnson, A.B. 1997. Zinc repletion with organic or inorganic forms of zinc and protein turnover in marginally zinc-deficient calves. J. Anim. Sci. 75, 3074–3081. Ganaie, A.H., Shanker, G., Bumla, N.A., Ghasura, R.S., Mir, N.A., Wani, S.A. and Dudhatra, G.B. 2013. Biochemical and physiological changes during thermal stress in bovines. J. Vet. Sci. Technol. 4(126), 126–132. Gerke, H.H., Molson, J.W. and Frind, E.O. 1998. Modelling the effect of chemical heterogeneity on acidification and solute leaching in overburden mine spoils. J. Hydrol. 209(1–4), 166–185. Gooneratne, S.R., Buckley, W.T. and Christensen, D.A. 1989. Review of copper deficiency and metabolism in ruminants. Can. J. Anim. Sci. 69, 819–845. Green, L.E., Hovers, K., French, N.P., Jore, S., Taylor, A. and Morgan, L. 1995. Sub capsular liver rupture in young lambs associated with vit. E deficiency. Vet. Rec. 25(136), 197–198. Haddad, M., Hervé, V., Ben Khedher, M.R., Rabanel, J.M. and Ramassamy, C. 2021. Glutathione: an old and small molecule with great functions and new applications in the brain and in Alzheimer’s disease. Antioxid. Redox Signal 35(4), 270–292. Harlod, E., James, A., Doglas, C., Cherly, L., Franklin, M. and Glenn, H. 2011. The merck veterinary manual, 11th ed. Rahaway, NJ: Merck & CO., Inc.. Hefnawy, A., Helal, M.A.Y., Sabry, I. and Abdelraoof, Y. 2018. The relationships between ruminal juice, urine, serum and fecal zinc in experimentally zinc deficient ossimi lambs. Assiut Vet. Med. J. 64, 25–31. Jenkins, S.J., Green, S.A. and Clark, P.A. 1982. Clinical chemistry reference values of normal domestic animals in various age groups. Cornell Vet. 72, 403. Jiwuba, P.C., Ikwunze, K., Jiwuba, L.C., Okoye, L.E., Amaduruonye, W., Ilo, S.U., Okah, U. and Ahamefule, F.O. 2022. Haematology and serum biochemistry of West African Dwarf goats fed Pleurotus tuber-regium-treated cassava root sievate-based diets. Trop. Anim. Health Prod. 54(4), 217. Kaneko, J.J., Harvey, J.W. and Bruss, M.L. 2008. Clinical biochemistry of domestic animals, 6th ed. London, UK: Elsevier Inc. Kendall, N.R., McMullen, S., Green, A. and Rodway, R.G. 2000. The effect of zinc, cobalt and selenium soluble glass bolus on trace element status and semen quality of ram lambs. Anim. Reprod. Sci. 62, 277–283. Kidd, M.T., Ferket, P.R. and Qureshi, M.A. 1996. Zinc metabolism with special reference to its role in immunity. World’s Poult. Sci. J. 52, 309–324. Kusiluka, L.J.M., Kambarage, D.M., Matthewman, R.W., Harrison, L.J.S. and Daborn, C.J. 1996. Coccidiosis of small ruminants in Tanzania. Small Rumin. Res. 21(2), 127–131. Lavari, A., Eidi, S. and Soltani, M. 2022. Molecular diagnosis of dermatophyte isolates from canine and feline dermatophytosis in Northeast Iran. Vet. Med. Sci. 8(2), 492–497. Lazzaro, L. 2005. Basic information on copper deficiency in daiy goats in southern California Saanendoah Dairy goats Winchester/Temecula, California. Lukaski, H.C. 2004. Vitamin and mineral status: effects on physical performance. Nutrition 20(7–8), 632–644. Mayorga Mc Donald, R. 2021. Control migratorio y derechos fundamentales en la Constitución chilena: algunas consideraciones para el proceso constituyente. Estud. Const. 