| Research Article | ||
Open Vet. J.. 2025; 15(9): 4242-4247
Open Veterinary Journal, (2025), Vol. 15(9): 4242-4247 Research Article Effect of valproic acid administration on motor coordination and sensory function in Mus musculus as an autism animal modelIzzatul Fithriyah1, Irwanto Irwanto2*, Yunias Setiawati3 and Widjiati Widjiati41Doctoral Program of Medical Science, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia 2Department of Child Health, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia 3Department of Psychiatry, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia 4Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia *Corresponding Author: Irwanto Irwanto. Department of Child Health, Faculty of Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: irwanto [at] fk.unair.ac.id Submitted: 30/06/2025 Revised: 20/08/2025 Accepted: 26/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
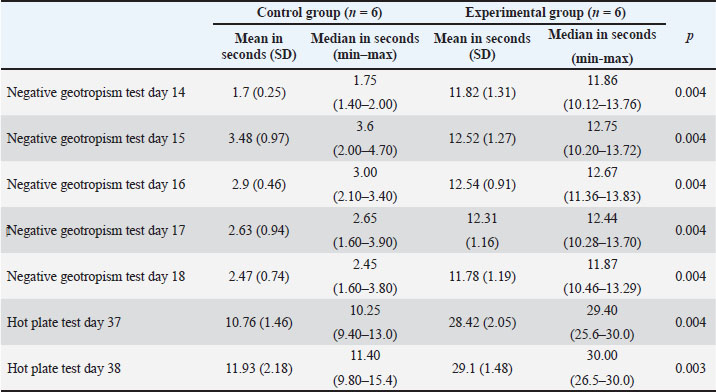
ABSTRACTBackground: The cause of autism spectrum disorder (ASD) is currently still unclear. Research on the etiology and biomolecular aspects of autism is needed to develop further prevention and therapy strategies. Animal models of autism are needed for further research. Aim: This study aimed to investigate the effect of valproic acid administration on motor coordination and sensory function in Mus musculus as an animal model of autism. Methods: This study used M. musculus that were ready to be mated and waited until they were pregnant. Randomization was carried out using the website random.org, and the participants were divided into two groups: the control and experimental groups. On embryo day 12.5, the control group was injected intraperitoneally with normal saline, and the experimental group was injected intraperitoneally with 600 mg/kg body weight of valproic acid. The offspring of the mice underwent autism symptom behavior test, motor coordination, and pain response. Results: There were significant differences in the negative geotropism test and hot plate test between the control and experimental groups (p < 0.05). The experimental group that was intraperitoneally injected with valproic acid takes longer to reorient on an inclined plane as part of motor coordination skills. The experimental group also provided a longer response time to heat stimuli on a hot plate, indicating an abnormal response to pain stimuli. Conclusion: Intraperitoneally injected M. musculus with valproic acid showed symptoms of autism, especially disorders in motor coordination and response to pain stimuli. Keywords: Animal model, Autism, Behavior, Mental health, Valproic acid. IntroductionAutism spectrum disorder (ASD) is a neurodevelopmental disorder characterized by impaired social communication and restricted and repetitive behaviors (Hirota and King, 2023). Autism is a spectrum disorder with various severities (Alpert, 2021). In its most severe forms, it can be completely disabling, requiring lifelong supportive care, often in a chronic health care institution. In milder forms, individuals with autism can lead normal, rich, and productive lives after learning coping strategies (Alpert, 2021). The incidence and prevalence of ASD are trending upwards worldwide. An estimated 1 in 100 children are diagnosed with ASD worldwide (Zeidan et al., 2022). The prevalence of ASD worldwide is 0.6% [95% confidence interval (CI): 0.4%–1%]. The prevalence of ASD in Asia, America, Europe, Africa, and Australia was 0.4% (95% CI: 0.1–1), 1% (95% CI: 0.8–1.1), 0.5% (95% CI: 0.2–1), 1% (95% CI: 0.3–3.1), and 1.7% (95% CI: 0.5–6.1), respectively (Salari et al., 2022). The cause of ASD remains unknown (Otaru and Lawrence, 2022). The etiology and pathophysiology of this disorder remain unclear (Li et al., 2021; Uddin et al., 2023; Jiao et al., 2024). Numerous environmental and genetic factors have been linked to the cause of ASD (Wong, 2022). Research in animal models is currently ongoing with the aim of modifying genetic risk factors and thereby helping to prevent autism in the future (Barik et al., 2023). Early identification of clear developmental differences may facilitate earlier interventions that may improve long-term quality of life (Cleary et al., 2023). The mechanisms influencing ASD development remain unclear (Uddin et al., 2023). Early interventions initiated before age 3 have a stronger positive impact than those initiated after age 5. Therefore, early diagnosis of ASD is very important. In addition to early diagnosis, a reliable set of ASD biomarkers is also useful in clinical