| Research Article | ||
Open Vet. J.. 2025; 15(9): 4235-4241
Open Veterinary Journal, (2025), Vol. 15(9): 4235-4241 Research Article Protective role of coenzyme Q10 against renal and hepatic toxicity induced by acetaminophen overdose in albino ratsIman Hussein Naser1*, Zahraa Abed al-Kareem1, Moayad Mijbil Ubaid2 and Shatha Hussein Kadhim11Department of Pharmacology and Toxicology, College of Pharmacy, University of Kerbala, Kerbala, Iraq 2Department of Biology/College of Basic Education/University of Sumer, Thi-Qar, Iraq *Corresponding Author: Iman Hussein Naser. Department of Pharmacology and Toxicology, College of Pharmacy, University of Kerbala, Kerbala, Iraq. Email: iman [at] uokerbala.edu.iq Submitted: 08/06/2025 Revised: 30/07/2025 Accepted: 06/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
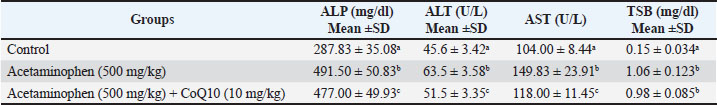
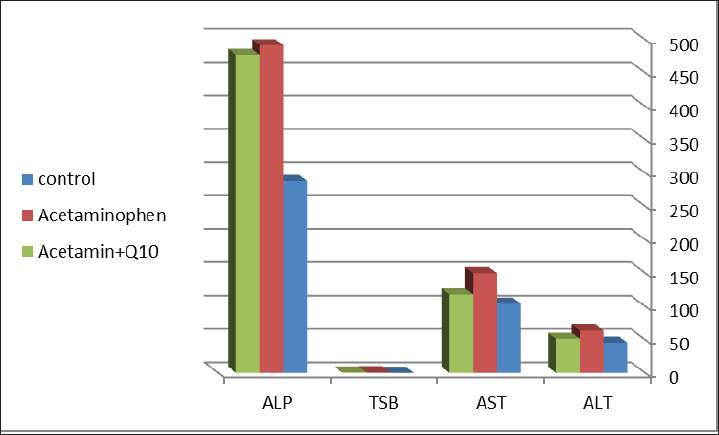
AbstractBackground: Acetaminophen has an antipyretic activity by inhibiting prostaglandin synthesis found in the brain, and this action is responsible for its analgesic effect, but overdose can cause hepatic and renal toxicity. Coenzyme Q10 has cytoprotective properties through antioxidant and anti-inflammatory effects. In addition, it plays a role in energy production in the mitochondria, making it important in reducing cellular damage by toxic agents. Aim: This study aimed to evaluate the potential protective effect of CoQ10 against acetaminophen toxicity on liver and kidney functions. Methods: Thirty adult male albino rats were randomly subdivided into three groups: control group, acetaminophen group, acetaminophen + CoQ10 group, acetaminophen in toxic dose (500 mg/kg) orally, and acetaminophen + CoQ10 group, drenched with coenzymeQ10 (10 mg/kg) orally, then acetaminophen in toxic dose (500 mg/kg). Dosing continued for 30 days. Blood was collected for biochemical analysis of hepatic and renal biochemical parameters. Results: Acetaminophen induced hepatic injury as measured by increased hepatic biochemical parameters “Total serum bilirubin (TSB), alkaline phosphatase (ALP), aspartate aminotransferase (AST), and alanine aminotransferase (ALT)” (p ≤ 0.05). The third group, which received acetaminophen plus CoQ10, had a significant decrease in hepatic biochemical markers(p ≤ 0.05). The drenched of acetaminophen increased (creatinine and albumin), whereas the treatment with CoQ10 reduced their levels. Conclusion: Coenzyme Q10 can reduce the toxicity of acetaminophen overdose by acting as an antioxidant agent that reduces tissue and organ damage. Keywords: Acetaminophen toxicity, Antioxidant, Hepatic enzymes, Nephrotoxicity, Oxidative stress. IntroductionAcetaminophen (Paracetamol®) is an antipyretic and analgesic agent that is used globally as an over-the-counter drug for patients of all ages. It was first marketed in the United Kingdom (Wongrakpanich et al., 2018). In 1956, this drug became common among the public and medical profession as an alternative to aspirin. Acetaminophen is characterized by safety, efficacy, and availability. It is widely available in markets and pharmacies and is effective in treating mild and moderate pain and even severe pain. It can be used in all patients, including those with hepatic or renal dysfunction and the elderly (Freo et al., 2021). The use of acetaminophen in high doses is the main cause of hepatotoxicity, which can be lethal to humans and those who were not given N-acetylcysteine, which is the antidote for acetaminophen toxicity (Marks et al., 2017). Acetaminophen toxicity is now the leading cause of acute liver failure in the United Kingdom and the United States. The main causes of acetaminophen hepatotoxicity are the formation of the drug’s oxidized metabolite and its reaction with glutathione (Chun et al., 2009). Many drugs that are used with high doses or long-term therapy can also damage the kidneys and cause liver injuries (Aziz et al., 2018; Ubaid,2019; Kadhim et al., 2022; Ubaid et al., 2022). The primary metabolic pathways for acetaminophen