19(2), 199–227. McDowell, L.R., Wilkinson, N., Madison, R. and Felix, T. 2007. Vitamins and minerals functioning as antioxidants with supplementation considerations. Florida Ruminant Nutrition Symposium. Gainesville, FL: Best Western Gateway Grand, 52(3), pp: 1–17. Mert, N., Gündüz, H., Akgündüz, V. and Akgündüz, M. 2003. Correlation between biochemical parameters and production traits in merino cross sheep III-glucose, alkaline phosphatase, ceruloplasmin. Turk. J. Vet. Anim. Sci. 27, 583–588. Mohammed, I.A.,., Gadi, J.A. and Al-Amery, M.A.Y.. 2013. Study of some minerals deficiency in grazing sheep in Thi-Qar Province. Al-Qadisiyah J. Vet. Med. Sci. 12(1), 106–112. Mozaffari, A. and Derakhshanfar, A. 2007. Zinc responsive dermatosis in an Iranian cross-breed ram. Iran. J. Vet. Res. 2, 182–183. Mueller, R.S., Rosenkrantz, W., Bensignor, E., Karaś-Tęcza, J., Paterson, T. and Shipstone, M.A. 2020. Diagnosis and treatment of demodicosis in dogs and cats: clinical consensus guidelines of the World Association for Veterinary Dermatology. Vet. Dermatol. 31(1), 5–27. Muller, A., Guaguère, E., Degorce-Rubiales, F. and Bourdoiseau, G. 2011. Dermatophytosis due to Microsporum persicolor: a retrospective study of 16 cases. Can. Vet. J. 52(4), 385. Murray, R.K., Granner, D.K., Mayes, P.A. and Rodwell, V.W. 1999. Harper’s biochemistry, 25th ed. New York, NY: McGraw Hill Lange, Citeseer. Nardoni, S., Mugnaini, L., Papini, R., Fiaschi, M. and Mancianti, F. 2013. Canine and feline dermatophytosis due to Microsporum gypseum: a retrospective study of clinical data and therapy outcome with griseofulvin. J. Mycol. Med. 23(3), 164–167. Nasser, M.H., Nassif, M., Nasr, M.Y. and Naia, A.A. 2000. Some biochemical alterations associating achromtrichia and alopecia in buffalo calves with a trial of treatment. J. Egypt. Vet. Med. Assoc. 60(30), 115–122. Nelson, L.S., Jacobs, F.A. and Brushmiller, J.G. 1985. Solubility of calcium and zinc in model solutions based on bovine and human milks. J. Inorg. Biochem. 24(4), 255–265. Niederman, C.N., Blodgett, D., Eversole, D., Schurig, G.G. and Thatcher, C.D. 1994. Effect of copper and iron on neutrophil function and humoral immunity of gestating beef cattle. J. Am. Vet. Med. Assoc. 204, 1796–1800. Nocklels, C.F. 1994. Micronutrient and immune response. In: Proceeding of the Montana Nutrition Conference Proceedings, Bozeman, Montana, p. 31 Ogawa, Y., Kawamura, T. and Shimada, S. 2016. Zinc and skin biology. Arch. Biochem. Biophys. 611, 113–119. Pond, W.G., Church, D.C., Pond, K.R. and Schoknecht, P.A. 2004. Basic animal nutrition and feeding. Hoboken, NJ: John Wiley & Sons. Postnov, D., Marsh, D.J., Cupples, W.A., Holstein-Rathlou, N.H. and Sosnovtseva, O. 2022. Synchronization in renal microcirculation unveiled with high-resolution blood flow imaging. eLife 11, e75284. Prohic, A., Doss, N., Hay, R.J., Diallo, M. and Gupta, A.K., 2021. Fungal skin infections (mycology). In Atlas of dermatology, dermatopathology and venereology: cutaneous infectious and neoplastic conditions and procedural dermatology. Cham, Switzerland: Springer International Publishing, pp. 77–111. Pugh, D.G. and Baird, N.N. 2012. Sheep & goat medicine-E-Book. St Louise, MO: Elsevier Health Sciences, Vol. 2. Radostits, O.M., Gay, C.C., Hinchcliff, K.W. and Constable, P.D. 2007. A textbook of the diseases of cattle, horses, sheep, pigs and goats. Vet. Med. 10, 2045–2050. Rock, M.J., Kincaid, R.L. and Carstens, G.E. 2001. Effects of prenatal source and level of dietary selenium on passive immunity and thermometabolism of newborn lambs. Small Rumin. Res. 40(2), 129–138. Rosolova, H., Mayer, O. and Reaven, G. 1997. Effect of variations in plasma magnesium concentration on resistance to insulin-mediated glucose disposal in non-diabetic subjects. J. Clin. Endocrinol. Metab. 