practice, pathogenic processes, treatment and intervention outcomes assessment, and identifying physiologically homogeneous groups of patients with ASD (Liu et al., 2022). No single biomarker, neuroimaging finding, or genetic mutation is sufficient for an accurate diagnosis of ASD. In recent years, inflammation and oxidative stress have been implicated in ASD pathogenesis. The prevention aspect needs to be developed since in the womb (Usui et al., 2023). Oxidative stress is involved in the pathogenesis of ASD, either directly or through its interaction with inflammation and mitochondrial dysfunction. Oxidative stress biomarkers appear to have good potential to be used as diagnostic tools in the early diagnosis of ASD (Kuźniar-Pałka, 2025). Oxidative stress may be implicated in the pathophysiology of ASD (Chen et al., 2021). This study aimed to investigate the effect of valproic acid administration on motor coordination and sensory function. The results of this study are expected to provide opportunities for in vivo research on aspects of etiology and pathogenesis in animal models of autism, so that earlier diagnosis can be made in ASD and can be continued with evidence-based therapy in ASD. Materials and MethodsMaterialsThis study used valproic acid salt (Sigma-Aldrich, Burlington, Massachusetts, United States), an injection syringe, a mouse cage, a gastric tube for mice, hot plate test equipment, standardized feed, and a board for the negative geotropism test. The mice used were healthy Mus musculus from a certified institution, aged adults (8–10 weeks), and weighed between 25 and 40 g. MethodsThis study was a true experimental post-test only control group design (randomized control group) study on animal models. This study divided mice into the control (n=6) and experimental (n=6) groups. Mice were given 2 weeks of adaptation time in a cage with a wood-shaving floor. The light-dark cycle was set, the room temperature was 22 ± 2°C, and the room humidity was 65% ± 5%. Access to food and drink was free. The next procedure was to mate female mice with male mice by placing 1 male mouse and 2 female mice in one cage. Vaginal plug checks were performed every morning. Mice that had obtained vaginal plugs were calculated by fertilization on day 1 (Embryo 1) and at E12.5 were given intraperitoneal injection of normal saline in the control group, and the experimental group was injected with 600 mg/kg body weight (mg/kg) valproic acid intraperitoneally. Mice were left to give birth with free feeding. Mice were allowed to give birth and care for their offspring until they were ready to be examined for autism symptoms, namely, the negative geotropism test on days 14–18 and the hot plate test on days 37–38. The geotropism test was conducted by placing mice face down along a 45° incline in a temperature-controlled environment. The latency to turn 180° such that the head faces up along the incline was recorded with a maximum of 30 seconds for each trial. A significant increase in the time required for reorientation was observed in the experimental group on day 14 compared to controls (p < 0.05). Tested on days 37–39, rats were individually placed on a hot plate (55.0 ± 0.3°C), and the latency of the first hind paw response was recorded. A hind paw response was defined as a paw shake or paw lick. A cut-off time of 30 seconds was maintained. The nociceptive index was tested on the hot plate to determine the pain threshold to thermal stimuli on days 37–39. Statistical analysisThe Statistical Package for the Social Sciences version 25 (IBM Corp., Armonk, New York) was used for data analysis. The results were tested using the Mann–Whitney test. Ethical approvalThis research has been reviewed and received ethical approval from the Animal Care and Use Committee of the Animal Ethics Commission, Faculty of Veterinary Medicine, Universitas Airlangga, with No. 2.KEH.021.02.2024. ResultsThe results of the examination, as explained in Table 1, show a significant difference in the negative geotropism test on the examination on days 14, 15, 16, 17, and 18. In the control group that did not receive valproic acid injection, it took less time to turn up and correctly reposition on the inclined board. The experimental group of mice that received valproic acid injection showed a longer time to reposition on the inclined plane. This means that mice that received valproic acid injection have a poor coordination system, which is a symptom of autism. Table 1. Results of the negative geotropism and hot plate tests on an animal model of autism.
