| Research Article | ||
Open Vet. J.. 2025; 15(8): 3770-3779 Open Veterinary Journal, (2025), Vol. 15(8): 3770-3779 Research Article Effects of nanoparticle antibiotics on cytotoxicity and in vitro antibacterial properties of Escherichia coli isolated from respiratory tract infection in chickensManh Tuong Nguyen, Ha Thi Thanh Nguyen and Thanh Trung Nguyen*Department of Pharmacology, Toxicology, Internal Medicine and Diagnostics, Faculty of Veterinary Medicine, Vietnam National University of Agriculture, Hanoi, Vietnam *Corresponding Author: Thanh Trung Nguyen,. Department of Pharmacology, Toxicology, Internal Medicine and Diagnostics, Faculty of Veterinary Medicine, Vietnam National University of Agriculture, Hanoi, Vietnam. Email: nguyenthanhtrung [at] vnua.edu.vn Submitted: 14/05/2024 Revised: 03/07/2025 Accepted: 08/07/2025 Published: 31/08/2025 © 2025 Open Veterinary Journal
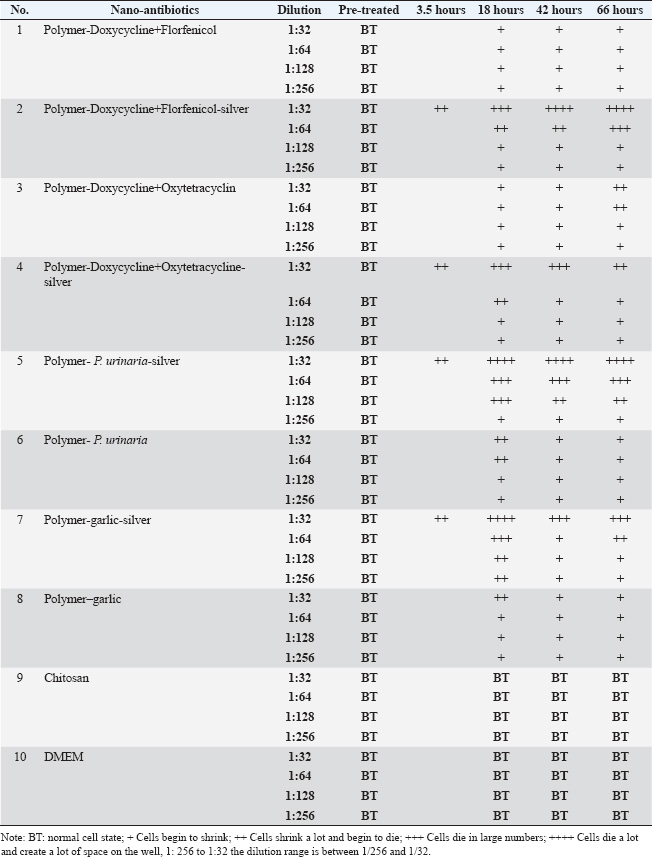
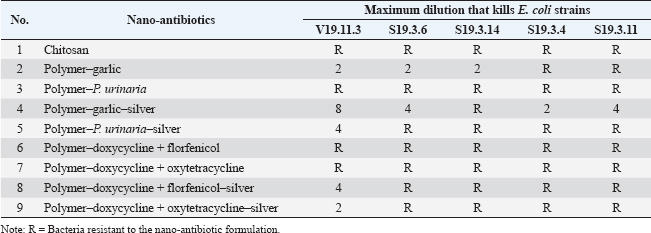
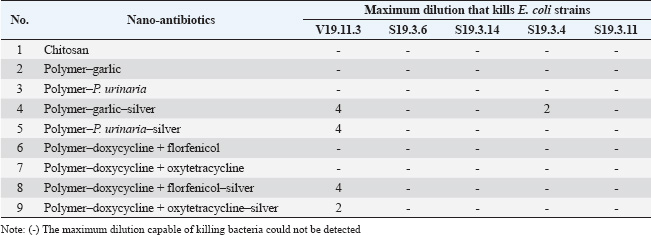
ABSTRACTBackground: Respiratory infections in poultry, particularly those caused by Escherichia coli, are a major concern in veterinary medicine due to their economic impact and increasing antibiotic resistance. Nanotechnology-based antimicrobial delivery systems have emerged as promising alternatives to conventional treatments. Aim: This study aimed to evaluate the cytotoxicity and antibacterial activity of various nano-antibiotic formulations—comprising antibiotics, herbal extracts, and silver nanoparticles—against E. coli strains isolated from chickens with respiratory disease. Method: The nanoantibiotic formulations were developed by the Department of Biomedical Nanomaterials, Institute of Materials Science, Vietnam, and included combinations such as polymer–garlic, polymer–Phyllanthus urinaria, polymer–garlic–silver, polymer–P. urinaria–silver, polymer–doxycycline + florfenicol, polymer–doxycycline + oxytetracycline, and their respective silver-conjugated forms, along with chitosan. Cytotoxicity was assessed using Vero cells, and antibacterial activity was determined in vitro using the dilution method in 96-well flat-bottom plates. Results: The study found that cytotoxic effects were observed at dilutions of 1:32 and 1:64 when the formulations combined antibiotics, herbal extracts, and nanosilver. Importantly, this combination significantly enhanced bactericidal activity compared with formulations containing only antibiotics or herbal extracts. Conclusion: The integration of nanosilver with antibiotics and herbal extracts in nanoparticle systems shows promising antibacterial efficacy against E. coli, with tolerable cytotoxicity at appropriate dilutions. These findings suggest the potential of such nanoformulations as effective alternatives in managing bacterial respiratory infections in poultry. Keywords: In vitro nanoparticle antibiotic, Complex respiratory bacteria, E.coli, Chicken. IntroductionCurrently, respiratory diseases caused by bacteria such as Escherichia coli, Salmonella, and Pasteurella multocida in livestock—particularly in poultry—have become a persistent problem (Yehia et al., 2023). These diseases result in significant economic losses by reducing meat and egg production. Furthermore, the abuse and widespread use of critically important antibiotics in livestock, aquaculture, and food production has become a serious issue (Shao et al., 2021). This practice increases the prevalence of antibiotic-resistant bacteria in livestock, poultry, aquatic animals, and animal-derived food products in Vietnam (Carrique-Mas et al., 2015). Moreover, antibiotic resistance is one of the most pressing global health concerns. A recent study estimated that approximately 700,000 people die each year due to antibiotic-resistant infections—more than half of the number of deaths caused by traffic accidents (O’Neill, 2014; Naghavi et al., 2024). The same study projected that by 2050, without effective interventions, antibiotic resistance could result in 10 million deaths annually—exceeding the number of deaths caused by cancer (O’Neill, 2014; Naghavi et al., 2024). In recent years, with