| Research Article | ||
Open Vet. J.. 2025; 15(9): 4190-4198
Open Veterinary Journal, (2025), Vol. 15(9): 4190-4198 Research Article Molecular detection and genetic relatedness of enteric bacteria from Iraqi dairy productsHuda Hassan Gaber and Orooba Meteab Faja*Department of Public Health, College of Veterinary Medicine, University of Al-Qadisiyah, Al Diwaniyah, Iraq © 2025 Open Veterinary Journal
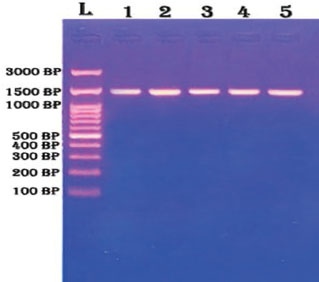
ABSTRACTBackground: Raw milk and unpasteurized dairy products are still widely consumed in Iraq, particularly in rural areas. These products may carry harmful bacteria, including those resistant to antibiotics. Although many studies have focused on common pathogens, few have used molecular methods to detect and compare local bacterial strains with international strains. This lack of data creates a gap in understanding the public health risk and genetic diversity of these bacteria in Iraqi dairy products. Aim: This study aimed to detect bacteria in local dairy products from Al-Diwaniyah City, Iraq. Methods: This study was observational. Milk, yogurt, and cheese samples were taken. A total of 150 samples were collected: 60 milk, 45 yogurts, and 45 cheese samples. They were collected from farms, markets, and shops. Each sample was tested in the laboratory. The objectives of this study were to detect and identify the presence of bacteria in local dairy products, including milk, yogurt, and cheese, collected from Al-Diwaniyah City, Iraq. This study aimed to use both biochemical methods, such as the VITEK®2 system, and molecular tools, such as polymerase chain reaction and 16S rRNA sequencing, to accurately classify the bacterial species. Another goal was to compare the genetic sequences of local isolates with those of international strains using Basic Local Alignment Search Tool analysis and phylogenetic tree construction. In addition, we evaluated the protein patterns of the bacterial isolates using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) to understand their enzymatic activity. These objectives were designed to highlight the risks of contamination in unpasteurized dairy products and support better food safety practices using molecular evidence. Results: Klebsiella pneumoniae, Klebsiella aerogenes, Enterobacter cloacae, Escherichia coli, and Enterococcus cecorum were found. These bacteria were very similar to those from other countries, such as India and Egypt. Gene sequences were matched with those in the GenBank database. Some gene regions were the same in all bacteria. Other regions exhibited changes. SDS-PAGE showed clear protein bands between 20 and 70 kDa, confirming diverse peptide profiles among the bacterial isolates. These changes help to separate the bacterial species. Conclusion: Dairy products can carry serious bacteria. These bacteria can be studied by examining their genes. Regular checking of food is needed. Molecular tools can help identify and track these bacteria. Keywords: Bacteria, Dairy, Iraq, PCR, Phylogeny. IntroductionThe One Health approach is important in understanding and controlling diseases that occur between animals, humans, and the environment. It connects veterinary, medical, and environmental sciences to fight health threats, such as zoonotic diseases, antimicrobial resistance, and unsafe food. Many foodborne bacteria come from animals or the environment; therefore, this method is highly relevant in dairy safety studies. However, many medical professionals remain unaware of this approach, which limits its use in public health planning. Increasing awareness and training on One Health are needed, especially in regions where animal contact and raw food consumption are common. This study supports the idea that a better understanding of bacterial contamination in dairy can help improve food safety using the One Health concept (Meurer et al., 2024). Foodborne bacteria remain a growing global threat. Due to their nutrient content and perishable nature, dairy products are prone to contamination. Contaminated dairy products can cause severe infections or spread resistant bacteria (Krishnamoorthy et al., 2024). Livestock is the main source of zoonotic pathogens in these products (Abebe et al., 2020). These pathogens include Escherichia coli, Salmonella spp., Listeria spp., and others. Infected animals and unclean equipment contribute to the risk of infection. Unpasteurized dairy products are especially hazardous. Consumption of such products continues despite health warnings. Human cases of brucellosis and other zoonotic infections have been linked to raw dairy intake of unpasteurized milk and dairy products, which may contain live bacteria transmitted from infected animals (Yu et al., 2022). Even modern packaging does not fully prevent microbial contamination (Xie et al., 2024b). New pathogens have recently emerged from dairy sources. These include non-O157 STEC (Shiga toxin-producing E. coli), Aeromonas, and multi-resistant Enterobacteriaceae (Alharbi et al., 2022; Carusi et al., 2024). Many of these bacteria carry antibiotic resistance gene. Once these bacteria enter the food chain, they can quickly spread to consumers. Resistance genes can be transferred between bacterial strains, even across species. Gut colonization can occur silently, especially in people with a compromised immune system. The role of gut bacteria in health is now better