The hot plate test on days 37–38 also showed a significant difference in sensory function between the control and experimental groups. The hot plate test in the experimental group with valproic acid injection showed that mice took longer to respond to heat stimuli at a temperature of 50°C. This indicates that the experimental mice have higher pain stimuli. This is also a symptom of autism. DiscussionIn this study, significant differences were found in autism symptoms between the control and valproic acid-injected groups. In the embryonic period, a single intraperitoneal injection of valproic acid into 12.5-day-old female mice caused relevant autism symptoms in their offspring, brain structure, and biomarkers (Dana et al., 2020). The negative geotropism test indicates that the motor skill development process requires a good balance between sensory and motor coordination. Motor coordination develops in the first 3 weeks after birth, that is, the 13th to 30th day after birth, which is a critical period for assessing this skill. Visual, tactile, and proprioceptive reflexes need to work together for better motor performance (Zhang et al., 2019). In the experimental group that received valproic acid injection, the time required for reorientation back on the inclined plane was significantly prolonged, indicating that the ability of mice in motor coordination is less well developed. Motor disorders, some of which are related to core behavioral characteristics, are often found in children with autism (Wieting et al., 2024). Symptoms of autism include disturbances in fine and gross motor skills, poor balance, poor rhythm, greater variation in gait control, and weakness (da Silva et al., 2025). In this study, the hot plate test also showed a significant difference in the response time of the hind legs of mice when placed on the hot plate. Pain perception occurs due to the activation of the peripheral afferent pathway and through the sensory network that transmits impulses between the spinal cord and brain when exposed to mechanical, chemical, and electrical stimuli. In animals exposed to valproic acid, the nociceptive threshold increases. During pregnancy, valproic acid exposure can cause injury to the pain modulation pathway in the fetus, which can be seen in the first month of postnatal life (Zhang et al., 2019). Individuals with ASD have altered sensory processing but are unable to communicate their experiences effectively. Mice demonstrate mixed nociceptive responses with hyporesponsiveness to mechanical/thermal stimuli and intraplantar injections of formalin and capsaicin, while demonstrating hypersensitivity to acetic acid testing. Pain responses can vary depending on the sensation, mirroring findings in individuals with ASD (Martin et al., 2022). Pregnancy environmental and genetic risk factors can influence the neonatal inflammatory response, thereby altering postnatal brain development. Genetic and environmental factors can directly induce chronic neuroinflammation, which can modulate neural function and immune responses through glial activation or directly affect neural function. Valproic acid, an environmental risk factor, induces activation in multiple brain regions, with persistent glial activation in the hippocampus and cerebellum. The hippocampus and cerebellum are two brain regions associated with autism-like behaviors, that is, restricted social interaction and repetitive behaviors. Several studies have suggested that cerebellar inflammation causes altered social behavior in adult mice, as the cerebellum is thought to be involved in executive and cognitive functions (Mokra et al., 2023). Valproic acid can produce embryonic variation because the fetal brain is more susceptible to reactive oxygen species (ROS) than other