in hepatocytes are glucuronidation and sulfation. These two mechanisms will be saturated in high dosages, and more acetaminophen is metabolized to N-acetyl-P-benzoquinone imine (NAPQI) by cytochrome P450. NAPQI is a toxic metabolite that is normally detoxified into nontoxic cysteine and mercapturic acid compounds by the glutathione pathway, which are readily excreted by the kidneys. Acetaminophen overdose decreases glutathione stores to 30% of average levels (Du et al., 2014). NAPQI levels in hepatocytes rise and bind to hepatic macromolecules, resulting in irreversible hepatic cell damage (Popiolek et al., 2021). Acetylcysteine can replenish glutathione concentration in the liver by inducing its synthesis since it releases the cysteine molecules, which are considered as the rate-limiting step in the synthesis of glutathione (Mazraati and Minaiyan, 2018; Paschalis et al., 2018). Acetylcysteine has a half-life of approximately 6.25 hours, and its bioavailability differs in scientific references from 4% to 10% (Schwalfenberg, 2021). Previous studies evaluated appropriate agents that could reduce the toxicity of acetaminophen and other toxins Millman and Grundon, (1969). These agents include curcumin, honey, silymarin, and zingiber. These food-derived agents play potential roles in reducing the adverse effects of acetaminophen but are not available in pharmaceutical dosage forms, such as capsules or liquids, which patients prefer. These agents may require a longer time to exert their therapeutic effects or may interact with food or other medicines (Eugenio-Pérez, et al., 2016; Khalid and Azmat, 2025). Other recent studies have examined aqueous extracts of some herbal plants, such as Moringa oleifera (Khalid et al., 2024; Fakhira and Hamdat, 2025). Therefore, in this study, we aimed to identify agents that have a potential antioxidant role and are available for all patients of all ages and preferences with fewer side effects and interactions. It is important to reduce the adverse effects of acetaminophen and other agents by using available, economic, and effective antioxidant agents, such as CoQ10, which is present in variable amounts in various organelles and in the center of the lipid bilayers of distinct cellular membranes (Crane 2001). Higher concentrations can also be found in cells with high energy requirements, such as those in the heart, liver, muscles, and pancreas. It is also found in the ER, Golgi apparatus, peroxisomes, lysosomes, and vesicle membranes (Casagrande et al., 2018; Appiah et al., 2020). Numerous studies have highlighted the role of CoQ10 in the maintenance of human health status. It is present in the cellular membrane, protecting cells from oxidative stress and maintaining cellular stability by acting as a free radical scavenger Ebadi et al. (2001). Conversely, CoQ10 is predominant in the mitochondria and helps synthesize ATP, supplying cells with their energy requirements. In addition, it plays a vital role in the metabolism of sulfides and pyrimidines. It also plays a role in the expression of some inflammatory genes (Mantle et al., 2023). It is available for green vegetables and seafood in addition to pharmaceuticals that could be easily obtained at a low cost. Therefore, in this study, we aimed to evaluate the protective effect of CoQ10 against hepatic and renal toxicity induced by acetaminophen overdose. Materials and MethodsMaterialsAcetaminophen tablets (DOLOROK®) were obtained from UROK PHARMA, Iraq. Normal saline was obtained from Pioneer, Iraq. CoQ10 (Ultra Co-Q10 50 mg) was obtained from VITABIOTICS Company, UK. Doses calculationAcetaminophen tablets were ground and dissolved in 5 ml normal saline, then 1 ml each contained 100 mg of the drug, which was drenched in each animal. CoQ10 (50 mg table), each tablet was ground and dissolved in 5 ml of normal saline. To achieve the dose (10 mg/kg), each animal should receive 2 mg because the average weight is 200 mg. Therefore, each animal was drenched with 0.2 ml of the solution. AnimalsThe