| Review Article | ||
Open Vet. J.. 2025; 15(8): 3388-3398 Open Veterinary Journal, (2025), Vol. 15(8): 3388-3398 Review Article Antiviral effects of herbal extracts against avian influenza and Newcastle disease virusesMohamed Mohsen Ahmed Elsherbini*,Ahmed Abd El-Samie Hassan Ali, Fatma Mohamed Abdalla Ahmed and Gamilat KotbDepartment of Virology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt *Corresponding Author: Mohamed Mohsen Ahmed Elsherbini. Department of Virology, Faculty of Veterinary Medicine, Zagazig University, Zagazig, Egypt. Email: mohamedmohsen3918 [at] gmail.com Submitted: 26/05/2025 Revised: 15/07/2025 Accepted: 22/07/2025 Published: 31/08/2025 © 2025 Open Veterinary Journal
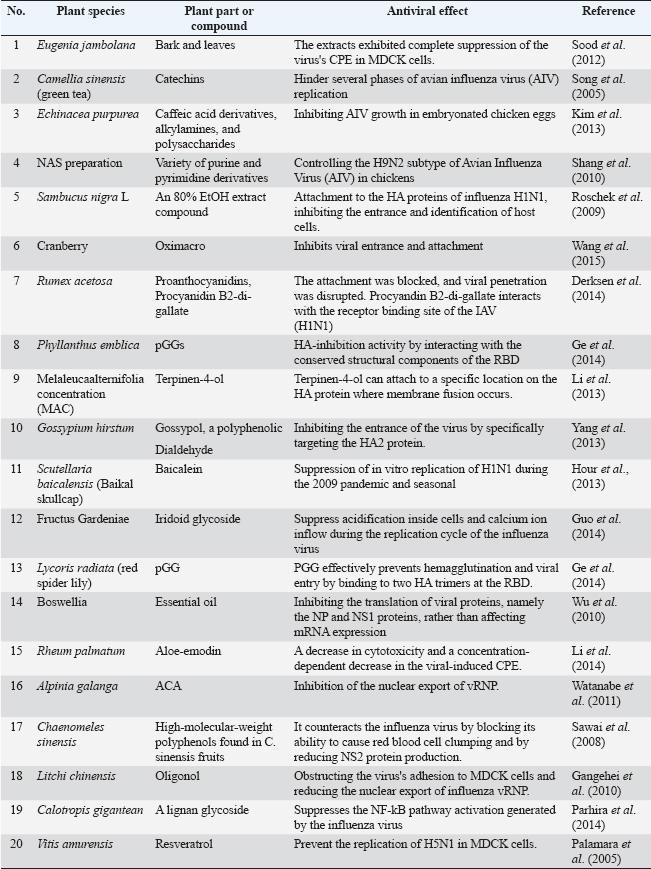
ABSTRACTMedicinal herbs have long been used for their ability to manage viral infections and other diseases. Plants are beneficial for treating several bacterial and viral infections in humans and animals. Plants play a crucial role in providing pharmacological and medicinal products due to their capacity to produce chemicals with antiviral properties via secondary metabolism. This could reduce the need for chemotherapy treatments in poultry. Recent research indicates that natural products have a significant promise as a source of novel antiviral agents. Additional ethnobotanical research and laboratory experiments are being conducted to identify plants with the potential to enhance avian influenza virus and Newcastle Disease management. This review sheds light on plants exhibiting antiviral effects against avian influenza virus and 21 Newcastle disease viruses. Keywords: Avian influenza virus, Newcastle disease virus, Management, Herbal plants. IntroductionViruses have a substantial mutation rate, resulting in the constant appearance of novel strains that are resistant to antiviral treatments. Thus, it is crucial to consistently investigate and create novel antiviral substances. In recent decades, there has been a growing interest in the quest for plant-derived pharmaceutical compounds that might effectively combat animal viruses with high fatality rates or that result in significant economic losses. The poultry business is a crucial agricultural sector that supplies food to approximately 7 billion people globally. The demand for chicken meat has been consistently rising and is projected to reach 131,607 thousand tons by 2026. The chicken business is affected by a range of pathogenic microorganisms, including viruses, bacteria, and protozoa. The most formidable diseases among them are viral infections that persist via genetic modifications, such as mutations, recombinations, or co-evolution with vaccinations (Yasmin et al., 2020). Although vaccinations have proven effective in managing several viral infections, some diseases may only be managed with the use of antiviral chemotherapy. The acceptance of antiviral medications has been gradual, mostly due to the toxicity associated with the previous generations of these agents. Unlike the progress made in the creation of antibiotics, efforts to create antiviral medications have encountered several challenges. Viruses rely only on cellular metabolic processes, which means that they have a restricted number of enzyme systems and building components that may be targeted by drugs. In addition, unlike a substance that inhibits the growth of bacteria, a potent antiviral medication must not only exhibit significant selectivity in its ability to combat viruses but also permanently hinder viral replication to prevent cell death caused by the viral infection and promote the resumption of normal cellular processes. A significant number of infectious viral illnesses continue to result in substantial fatality rates. Despite significant advancements in antiviral chemotherapy, there is a continuing need for antiviral medicines. The emergence of drug-resistant viruses during treatment is a possible obstacle to successful therapy. In addition, novel viral pathogens may be identified. Over a considerable period of time, phytochemicals have been recognized for their ability to suppress viruses. These antiviral chemicals may be obtained from sources, such as higher plants, which have been far less investigated than conventional sources for different reasons. The first antiviral medication with clinical efficacy was methylisatin-thiosemicarbazone (Methisazone), which showed effectiveness in treating dermatitis vaccinatum and preventing smallpox, particularly the smallpox virus. Methisazone, administered at a dosage of 25 mg/kg, exerted inhibitory effects against the variola virus. Subsequently, it was effectively used for treating dermatitis vaccinatum and as a preventive and therapeutic measure against smallpox. The proliferation of drug-resistant viruses in medical therapy is a significant challenge for achieving successful therapeutic outcomes. In addition, novel viral pathogens may also be discovered. Consequently, there is a significant need for readily available antiviral drugs that are affordable and have low adverse effects. Therefore, exploring traditional medicines as potential new antiviral agents is essential, given that several ancient remedies, which consist of various plant metabolites, have strong antiviral properties (Mukherjee, 2019). This review will discuss the antiviral effect of herbal plants against the most severe avian infections affecting the poultry industry caused by viruses, including the avian influenza