the advancement of nanotechnology, silver production at the nanoscale has become possible, elevating its applications to a new level (Elzein, 2024). Research has shown that at a nanoscale size (ranging from 1 to 100 nm), the antibacterial activity of silver increases by approximately 50,000 times compared to bulk silver. As a result, just 1 g of nanosilver can disinfect hundreds of square meters of surface area (Nguyen and Nguyen, 2013). Today, silver is incorporated into various materials in different forms—such as salts, fixed ions, or metal nanoparticles—and is widely used in many fields, including biomedicine and pharmaceuticals (Ansari et al., 2014; Zhang et al., 2016; Slavin et al., 2017). In traditional Vietnamese medicine, the use of plant-based antibiotics derived from garlic (Allium sativum L.) and Phyllanthus urinaria L. to treat diseases in livestock and poultry has demonstrated high effectiveness and a lower tendency for drug resistance development. Compared with synthetic antibiotics or antibiotics derived from fungi, the development of resistance to plant-based antibiotics develops more slowly, and bacteria tend to be more sensitive to them (Trinh et al., 2016; Li et al., 2024). For instance, although Helicobacter pylori is resistant to most conventional antibiotics (Tshibangu-Kabamba and Yamaoka, 2021), P. urinaria has shown antibacterial activity against this bacterium (Lai et al., 2008). Additionally, the application of nanotechnology in the production of herbal-based products represents a new trend that enhances the value of Vietnamese medicinal plants in the context of global integration (Dewi et al., 2022). A recent study synthesized silver nanoparticles using tea leaf extract and successfully applied them to inhibit the growth of E. coli and Staphylococcus aureus (Lam Xuan Huong Nguyen, 2014). Moreover, a combination of nano chitosan, turmeric essential oil, and nanosilver significantly improved the antibacterial efficacy against Bacillus cereus and Listonella damsela (Thi Kim Cuc Nguyen, 2014). However, studies evaluating the cytotoxicity and antibacterial activity of nanosilver when combined with antibiotics and herbal extracts are lacking. Therefore, to enhance the in vitro antibacterial effects of medicinal herbs and antibiotics while leveraging the advantages of nanotechnology and minimizing antibiotic resistance, the purpose of this study is to evaluate the cytotoxicity and investigate the in vitro antibacterial activity of herbal extracts, antibiotics, and nanosilver—individually and in combination. The goal of this study is to lay the groundwork for the development of herbal-based preparations for the prevention and treatment of respiratory diseases in chickens. Materials and MethodsMaterialsThe nanoantibiotic system used in this study was provided by the Department of Biomedical Nanomaterials, Institute of Materials Science, Vietnam, and consisted of the following formulations: polymer–garlic, polymer–P.urinaria, polymer–garlic–silver, polymer–P. urinaria–silver, polymer–doxycycline + florfenicol, polymer–doxycycline + oxytetracycline, polymer–doxycycline + florfenicol–silver, polymer–doxycycline + oxytetracycline–silver, and chitosan. Chemicals and materials used for the cytotoxicity test on the Vero cell line included 10% fetal bovine serum (FBS), amphotericin B (0.25 g/ml), penicillin (100 units/ml), streptomycin (100 mg/ml), Dulbecco’s Modified Eagle Medium (DMEM), phosphate-buffered saline (1× PBS), and 2× trypsin. The following reagents and materials were used for the bactericidal activity assay: peptone medium, Luria-Bertani (LB) medium, 1% BaCl2 and 1% H2SO4 solution, and sterile 96-well flat-bottom microplates. The E. coli strains used in these experiments were randomly isolated from the respiratory tract infections in chickens in the field. The isolates were named 19.11.3, S19.3.6, S19.3.14, S193.3.4, and S19.3.11, and were obtained from the Faculty of Veterinary Medicine, Vietnam National University of Agriculture. Vero cell preparationVero cells were cultured in DMEM supplemented with 10% FBS, 100 units/ml penicillin, 100 