understood. Metabolites such as phenylacetylglutamine affect heart and brain health. This underscores the need for early microbial detection in foods (De Alcântara Rodrigues et al., 2020; Krishnamoorthy et al., 2024). Traditional methods for identifying pathogens are often slow. Some require several days to yield results. This delay is critical for products such as milk with a short shelf life. New tools, such as polymerase chain reaction (PCR), biosensors, and omics, have reduced detection times. DNA-based methods, especially 16S rRNA gene analysis, allow species-level identification. These methods are accurate, fast, and widely used. They also help in tracking bacterial evolution. Understanding phylogenetic relationships supports better control strategies. Some pathogens evolve quickly due to environmental pressure (Ferone et al., 2020). Bacteriophages, bacteriocins, and essential oils are being tested as natural antimicrobial agents (Falleh et al., 2020; Diacon et al., 2022; Wu et al., 2023a, 2023b). They are considered safe and eco-friendly alternatives to synthetic chemicals. Nisin is a natural peptide that is widely used in dairy preservation. Lauric arginate also shows strong antibacterial action at low doses (Ma et al., 2020; Anyanwu et al., 2021). These natural compounds reduce pathogen load without changing the taste or texture of food. However, regulatory and consumer acceptance issues remain. Other solutions include non-thermal processing and intelligent packaging. These approaches aim to maintain food safety without compromising nutrition (Xie et al., 2024a, 2024b). Food safety also depends on bacterial adaptation monitoring. Some bacteria form biofilms or produce exopolysaccharides that shield them from treatment. Other researchers use siderophores to capture iron, enhancing survival and virulence. These features complicate the cleaning and disinfection procedures. The role of the gut microbiome in overall health further complicates the issue (Ozsoy et al., 2022; Wu et al., 2023a, 2023b; Cui et al., 2024). Microbial metabolites affect obesity, diabetes, and immune responses (Abd-Alhassen et al., 2021; Ghazi et al., 2024). Contaminated dairy items can alter gut balance and trigger illness. Therefore, precise and fast detection is crucial. Molecular tools, such as 16S rRNA sequencing, are key in tracking these threats (Karim et al., 2018; Yaseen et al., 2020). This study aimed to detect bacteria in local dairy products from Al-Diwaniyah City, Iraq, from September 25 to December 28, 2024. Materials and MethodsStudy areaThis observational study was conducted in the Department of Public Health Laboratory, College of Veterinary Medicine, University of Al-Qadisyah, Al-Diwaniyah City, Iraq. Sample collectionMilk, yogurt, and cheese samples were collected from different areas in the province of Al-Diwaniyah, Iraq. A total of 150 samples were collected: 60 milk, 45 yogurts, and 45 cheese samples. Sources included local markets, buffalo farms, and the Taj Al-Nahreen Dairy Company. The collection lasted from September 25 to December 28, 2024. Each day, 10 samples were collected using sterile techniques. Two were from the company, one from a local shop, and seven from various buffalo farms. A total of 150 samples were collected: 30 from the company, 15 from the local shop, and 105 from buffalo farms. Samples were collected in 10 ml sterile containers and immediately placed in ice-filled boxes. The samples were transported to the laboratory within 3 hours to ensure integrity. Bacterial culture and isolationSamples were processed in a laminar flow hood in the laboratory. Each sample was shaken and streaked onto multiple media plates. Blood agar, MacConkey agar, Eosin Methylene Blue (EMB) agar, Mannitol Salt Agar, and Nutrient agar were used as media. All media were prepared according to the manufacturer’s instructions. After plating, the dishes were incubated at 37°C for 24–48 hours. The morphology, pigmentation, and hemolytic activity of colonies were examined. The suspected colonies were picked and subcultured for purification. The purified colonies were maintained for further testing. Each isolate was transferred onto nutrient agar slants. The slants were stored at 4°C. A backup set was stored in glycerol stocks at −20°C. The preserved isolates were used for biochemical and molecular identification. Only pure cultures were used for DNA extraction and PCR assays. Biochemical identification using VITEK®2The VITEK®2 Compact System was used for biochemical analysis. This system works automatically and uses bacteria identification cards. The isolates were suspended in a 0.45% NaCl solution. The turbidity was adjusted to match the standard of McFarland 0.5. Each suspension was loaded into the sample port along with an ID card. The machine automatically read, incubated, and interpreted the results. The output data were printed and saved for each isolate. DNA extraction from bacterial isolatesGenomic DNA was extracted using the Presto™ Mini gDNA Bacteria Kit. For Gram-negative bacteria, 1 ml of culture was pelleted and treated with GT buffer and proteinase K. The mixture was incubated at 60°C for 10 minutes. For Gram-positive isolates, the pellet was treated with lysozyme in Gram+ buffer before proteinase K was added. After a 30-minute incubation at 37°C, the sample was processed similarly to Gram-negative