fetal organs, which may be related to the immature antioxidant system. ROS play an important role in cell apoptosis (Taleb et al., 2021). The cycle of hypoxia and oxidative stress plays a key role in proper fetal development during pregnancy. An imbalance in the amount of oxidative molecules due to endogenous or exogenous factors can overwhelm the immune system and lead to excessive ROS production (Sebastiani et al., 2022). Oxidative stress and impaired antioxidant defenses play an important role in the severity of ASD (Shareef et al., 2025). Elevated malondialdehyde (MDA) levels indicate high oxidative stress. Lipid peroxidation is an important part of oxidative stress and can be described as a process in which free radicals attack lipids containing carbon–carbon double bonds, especially polyunsaturated fatty acids. Lipids are oxidized by enzymes or by ROS attack. The process produces various classic biomarkers of lipid peroxidation, such as MDA, 4-hydroxynonenal (4-HNE), and F2-isoprostane. MDA is known for its cytotoxicity, which occurs when it forms adducts with membrane proteins. MDA is also involved in DNA damage and mutation, leading to cell cycle arrest. In several studies of children with ASD, elevated MDA levels in the blood have been observed as a hallmark of lipid peroxidation. Increased MDA levels have a sensitivity of 90.4% and a specificity of 93.7% (Altun et al., 2018). MDA is not only a general indicator of oxidative stress but also has toxic effects on various cell types, including red blood cells, endothelial cells, fibroblasts, and hippocampal neurons (Nasrallah and Alzeer, 2022). Therefore, MDA may interfere with the pathophysiology of ASDs, as it is a highly neurotoxic compound that has a longer lifespan in the body than ROS. This compound damages brain function and causes cell death through two mechanisms: apoptosis and necrosis (Yui et al., 2020). Higher levels of IL-6 have been reported in the hippocampus and whole brain homogenates in an animal model of autism using Valproic acid (VPA) (Deckmann et al., 2019). Following the success of VPA using the above repertoire of behavioral tests, other causative agents can be studied to investigate new therapeutic drugs for autism (Chaliha et al., 2020). IL-6 signaling components are dysregulated in individuals with ASD (Nadeem et al., 2020). Studies have shown the critical involvement of IL-6 in triggering core symptoms associated with proinflammatory responses in the maternal immune activation model of autism (Sarieva et al., 2023). This study found significant differences in sensory and motor domains between mice injected with valproic acid as an autism model in the animal and control groups. However, our study has a few limitations. Our study did not include other aspects, such as social interaction and stereotypic behaviors, which are usually associated with ASD. Adding these characteristics in future studies might help shed light on the biological underpinnings of autism and improve the treatment for ASD. ConclusionThe offspring of mice injected with valproic acid intraperitoneally during pregnancy showed autism symptom behavior, namely a prolonged negative geotropism test indicating impaired motor skills that require a balance between sensory and motor organization, and a hot plate test, which is a manifestation of sensory processing disorders, which are symptoms of autism. Further research is needed to examine the behavior of more specific autistic mice, such as those with autism symptoms in humans. Research on the biomolecular aspects of autism in test animals is very useful for further scientific development, namely, earlier diagnosis, prevention, and curative. AcknowledgmentsThe authors would like to thank the Faculty of Medicine, Universitas Airlangga, and