virus (AIV) and Newcastle disease virus (NDV). The AIVThe Orthomyxoviridae family includes the AIV. The virus encodes at least 11 proteins through its negative, single-stranded, eight-segmented RNAs. Virus subtypes are defined by the surface hemagglutinin (HA) and neuraminidase (NA) proteins, which are in charge of virus attachment and release, respectively (Abdelwhab and Hafez, 2012). Based on its disease-causing potential in chickens, the virus is classified as either highly pathogenic AIV (HPAIV) or low pathogenic AIV. Numerous subtypes of AIV, such as H5, H7, and H9, co-circulate, rendering the vaccine useless. Hence, vaccination against several HA subtypes is essential. Immunological pressure, which accelerates the virus’s evolution rate, is another factor that reduces vaccination effectiveness (Yasmin et al., 2020). Table 1 summarizes the antiviral activity of some medicinal plants against AIV in birds. Eugenia jambolanaEugenia jambolana is a large perennial. Therapeutic compounds in tree bark and leaves may be used to treat chronic diarrhea, dysentery, sore throat, and bacterial and viral infections. The antiviral activity of crude leaf and bark extracts against HPAIV H5N1 was tested. The extracts completely suppressed the virus’s cytopathic effect (CPE) in Madine-Darby Canine Kidney (MDCK) cells and reduced viral production in embryonated eggs by approximately 99% (Sood et al., 2012). Camellia sinensis (green tea)Green tea’s main phenolic ingredient, catechins, is antiviral and antibacterial. Song et al. (2005) found that Epigallocatechin-3-O-gallate (EGCG), epicatechin gallate (ECG), and epigallocatechin inhibited numerous stages of AIV replication. EGCG and ECG effectively inhibit virus binding by attaching to the hemagglutination protein. Kim et al. (2013) found that EGCG did not affect protein receptor binding. However, it prevented virus–cellular membrane hemifusion. Catechins may also inhibit AIV RNA polymerase endonuclease activity, thereby reducing RNA synthesis (Kuzuhara et al., 2009). Pure EchinaceaEchinacea contains polysaccharides, alkylamines, and caffeic acid derivatives. These chemicals have antibacterial, antioxidant, and immunomodulatory properties in laboratory experiments and in real species. This compound was tested for its ability to prevent AIV growth in embryonated chicken eggs. Researchers found that the extract neutralized AIV when mixed with the virus before its introduction into chicken embryos. They obtained similar results when they used quantitative real time polymerase chain reaction (qRT-PCR) to determine the viral concentration. The extract’s antiviral efficacy was ineffective when the virus penetrated the cells, showing that it inhibits the virus’s receptor-binding activity (Kuzuhara et al., 2009). NAS prepNAS contains herbs Folium isatidis and other compounds. They deliberately infected 28-day-old hens with H9N2 and administered varying dosages of NAS 48 hours later for 4 days. AIV-free hens were shown on the seventh day after infection, 5 days after the first medication. In contrast, the positive control and adamantanamine control groups had detectable virus 9 days after infection. NAS may be a good treatment option for chickens with the H9N2 subtype of AIV (Shang et al., 2010). Sumac nigra LSambucus nigra L, also known as black elderberry, exhibits antibacterial, antioxidant, and anti-inflammatory effects in vitro and in vivo (Krawitz et al., 2011). Elderberry contains phenolic acids, flavonoids, catechins, and proanthocyanidins. Laboratory investigations have demonstrated that certain flavonoids suppress influenza virions (Roschek et al., 2009). Karimi et al. (2014) found that Echinacea inhibited viral penetration. When the extract and virus were mixed before inoculation, the chemical prevented viral penetration. After the virus penetrated the cells, no effect was observed. Using qRT–PCR to target the viral gene, the virus’s titer was significantly reduced. CranberryLuganini et al. (2018) examined the effect of cranberry extract affects influenza. They found that the novel cranberry extract Oximacro inhibits influenza subtypes A and B. They explored in vitro how blocks have an entrance and adhesion have causing virucidal effects. Nutritional immunoadjuvants and plant extracts have enhanced hens’ immune responses to inactivated AIV vaccines. Additionally, adding stems and leaves of ginseng saponins to drinking water increased the humoral immune response. Serum antibody levels also increased significantly when H. perforatum L added orally to drinking water. Fermented ginseng extract protected mice from H1N1, H3N2, H5N1, and H7N9 influenza virus subtypes (Zhai et al., 2011). Rumex acetosaProanthocyanidin-rich Rumex acetosa (sorrel) extract contains the main active ingredient, procyanidin B2-di-gallate. Derksen et al. (2014) found that this extract could block viral entry into host cells. Additionally, computerized docking tests showed that procyanidin B2-di-gallate interacted with the receptor-binding area of the HA protein of an oseltamivir-resistant H1N1 virus in the 2009 pandemic. The second ECG unit in procyanidin B2-di-gallate has a galloyl group close to a highly conserved Trp153 residue’s aromatic side chain. The HA protein’s sialic acid-binding pocket hosts this interaction. The closeness between the extract and residue increases the possibility of subsequent interactions. Table 1. Antiviral activity of some medicinal plants against avian influenza.