bacteria. After lysis, 200 µl of ethanol was added to each lysate. The mixture was applied to a generation diversity column placed in a 2-ml collection tube. The tube was centrifuged for 2 minutes at 14,000 × g. The column was washed first with W1 buffer and then with wash buffer. The final spin dried the column. The DNA was eluted in 50 µl of nuclease-free water and stored at −20°C. We evaluated the quality and quantity of DNA using a NanoDrop Lite Spectrophotometer. Two microliters of each sample was loaded on the pedestal. The absorbance at 260 and 280 nm was recorded. The purity ratio was calculated. Samples with ratios between 1.8 and 2.0 were selected for PCR. DNA was used immediately or kept on ice until use. PCRPCR primer designThe 16S rRNA gene was identified. The primers were designed according to the published sequences. The forward primer sequence was AGA GTT TGA TCC TGG CTC AG. The reverse primer was CTA CGG CTA CCT TGT TAC GA. These primers produced an expected band of 1,500 base pairs. The primers were obtained from Macrogen (Korea). PCR reactions were performed using Maxime PreMix tubes. Each 50 µl reaction contained 25 µl master mix, 3 µl of each primer, 4 µl of DNA template, and 15 µl of nuclease-free water. All components were gently mixed. Tubes were briefly vortexed and centrifuged. The prepared reactions were transferred to the thermal cycler. Thermal cycling was performed in a Bio-Rad T100 thermocycler. The initial denaturation step was performed at 95°C for 5 minutes. This was followed by 30 cycles of denaturation at 95°C for 1 minute, annealing at 55°C for 1 minute, and extension at 72°C for 90 s. A final extension at 72°C was run for 7 minutes. Tubes were kept at 4°C until analysis. The PCR products were resolved on a 1.5% agarose gel. The gel was prepared using TBE buffer and stained with ethidium bromide. A DNA ladder was used to estimate the size of the product. Three microliters of each product was loaded into the wells. Electrophoresis was performed at 85 V for 90 min. Bands were visualized under UV light. The clear bands at 1,500 bp were considered positive. Only products with sharp single bands were sent for sequencing. Faint or multiple bands were excluded. The gels were photographed and stored for documentation. Band intensity and clarity were used as quality indicators. DNA sequencingThe selected PCR products were purified and sent for sequencing. Sanger sequencing was performed at the Korea-based Macrogen Corporation. The sequencing results were returned as Fast-All (FASTA) files. Each file represents a single isolate. Sequences were compared using the NCBI Basic Local Alignment Search Tool (BLAST). The goal was to identify closely related strains. Only matches with >97% identity were accepted. The results provided GenBank accession numbers and organism names. These were recorded for each isolate. Phylogenetic trees were constructed using the MEGA version 6 software. Sequences were aligned using ClustalW2. The neighbor-Joining method was used to infer the tree structure. Bootstrap analysis with 1,000 replicates of the tested tree reliability. The local isolates were compared with the reference strains from India, Egypt, Brazil, and China. All sequences were aligned to detect conserved and variable regions. Color-coded alignments were used to visualize base pair differences. Conserved regions were observed between positions 5–20 and 45–70. Mutations were mostly observed at positions 30 and 90. Gaps and insertions were common in E. coli and Klebsiella. The conserved sites are likely related to essential functions. The variable regions showed point mutations and indel. These findings may explain species-specific traits. The presence of high similarity between local and foreign strains suggested a global circulation of strains. Each gel result was saved as an image. These images were annotated with the number of samples. A logbook recording gel run conditions and outcomes. Documentation helped track performance and troubleshoot errors. Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)SDS-PAGE was used to separate the proteins from the bacterial samples. This method enables the identification of proteins based on their size. Several chemical solutions were prepared prior to the process started. These included acrylamide mix, buffers, and staining agents. A 30% acrylamide–bisacrylamide solution was prepared using 29.2 g of acrylamide and 0.8 g of bisacrylamide in 100 ml of distilled water. The solution was filtered and stored at 4°C in a dark bottle. Tris-HCl buffers were used to control the pH during the gel run. One buffer was adjusted to pH 8.8 for the separation gel. The pH was set to 6.8 for the stacking gel. A 10% SDS solution was prepared by dissolving the SDS powder in water. A fresh 10% ammonium persulfate solution was prepared each time. The SAB sample buffer included SDS, β-mercaptoethanol, glycerol, bromophenol blue, and Tris. These helped denature proteins and track their movement. The running buffer contained Tris, glycine, and sodium dodecyl sulfate. It was made at 10× concentration and diluted before use. Coomassie Brilliant Blue, methanol, acetic acid, and water were used as the staining solution. A separate destaining solution was used to remove the background color from the gel. A mixture of acrylamide, Tris buffer, SDS, APS, and TEMED was prepared to make the