mg/ml streptomycin, and 0.25 g/ml amphotericin B. According to a previous study, the toxicity of silver nanoparticles ranges from 0.01 to 10 mg/ml after 24 hours of exposure (Kim et al., 2011). Based on this reference, the test samples were prepared at dilutions of 1:32, 1:64, 1:128, and 1:256. Nine nanoantibiotic formulations were then applied to the Vero cells. The culture medium was removed from the prepared cell culture plates, the cells were washed with PBS, and 100 µl of each diluted sample was added. Infected cells were incubated at 37°C with 5% CO2 for designated time points. The cellular changes and cytotoxic effects were monitored daily using a microscope. Bacterial culture methodEscherichia coli strains were cultured on LB agar medium at 37°C for 24 hours. Single colonies were selected and suspended in peptone medium, then adjusted to a turbidity equivalent to 0.5 on the McFarland standard scale. In vitro bacterial inhibition assay (MIC)The minimum inhibitory concentration (MIC) of the test substances was determined using a two-fold serial dilution method in a sterile flat-bottom 96-well microplate. Each test substance was assessed in quadruplicate. Initially, 100 µl of peptone solution was added to all wells. Subsequently, 100 µl of each active substance was added to the wells in column 1, with one substance per well. Serial two-fold dilutions were performed across columns 1–10 by thoroughly mixing the solution in column 1, transferring 100 µl to column 2, and continuing the process until column 10, where 100 µl was discarded. This resulted in a dilution series ranging from 1/2 to 1/1024. Subsequently, 20 µl of the previously prepared E. coli suspension (adjusted to 0.5 OD on the McFarland scale) was added to each well. Three types of control wells were included for each substance: a substance-only control (no bacteria), a bacteria-only control (no substance), and a medium-only control (no bacteria, no substance). The microplates were incubated at 37°C, and optical density (OD) was measured at 595 nm at 0, 3, 5, 7, and 24 hours to evaluate bacterial growth inhibition. In vitro bactericidal activity assessment (MBC)The minimal bactericidal concentration (MBC) was determined using the spread plate method. From the MIC assay, 100 μl of the solution from wells showing no visible bacterial growth was spread onto LB agar plates and incubated at 37°C for 24 hours. Bacterial survival was assessed after incubation. MBC was defined as the lowest concentration of the nanoantibiotic solution at which no bacterial colonies were observed, indicating complete bactericidal activity. Data analysisCellular changes were monitored using a microscope at specific time points. At the end of the observation period, the results were considered valid only if the following conditions were simultaneously met: (i) the environmental control well showed no turbidity, (ii) the active ingredient control well remained clear, and (iii) the bacterial control well exhibited turbidity. The inhibitory effect of each active ingredient was assessed by calculating the change in turbidity over time, expressed as the ratio of the OD at each subsequent time point to the OD at time zero. The minimum effective dilution was defined as the highest dilution at which bacterial growth was inhibited, indicated by the absence of a significant increase in turbidity. Data were processed and analyzed using Microsoft Excel and PrismGraph version 4.0 (GraphPad, San Diego, CA). Ethical approvalNot needed for this study. ResultsEvaluation of the toxicity of nano-antibiotics on Vero cellsTo assess the cytotoxicity of the nine nanoantibiotic samples, sterility testing was first conducted on LB agar plates. After 24 hours of incubation at 37°C, no bacterial colonies were observed (data not shown), confirming that the samples met sterility standards for in vitro testing. According to a previous study, serum can affect nano-antibiotics (Hansen and Thünemann, 2015). Therefore, following sterility confirmation, the samples were tested on Vero cell lines without serum addition. Vero cells were cultured in serum-free DMEM under