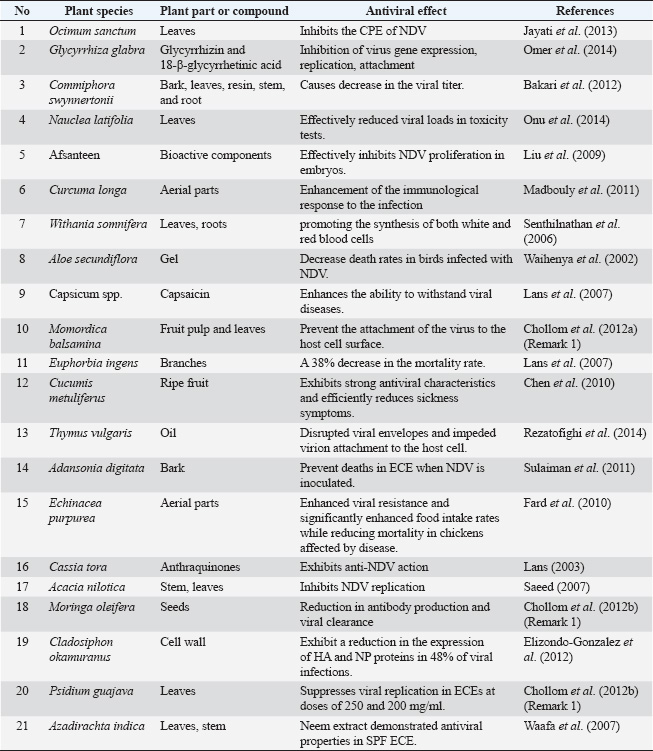
Phyllanthus emblicaGooseberry (Phyllanthus emblica) yields poly-galloyl glucoses (pGGs). A pGG analog had HA-inhibition action by interacting with receptor binding domain (RBD) conserved structural components (Ge et al., 2014). They also found that the galloyl substituents in pGG analogs affect inhibition. The most effective suppression was achieved by attaching penta-galloyl-glucose to two HA trimers at their conserved RBD (loop 130, loop 220, and 190-a-helix). In silico modeling showed that pGG acts as a molecular glue by aggregating viral particles, protecting host cells against viral entry. The antiviral effects of pGGs were observed against H1, H3, H5, and H7 HA protein subtypes. This suggests that pGG analogs could prevent many influenza virus infections. Potency of Melaleuca alternifoliaMelaleuca alternifolia concentration (MAC), an essential oil from native Australian tea tree leaves or terminal branches, inhibited the influenza virus. MAC inhibits the 2009 pandemic H1N1 virus. The primary bioactive component terpinen-4-ol binds to HA’s membrane fusion site. Terpinen-4-ol’s hydrogen bonds with HA2 residues I-56 and N-60 boost binding affinity. The virus cannot enter the host cell because this stabilizes HA’s neutral pH structure (Li et al., 2013). Gossypium hirsutumGossypium hirsutum (upland cotton) pigment glands contain gossypol, a rich yellow polyphenolic dialdehyde. Yang et al. (2013) investigated whether chiral gossypol derivatives and their analogs could inhibit hemagglutination by interfering with H5 HA adsorption to RBCs. A simple amino acid modification increased the efficiency of the less active chiral gossypol more efficient against H5N1, exceeding 1-adamantylamine. The researchers hypothesized that chiral gossypol derivatives and their analogs targeted the HA2 protein to prevent viral entry into cells. Baikal skullcapScutellaria baicalensis (Baikal skullcap) extracts exhibit antiviral properties against various influenza viruses, including pandemic 2009 H1N1, seasonal H1N1, and H3N2. The IC50 values for suppressing NA activity were as follows: seasonal 2007 H1N1 (73.16 μg/ml), 2009 H1N1 (176.57 μg/ml), 2009 H3N2 (306.96 μg/ml), pandemic 2009 H1N1 (388.98 μg/ml), and PR8 H1N1. The high baicalein content in ethyl acetate extracts may explain the strain variation. This chemical strongly inhibits the in vitro activity and replication of various influenza viruses, including the pandemic 2009 H1N1 strain and seasonal H1N1 and H3N2 strains. Baicalein, produced from Scutellaria baicalensis, interacts with NA1 via hydrophobic interactions with Ile222, Arg224, and Ser246. This interaction inhibits the 2009 pandemic and seasonal H1N1 reproduction in vitro (Hour et al., 2013). Fruit of gardeningDespite M2 inhibitor resistance, acidification pathway modification can be used to develop antiviral drugs. Iridoid glycoside from Fructus Gardeniae reduces cell acidification and calcium ion influx during influenza virus replication (Guo et al, 2014). Red spider lily (Lycoris radiata)The antiviral activities of Amaryllidaceae alkaloids from Lycoris radiata (red spider lily) bulbs were tested in vitro against the H5N1 highly pathogenic AIV (He et al., 2013). Alkaloids such as lycorine (AA1), hippeastrine (AA2), hemanthamine (AA3), and 11-hydroxyvittatine (AA4) are antiviral. The intracellular position of nucleoprotein (NP) showed that AA1 and AA3 prevented the development of the ribo NP (RNP) complex in the nucleus during the early stages of single- and multiround viral replication. BoswelliaWu et al. (2010) found antiviral activities in Boswellia carteri (frankincense) and Boswellia serrata (Indian frankincense) essential oil. Essential oils decreased influenza infectivity depending on their dosage. The virus released 90% and 40% fewer viral particles from MDCK cells when treated with oil dilutions of 1:4,000 and 1:6,000, respectively. Rather than influencing mRNA expression, blocking viral protein translation, specifically NP and NS1, is thought to provide antiviral action. Rheum palmatumAloe-emodin is found in the Chinese rhubarb, Rheum palmatum. Aloe-emodin, an anthraquinone derivative, concentration-dependently decreased cytotoxicity and viral-induced cytopathic impact. It also suppressed the influenza virus reproduction in MDCK cells. Aloe-emodin restored STAT1 phosphorylation in the interferon stimulation response element-driven promoter and RNA-dependent protein kinase and 2050,-oligoadenylate synthetase expression in cells transfected with NS1-inhibited STAT1-mediated antiviral responses. Aloeemodin may help persons with influenza (Li et al., 2014). Alpine galangaValerianae radix (valerians) valtrate and Alpinia galanga 10-acetoxychavicol acetate (ACA) suppressed the influenza virus multiplication. These inhibitors block the nuclear export of vRNP. Thus, inhibitors stop virus spread (Watanabe et al., 2011). Chaenomeles sinensisSawai et al. (2008) found that adding 50 mg/ml of ethanol extract from Chaenomeles sinensis (flowering quince) to the culture medium after inoculating the influenza virus selectively suppressed viral NS2 protein synthesis. This fruit contains high-molecular-weight polyphenols. Sinensis reduces hemagglutination and NS2 protein synthesis, thereby fighting influenza. All known herbal inhibitors target viral proteins such as polymerases, NP, NS1, and NS2. Litchi chinensisOligonol from Litchi chinensis (lychee fruit) inhibits influenza virus multiplication. This is done by blocking the virus’s adherence to MDCK cells and lowering the nuclear export of influenza vRNP. Suppressing ERK activation with a dominant negative mutant or N-acetyl-cysteine inhibits RNP nuclear export. Trifolium pretense (red clover) bioactive isoflavone biochanin A disrupts H5N1 virus-induced Akt and ERK 1/2 activation. It does not influence JNK or p38 phosphorylation (Gangehei et al., 2010). Giant calotropisA lignan glycoside from Calotropis gigantea (Asclepiadaceae) latex reduced influenza virus-induced NF-kB pathway activation in a dose-dependent manner. In addition, vRNP transport out of the nucleus was blocked (Parhira et al., 2014). Vitis amurensisVitis amurensis, or Amur grape, inhibited NAs from influenza A/PR/8/34 (H1N1), 2009 pandemic H1N1, and novel oseltamivir-resistant H1N1 (H274Y) strains. Resveratrol from Vitis amurensis inhibits H5N1 replication in MDCK cells. It blocks the movement of viral RNP (vRNP) between the nucleus and cytoplasm and reduces late viral protein synthesis. This inhibition is due to the drug’s capacity to inhibit PKC and its pathway (Palamara et al., 2005). NDVNDV is a member of the Avulavirus genus, Paramyxovirinae subfamily, Paramyxoviridae family, and Mononegavirales order. Avian paramyxovirus type 1 is the official classification. This virus infects more than half of the bird orders and is one of the most common causes of viral disease in birds. There are three pathotypes of the virus that are characterized by their virulence: the asymptomatic lentogenic strain, which is used in vaccines, the respiratory mesogenic strain, which causes moderate mortality, and the neurological velogenic strain, which causes 100% mortality due to gastrointestinal lesions (viscerotropic) or neurological infection (Yusoff and Tan, 2001). The spread of Newcastle Disease (ND) is prevented by using vaccines on a global scale. Birds may experience less severe symptoms after receiving a vaccine; however, the virus can still infect birds through shedding and reproduction (Chukwudi et al., 2012). During the first week of life, the concentrations of anti-NDV antibodies protect the chicks from illness, which effectively preserve the levels of mother antibodies against NDV in the offspring. Despite extensive vaccination campaigns, outbreaks continue (Shabbir et al., 2024). To combat this, poultry producers employ a combination of live and inactivated vaccines in their flocks (Xiao et al., 2013). Because of geographical constraints in African and Asian nations, the World Health Organization (WHO) estimates that 80% of the world’s population uses traditional healthcare techniques (WHO, 2008). Prevention, treatment, and diagnosis of illness are all aspects of traditional medicine that draw on indigenous peoples’ knowledge, experience, and cultural beliefs. Due in large part to the lack of serious side effects, the medical use of plants and herbs is commonplace over the globe. Experimental evidence suggests that several medicinal plant species may be useful in combating NDV due to their antiviral characteristics. Table 2 summarizes the antiviral activity of some medicinal plants against NDV in birds. Ocimum sanctumIn India and tropical and subtropical countries, Sanctum is grown, often known as Holy basil or Tulsi, a sacred plant. Several ancient civilizations depicted therapy. Antiviral power of O. sanctum with a hot aqueous leaf extract of O. sacred to chicken embryo fibroblast monolayer culture. The hemagglutination assay measured the viral load in the media, whereas a monolayer of chicken embryo fibroblasts examined NDV’s cytopathic properties. According to Jayati et al. (2013), a dose of 10 mg/ml or less of hot aqueous leaves extract suppresses NDV’s CPE and reduces NDV proliferation in fibroblasts. Glycyrrhiza glabraLicorice, Mulethi, and sweetwood are native to Asia and Europe. It flavors tobacco, beverages, and confections. The soft, fibrous primary taproot has the plant’s medicinal properties. Embryonated chicken egg (ECE) hemagglutination inhibition results showed that an aqueous extract of G. glabra at 60 mg/100 ml inhibited the virus (Omer et al., 2014). Over 300 flavonoids and 20 triterpenoids are present (Wang et al., 2015). Only two triterpenoids, glycyrrhizin and 18-β-glycyrrhetinic acid, exhibit antiviral effects (Feng, 2013). Suppressing virus gene expression and replication, attachment force and stress, and HMGB1 binding to DNA can reduce viral activity. In addition, they can improve host cell function by suppressing IκB breakdown, promoting T lymphocyte proliferation, and minimizing host cell death (Omer et al., 2014). Commiphora swynnertoniiCommiphora swynnertonii grows in tropical and subtropical Asia and northeastern Africa. Commiphora swynnertonii has spines, light gray bark, and brownish resinous exudate, giving it a shrub-like appearance. Commiphora swynnertonii root, bark, leaves, resin, and stem were tested for NDV treatment in ECEs in ovo. Five of the seven egg groups received C extracts. The other two groups were positive and negative controls. From 5 days after injection, embryos were regularly checked and weighed. Some eggs have also hatched (Moshi et al., 2010). Table 2. Antiviral activity of some medicinal plants against NDV.
Allantoic fluid from eggs and blood from hatched chicks were used in hemagglutination and inhibition experiments. Eggs treated with the extract had a higher average weight and survival rate than the infected control group. Extract reduced viral titer. Eggs treated with resin extract had no viruses in their allantoic fluid. Since no antibodies were found in the bloodstream of the chicks, bark and root extracts were thought to kill the virus (Bakari et al., 2012). Nauclea latifoliaDeciduous shrub or small tree Nauclea latifolia is found in African tropical forests. The dried powder of N. latifolia is antiviral against ND in wild species. The EID50/ml concentration was determined via end-point analysis. Literature shows that three doses of hot aqueous and ethanolic Nauclea latifolia decreased virus levels in toxicity tests. Both extracts were harmful to chicken eggs at 125 mg/ml. The antiviral activity of ethanolic extracts was higher than that of hot water extracts (Onu et al., 2015). AfsanteenThe Asteraceae family includes Afsanteen (Artemisia annua L). It is native to temperate Asia and North America. There is little information about A. annua’s antiviral activity. Anti-NDV activity has been shown. Compounds were extracted by decoction. Ethanolic extracts with bioactive components suppressed the growth of NDV in embryos without harming them (Liu et al., 2009). Curcuma longaCurcuma longa, also known as turmeric, is an ancient coloring spice. It has long been used as a medicine worldwide. Curcumin, a