resolving gel. This was poured between the glass plates. The stacking gel was layered on top. A comb was placed to create wells for loading the samples. The protein samples were prepared by mixing with SAB buffer and boiling for 3 minutes. They were then cooled and loaded into the wells. The electrophoresis unit was initially set at 15 mA and then raised to 30 mA at 120 volts. The gel was run until the dye reached the bottom. The gel was removed and stained overnight in Coomassie solution after the run. The excess stain was washed off using the destaining solution. Clear bands were observed, showing the separated proteins. These protein bands were used to analyze the peptide composition of casein. The SDS-PAGE method helped to evaluate the peptides’ molecular weight and purity. This step is important for confirming the presence of active protein fractions. SDS-PAGE was used to study the protein profiles of the bacterial isolates. It helped identify the size and intensity of CdPs. This allowed us to compare enzymatic activity between species and confirm the presence of functional proteins, supporting the biochemical behavior of the detected bacteria. Ethical approvalNo human or animal subjects were directly involved. This ensured compliance with ethical research standards. ResultsBacteria were successfully isolated from all 150 dairy samples. Colonies appeared after 24–48 hours of incubation. Growth was observed on blood agar, MacConkey’s medium, and EMB media. The most frequent colonies exhibited characteristics of lactose-fermenting Gram-negative bacteria. Pink colonies were dominant on MacConkey agar, indicating lactose fermentation. Non-lactose fermenters also appeared as colorless colonies. These phenotypes helped guide presumptive identification. EMB plates revealed a metallic green sheen in several E. coli colonies. Blood agar revealed both alpha and gamma hemolytic reactions. Colonies with clear zones suggest complete hemolysis, likely caused by Klebsiella or Enterococcus strains. Hemolysis patterns were noted and matched with further results. Biochemical identification using the VITEK®2 system confirmed the presence of five major bacterial species. These bacteria were Klebsiella pneumoniae, Klebsiella aerogenes, Enterobacter cloacae, E. coli, and Enterococcus cecorum. Each isolate exhibited distinct biochemical fingerprints. Of the 150 samples, 48 yielded K. pneumoniae. This species was mostly found in raw milk. Yogurt and cheese had a lower incidence. These isolates exhibited consistent citrate use and urease production. Klebsiella aerogenes was isolated from 32 samples. It shared several biochemical traits with K. pneumoniae but differed in motility and the indole test. Most isolates were obtained from cheese samples, suggesting a dairy-related niche. Enterobacter cloacae was detected in 28 samples. This species exhibited a positive arginine dihydrolase reaction and was motile. Colonies were found in milk and yogurt samples. Their resistance pattern was noted for future testing. Escherichia coli was confirmed in 26 samples. The isolates were indole-positive and showed glucose-induced gas production. Colonies matched the typical morphology observed on EMB agar. Most E. coli was recovered from yogurt. Enterococcus cecorum was detected in 16 samples. These were Gram-positive cocci with no catalase activity. Colonies grew well on blood agar and showed gamma hemolysis. This organism was more frequent in the cheese samples. DNA extraction was successful for all isolates. The concentration of genomic DNA ranged from 50 to 250 ng/μl. The purity (260/280 ratio) was between 1.8 and 2.0, indicating good quality. No contamination was observed. PCR amplification targeted the 16S rRNA gene. All five bacterial species yielded clear bands at 1,500 bp. These results were consistent across the different samples. The gel electrophoresis results are shown in Figure 1.
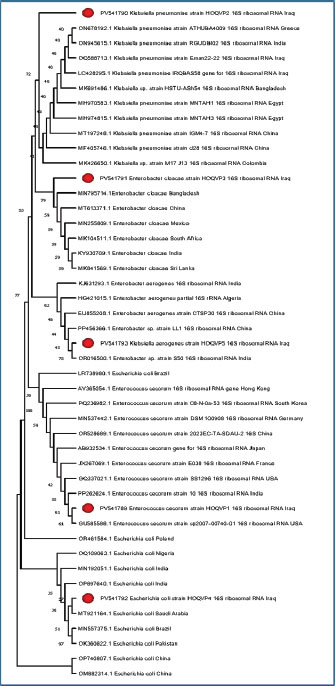
Fig. 1. Agarose gel electrophoresis of PCR products targeting the 16s rRNA gene. The gel was run on 1.5% agarose and stained with ethidium bromide. Lane (L) represents the DNA ladder (marker), whereas lanes 1–5 show clear amplification products of approximately 1,500 base pairs, indicating successful target gene amplification in all samples. All lanes exhibited a single sharp band. Lane L contains the DNA ladder. Lanes 1–5 represent the five species. The presence of a single band confirmed target amplification. No primer–dimer formation was detected. Selected PCR products were sent for sequencing. FASTA files were returned and analyzed using BLAST. The identity percentage ranged from 97% to 100%. GenBank accession numbers were recorded for all matches. Klebsiella pneumoniae strains shared 99%–100% identity with isolates from India and China. Escherichia coli matched strains from Egypt and Brazil. The identity scores suggest high conservation of the 16S rRNA gene across regions. The phylogenetic tree built using the MEGA software showed clustering by genus. The Klebsiella strains formed one main clade. Enterobacter and E. coli appeared in the adjacent branches. Enterococcus was distant from the others. The bootstrap values for the major branches were above 90%, confirming tree reliability. The tree topology revealed close evolutionary relationships among the isolates. The visual structure is provided in Figure 2.