current study was conducted in November 2024 at the Pharmacy College of the University of Karbala in accordance with all the animal ethics committee recommendations. Thirty male albino rats were used in this study. The animal was 6 weeks old and weighed 200 g on average when taken from the animal house of the college’s pharmacology department. To ensure uniform rat growth and performance, all rats were kept in a typical light-dark cycle, fed a standard laboratory diet, and had free access to water throughout the experimental period. Rats were randomly separated into three groups as follows: a-Control group: Apparently healthy and adult rats. Standard food and normal saline were administered to each rat for 30 days. b-Acetaminophen group: Rats were orally drenched with 500 mg/kg/day (100 mg/ml) for 30 days by oral gavage (Hosny et al., 2019). c-CoQ10 +Acetaminophen group: Rats were orally administered 10 mg/kg/day (10 mg/ml) of synthetic CoQ10 (Kiremitli et al., 2021) (0.2 ml containing 2mg of CoQ10), and after 10 minutes, the animals were orally administered 500 mg/kg/day Acetaminophen for 30 days. All drugs were given to the animals between 9:00 and 10:00 a.m. daily. After the last dose, on day 30 (11:00 a.m.), after 1 hour of dosing, all animals were anesthetized with chloroform, and 5 ml of blood was collected from their hearts for further processing. Blood samples were then centrifuged at 3,000 rpm for 5 minutes to collect the plasma and sent to the specialist for biochemical analysis of liver and kidney function parameters. Laboratory measurementRenal and hepatic functions were assessed by measuring the primary markers of these main organ functions, including serum creatinine, blood urea, and total serum bilirubin (for renal assessment) and alkaline phosphatase (ALP), alanine transaminase (ALT), and aspartate aminotransferase (AST) for liver assessment. (Kaplan et al., 1965). Data analysisThe results of the biochemical tests were analyzed using one-way analysis of variance using SPSS version 27, followed by a post-hoc test (Turkey’s HSD) to determine the significance of differences between groups. Data are expressed as mean ±standard deviation. Different superscript letters (a, b) indicate statistically significant differences between groups at p < 0.0. Ethical approvalThe Scientific and Ethical Committee of Pharmacy College, University of Karbala, approved the present study on July 21, 2024 (Ref:2024An.17). The study was conducted in accordance with the Declaration of Helsinki. ResultsTable 1 shows the effect of acetaminophen overdose and CoQ10 on liver function parameters. The results in the table revealed a significant increase (p ≤ 0.05) in ALT, AST, and ALP (45.6, 104, and 287.83 U/L and 287.83 mg/dl). The values of ALP, ALT, and AST were reduced significantly (p 0.05) in the acetaminophen + CoQ10 group compared to the control group (51.5 U/L, 118 U/L, and 477 mg/dl). The table also clarifies that the values of ALP, ALT, and AST were reduced significantly (p ≤ 0.05) (51.5 U/L, 118 U/L, and 477 mg/dl), respectively, in Acetaminophen+CoQ10 group compared to the acetaminophen group, while bilirubin showed no significant change after CoQ10 use (Fig. 1). Table 1. Effect of CoQ10 on liver function biomarkers after ACE toxicity.
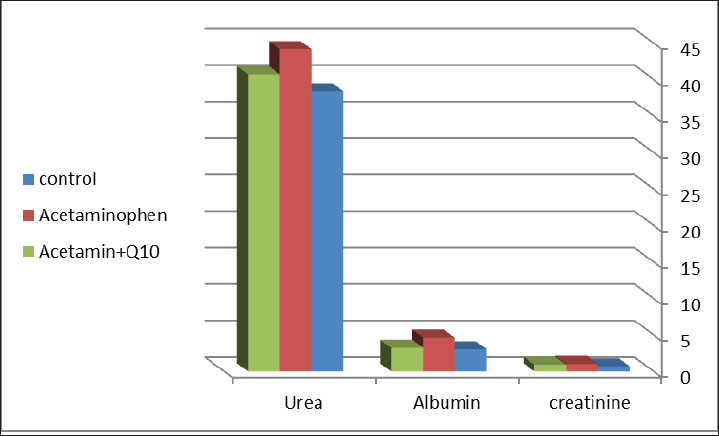
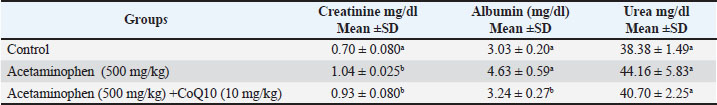
Fig. 1. Differences in liver function parameters between the control, acetaminophen, and acetaminophen + CoQ10 groups. Table 2 illustrates the effect of acetaminophen toxicity and CoQ10 on kidney function tests. The findings revealed a significant increase (p ≤ 0.05) in creatinine and albumin (1.04 m/dl and 4.63 mg/dl, respectively) in the acetaminophen group compared with the control group (0.7 mg/dl and 3.03 mg/dl, respectively), while urea showed no significant change. The table also revealed a significant reduction in creatinine and albumin values (0.93 mg/dl and 3.24 mg/dl, respectively) in the third group (Acetaminophen and CoQ10) as expressed in Figure 2.