the Faculty of Veterinary Medicine, Universitas Airlangga. Conflict of interestThe authors declare no conflict of interest. FundingNone. Author contributionsIF: Contributed to conceptualization, methodology, work practices, manuscript writing and drafting, editing, and manuscript revision. II: Contributed to conceptualization, methodology, supervision, manuscript draft writing, editing, and manuscript revision. YS: Contributed to conceptualization, methodology, supervision, manuscript draft writing, editing, and manuscript revision. WW: Contributed to the conceptualization, methodology, and supervision of the study, writing the manuscript draft, editing, and revising the manuscript. All authors have revised and approved the manuscript for publication. Data availabilityAll data supporting this study’s findings are available within the manuscript. ReferencesAlpert. 2021. Autism: a spectrum disorder. Am. J. Med. 134(6), 701–702; doi:10.1016/j.amjmed.2020.10.022 Altun, H., Şahin, N., Kurutaş, E.B., Karaaslan, U., Sevgen, F.H. and Fındıklı, E. 2018. Malondialdehyde assessment of malondialdehyde levels, superoxide dismutase, and catalase activity in children with autism spectrum disorders. Psychiatry Clin. Psychopharmacol. 28(4), 408–415; doi:10.1080/24750573.2018.1470360 Barik, S., Patnaik, L. and Pattanaik, S. 2023. Autism spectrum disorders: preventive aspects-a review on the preventive aspects. In National Journal of Community Medicine. MedSci Publications, 14, pp: 391–8. https://doi.org/10.55489/njcm.140620232975 Chaliha, D., Albrecht, M., Vaccarezza, M., Takechi, R., Lam, V., Al-Salami, H. and Mamo, J. 2020. Systematic review of the valproic-acid-induced rodent model of autism. In Developmental Neuroscience, ed. Karger AG., 42(1), pp 12–48. doi:10.1159/000509109 Chen, L., Shi, X.J., Liu, H., Mao, X., Gui, L.N., Wang, H. and Cheng, Y. 2021. Oxidative stress marker aberrations in children with autism spectrum disorder: a systematic review and meta-analysis of 87 studies (N=9109). Translational Psychiatry 11(1), 1–10; doi:10.1038/s41398-020-01135-3 Cleary, D.B., Maybery, M.T., Green, C. and Whitehouse, A.J.O. 2023. The first six months of lifeFirst Six Months of Life: a systematic review of early markers associated with later autismSystematic Review of Early Markers Associated with Later Autism. In neuroscience and biobehavioral reviews (Vol. 152). Elsevier Ltd; doi: 10.1016/j.neubiorev.2023.105304 da Silva, S.H., Felippin, M.R., de Oliveira Medeiros, L., Hedin-Pereira, C. and Nogueira-Campos, A.A. 2025. A scoping review of the motor impairments in autism spectrum disorder. Neurosci. Biobehav. Rev. 169(2025), 1–7; doi: 10.1016/j.neubiorev.2025.106002 Dana, H., Tahtasakal, R. and Sener, E.F. 2020. Animal models of autism: a perspective from the mechanisms of autophagy. J. Translational Genet. Genom. 4, 251–262; doi:10.20517/jtgg.2020.25 Deckmann, I., Schwingel, G.B., Fontes-Dutra, M., Bambini-Junior, V. and Gottfried, C. 2019. Neuroimmune alterations in autism: a translational analysis offocusing on the animal model of autism induced by prenatal exposure to valproic acid. NeuroImmunoModulation 25(5–6), 285–299; doi:10.1159/000492113 Hirota, T. and King, B.H. 2023. Autism spectrum disorder: a review. JAMA 329(2), 157–168; doi:10.1001/jama.2022.23661 Jiao, D., Xu, Y., Tian, F., Zhou, Y., Chen, D. and Wang, Y. 2024. Animal establishment of animal models and behavioral studies for autism spectrum disorders. J. Int. Med. Res. 52(4), 1–11; doi:10.1177/03000605241245293 Kuźniar-Pałka, A. 2025. The role of oxidative stress in autism spectrum disorder pathophysiology, diagnosis and treatment. Biomedicines 13(2), 388; doi:10.3390/biomedicines13020388 Li, Z., Zhu, Y.X., Gu, L.J. and Cheng, Y. Understanding autism spectrum disorders using