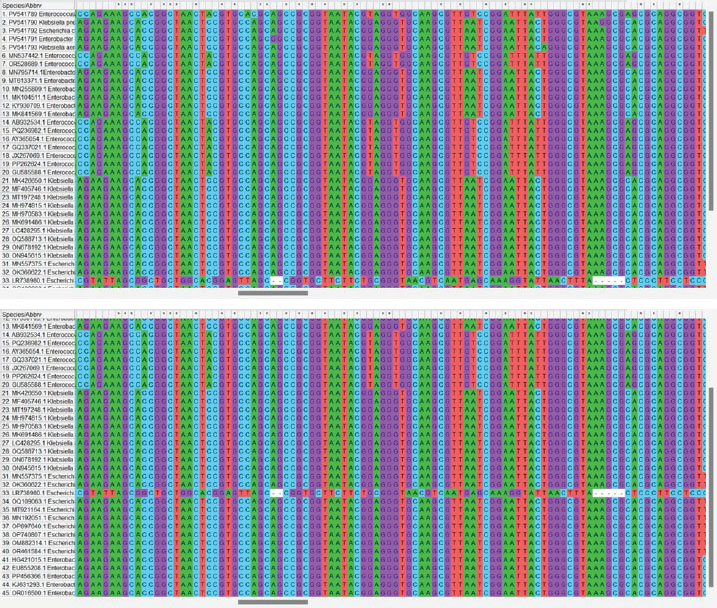
Fig. 2. Phylogenetic tree constructed from 16S rRNA gene sequences illustrating the genetic relationships between bacterial strains isolated from Iraq and reference strains from various countries. The tree highlights the high degree of similarity among the isolates, with GenBank accession numbers provided for accurate identification. The multiple sequence alignment included 45 sequences. These represent local isolates and international reference strains. The alignment length was 100–150 bp. Gaps were introduced for proper alignment. Conserved regions were observed between nucleotide positions 5–20 and 45–70. These sequences exhibited no variation across genera. They may represent the essential domains of the 16S gene. These regions are highlighted in Figure 3.
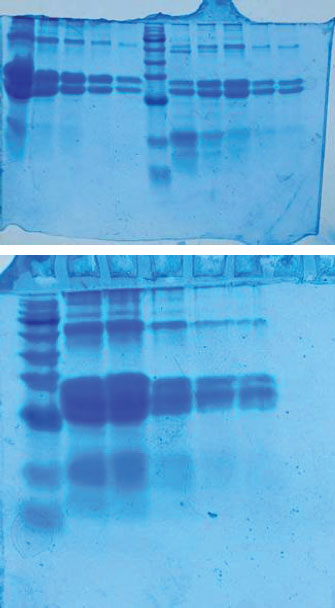
Fig. 3. Color-coded nucleotide alignment results are displayed for visual comparison. Color schemes represent different nucleotides (A, T, C, and G), thereby helping to distinguish between conserved and variable sites. Variable regions occurred around positions 30 and 90. Substitutions such as A→T and C→G were observed. Some isolates showed insertions or deletions. Escherichia coli and Klebsiella exhibited the highest variability. The genetic similarity between Iraqi and foreign strains was high. The gel documentation showed consistent results across the samples. No contamination bands were observed. Each gel image was annotated and saved. Based on sequencing data (Accession No.: PV541790.1, PV541791.1, PV541792.1, and PV541793.1), strain designations were assigned. For example, K. pneumoniae isolate HOQVP2 matched strain MT843920.1 from India. Each strain was compared with the closest match. The percentage identity and E-value were used as criteria. BLAST hits with the highest alignment scores were considered reliable. Phylogenetic comparison showed clear genus-level clustering. Enterococcus cecorum formed a distinct clade. It is distant from the other four species. SDS-PAGE was used to study the protein content of the bacterial peptide extracts. The gel helped to show the size and pattern of proteins from different isolates. All samples showed clear bands on the gel. These bands were stained with Coomassie Brilliant Blue. Most of the visible bands were between 20 and 70 kDa. The first lane likely contained a protein marker used to measure the band sizes. Each bacterial sample exhibited a unique pattern. Some lanes had more bands than others. This indicates that the protein content was not the same in every strain. Some isolates had strong thick bands, while others had faint or few bands. The casein peptide samples exhibited more intense bands than the whey samples. This means that they may contain more protein or more stable peptides. The number and position of the bands indicate different types of proteins in the extracts. Klebsiella and Enterobacter isolates showed complex banding. This may reflect the strong protein activity of these strains. In contrast, E. coli and Enterococcus had simpler profiles. The gel run was clean with no smearing or broken bands. This confirms that the procedure worked well. These findings support the hypothesis that different bacteria produce different peptide patterns. This helps explain the biochemical results and proves that protein extraction was successful (Fig. 4).