Fig. 2. Differences in renal function parameters between the control, acetaminophen, and acetaminophen + CoQ10 groups. Table 2. Effect of CoQ10 on kidney function after acetaminophen toxicity.
DiscussionThe current study demonstrated the effectiveness of CoQ10 in reducing the toxicity of acetaminophen and improving the liver and kidney efficiency in laboratory rats. Tables 1 and 2 present the concentrations of liver and kidney indicators (ALT, AST, ALP, TSB, creatinine, urea, and albumin) of the experimental groups. There was a significant increase in liver enzyme and renal function biomarker levels in the acetaminophen group compared with the control group. This may be due to the effect of this drug on the liver and kidney cells, causing severe damage at this dose. This damage releases large amounts of these enzymes, which are transported to the intercellular fluids, and their concentrations increase in the bloodstream. The increase in AST and ALT concentrations indicates hepatocyte necrosis, whereas ALP levels may result from damage to the canalicular membrane or biliary epithelial cells (Parmar et al., 2010). The findings also revealed a considerable rise in total serum bilirubin, which agreed with the findings of Al-Jawad et al. (2019), who found the same as our observations. Regarding the kidneys, creatinine and albumin levels were also significantly increased (Table 2), indicating that acetaminophen overdose negatively impacted the kidneys and produced nephrotoxicity. Acetaminophen intoxication is uncommonly linked to renal impairment; instead, hepatotoxicity is typically the cause. Based on human research, clinical presentation, and imaging, most patients with ARI display a pattern of acute tubular necrosis or damage. The extent of injury was related to the dosage of acetaminophen (Khan et al., 2021). The study also revealed the protective effect of CoQ10 against ACE toxicity. The results of the third group of the study (Acetaminophen overdose and CoQ10) revealed that CoQ10 caused the plasma concentrations of hazardous liver and kidney biomarkers to return to approximately control levels. Significant reductions in AST, ALT, and ALP levels were induced by CoQ10. Additionally, it lowers the levels of creatinine and albumin. These findings demonstrated that acetaminophen-induced liver damage can be reversed by Coenzyme Q10 therapy, as stated in previous research. Martelli et al. (2020) also proved our findings by showing that Coenzyme Q10 is an internal antioxidant that protects cells from pro-oxidant oxidative damage by reducing oxidative damage and the regeneration of other antioxidant activities. The content of CoQ10 is higher in organs such as the kidneys, heart, and liver because these organs have high metabolic rates and require CoQ10 as an adequate energy source. CoQ10 can withstand oxidation-reduction cycles. It has anti-inflammatory properties that protect against tissue injury by preventing the synthesis of pro-inflammatory cytokine factors (Samimi et al., 2024). Sutken et al. (2007) demonstrated that CoQ10 protects liver tissue against toxins and enhances the antioxidant protection of liver membranes in long-lived rats. CoQ10 has hydrophobic properties and a large molecular weight, making its absorption after oral administration limited. Therefore, special formulations are important to improve its bioavailability. CoQ10 has a half-life of approximately 33 hours and a T max of about 6 hours. Ingestion of large doses of CoQ10 can reach different parts of the body, such as the heart and brain, giving this agent a promising future in treating diverse diseases and improving cardiac and cerebral health (Bhagavan and Chopra, 2006). Numerous studies on various animal models have shown that CoQ10 might slow or stop the progression of hepatocyte cirrhosis following exposure to various types of toxic chemicals (Kettawan et al., 2007; Li et al., 2016; Zhao et al., 2022,). In a rat model of renal injury caused by acetate, a