major component of turmeric, strongly inhibits many viruses (Dairaku et al., 2010). Test of Curcumin’s antiviral activity revealed prominent upregulation of the immune responses in experimental birds that received curcumin (Madbouly et al., 2011). Withania somniferaWorldwide, Withania somnifera, a Solanaceae herb, is used in herbal medicine and is well-known as Asgandha or around. According to research, this herb boosts white and red blood cell formation. The addition of Withania aqueous extract to chicken drinking water improved hemoglobin, body weight, and total lymphocyte count (Senthilnathan et al., 2006). Aloe secundifloraAloe secundiflora reduces NDV-infected avian deaths. Application of A. secundiflora therapy or pretreatment may reduce death rates by 21.6%–31.6%. Aloe-gel secundiflora contains antiviral polysaccharides and anthraquinone glycosides. Anthraquinones can damage influenza and NDV protective coatings (Waihenya et al., 2002). Capsicum spp.Capsicum species is used with other botanicals to treat numerous diseases. Capsaicin, found in Capsicum spp., may help chickens fight viruses, particularly NDV (Lans et al., 2007). Momordica balsaminaThis plant is Cucurbitaceae. It comes from Northern Nigeria. According to phytochemical analysis, the fruit, leaves, and seeds contained lectins, steroids, saponins, glycosides, and tannins. Alkaloids, flavonoids, saponins, and tannins are new antivirals (Jassim and Naji, 2003). To prove the antiviral activity of M. balsamina fruit and leaf extracts. Balsamina used chicken embryo fibroblastic cell lines. The results showed that both extracts inhibited infection at 10 and 20 mg/ml. Further testing showed that the extract prevented viral attachment to host cells (Chollom et al., 2012a). Euphorbia ingensEuphorbia ingens is a South African arid-region Euphorbiaceae species. The candelabra tree is another name. Feeding crushed and soaking E. ingens branches reduced NDV-infected hen mortality by 38.4% in their drinking water following overnight soaking (Lans et al., 2007). Cucumis metuliferusThe Cucurbitaceae family includes the horned melon or kiwano. Ripe fruit has a yellow to orange exterior and lime green, gelatinous, and acidic flesh like a cucumber. This plant has unique medicinal qualities due to its phytochemicals. The fruit pulp of C. metuiferus contains antiviral alkaloids. Kiwano extracts were given to NDV-infected hens to prove their antiviral properties. Alkaloids in this plant have significant antiviral activities and relieve disease symptoms at 60 mg/kg (Chen et al., 2010). Thymus vulgarisThymus vulgaris is commonly called thyme. This species is found in the Mediterranean, North Africa, and Asia. It grows to 50 cm tall with woody, spreading branches. Flower colors range from purple to pink. This product contains antioxidant and antibacterial essential oils and bioactive compounds. These chemicals inhibit fungi, yeasts, viruses, and bacteria. Thymus vulgaris fights HSV-1/HSV-2 and NDV. The plant’s essential oils ruptured viral envelopes and prevented virion attachment to host cells (Rezatofighi et al., 2014). Adansonia digitataThe species Adansonia digitata is called the “Baobab tree” regionally. Africa caused the Malvaceae plant. Africa uses this herb to treat infectious diseases. The bark, fruit pulp, leaves, and seeds are medicinal and nutritious. The antiviral properties of A. digitata root bark methanolic extract were tested with 175 specific antibodies in NDV-infected ECEs. The death rate was assessed after 24 hours of incubation after a 2-hour viral exposure to eight extract dosages. The extract was inoculated with the highest concentration, whereas the virus was infected with 100 EID50. The extract was used as the negative control and the virus as the positive control. The virus alone and viral suspensions of 5 and 2 mg/ml extract killed all eggs 72 hours after inoculation. No fatalities were observed in eggs implanted with 250 and 200 mg/ml virus/extract solutions or the extract alone. Methanolic root extract of A. digitata inhibits NDV in ovo at concentrations of 200 and 250 mg/ml (Sulaiman et al., 2011). Echinacea purpureaThe purple coneflower is Echinacea purpurea, a Compositae plant. The ethanolic extract contains isoleucine, lysine, glutamic acid, proline, serine, phenylalanine, and threonine. These amino acids significantly affect NDV clearance. E. purpurea improves viral resistance, food consumption, and mortality in infected poultry (Fard et al., 2010). Cassia toraCassia tora is a South-East Asian dicotyledonous leguminous. It was reported to have clear anti-NDV effects (Lans et al., 2007). Acacia niloticaAcacia nilotica, a monopodial plant, grows in tropical and subtropical Asia, Africa, Australia, and Kenya. Kikar, the plant, has significant tannin content and medicinal benefits. Methanol extract of the plant is antiviral against chicken viruses. The hemagglutination test was used to measure viral viability reduction with and without the extract. Cytotoxicity of each extract was determined by CPEs, a methanolic extract. At 40 µg/ml, A. nilotica exhibited nontoxic and significant inhibitory effects against the tested virus in Vero cells. The statistics suggest A. nilotica substantially suppresses NDV replication (Saeed, 2007). Moringa oleiferaMoringa oleifera, the “miracle tree,” has all the amino acids, vitamins, calcium, and other minerals in high concentrations in its leaves. Sohanjana is another name for the Moringa plant. The plant is Pakistani and Indian. Southern Punjab may have caused Moringa. Aqueous M. oleifera M. seed extract. was tested for anti-NDV activity in ovo. The concentration-dependent method reduced viral clearance and antibody generation. As the “miracle tree,” the extract strengthened the immune system by containing all the necessary amino acids, vitamins, calcium, and other minerals in high concentrations in its leaves. This Moringacea plant is also called Sohanjana (Ashraf et al., 2018). The extract also boosted immunity (Chollom et al., 2012b). Cladosiphon okamuranusCladosiphon okamuranus is an edible seaweed found in Okinawa, Japan. Fucoidan in this