animal models: applications, insights, and perspectives. In Zoological Research, 2021 Science China Press, 42(6), pp 800–823. doi:10.24272/J.ISSN.2095-8137.2021.251 Liu, X., Lin, J., Zhang, H., Khan, N. U., Zhang, J., Tang, X., Cao, X. and Shen, L. 2022. Oxidative stress in autism spectrum disorder—current progress of mechanisms and biomarkers. Front. Psychiatry 13, 813304; doi: 10.3389/fpsyt.2022.813304 Martin, L.J., Poulson, S.J., Mannan, E., Sivaselvachandran, S., Cho, M., Setak, F. and Chan, C. 2022. Altered nociceptive behavior and emotional contagion of pain in mouse models of autism. Genes. Brain Behav. 21(1), 1–12; doi:10.1111/gbb.12778 Mokra, D., Joskova, M. and Mokry, J. 2023. Therapeutic effects of green tea polyphenol (-epigallocatechin-3-gallate) on in relation to molecular pathways controlling inflammation, oxidative stress, and apoptosis. Int. J. Mol. Sci. 24(1), 1–26; doi:10.3390/ijms24010340 Nadeem, A., Ahmad, S.F., Attia, S.M., AlAL-Ayadhi, L.Y., Al-Harbi, N.O. and Bakheet, S.A. 2020. Dysregulation ofin IL-6 receptors is associated with upregulated IL-17A-related related signaling in CD4+ T cells of children with autismautism. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 97; doi:10.1016/j.pnpbp.2019.109783 Nasrallah, O. and Alzeer, S. 2022. Measuring some oxidative stress biomarkers in autistic Syrian children and their siblings some oxidative stress biomarkers in autistic syrian children and their siblings: a case-control study case-control Study. Biomarker Insights 17, 1–8; doi:10.1177/11772719221123913 Otaru, S. and Lawrence, D.A. 2022. Autism: genetics, environmental stressors, maternal immune activation, and the male bias in autism. Explor. Neuroprot. Ther. 2, 141–161; doi:10.37349/ent.2022.00025 Salari, N.N., Rasoulpoor S., Shohaimi, S., Jafarpour, S.S., Abdoli, N.N., Khaledi-Paveh, B. and Mohammadi, M. 2022. Global the global prevalence of autism spectrum disorder: a comprehensive systematic review and meta-analysis. Italian J. Pediatrics 48(1), 1–16; doi:10.1186/s13052-022-01310-w Sarieva, K., Kagermeier, T., Khakipoor, S., Atay, E., Yentür, Z., Becker, K. and Mayer, S. 2023. A human brain organoid model of maternal immune activation identifies radial glia cells as selectively vulnerable. Mol. Psychiatry 28(12), 5077–5089; doi:10.1038/s41380-023-01997-1 Sebastiani, G., Navarro-Tapia, E., Almeida-Toledano, L., Serra-Delgado, M., Paltrinieri, A.L., García-Algar. and Andreu-Fernández, V. 2022. Effects of antioxidant intake on fetal development and maternal/neonatal health during pregnancy antioxidant Intake on Fetal Development and Maternal/Neonatal Health during Pregnancy. Antioxidants 11(4), 1–36; doi:10.3390/antiox11040648 Shareef, A.A., Kheder, A.H., Albarzinji, N., Karim, K.J., Smail, S.W., Mahmood, A.A. and Amin, K. 2025. Oxidative markers and SOD variant: predictors of autism severity and susceptibility. Future Sci. OA. 11(1), 1–15; doi:10.1080/20565623.2025.2483628 Taleb, A., Lin, W., Xu, X., Zhang, G., Zhou, Q.G., Naveed, M., Meng, F., Fukunaga, K. and Han, F. 2021. Emerging mechanisms of valproic acid-induced neurotoxic events in autism and its implications for pharmacological treatment of autism. Biomed. Pharmacotherapy 137,1–8; doi:10.1016/j.biopha.2021.111322 Uddin, M.N., Mondal, T., Yao, Y., Manley, K. and Lawrence, D.A. 2023. Oxidative stress and neuroimmune protein expression proteins in a mouse model of autism. Cell Stress Chaperones 28(2), 201–217; doi:10.1007/s12192-023-01331-2 Usui, N., Kobayashi, H. and Shimada, S. 2023. Neuroinflammation and oxidative stress in the pathogenesis of autism spectrum disorder. Int. J. Mol. Sci. 24(6), 1–22; doi:10.3390/ijms24065487 Wieting, J., Baumann, M.V., Deest-Gaubatz, S., Bleich, S., Eberlein, C.K., Frieling, H. and Deest, M. 2024. Examination of structured neurological soft signs reveals motor coordination deficits in adults diagnosed with high-functioning autism. Scientific Rep. 14(1), 1–10; doi:10.1038/s41598-024-66723-5 Wong, R.S.Y. 2022. Neuroinflammation in autism spectrum disorders: a potential target for mesenchymal stem cell-based therapy. Egypt. J. Neurol. Psychiatry Neurosurg. 