study found that CoQ10 may initiate the antioxidant mechanism and prevent inflammatory and apoptotic events (Al-Megrin et al., 2020). Additionally, a meta-analysis of research has demonstrated that CoQ10 injection may dramatically reduce the levels of inflammatory mediators, such as C-reactive protein, tumor necrosis factor-alpha, and interleukin 6 (Zhai et al., 2017). An important property of CoQ10 is its ability to reduce structural damage in cells, decrease the rate of spontaneous cell death, and eliminate free radicals in the body (Liu et al., 2020). Liu et al. (2020) demonstrated the processes of inhibiting free radicals in cells, preventing kidney parts from necrosis and self-death, and reducing the huge numbers of white blood cells. The results of our study demonstrated the positive effect of CoQ10 against various oxidizing agents, such as aminoglycosides, acetaminophen, and other commonly used agents, whose quantities accumulate in the liver and kidneys if used daily and for long periods (Ashkani Esfahani et al., 2016; Liu et al., 2021). The therapeutic properties of this treatment led to an improvement in the functioning of various body organs and increased immunity and the body’s resistance to diseases. It has been observed in some studies that there is a significant improvement in patients with kidney disease when they take CoQ10 for a certain period (Drovandi et al., 2022). They noticed that the rates of liver and kidney enzymes in the bloodstream decreased, and the effect of oxidizing factors in the cells was reduced. CoQ10 has been proven to possess individual metabolic pathways, “Nrf2/HO-1” that improve health and reduce oxidative factors, in addition to possessing important proteins, such as “caspase-3, p53, and PON1” which are attributed to protecting important organs in the body, such as the liver and kidneys (Zhao et al., 2022). CoQ10 is effective against different diseases, such as cardiovascular disease, aging, and overall health (Gasmi et al., 2022). In addition, preclinical and clinical assessment of the pharmacokinetic profile of CoQ10 indicate that it has a high degree of safety and bioavailability with tolerated side effects such as mild nausea and vomiting (Hidaka et al., 2008). The present study’s findings include the evaluation of the effectiveness of the biochemical parameters in the diagnosis of acetaminophen side effects. The present study has some limitations that could be addressed in future work. These limitations include assessments of other renal and hepatic biomarkers and oxidative stress markers, evaluation of blood parameters, and histological examination to prove the biochemical results, which were not available in this study. The authors plan to include other antioxidant agents in the study and increase the sample size in addition to assessing more sensitive markers for oxidative stress, such as MDA, GSH, and SOD. ConclusionThe findings of our study showed a positive protective effect of CoQ10 on hepatic function tests. It can reduce ACE-induced toxicity. Moreover, CoQ10 protects the kidneys against acetaminophen toxicity by reducing the levels of creatinine and albumin. In the future, further studies, including human trials, will be conducted to understand the actual mechanism of action of CoQ10. AcknowledgmentsWe would like to thank the leader of the animal house in Pharmacy College, University of Kerbala, and all other pathologists and chemists who helped us in the work. Conflict of interestThe authors declare no conflicts of interest to be included. FundingSelf-funding study. Authors’ contributionsIman Hussein Naser and Zahraa Abed al-kareem conceived, planned, and conducted the experiments and contributed to sample preparation. Moayad Mijbil Ubaid and Shatha Hussein Kadhim contributed to the statistical analysis of data and interpretation of the results. Iman Hussein Naser took the lead in writing the manuscript. All authors provided critical feedback and helped in the planning of the