species inhibits the growth of the NDV (La Sota strain) virus and reduces its reproduction between 0 and 60 minutes after inoculation, according to studies. It decreases HA and NP protein expression in 48% of viral infections (Elizondo-Gonzalez et al., 2012). GuajavaCommon guava, also known as Psidium guajava, is found globally. Tropical yard gardens use trees for shade. Plant leaves contain pharmacologically beneficial substances such as alkaloids, tannins, flavonoids, saponins, and others, which may explain their many local sickness treatment benefits. Psidium guajava leaf extract was tested in ovo for NDV antiviral activity. The extracts suppressed viral replication in ECEs at concentrations of 250 and 200 mg/ml. The extract prevented antibodies in hatched chicks and enhanced embryo viability proportionally to dosage (Chollom et al., 2012a). Azadirachta indicaThe Meliaceae plant Azadirachta indica is hardy. Sindh, lower Baluchistan, Southern Punjab, and Southern North-West Frontier Province are its native habitats (Durrani et al., 2008). It is native to India and possibly Africa. Neem (Azadirachta indica) is a fast-growing tree that grows to 15–20 m. It grows in tropical and semitropical regions. Azadirachta nimbin, nimbidin, azadirachtin, and quercetin are among the 140 chemicals found in indica (Makeri et al., 2007). These chemicals have antihelminth, antiprotozoal, antioxidant, antifungal, antimicrobial, spermicidal, and insecticidal activities (Bonsu et al., 2012). However, virus inhibition techniques show that antiviral compounds are insufficient for some viruses. The leaf methanolic extract and chloroform and hexane seed extracts have antiviral effects. These extracts are also highly cytotoxic to the host. Egg concentrations above 3–4 μg significantly inhibit NDV. Azadirachta’s IC50 and TC50 were calculated. The levels of A. indica in chicken embryos were 4 μg/egg and 300 μg/egg (Wafaa et al., 2007). ConclusionThe data reported in this research emphasize the use of medicinal plants as a means to fight ND and AIV. This document examines 41 plants and their respective components, as well as the diverse extracts used to combat NDV and AIV. In order to discover more effective and less harmful treatments for NDV and AIV, it is crucial to use innovative antiviral medications derived from the bioactive compounds found in plants. Significant focus was given to the potential abilities of plants containing active compounds that have antiviral properties against NDV and AIV. Regarding the in vitro tests. By using optimization techniques and employing a suitable methodology, it is possible to uncover potential compounds that exhibit promising antiviral properties against NDV and AIV can be identified, leading to the development of new antiviral drugs. AcknowledgementNot applicable. Conflict of interests:The authors have not declared any conflict of interest. FundingNot applicable. Ethical approvalNot applicable. Authors’ contributionsAll authors contributed equally. Data availabilityAll data were presented in the study. ReferencesAbdelwhab, E.M. and Hafez, H.M. 2012. Insight into alternative approaches for control of avian influenza in poultry, with emphasis on highly pathogenic H5N1. Viruses 4(11), 3179–3208. Ashraf, A., Mahboob, S., Andleeb, R., Ijaz, M.U. and Shah, M.S. 2018. Status updates of Newcastle disease and amelioration effects of medicinal plants against Newcastle disease virus: a review. Acta Virol. 62(1), 3–15. Bakari, G.G., Max, R.A., Mdegela, R.H., Phiri, E.C. and Mtambo, M.M. 2012. Antiviral activity of crude extracts from Commiphora swynnertonii against Newcastle disease virus in ovo. Trop. Anim. Health Prod. 44, 1389–1393. Bonsu, F.R.K., Kagya-Agyemang, J.K., Kwenin, W.K.J. and Zanu, H.K. 2012. Medicinal response of broiler chickens to diets containing neem (Azadirachta indica) leaf meal, haematology and meat sensory analysis. World Appl. Sci. J. 19(6), 800–805. Chen, Y., Wang, D., Hu, Y., Guo, Z., Wang, J., Zhao, X., Fan, Y., Guo, L., Yang, S., Sai, F. and Xing, Y. 2010. Astragalus polysaccharide and oxymatrine can synergistically improve the immune efficacy of Newcastle disease vaccine in chicken. Inter. J. Biol. Macromol. 46(4), 425–428. Chollom, S.C., Agada, G.O.A., Gotep, J.G., Mwankon, S.E., Dus, P.C., Bot, Y.S., Nyango, D.Y., Singnap, C.L., Fyaktu, E.J. and Okwori, A.E.J. 2012a. Investigation of aqueous extract of Moringa oleifera lam seed for antiviral activity against Newcastle disease virus in ovo. J. Med. Plants Res. 6(22), 3870–3875. Chollom, S.C., Olawuyi, A.K., Danjuma, L.D., Nanbol, L.D., Makinde, I.O., Hashimu, G.A., Alesa, M.U., Esilonu, J.T., Ogundeji, E.B. and Kwatjel, J.S. 2012b. Antiviral potential of aqueous extracts of some parts of Momordica balsamina plant against Newcastle disease virus. J. Adv. Pharm. Edu. Res. 2(3), 82–92. Chukwudi, O.E., Chukwuemeka, E.D. and Mary, U. 2012. Newcastle disease virus shedding among healthy commercial chickens and its epidemiological importance. Pak. Vet. J. 32(3), 354–356. Dairaku, I., Han, Y., Yanaka, N. and Kato, N. 2010. Inhibitory effect of curcumin on IMP dehydrogenase, the target for anticancer and antiviral chemotherapy agents. Biosci. Biotechnol. Biochem. 74(1), 185–187. Derksen, A., Hensel, A., Hafezi, W., Herrmann, F., Schmidt, T.J., Ehrhardt, C., Ludwig, S. and Kühn, J. 2014. 3-O-galloylated procyanidins from Rumex acetosa L. inhibit the attachment of influenza A virus. PLoS One 9(10), e110089. Durrani, F.R., Sultan, A., Jan, M., Chand, N. and Durrani, Z. 2008. Immunomodulatory and growth promoting effects of neem (Azadirachta indica) leaves infusion in broiler chicks. Sarhad J. Agricult. 