appropriate conditions and prewarmed at 37°C for 30 minutes before sample application. Table 1 and Figure 1 show that the cytotoxicity of the samples varied depending on the dilution. Notably, samples 2, 4, 5, and 7—each containing nanosilver—exhibited relatively strong cytotoxicity at a dilution of 1:32, with effects appearing as early as 3.5 hours post-infection. Cytotoxic manifestations included cell shrinkage and gradual detachment from the culture well surface. In contrast, no morphological changes were observed in the remaining samples (Fig. 2). The samples were washed with PBS to further assess reversibility, and fresh DMEM supplemented with 10% FBS was added. Subsequent observations at 18, 42, and 66 hours post-infection showed a reduction in cytotoxicity for samples 2, 4, 5, and 7—particularly at the 1:32 dilution—with cells gradually returning to normal morphology. No significant differences were observed in the other dilutions. No notable cytotoxic effects were detected in the remaining samples throughout the observation period. Overall, samples 2, 4, 5, and 7, which contain nanosilver, demonstrated cytotoxicity at dilutions of 1:32 and 1:64, respectively. The remaining samples showed cytotoxicity only at dilutions greater than 1:32. Evaluation of minimal bacterial inhibition in vitroTable 2 presents the results of the in vitro minimal bacterial inhibition assay. These findings indicate that the incorporation of nanosilver enhances the antibacterial activity of herbal extracts such as garlic and P. urinaria. Specifically, nano garlic alone, at a 1:2 dilution, exhibited inhibitory effects against E. coli, while resistance to P. urinaria was resistant. However, when these herbal extracts were combined with nanosilver, both garlic and P. urinaria retained the ability to inhibit the E. coli strain V19.11.3, even at this and lower concentrations. Notably, this combination also improved the antibacterial activity against other E. coli strains, including S19.3.6, S19.3.14, S19.3.4, and S19.3.11. A similar enhancement was observed when nanosilver was combined with conventional antibiotics such as Polymer-Doxycycline+Oxytetracycline and Polymer-Doxycycline+Florfenicol (Table 2). Overall, the presence of nanosilver significantly increased antibacterial efficacy. Additionally, turbidity associated with bacterial growth in nano-antibiotic-treated wells showed a time-dependent pattern, with noticeable increases occurring after 7 hours of incubation (Fig. 2). To investigate the prevalence of drug-resistant E. coli strains, Table 3 presents the results. The data indicate that most commonly used antibiotics and P. urinaria showed resistance in most E. coli strains tested. Only garlic remained effective, with a sensitivity rate of 3 of 5 strains (60%). The combination of nanosilver with antibiotics or herbal extracts significantly enhanced their bactericidal efficacy. When garlic was combined with nanosilver, the sensitivity rate increased from 3/5 strains (60%) to 4/5 strains (80%). A similar enhancement in sensitivity (to 1/5 strains, or 20%) was observed with P. urinaria and the antibiotics Doxycycline, Florfenicol, and Oxytetracycline when combined with nano silver. Evaluation of minimal bactericidal activity in vitroFor the samples with determined MIC, the MBC was evaluated using the spread plate method. Specifically, 100 μl of the test solution from wells that did not show visible bacterial growth was spread onto LB agar plates and incubated at 37°C. After 24 hours, bacterial survival was assessed after 24 hours. The results are presented in Table 4. As shown, the MBC for the nano-antibiotics Polymer-Garlic-Silver, Polymer-Phyllanthus urinaria-Silver, and Polymer-Doxycycline-Florfenicol-Silver was at a dilution of 1:4 for E. coli strain V19.11.3. Meanwhile, Polymer-Doxycycline-Oxytetracycline-Silver and Polymer-Garlic-Silver exhibited MBC at a dilution of 1:2 for strains V19.11.3 and S19.3.4. These findings indicate that the combination of nanosilver with herbal or antibiotic components enhances bactericidal activity. Table 1. Cytotoxicity of nano-antibiotics on Vero cells.