82, 3783–3785. Saleh, M.A., Abou El-ela, A. and Osman, F.A. 2007. Trace elements variation in blood serum of sheep suffering from internal parasites in recently reclaimed areas (Darb Al-Arbaiyn) in the Western Egyptian desert. Assiut Vet Med J 53(112), 1–14. Sándor, E., Kolláth, I.S., Fekete, E., Bíró, V., Flipphi, M., Kovács, B., Kubicek, C.P. and Karaffa, L. 2021. Carbon-source dependent interplay of copper and manganese ions modulates the morphology and itaconic acid production in Aspergillus terreus. Front. Microbiol. 12, 680420. Scott, P. 2006. Trace element deficiencies affecting adult sheep and their progeny. UK Vet. Livestock 11(1), 45–49. Scott, P.R. 2015. Sheep medicine. London, UK: CRC Press. Sharma, M.C., Joshi, C., Pathak, N.N. and Kaur, H. 2005. Copper status and enzyme, hormone, vitamin and immune function in heifers. Res. Vet. Sci. 79(2), 113–123. Singh, K., Kaur, S., Kumari, K., Singh, G. and Kaur, A. 2009. Alterations in lipid peroxidation and certain antioxidant enzymes in different age groups under physiological conditions. J. Hum. Ecol. 27, 143–147. Smith, B.P. 2014. Large animal internal medicine-E-Book (Vol. 4). Elsevier Health Sciences. Sobiech, P. and Kuleta, Z. 2002. Usefulness of some biochemical indicators in detection of early stages of nutritional muscular dystrophy in lambs. Small Rumin. Res. 45(2), 209–215. Suliman, H.B., Abdelrahim, A.I., Zakia, A.M. and Shommein, A.M. 1988. Zinc deficiency in sheep: field cases. Trop. Anim. Health Prod. 20(1), 47–51. Suttle, N.F. 2010. Mineral nutrition of Livestock, 4th ed. Wallingford, UK: Cabi Publishing,. Tag El Din, H.A., Yousif, H.M. and Rezk, R.A. 2022. Some hormonal changes related to some trace elements deficiency in ration of male lambs. Egypt J. Anim. Health 2(1), 13–20. Tefera, S. 2004. Investigation on ectoparasites of small ruminants in selected sites of Amhara regional state and their impact on the tanning industry. Addis Ababa, Ethiopia: Addis Ababa University, Faculty of Veterinary Medicine. Underwood, E.J. and Suttle, N.F. 2001. The mineral nutrition of Livestock, 3rd ed. London: Biddles Ltd., pp. 47–63. Underwood, E.J. and Shuttle, N.F. 2000. The mineral nutrition of livestock, 3rd ed. New York, NY: CABI Publishing, pp: 421–512. Van Beeck, F.L., Watson, A., Bos, M., Biourge, V. and Willemse, T. 2015. The effect of long-term feeding of skin barrier-fortified diets on the owner-assessed incidence of atopic dermatitis symptoms in Labrador retrievers. J. Nutr. Sci. 4, e5. Watson, T.D.G. 1998. Diet and skin disease in dogs and cats. J. Nutr. 128(12), 2783S–2789S. White, C. 2004. Copper deficiency in sheep and cattle. Farm note, Australia: Department of Agriculture. Youde, H. and Huaitao, C. 2001. Studies on the pathogenesis of shimao zheng (fleece-eating) in sheep and goats. Vet. Res. Commun. 25, 631–640. Yousef, M.I., El-Hendy, H.A., El-Demerdash, F.M. and Elagamy, E.I. 2002. Dietary zinc deficiency induced-changes in the activity of enzymes and the levels of free radicals, lipids and protein electrophoretic behavior in growing rats. Toxicology 175(1–3), 223–234. Zago, M.P. and Oteiza, P.I. 2001. The antioxidant properties of zinc interactions with iron and antioxidants. Free Rad. Biol. Med. 31, 266. | ||