Fig. 4. SDS-PAGE profile of the protein extracts from the bacterial isolates. Proteins were separated on a 12% polyacrylamide gel and stained with Coomassie Brilliant Blue. Lane M: protein molecular weight marker. Lanes 1–5: Peptide extracts from different bacterial isolates. Molecular markers: 20 and 70 kDa. Each lane shows a distinct pattern, reflecting the differences in protein content and molecular size among the isolates. DiscussionThe SDS-PAGE results showed a diverse range of protein bands across the tested bacterial isolates. Most bands were between 20 and 70 kDa. Casein-derived peptides produced more intense and complex patterns than whey peptides. These results suggest that the isolates hydrolyzed casein more efficiently. This observation is consistent with earlier findings that dairy-origin bacteria can actively break down casein into bioactive peptides (Wu et al., 2023a, 2023b). The distinct banding patterns observed in Klebsiella and Enterobacter support their proteolytic activity. Several studies have reported strong protein-processing traits in these genera, especially in nutrient-rich dairy environments (Abebe et al., 2020). The simpler patterns in E. coli and Enterococcus may reflect limited protease expression or lower enzymatic strength. Previous studies have shown that not all strains within these genera produce high levels of proteinases under the same conditions (Wu et al., 2023a, 2023b). The quality of band separation and absence of smearing in this study also confirm the purity of the peptide extracts and the accuracy of the SDS-PAGE method used. The presence of multiple bands suggests the production of peptides with varying sizes and possibly bioactive functions. Protein bands within the 20–70 kDa range may include casein phosphopeptide or bacteriocin-like proteins, which are commonly produced in fermented milk by lactic acid bacteria (Wu et al., 2023a, 2023b). Similar findings were reported by Wu et al. (2023a, 2023b), who identified peptide fractions in dairy products using electrophoretic separation (Wu et al., 2023a, 2023b). These proteins play roles in antimicrobial activity, nutrient transport, and host interactions. The presence of intense bands in casein-treated samples indicates that casein is a preferred bacterial protease substrate. This supports the work of Ma et al. (2020), who emphasized the structural richness of casein compared to whey proteins (Ma et al., 2020). The ability of these isolates to process casein into visible protein fragments aligns with studies on dairy-origin microbes used in probiotic formulations and food preservation (Xie et al., 2024b). Bacteria can target proteins from dairy sources to produce metabolites that affect both microbial competition and human health (Ozsoy et al., 2022; Krishnamoorthy et al., 2024). The distinct electrophoretic profiles observed in this study suggest strain-specific protein-processing capabilities, a finding also observed by researchers studying foodborne pathogens and their enzymatic behaviors (de Alcântara Rodrigues et al., 2020; Diacon et al., 2022). Comparing these findings with previous studies also highlights the potential value of these isolates in food biotechnology. Strains with clear and diverse peptide profiles may contribute to flavor development, bioactivity, or safety in dairy products. This is supported by the work of Ferone et al. (2020), who showed that protein fingerprinting can be used to track useful strains in dairy fermentation (Ferone et al., 2020). Enterobacteriaceae, such as Klebsiella, are sometimes overlooked because of their pathogenic potential. However, when well-characterized and isolated from non-clinical sources, they may offer useful peptide processing traits in controlled environments (Alharbi et al., 2022). Carusi et al. (2024) noted that not all food-associated Klebsiella strains are harmful, and some have industrial applications when safety is ensured (Carusi et al., 2024). The diversity of bands in our SDS-PAGE gel supports the hypothesis that strain-level variation is key. As shown in multiple reviews, peptide output can vary based on environmental factors, genetic regulation, and medium composition, even within the same genus (Falleh et al., 2020; Anyanwu et al., 2021). Our results mirror this complexity and highlight the potential for selecting specific strains for future use. Finally, the ability to extract, separate, and identify these peptide bands using sodium dodecyl sulfate-polyacrylamide gel electrophoresis underscores the method’s value in protein profiling. Gel-based protein separation has also been used to classify bacterial functionality in food systems (Cui et al., 2024; Xie et al., 2024a). Falleh et al. (2020) highlighted that such electrophoretic patterns can be used to monitor peptide purity and confirm protein expression under treatment conditions (Falleh et al., 2020). Our findings show that SDS-PAGE remains a reliable tool, especially when it is combined with well-designed culture methods. The distinct bands visualized here confirm the enzymatic potential of the isolates and suggest future steps for bioactivity assays or peptide sequencing. As discussed by Wu et al. (2023a, 2023b), clear banding not only confirms hydrolysis but