58(1), 1–13; doi:10.1186/s41983-022-00525-2 Yui, K., Imataka, G., Sasaki, H. and Shiroki, R. 2020. RoleThe role of lipid peroxidation in individuals with autism spectrum disorders. Metabolic Brain Dis. 35(7), 1101–1108; doi:10.1007/s11011-020-00585-4 Zeidan, J., Fombonne, E., Scorah, J., Ibrahim, A., Durkin, M.S., Saxena, S., Yusuf, A., Shih, A. and Elsabbagh, M. 2022. Global prevalence of autism: a systematic review updateupdate. Autism Res. 15(5), 778–790; doi:10.1002/aur.2696 Zhang, Q., Wu, H., Zou, M., Li, L., Li, Q., Sun, C., Xia, W., Cao, Y. and Wu, L. 2019. Folic acid improves abnormal behavior via mitigation of oxidative stress, inflammation, and ferroptosis in the BTBR T + tf / J mouse model of autism. J. Nutritional Biochem. 71, 98–109. | ||
| How to Cite this Article |
| Pubmed Style Fithriyah I, Irwanto I, Setiawati Y, Widjiati W. Effect of valproic acid administration on motor coordination and sensory function in Mus musculus as an autism animal model. Open Vet. J.. 2025; 15(9): 4242-4247. doi:10.5455/OVJ.2025.v15.i9.30 Web Style Fithriyah I, Irwanto I, Setiawati Y, Widjiati W. Effect of valproic acid administration on motor coordination and sensory function in Mus musculus as an autism animal model. https://www.openveterinaryjournal.com/?mno=267919 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i9.30 AMA (American Medical Association) Style Fithriyah I, Irwanto I, Setiawati Y, Widjiati W. Effect of valproic acid administration on motor coordination and sensory function in Mus musculus as an autism animal model. Open Vet. J.. 2025; 15(9): 4242-4247. doi:10.5455/OVJ.2025.v15.i9.30 Vancouver/ICMJE Style Fithriyah I, Irwanto I, Setiawati Y, Widjiati W. Effect of valproic acid administration on motor coordination and sensory function in Mus musculus as an autism animal model. Open Vet. J.. (2025), [cited January 25, 2026]; 15(9): 4242-4247. doi:10.5455/OVJ.2025.v15.i9.30 Harvard Style Fithriyah, I., Irwanto, . I., Setiawati, . Y. & Widjiati, . W. (2025) Effect of valproic acid administration on motor coordination and sensory function in Mus musculus as an autism animal model. Open Vet. J., 15 (9), 4242-4247. doi:10.5455/OVJ.2025.v15.i9.30 Turabian Style Fithriyah, Izzatul, Irwanto Irwanto, Yunias Setiawati, and Widjiati Widjiati. 2025. Effect of valproic acid administration on motor coordination and sensory function in Mus musculus as an autism animal model. Open Veterinary Journal, 15 (9), 4242-4247. doi:10.5455/OVJ.2025.v15.i9.30 Chicago Style Fithriyah, Izzatul, Irwanto Irwanto, Yunias Setiawati, and Widjiati Widjiati. "Effect of valproic acid administration on motor coordination and sensory function in Mus musculus as an autism animal model." Open Veterinary Journal 15 (2025), 4242-4247. doi:10.5455/OVJ.2025.v15.i9.30 MLA (The Modern Language Association) Style Fithriyah, Izzatul, Irwanto Irwanto, Yunias Setiawati, and Widjiati Widjiati. "Effect of valproic acid administration on motor coordination and sensory function in Mus musculus as an autism animal model." Open Veterinary Journal 15.9 (2025), 4242-4247. Print. doi:10.5455/OVJ.2025.v15.i9.30 APA (American Psychological Association) Style Fithriyah, I., Irwanto, . I., Setiawati, . Y. & Widjiati, . W. (2025) Effect of valproic acid administration on motor coordination and sensory function in Mus musculus as an autism animal model. Open Veterinary Journal, 15 (9), 4242-4247. doi:10.5455/OVJ.2025.v15.i9.30 |