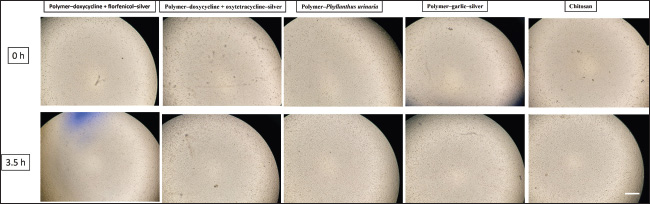
Fig. 1. Morphological changes in Vero cells 3.5 hours post-infection with nano-antibiotic (1:32 dilution); scale bar=1 mm.
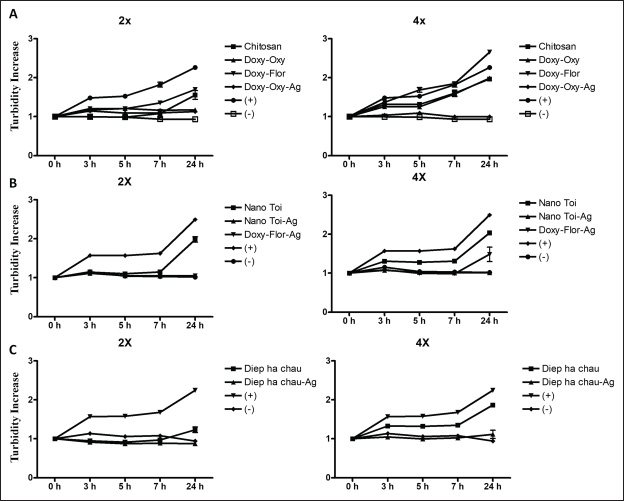
Fig. 2. Changes in turbidity over time at different nanoantibiotic dilutions against E. coli strain V19.11.3. (A) Chitosan, Polymer-Doxycycline+Oxytetracycline (Doxy-Oxy), Polymer-Doxycycline+Florfenicol (Doxy-Flor), and Polymer-Doxycycline+Oxytetracycline-silver (Doxy-Oxy-Ag); (+) Positive control; (−) Negative control. (B) Polymer-garlic (Nano-toi), Polymer-garlic-silver (Nano-Toi-Ag), and Polymer-Doxycycline+Florfenicol-silver (Doxy-Flor-Ag); (+) Positive control; (−) Negative control. (C) Polymer-Phyllanthus urinaria (Diep ha chau) and Polymer-Phyllanthus urinaria-silver (Diep ha chau -Ag); (+) Positive control, (−) Negative control. Note: For ease of interpretation, only two dilutions were selected for each nanoantibiotic: (i) the lowest dilution capable of inhibiting bacterial growth and (ii) the next higher dilution that did not inhibit bacterial growth (i.e., turbidity increased at 24 hours compared to 0 hours). A sample was considered to inhibit bacterial growth if the increase in turbidity was less than 1.5-fold. Table 2. Antibacterial activity of nano-antibiotic formulations against E. coli strains V19.11.3, S19.3.6, S19.3.14, S19.3.4, and S19.3.11.
Table 3. Antibacterial activity of nano-antibiotics against E. coli strains V19.11.3, S19.3.6, S19.3.14, S19.3.4, and S19.3.11.