research, results analysis, and manuscript evaluation. Data availabilityAll data supporting this study’s findings are available within the manuscript. ReferencesAl-Jawad, F.H., Al-Attar, Z. and Abbood, M.S. 2019. The protective effect of Nitroglycerin, N-Acetyl Cysteine and metoprolol in CCL4 induced animal model of acute liver injury. OAMJMS 7(11), 1739–1743. Al-Megrin, W.A., Soliman, D., Kassab, R.B., Metwally, D.M., Abdel Moneim, A.E. and El-Khadragy, M.F. 2020. Coenzyme Q10 activates the antioxidant machinery and inhibits the inflammatory and apoptotic cascades against lead acetate-induced renal injury in rats. Front. Physiol. 11, 64. Appiah, M.O., Asante-Badu, B., Zhao, J., Liu, H., Wang, J. and Lu, W. 2020. Possible protective mechanisms of coenzyme Q10 action on spermatozoa during cryopreservation or cooled-stored condition. Cryo. Lett. 41(5), 246–256. Ashkani-Esfahani, S., Bagheri, F., Emami, Y., Esmaeilzadeh, E., Azarpira, N., Hassanabadi, N., Keshtkar, M., Farjam, M., Koohi-Hosseinabadi, O. and Noorafshan, A. 2016. Protective effects of Co-enzyme Q10 on Thioacetamide-induced acute liver damage and its correlation with behavioral, biochemical, and pathological factors. IRCMJ 18(8), e29166. Aziz, N.D., Ouda, M.H. and Ubaid, M.M. 2018. Comparing the toxic effects of nonsteroidal anti-inflammatory drugs (celecoxib and ibuprofen) on heart, liver, and kidney in rats. AJPCRes 11(6), 482–485. Bhagavan, H.N. and Chopra, R.K. 2006. Coenzyme Q10: absorption, tissue uptake, metabolism and pharmacokinetics. Free Radic. Res. 40(5), 445–453. Casagrande, D., Waib, P.H. and Júnior, A.A.J. 2018. Mechanisms of action and effects of the administration of Coenzyme Q10 on metabolic syndrome. JNIM 13, 26–32. Chun, L.J., Tong, M.J., Busuttil, R.W. and Hiatt, J.R. 2009. Acetaminophen hepatotoxicity and acute liver failure. JCG 43(4), 342–349. Crane, F.L. 2001. Biochemical functions of coenzyme Q10. JANA 20(6), 591–598. Drovandi, S., Lipska-Ziętkiewicz, B.S., Ozaltin, F., Emma, F., Gulhan, B., Boyer, O., Trautmann, A., Xu, H., Shen, Q., Rao, J., Riedhammer, K.M., Heemann, U., Hoefele, J., Stenton, S.L., Tsygin, A.N., Ng, K.H., Fomina, S., Benetti, E., Aurelle, M., Prikhodina, L. and Schaefer, F. 2022. Oral Coenzyme Q10 supplementation leads to better preservation of kidney function in steroid-resistant nephrotic syndrome due to primary Coenzyme Q10 deficiency. KI 102(3), 604–612. Du, K., Williams, C.D., Mcgill, M.R. and Jaeschke, H. 2014. Lower susceptibility of female mice to acetaminophen hepatotoxicity: role of mitochondrial glutathione, oxidant stress and c-jun N-terminal kinase. Toxicol. Appl. Pharmacol. 281(1), 58–66. Ebadi, M., Govitrapong, P., Sharma, S., Muralikrishnan, D., Shavali, S., Pellett, L., Schafer, R., Albano, C. and Eken, J. 2001. Ubiquinone (coenzyme q10) and mitochondria in oxidative stress of parkinson's disease. Biol. Signals Recept. 10(3-4), 224–253. Eugenio-Pérez, D., Montes De Oca-Solano, H.A. and Pedraza-Chaverri, J. 2016. Role of food-derived antioxidant agents against acetaminophen-induced hepatotoxicity. Pharma. Biol. 54(10), 2340–2352. Fouad, A.A. and Jresat, I. 2012. Hepatoprotective effect of coenzyme Q10 in rats with acetaminophen toxicity. Environ. Toxicol. Pharmacol. 33(2), 158–167. Freo, U., Ruocco, C., Valerio, A., Scagnol, I. and Nisoli, E. 2021. Acetaminophen: a review of guideline recommendations. JCM 10, 3420. Gasmi, A., Bjørklund, G., Mujawdiya, P.K., Semenova, Y., Piscopo, S. and Peana, M. 2022. Coenzyme Q10 in aging and disease. Crit. Rev. Food Sci. Nutr. 64(12), 3907–3919. Hidaka, T., Fjii, K., Funahashi, I., Fukutomi, N. and Hosoe, K. 2008. Safety assessment of coenzyme Q10 (CoQ10). BioFactors 32(1-4), 199–208. Hosny Abd El Fadil., Edress, N., Khorshid, N. and Amin, N. 2019. Protective impact of curcumin against paracetamol-induced hepatotoxicity in rats. IJPRAS 8(1), 84–94. Kadhim, S.H., Mosa, A.U. and Ubaid, M.M. 2022. Hepatorenal protective activity of Artemisia against diclofenac toxicity in male rats. PAMJ 43, 192. Kaplan, A., Chaney, A.L., Lynch, R.L. and Meites, S. 1965. Urea nitrogen and urinary ammonia. Clin. Chem. 5, 245–256. Kettawan, A., Takahashi, T., Kongkachuichai, R., Charoenkiatkul, S., Kishi, T. and Okamoto, T. 2007. Protective effects of coenzyme Q10 on decreased oxidative stress resistance induced by simvastatin. J. Clin. Biochem. Nutr. 40, 194–202. Khalid, F. and Azmat, H. 2025. Restoration of skin mucosal immune responses, cytogenotoxicity and histological alterations in arsenic exposed Labeo rohita by Moringa oleifera supplementation. Fish Shellfish Immunol. 