24(4), 655–659. Elizondo-Gonzalez, R., Cruz-Suarez, L.E., Ricque-Marie, D., Mendoza-Gamboa, E., Rodriguez-Padilla, C. and Trejo-Avila, L.M. 2012. In vitro characterization of the antiviral activity of fucoidan from Cladosiphon okamuranus against Newcastle disease virus. Virol. J. 9, 1–9. Fard, M.H.B., Feizi, A. and Bijanzad, P. 2010. Effect of Echinacea purpurea dried extract effect on producing antibody from Newcastle disease vaccine in broilers by HI test. J. Vet. Res., 65, 119–122. Gangehei, L., Ali, M., Zhang, W., Chen, Z., Wakame, K. and Haidari, M. 2010. Oligonol a low molecular weight polyphenol of lychee fruit extract inhibits proliferation of influenza virus by blocking reactive oxygen species-dependent ERK phosphorylation. Phytomedicine 17(13), 1047–1056. Ge, H., Liu, G., Xiang, Y.F., Wang, Y., Guo, C.W., Chen, N.H., Zhang, Y.J., Wang, Y.F., Kitazato, K. and Xu, J. 2014. The mechanism of poly-galloyl-glucoses preventing Influenza A virus entry into host cells. PLoS One 9(4), e94392. Guo, S., Gao, Y., Jin, Y., Tian, X. and Cui, X. 2014. The inhibitory effect of iridoid glycoside extracted from Fructus Gardeniae on intracellular acidification and extracellular Ca2+ influx induced by influenza A virus. Exper. Biol. Med. 239(8), 986–997. He, J., Qi, W.B., Wang, L., Tian, J., Jiao, P.R., Liu, G.Q., Ye, W.C. and Liao, M. 2013. Amaryllidaceae alkaloids inhibit nuclear-to-cytoplasmic export of ribonucleoprotein (RNP) complex of highly pathogenic avian influenza virus H5N1. Influenza Other Respir Viruses 7(6), 922–931. Hour, M.J., Huang, S.H., Chang, C.Y., Lin, Y.K., Wang, C.Y., Chang, Y.S. and Lin, C.W. 2013. Baicalein, ethyl acetate, and chloroform extracts of Scutellaria baicalensis inhibit the neuraminidase activity of pandemic 2009 H1N1 and seasonal influenza A viruses. Evid. Based Complem. Altern. Med. 2013(1), 750803. Jassim, S.A. and Naji, M.A. 2003. Novel antiviral agents: a medicinal plant perspective. J. Appl. Microbiol. 95, 412–427. Jayati, B.A., Kumar, A., Goel, A., Gupta, S. and Rahal, A. 2013. In vitro antiviral potential of Ocimum sanctum leaves extract against New Castle Disease virus of poultry. Inter. J. Microbiol. Immunol. Res. 2(7), 51–55. Karimi, S., Mohammadi, A. and Dadras, H. 2014. The effect of Echinacea purpurea and Sambucus nigra L. on H9N2 avian influenza virus in infected chicken embryo. Veter. Arhiv. 84(2), 153–165. Kim, M., Kim, S.Y., Lee, H.W., Shin, J.S., Kim, P., Jung, Y.S., Jeong, H.S., Hyun, J.K. and Lee, C.K. 2013. Inhibition of influenza virus internalization by (−)-epigallocatechin-3-gallate. Antiviral Res. 100(2), 460–472. Krawitz, C., Mraheil, M.A., Stein, M., Imirzalioglu, C., Domann, E., Pleschka, S. and Hain, T. 2011. Inhibitory activity of a standardized elderberry liquid extract against clinically-relevant human respiratory bacterial pathogens and influenza A and B viruses. BMC Complem. Altern. Med. 11, 1–6. Kuzuhara, T., Iwai, Y., Takahashi, H., Hatakeyama, D. and Echigo, N. 2009. Green tea catechins inhibit the endonuclease activity of influenza A virus RNA polymerase. PLoS Curr. 1, 1052. Lans, C., Khan, T.E., Curran, M.M. and McCorkle, C.M. 2007. Ethnoveterinary medicine: potential solutions for large-scale problems?. In Veterinary herbal medicine. Eds.,Wynn, S.G. and Fougere, B.J. St. Louis, MO: Mosby, pp: 17–32. Li, S.W., Yang, T.C., Lai, C.C., Huang, S.H., Liao, J.M., Wan, L., Lin, Y.J. and Lin, C.W. 2014. Antiviral activity of aloe-emodin against influenza A virus via galectin-3 up-regulation. Europ. J. Pharmacol. 738, 125–132. Li, X., Duan, S., Chu, C., Xu, J., Zeng, G., Lam, A.K.Y., Zhou, J., Yin, Y., Fang, D., Reynolds, M.J. and Gu, H. 2013. Melaleuca alternifolia concentrate inhibits in vitro entry of influenza virus into host cells. Molecules 18(8), 9550–9566. Liu Yali, L.Y., Yan GenQiang, Y.G., Chen GuoHui, C.G. and Zhang Jing, Z.J. 2009. Efficacy trials of crude extraction from Artemisia annul L. against Newcastle disease virus in vivo in Xinjiang. App. Sci. 3, 176–178. Luganini, A., Terlizzi, M.E., Catucci, G., Gilardi, G., Maffei, M.E. and Gribaudo, G. 2018. The cranberry extract oximacro® exerts in vitro virucidal activity against influenza virus by interfering with hemagglutinin. Front. Microbiol. 9, 1826. Madbouly, H.M., Saif, M.A. and Hussein, A.S. 2011. Curcuma longa for protecting chicks against Newcastle disease virus infection and immunosuppressive effect of Marek’s disease viral vaccine. Int. J. Virol. 7, 176–183. Makeri, H.K., Maikai, V.A. and Nok, J.A. 2007. Effect of topical application of neem seed (Azadiracta indica) extract on sheep infested with Amblyomma variegatum. Afri. J. Biotechnol. 6, 20. Moshi, M.J., Innocent, E., Magadula, J.J., Otieno, D.F., Weisheit, A., Mbabazi, P.K. and Nondo, R.S.O. 2010. Brine shrimp toxicity of some plants used as traditional medicines in Kagera Region, north western Tanzania. Tanz. J. Health Res. 12(1), 63–67. Mukherjee, P.K. 2019. Antiviral evaluation of herbal drugs. In Quality control and evaluation of herbal drugs, pp. 599. Omer, M.O., AlMalki, W.H., Shahid, I., Khuram, S., Altaf, I. and Imran, S. 2014. Comparative study to evaluate the anti-viral efficacy of Glycyrrhiza glabra extract and ribavirin against the Newcastle disease virus. Pharmacog. Res. 6(1), 6. Onu, U., Nwiyi, P. and Erumaka, I. 2015. Antiviral effects of Nauclea latifolia on Newcastle disease virus (NDV). Sky J. Microbiol. Res. 3(1), 1–5. Palamara, A.T., Nencioni, L., Aquilano, K., De Chiara, G., Hernandez, L., Cozzolino, F., Ciriolo, M.R. and Garaci, E. 2005. Inhibition of influenza A virus replication by resveratrol. J. Infect. Dis. 191(10), 1719–1729. Parhira, S., Yang, Z.F., Zhu, G.Y., Chen, Q.L., Zhou, B.X., Wang, Y.T., Liu, L., Bai, L.P. and Jiang, Z.H. 2014. In vitro anti-influenza virus activities of a new lignan glycoside from the latex of Calotropis gigantea. PLoS One 9(8), e104544. Rezatofighi, S.E., Seydabadi, A. and Nejad, S.M.S. 2014. Evaluating the efficacy of Achillea millefolium and Thymus vulgaris extracts against Newcastle disease virus in Ovo. Jund. J. Microbiol. 7(2), e9016. Roschek Jr, B., Fink, R.C., McMichael, M.D., Li, D. and Alberte, R.S. 2009. Elderberry flavonoids bind to and prevent H1N1 infection in vitro. Phytochemistry 70(10), 1255–1261. Saeed, A.M.M. 2007. The antiviral effect of acacia nilotica extracts against the mesogenic strain of Newcastle disease virus. Doctoral Dissertation, Department of Medicine Pharmacology and Toxicology, Faculty of Veterinary Medicine, University of Khartoum, Khartoum, Sudan. Sawai, R., Kuroda, K., Shibata, T., Gomyou, R., Osawa, K. and Shimizu, K. 2008. Anti-influenza virus activity of Chaenomeles sinensis. J. Ethnopharmacol. 