can also guide the identification of peptides with health-promoting or preservative effects (Wu et al., 2023a, 2023b). These results provide a basis for selecting isolates for further study in functional food applications. The presence of pathogenic bacteria in food poses a serious public health risk. These bacteria can cause foodborne illness outbreaks, leading to hospitalizations and sometimes death. The danger is even higher in vulnerable groups like children, the elderly, or people with weak immunity. Some strains may also carry antibiotic resistance, making it difficult to treat infections. When contaminated dairy products are consumed raw or without proper storage, the spread of disease increases. Monitoring and controlling these bacteria in food helps prevent illness, reduce healthcare costs, and protect community health (Cui et al., 2021; Corcionivoschi et al., 2025; Urban-Chmiel et al., 2025). The presence of several pathogenic bacteria, including K. pneumoniae, E. coli, and E. cloacae, was confirmed by molecular analysis. These bacteria cause serious foodborne infections and may carry resistance genes. Their detection in raw dairy highlights a public health risk. The use of PCR and 16S rRNA sequencing allowed for precise identification and confirmed genetic similarity to global strains. The visual evidence from the gel, along with the known properties of the protein bands, aligns with other work showing how gut microbiota-derived peptides or dairy-associated enzymes may influence host health or food quality (Ma et al., 2020; Ozsoy et al., 2022; Krishnamoorthy et al., 2024). The SDS-PAGE results validated that different isolates produced unique and functionally relevant peptide patterns. This study has some limitations. The sample size was limited to one region, which may not reflect all of Iraq. Only a few bacterial species were analyzed, and their antibiotic resistance profiles were not included in the analysis. Furthermore, there was no follow-up on possible human infections linked to the contaminated dairy. These gaps should be addressed in future research to provide a fuller picture of public health risks. ConclusionThis study confirmed that dairy-derived bacterial isolates show distinct protein patterns when grown on casein and whey. SDS-PAGE revealed clear bands between 20 and 70 kDa, with stronger and more diverse bands in casein samples. This suggests that casein is a better substrate for bacterial protease activity. Each isolate produced a unique banding profile, reflecting strain-level variation in enzymatic function. These findings support the role of specific dairy bacteria in protein hydrolysis and bioactive peptide production. The results may help guide the future use of these strains in food processing or probiotic development. AcknowledgmentsThe authors thank the College of Veterinary Medicine, University of Al-Qadisiyah, for their support in this study. Conflict of interestThe authors declare no conflicts of interest. FundingThe authors self-funded the study. No external funding source is available. Authors’ contributionsAll authors participated in the study. Data availabilityData are available when requested by the corresponding author. ReferencesAbd-Alhassen, J.K., Janabi, A.H.D. and Aboktifa, M.A. 2021. Antioxidant and antimicrobial evaluation of lycopene isolated from watermelon. Biochem. Cell. Arch. 21, 2905–2910. Abebe, E., Gugsa, G. and Ahmed, M. 2020. Review on major food-borne zoonotic bacterial pathogens. J. Trop. Med. 2020, 1–19. Alharbi, M.G., Al-Hindi, R.R., Esmael, A., Alotibi, I.A., Azhari, S.A., Alseghayer, M.S. and Teklemariam, A.D. 2022. The “big six”: hidden emerging foodborne bacterial pathogens. Trop. Med. Infect. Dis. 7(11), 356. Anyanwu, M.U., Okpala, C.O.R., Chah, K.F. and Shoyinka, V.S. 2021. Prevalence and traits of mobile colistin resistance gene harbouring isolates from different ecosystems in Africa. BioMed. Res. Int. 2021, 6630379. Carusi, J., Kabuki, D.Y., De Seixas Pereira, P.M. and Cabral, L. 2024. Aeromonas spp. in drinking water and food: occurrence, virulence potential and antimicrobial resistance. Food Res. Int. 175, 113710. Corcionivoschi, N., Balta, I., Mccleery, D., Bundurus, I., Pet, I., Calaway, T., Nichita, I., Stef, L. and Morariu, S. 2025. Mechanisms of pathogenic Escherichia coli attachment to meat. Foodborne Pathog. Dis. 22(5), 339–349. Cui, F., Fan, R., Wang, D., Li, J. and Li, T. 2024. Research progress on iron uptake pathways and mechanisms of foodborne microorganisms and their application in the food sector. Crit. Rev. Food Sci. Nutr. 64(24), 8892–8910. Cui, Y., Luo, L., Wang, X., Lu, Y., Yi, Y., Shan, Y., Liu, B., Zhou, Y. and Lü, X. 2021. Mining, heterologous expression, purification, antibactericidal mechanism, and application of bacteriocins: a review. Compr. Rev. Food Sci. Food Saf. 20(1), 863–899; doi: 10.1111/1541-4337.12658 De Alcântara Rodrigues, I., Ferrari, R.G., Panzenhagen, P.H.N., Mano, S.B. and Conte-Junior, C.A. 2020. Antimicrobial resistance genes in bacteria from animal-based foods. Adv. Appl. Microbiol. 