161(110237), 110237. Khalid, F., Azmat, H., Khan, N. and Saima. 2024. Ameliorative effects of Moringa oleifera leaf extract against arsenic induced histo-biochemical alterations in Labeo rohita. Ecotoxicol. Environ. Saf. 287(117258), 117258. Khan, Z., Abumedian, M., Ibekwe, M., Musa, K. and Mlawa, G. 2021. Acute renal impairment in patients due to Acetaminophen overdose in the absence of hepatic impairment. Cureus 13(12), e20727. Kiremitli, T., Kiremitli, S., Akselim, B., Yilmaz, B., Mammadov, R., Tor, I., Yazici, G. and Gulaboglu, M. 2021. Protective effect of Coenzyme Q10 on oxidative ovarian and uterine damage induced by methotrexate in rats. Hum. Exp. Toxicol. 40(9), 1537–1544. Li, L., Du, J., Lian, Y., Zhang, Y., Li, X., Liu, Y., Zou, L. and Wu, T. 2016. Protective effects of coenzyme Q10 against hydrogen peroxide-induced oxidative stress in PC12 cell: the role of Nrf2 and antioxidant enzymes. Cell. Mol. Neurobiol. 36, 103–111. Liu, Z., Li, Y., Li, C., Yu, L., Chang, Y. and Qu, M. 2021. Delivery of coenzyme Q10 with mitochondria-targeted nanocarrier attenuates renal ischemia-reperfusion injury in mice. Mat. Sci. Eng. C-Mater. Biol. Appl. 131, 112536. Liu, Z., Liu, X., Yang, Q., Yu, L., Chang, Y. and Qu, M. 2020. Neutrophil membrane-enveloped nanoparticles for the amelioration of renal ischemia-reperfusion injury in mice. Acta Biomater. 104, 158–166. Mantle, D., Lopez-Lluch, G. and Hargreaves, I.P. 2023. Coenzyme Q10 metabolism: a review of unresolved issues. Int. J. Mol. Sci. 24, 2585. Marks, D.J., Dargan, P.I., Archer, J.R., Davies, C.L., Dines, A.M., Wood, D.M. and Greene, S.L. 2017. Outcomes from massive Acetaminophen overdose: a retrospective observational study. Br. J. Clin. Pharmacol. 83, 1263–1272. Martelli, A., Testai, L., Colletti, A. and Cicero, A.F.G. 2020. Coenzyme Q10: clinical applications in cardiovascular diseases. Antioxidants (Basel). 9(4), 341. Mazraati, P. and Minaiyan, M. 2018. Hepatoprotective effect of Metadoxine on Acetaminophen-induced liver toxicity in mice. Adv. Biomed. Res. 7, 67. Millman, M. and Grundon, W. 1969. Use of acetylcysteine in bronchial asthma and emphysema. J. Asthma 6(4), 199–209. Parmar, S.R., Vashrambhai, P.H. and Kalia, K. 2010. Hepatoprotective activity of some plants extract against Acetaminophen induced hepatotoxicity in rats. J. Herbal Med. Toxicol. 4(2), 101–106. Paschalis, V., Theodorou, A.A., Margaritelis, N.V., Kyparos, A. and Nikolaidis, M.G. 2018. N-acetylcysteine supplementation increases exercise performance and reduces oxidative stress only in individuals with low levels of glutathione. Free Radic. Biol. Med. 115, 288–297. Popiolek, I., Hydzik, P., Jagielski, P., Zrodlowska, M., Mystek, K. and Porebski, G. 2021. Risk factors for hepatotoxicity due to Acetaminophen overdose in adults. Medicina 57, 752. Saadi, T.A., Assaf, Y., Farwati, M., Turkmani, K., Al-Mouakeh, A., Shebli, B., Khoja, M., Essali, A. and Mohammed E Madmani. 2021. Coenzyme Q10 for heart failure. CDSR 26(1), 81–87. Samimi, F., Namiranian, N., Sharifi-Rigi, A., Siri, M., Abazari, O. and Dastghaib, S. 2024. Coenzyme Q10: a key antioxidant in the management of diabetes‐induced cardiovascular complications—an overview of mechanisms and clinical evidence. Int. J. Endocrinol. 2024, 2247748. Schwalfenberg, G.K. 2021. N-Acetylcysteine: a review of clinical usefulness (an old drug with new tricks). J. Nutr. Metab. 