118(1), 108–112. Senthilnathan, P., Padmavathi, R., Banu, S.M. and Sakthisekaran, D. 2006. Enhancement of antitumor effect of paclitaxel in combination with immunomodulatory Withania somnifera on benzo (a) pyrene induced experimental lung cancer. Chem. Biol. Inter. 159(3), 180–185. Shabbir, M.Z., Mahmood, S., Ul-Rahman, A., Banyard, A.C. and Ross, C.S. 2024. Genomic diversity and evolutionary insights of avian paramyxovirus-1 in avian populations in Pakistan. Viruses 16(9), 1414. Shang, R.F., Liang, J.P., Na, Z.Y., Yang, H.J., Lu, Y., Hua, L.Y., Guo, W.Z., Cui, Y. and Wang, L. 2010. In vivo inhibition of NAS preparation on H9N2 subtype AIV. Virolog. Sin. 25, 145–150. Song, J.M., Lee, K.H. and Seong, B.L. 2005. Antiviral effect of catechins in green tea on influenza virus. Antivir. Res. 68(2), 66–74. Sood, R., Swarup, D., Bhatia, S., Kulkarni, D.D., Dey, S., Saini, M. and Dubey, S.C. 2012. Antiviral activity of crude extracts of Eugenia jambolana Lam. against highly pathogenic avian influenza (H5N1) virus. Ind. J. Exper. Biol. 50(3), 179–186. Sulaiman, L.K., Oladele, O.A., Shittu, I.A., Emikpe, B.O., Oladokun, A.T. and Meseko, C.A. 2011. In-ovo evaluation of the antiviral activity of methanolic root-bark extract of the African Baobab (Adansonia digitata Lin). Afr. J. Biotechnol. 10(20), 4256–4258. Wafaa, A.H., Howaida, I.A., Hassan, A. and El-Safty, M.M. 2007. Chemical composition and ‘in vitro’ antiviral activity of Azadirachta indica A. Juss (Neem) leaves and fruits against Newcastle disease virus and infectious bursal disease virus. Aust. J. Basic Appl. Sci. 1, 801–812. Waihenya, R.K., Mtambo, M.M.A. and Nkwengulila, G. 2002. Evaluation of the efficacy of the crude extract of Aloe secundiflora in chickens experimentally infected with Newcastle disease virus. J. Ethnopharmacol. 79(3), 299–304. Wang, L., Yang, R., Yuan, B., Liu, Y. and Liu, C. 2015. The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharm. Sin. B 5(4), 310–315. Watanabe, K., Takatsuki, H., Sonoda, M., Tamura, S., Murakami, N. and Kobayashi, N. 2011. Anti-influenza viral effects of novel nuclear export inhibitors from Valerianae Radix and Alpinia galanga. Drug Discov. Therap. 5(1), 26–31. WHO, World Health Organization. 2008. Chapter 4. Public policies for the public›s health. pp: 63–76. Wu, S., Patel, K.B., Booth, L.J., Metcalf, J.P., Lin, H.K. and Wu, W. 2010. Protective essential oil attenuates influenza virus infection: an in vitro study in MDCK cells. BMC Compl. Alter. Med. 10, 1–13. Xiao, S., Paldurai, A., Nayak, B., Mirande, A., Collins, P.L. and Samal, S.K. 2013. Complete genome sequence of a highly virulent Newcastle disease virus currently circulating in Mexico. Gen. Announc. 1(1), 10–1128. Yang, J., Chen, G., Li, L.L., Pan, W., Zhang, F., Yang, J., Wu, S. and Tien, P. 2013. Synthesis and anti-H5N1 activity of chiral gossypol derivatives and its analogs implicated by a viral entry blocking mechanism. Bioorgan. Med. Chem. Lett. 23(9), 2619–2623. Yasmin, A.R., Chia, S.L., Looi, Q.H., Omar, A.R., Noordin, M.M. and Ideris, A. 2020. Herbal extracts as antiviral agents. In Feed additives. Eds., Florou-Paneri, P., Christaki, E. and Giannenas I. Cambridge, MA: Academic Press, Elsevier, pp: 115–132; doi: 10.1016/B978-0-12-814700-9.00007-8 Yusoff, K., and Tan, W.S. 2001. Newcastle disease virus: macromolecules and opportunities. Avian Pathol. 30(5), 439e455. Zhai, L., Li, Y., Wang, W. and Hu, S. 2011. Enhancement of humoral immune responses to inactivated Newcastle disease and avian influenza vaccines by oral administration of ginseng stem-and-leaf saponins in chickens. Poultry Sci. 90(9), 1955–1959. | ||
| How to Cite this Article |
| Pubmed Style Elsherbini MMA, Ali AAEH, Ahmed FMA, Kotb G. Antiviral effects of herbal extracts against avian influenza and Newcastle disease viruses. Open Vet. J.. 2025; 15(8): 3388-3398. doi:10.5455/OVJ.2025.v15.i8.3 Web Style Elsherbini MMA, Ali AAEH, Ahmed FMA, Kotb G. Antiviral effects of herbal extracts against avian influenza and Newcastle disease viruses. https://www.openveterinaryjournal.com/?mno=267138 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i8.3 AMA (American Medical Association) Style Elsherbini MMA, Ali AAEH, Ahmed FMA, Kotb G. Antiviral effects of herbal extracts against avian influenza and Newcastle disease viruses. Open Vet. J.. 2025; 15(8): 3388-3398. doi:10.5455/OVJ.2025.v15.i8.3 Vancouver/ICMJE Style Elsherbini MMA, Ali AAEH, Ahmed FMA, Kotb G. Antiviral effects of herbal extracts against avian influenza and Newcastle disease viruses. Open Vet. J.. (2025), [cited January 25, 2026]; 15(8): 3388-3398. doi:10.5455/OVJ.2025.v15.i8.3 Harvard Style Elsherbini, M. M. A., Ali, . A. A. E. H., Ahmed, . F. M. A. & Kotb, . G. (2025) Antiviral effects of herbal extracts against avian influenza and Newcastle disease viruses. Open Vet. J., 15 (8), 3388-3398. doi:10.5455/OVJ.2025.v15.i8.3 Turabian Style Elsherbini, Mohamed Mohsen Ahmed, Ahmed Abd El-samie Hassan Ali, Fatma Mohamed Abdalla Ahmed, and Gamilat Kotb. 2025. Antiviral effects of herbal extracts against avian influenza and Newcastle disease viruses. Open Veterinary Journal, 15 (8), 3388-3398. doi:10.5455/OVJ.2025.v15.i8.3 Chicago Style Elsherbini, Mohamed Mohsen Ahmed, Ahmed Abd El-samie Hassan Ali, Fatma Mohamed Abdalla Ahmed, and Gamilat Kotb. "Antiviral effects of herbal extracts against avian influenza and Newcastle disease viruses." Open Veterinary Journal 15 (2025), 3388-3398. doi:10.5455/OVJ.2025.v15.i8.3 MLA (The Modern Language Association) Style Elsherbini, Mohamed Mohsen Ahmed, Ahmed Abd El-samie Hassan Ali, Fatma Mohamed Abdalla Ahmed, and Gamilat Kotb. "Antiviral effects of herbal extracts against avian influenza and Newcastle disease viruses." Open Veterinary Journal 15.8 (2025), 3388-3398. Print. doi:10.5455/OVJ.2025.v15.i8.3 APA (American Psychological Association) Style Elsherbini, M. M. A., Ali, . A. A. E. H., Ahmed, . F. M. A. & Kotb, . G. (2025) Antiviral effects of herbal extracts against avian influenza and Newcastle disease viruses. Open Veterinary Journal, 15 (8), 3388-3398. doi:10.5455/OVJ.2025.v15.i8.3 |