112, 143–183. Diacon, A.H., Guerrero-Bustamante, C.A., Rosenkranz, B., Rubio Pomar, F.J., Vanker, N. and Hatfull, G.F. 2022. Mycobacteriophages to treat tuberculosis: dream or delusion?. Respiration 101(1), 1–15. Falleh, H., Ben Jemaa, M., Saada, M. and Ksouri, R. 2020. Essential oils: a promising eco-friendly food preservative. Food Chem. 330, 127268. Ferone, M., Gowen, A., Fanning, S. and Scannell, A.G.M. 2020. Microbial detection and identification methods: bench top assays to omics approaches. Compreh. Rev. Food Sci. Food Saf. 19(6), 3106–3129. Ghazi, A.M., Ali Al-bayati, M.A. and Janabi, A.H. 2024. Metabolomics-detected alterations generated by phytosomal propolis and phytosomal Lycopene in male rats with induced benign prostatic hyperplasia. Iraqi J. Vet. Sci. 38(Suppl I–IV), 7–15. Karim, S., Mansour, K., Janabi, A. and Al-Nakeeb, N. 2018. First phylogenetic characterization of Pseudocowpox virus from cattle in Al-Qadisiyah province/ Iraq. Iraqi J. Vet. Sci. 33(1), 123–126. Krishnamoorthy, N.K., Kalyan, M., Hediyal, T.A., Anand, N., Kendaganna, P.H., Pendyala, G., Yelamanchili, S.V., Yang, J., Chidambaram, S.B., Sakharkar, M.K. and Mahalakshmi, A.M. 2024. Role of the gut bacteria-derived metabolite phenylacetylglutamine in health and diseases. ACS Omega 9(3), 3164–3172. Ma, Q., Davidson, P.M. and Zhong, Q. 2020. Properties and potential food applications of lauric arginate as a cationic antimicrobial. Int. J. Food Microbiol. 315, 108417. Ozsoy, S., Sultanoglu, N. and Sanlidag, T. 2022. The role of Mediterranean diet and gut microbiota in type-2 diabetes mellitus associated with obesity (diabesity). J. Prev. Med. Hygiene 63(2 Suppl 3), E87–E92. Urban-Chmiel, R., Osek, J. and Wieczorek, K. 2025. Methods of controlling microbial contamination of Food. Pathogens 14(5), 492. Wu, J., Han, X., Ye, M., Li, Y., Wang, X. and Zhong, Q. 2023a. Exopolysaccharides synthesized by lactic acid bacteria: biosynthesis pathway, structure-function relationship, structural modification and applicability. Crit. Rev. Food Sci. Nutr. 63(24), 7043–7064. Wu, M., Ma, Y., Dou, X., Zohaib Aslam, M., Liu, Y., Xia, X., Yang, S., Wang, X., Qin, X., Hirata, T., Dong, Q. and Li, Z. 2023b. A review of potential antibacterial activities of nisin against Listeria monocytogenes: the combined use of nisin shows more advantages than single use. Food Res. Int. 164, 112363. Xie, D., Ma, H., Xie, Q., Guo, J., Liu, G., Zhang, B., Li, X., Zhang, Q., Cao, Q., Li, X., Ma, F., Li, Y., Guo, M. and Yin, J. 2024a. Developing active and intelligent biodegradable packaging from food waste and byproducts: a review of sources, properties, film production methods, and their application in food preservation. Compreh. Rev. Food Sci. Food Saf. 23(3), 13334. Xie, Y., Zhang, J., Zhang, P., Regenstein, J.M., Liu, D. and Zhou, P. 2024b. Improving the microbiological safety and quality of aquatic products using nonthermal processing. Compreh. Rev. Food Sci. Food Saf. 23(3), 13368. Yaseen, M.M., Karawan, A.C., Alfatlawi, M.A.A. and Janabi, A.H.D. 2020. The role of gut bacterial cytochrome-P450 of mosquito larvae in degradation of temephos insecticide. Ann. Trop. Med. Public Health 23(1), S412. Yu, J., Li, S., Wang, L., Dong, Z., Si, L., Bao, L. and Wu, L. 2022. Pathogenesis of Brucella epididymoorchitis-game of Brucella death. Crit. Rev. Microbiol. 48(1), 96–120. | ||
| How to Cite this Article |
| Pubmed Style Gaber HH, Faja OM. Molecular detection and genetic relatedness of enteric bacteria from Iraqi dairy products. Open Vet. J.. 2025; 15(9): 4190-4198. doi:10.5455/OVJ.2025.v15.i9.24 Web Style Gaber HH, Faja OM. Molecular detection and genetic relatedness of enteric bacteria from Iraqi dairy products. https://www.openveterinaryjournal.com/?mno=266677 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i9.24 AMA (American Medical Association) Style Gaber HH, Faja OM. Molecular detection and genetic relatedness of enteric bacteria from Iraqi dairy products. Open Vet. J.. 2025; 15(9): 4190-4198. doi:10.5455/OVJ.2025.v15.i9.24 Vancouver/ICMJE Style Gaber HH, Faja OM. Molecular detection and genetic relatedness of enteric bacteria from Iraqi dairy products. Open Vet. J.. (2025), [cited January 25, 2026]; 15(9): 4190-4198. doi:10.5455/OVJ.2025.v15.i9.24 Harvard Style Gaber, H. H. & Faja, . O. M. (2025) Molecular detection and genetic relatedness of enteric bacteria from Iraqi dairy products. Open Vet. J., 15 (9), 4190-4198. doi:10.5455/OVJ.2025.v15.i9.24 Turabian Style Gaber, Huda Hassan, and Orooba Meteab Faja. 2025. Molecular detection and genetic relatedness of enteric bacteria from Iraqi dairy products. Open Veterinary Journal, 15 (9), 4190-4198. doi:10.5455/OVJ.2025.v15.i9.24 Chicago Style Gaber, Huda Hassan, and Orooba Meteab Faja. "Molecular detection and genetic relatedness of enteric bacteria from Iraqi dairy products." Open Veterinary Journal 15 (2025), 4190-4198. doi:10.5455/OVJ.2025.v15.i9.24 MLA (The Modern Language Association) Style Gaber, Huda Hassan, and Orooba Meteab Faja. "Molecular detection and genetic relatedness of enteric bacteria from Iraqi dairy products." Open Veterinary Journal 15.9 (2025), 4190-4198. Print. doi:10.5455/OVJ.2025.v15.i9.24 APA (American Psychological Association) Style Gaber, H. H. & Faja, . O. M. (2025) Molecular detection and genetic relatedness of enteric bacteria from Iraqi dairy products. Open Veterinary Journal, 15 (9), 4190-4198. doi:10.5455/OVJ.2025.v15.i9.24 |