13. Sutken, E., Aral, E., Ozdemir, F., Uslu, S., Alatas, O. and Colak, O. 2007. Protective role of melatonin and coenzyme Q10 in ochratoxin A toxicity in rat liver and kidney. Int. J. Toxicol. 26(1), 81–87. Ubaid, M.M. 2019. Using of omega 3 to reduce the toxic Effect of antituberculosis therapy on hepatorenal function and some of blood parameters of albino male rats. Indian J. Public Health 10(11), 2101. Ubaid, M.M., Kadhim, S.H. and Al-Kareem, Z.A. 2022. Protective effect of cinnamon oil against ciprofloxacin toxicity on liver and kidney of male Wistar rats. J. Appl. Nat. Sci. 14(4), 1430–1434. Wongrakpanich, S., Wongrakpanich, A., Melhado, K. and Rangaswami, J. 2018. A comprehensive review of non-steroidal anti-Inflammatory drug use in the elderly. Aging Dis. 9(1), 143–150. Zhai, J., Bo, Y., Lu, Y., Liu, C. and Zhang, L. 2017. Effects of coenzyme Q10 on markers of inflammation: a systematic review and meta-analysis. PLos One 12(1), 170172. Zhang, W., Jones, A. and Doherty, M. 2004. Does acetaminophen (acetaminophen) reduce the pain of osteoarthritis? A meta-analysis of randomized controlled trials. Ann. Rheum. Dis. 63(8), 901–907. Zhao, S., Wu, W., Liao, J., Zhang, X., Shen, M., Li, X., Lin, Q. and Cao, C. 2022. Molecular mechanisms underlying the renal protective effects of coenzyme Q10 in acute kidney injury. Cell. Mol. Biol. Lett. 27(1), 57. | ||
| How to Cite this Article |
| Pubmed Style Naser IH, Al-kareem ZA, Ubaid MM, Kadhim SH. Protective role of coenzyme Q10 against renal and hepatic toxicity induced by acetaminophen overdose in albino rats. Open Vet. J.. 2025; 15(9): 4235-4241. doi:10.5455/OVJ.2025.v15.i9.29 Web Style Naser IH, Al-kareem ZA, Ubaid MM, Kadhim SH. Protective role of coenzyme Q10 against renal and hepatic toxicity induced by acetaminophen overdose in albino rats. https://www.openveterinaryjournal.com/?mno=266904 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i9.29 AMA (American Medical Association) Style Naser IH, Al-kareem ZA, Ubaid MM, Kadhim SH. Protective role of coenzyme Q10 against renal and hepatic toxicity induced by acetaminophen overdose in albino rats. Open Vet. J.. 2025; 15(9): 4235-4241. doi:10.5455/OVJ.2025.v15.i9.29 Vancouver/ICMJE Style Naser IH, Al-kareem ZA, Ubaid MM, Kadhim SH. Protective role of coenzyme Q10 against renal and hepatic toxicity induced by acetaminophen overdose in albino rats. Open Vet. J.. (2025), [cited January 25, 2026]; 15(9): 4235-4241. doi:10.5455/OVJ.2025.v15.i9.29 Harvard Style Naser, I. H., Al-kareem, . Z. A., Ubaid, . M. M. & Kadhim, . S. H. (2025) Protective role of coenzyme Q10 against renal and hepatic toxicity induced by acetaminophen overdose in albino rats. Open Vet. J., 15 (9), 4235-4241. doi:10.5455/OVJ.2025.v15.i9.29 Turabian Style Naser, Iman Hussein, Zahraa Abed Al-kareem, Moayad Mijbil Ubaid, and Shatha Hussein Kadhim. 2025. Protective role of coenzyme Q10 against renal and hepatic toxicity induced by acetaminophen overdose in albino rats. Open Veterinary Journal, 15 (9), 4235-4241. doi:10.5455/OVJ.2025.v15.i9.29 Chicago Style Naser, Iman Hussein, Zahraa Abed Al-kareem, Moayad Mijbil Ubaid, and Shatha Hussein Kadhim. "Protective role of coenzyme Q10 against renal and hepatic toxicity induced by acetaminophen overdose in albino rats." Open Veterinary Journal 15 (2025), 4235-4241. doi:10.5455/OVJ.2025.v15.i9.29 MLA (The Modern Language Association) Style Naser, Iman Hussein, Zahraa Abed Al-kareem, Moayad Mijbil Ubaid, and Shatha Hussein Kadhim. "Protective role of coenzyme Q10 against renal and hepatic toxicity induced by acetaminophen overdose in albino rats." Open Veterinary Journal 15.9 (2025), 4235-4241. Print. doi:10.5455/OVJ.2025.v15.i9.29 APA (American Psychological Association) Style Naser, I. H., Al-kareem, . Z. A., Ubaid, . M. M. & Kadhim, . S. H. (2025) Protective role of coenzyme Q10 against renal and hepatic toxicity induced by acetaminophen overdose in albino rats. Open Veterinary Journal, 15 (9), 4235-4241. doi:10.5455/OVJ.2025.v15.i9.29 |