| How to Cite this Article |
| Pubmed Style Mohamed AAM, Eissa AME, Gouda SM, Ali ASM. Hematological, biochemical, and antioxidant status in sheep with skin disorders suffering from zinc, copper, and vitamin A deficiencies. Open Vet. J.. 2025; 15(8): 3527-3540. doi:10.5455/OVJ.2025.v15.i8.15 Web Style Mohamed AAM, Eissa AME, Gouda SM, Ali ASM. Hematological, biochemical, and antioxidant status in sheep with skin disorders suffering from zinc, copper, and vitamin A deficiencies. https://www.openveterinaryjournal.com/?mno=268708 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i8.15 AMA (American Medical Association) Style Mohamed AAM, Eissa AME, Gouda SM, Ali ASM. Hematological, biochemical, and antioxidant status in sheep with skin disorders suffering from zinc, copper, and vitamin A deficiencies. Open Vet. J.. 2025; 15(8): 3527-3540. doi:10.5455/OVJ.2025.v15.i8.15 Vancouver/ICMJE Style Mohamed AAM, Eissa AME, Gouda SM, Ali ASM. Hematological, biochemical, and antioxidant status in sheep with skin disorders suffering from zinc, copper, and vitamin A deficiencies. Open Vet. J.. (2025), [cited January 25, 2026]; 15(8): 3527-3540. doi:10.5455/OVJ.2025.v15.i8.15 Harvard Style Mohamed, A. A. M., Eissa, . A. M. E., Gouda, . S. M. & Ali, . A. S. M. (2025) Hematological, biochemical, and antioxidant status in sheep with skin disorders suffering from zinc, copper, and vitamin A deficiencies. Open Vet. J., 15 (8), 3527-3540. doi:10.5455/OVJ.2025.v15.i8.15 Turabian Style Mohamed, Alshimaa Atef Mustafa, Aziza Mohamed Elsayed Eissa, Shimaa Mohamed Gouda, and Ahmed Shehta Mohamed Ali. 2025. Hematological, biochemical, and antioxidant status in sheep with skin disorders suffering from zinc, copper, and vitamin A deficiencies. Open Veterinary Journal, 15 (8), 3527-3540. doi:10.5455/OVJ.2025.v15.i8.15 Chicago Style Mohamed, Alshimaa Atef Mustafa, Aziza Mohamed Elsayed Eissa, Shimaa Mohamed Gouda, and Ahmed Shehta Mohamed Ali. "Hematological, biochemical, and antioxidant status in sheep with skin disorders suffering from zinc, copper, and vitamin A deficiencies." Open Veterinary Journal 15 (2025), 3527-3540. doi:10.5455/OVJ.2025.v15.i8.15 MLA (The Modern Language Association) Style Mohamed, Alshimaa Atef Mustafa, Aziza Mohamed Elsayed Eissa, Shimaa Mohamed Gouda, and Ahmed Shehta Mohamed Ali. "Hematological, biochemical, and antioxidant status in sheep with skin disorders suffering from zinc, copper, and vitamin A deficiencies." Open Veterinary Journal 15.8 (2025), 3527-3540. Print. doi:10.5455/OVJ.2025.v15.i8.15 APA (American Psychological Association) Style Mohamed, A. A. M., Eissa, . A. M. E., Gouda, . S. M. & Ali, . A. S. M. (2025) Hematological, biochemical, and antioxidant status in sheep with skin disorders suffering from zinc, copper, and vitamin A deficiencies. Open Veterinary Journal, 15 (8), 3527-3540. doi:10.5455/OVJ.2025.v15.i8.15 |