DiscussionTo the best of our knowledge, this is the first study to demonstrate that combining nano silver with conventional antibiotics and herbal extracts significantly enhances antibacterial activity against E. coli strains isolated from the respiratory tract of chickens. Nano silver, recognized for its broad-spectrum antimicrobial properties, synergizes effectively with traditional antibiotics, such as doxycycline, florfenicol, and oxytetracycline, as well as herbal extracts like garlic and P. urinaria. Notably, these combinations improved both the MIC and MBC in vitro. This observed synergistic effect is likely due to the unique physicochemical characteristics of silver nanoparticles. Their small size and high surface area allow for enhanced bacterial cell interaction. Silver ions (Ag+) bind to peptidoglycan in the bacterial cell wall, disrupting membrane integrity and inhibiting key cellular processes, such as oxygen transport. This disruption facilitates the penetration and activity of co-administered antimicrobial agents, including plant-derived compounds (Patra and Baek, 2017). These results are consistent with previous findings reporting enhanced antibacterial activity when nano silver is combined with plant extracts such as Piper betle (Usha Rani and Rajasekharreddy, 2011; Lagashetty et al., 2019), Wedelia chinensis (Paul Das et al., 2018), Clerodendrum viscosum (Sahoo et al., 2020; Vanlalveni et al., 2021), Excoecaria cochinchinensis (Bautista-Guzman et al., 2021), Polyalthia longifolia (Yaseen et al., 2021; Dashora et al., 2022), and Caesalpinia sappan (Jun et al., 2015; Moteriya and Chanda, 2018). Table 4. MBCs of nano-antibiotic formulations against E. coli strains: V19.11.3, S19.3.6, S19.3.14, S19.3.4, and S19.3.11.
Importantly, some E. coli strains that were initially antibiotics-resistant or herbal extracts alone showed sensitivity when treated with nanosilver combinations. This highlights the potential of nanoformulations to help overcome antimicrobial resistance—a growing challenge in both veterinary and human medicine. In Vietnam, where antibiotic usage in animal husbandry remains high, the emergence of antibiotic-resistant bacteria is particularly concerning. A study by Yamaguchi et al. (2015) identified antimicrobial residues in 17.3% of chicken, 8.8% of pork, and 7.4% of beef samples (Yamaguchi et al., 2015). Additionally, Carrique-Mas et al. (2020) reported antibiotic usage rates in Vietnam reaching 261.7 mg/kg for humans and 247.3 mg/kg for animals—substantially higher than in Europe (Carrique-Mas et al., 2020). These trends underscore the urgent need for alternative strategies, such as the nanoantibiotic and nano-herbal combinations explored in this study. Although we obtained promising in vitro results, this study tests only a small number of bacterial strains. Further research involving a broader spectrum of clinical isolates and comprehensive in vivo studies is essential to validate the observed antibacterial effects. In addition, the potential long-term impacts on animal health, food safety, and the environment should be carefully assessed before recommending widespread application in poultry production. Furthermore, we acknowledge this limitation and will include a silver-only control in our future experiments to clarify the contribution of silver to the antimicrobial and cytotoxic effects observed in our nanoformulations. ConclusionTaken together, the combination of nano silver with antibiotics and herbal extracts offers a promising strategy to enhance antibacterial efficacy and address the growing issue of antimicrobial resistance in veterinary medicine. This approach holds particular relevance for combating E. coli infections in poultry. However, future studies are warranted to explore its full potential and practical application under field conditions. AcknowledgmentsWe would like to thank the anonymous reviewers for their thoughtful comments and efforts towards improving our manuscript. Conflict of interestThe authors declare no conflict of interest. FundingVietnam National University of Agriculture partially funds this study under project code SV2022-09-38. Authors’ contributionsT.T.N, M.T.N. collected the data, performed statistical analysis, and drafted the manuscript. T.T.N, M.T.N, H.T.T.N. conceived of and participated in the design of the study, wrote the manuscript, and reviewed the manuscript. All authors read and approved of the submitted version. Data availabilityThe data will be made available on reasonable request. ReferencesAnsari, M.A., Khan, H.M., Khan, A.A., Ahmad, M.K., Mahdi, A.A., Pal, R. and Cameotra, S.S. 2014. Interaction of silver nanoparticles with Escherichia coli and their cell envelope biomolecules. J. Basic Microbiol. 54(9), 905–915. Carrique-Mas, J.J., Trung, N.V., Hoa, N.T., Mai, H.H., Thanh, T.H., Campbell, J.I., Wagenaar, J.A., Hardon, A., Hieu, T.Q. and Schultsz, C. 2015. Antimicrobial usage in chicken production in the Mekong Delta of Vietnam. Zoonoses. Public Health 62(s1), 70–78. Carrique-Mas, J.J., Choisy, M., Van Cuong, N., Thwaites, G., and Baker, S. 2020. An estimation of total antimicrobial usage in humans and animals in Vietnam. Antimicrob. Resist. Infect. Control 9(1), 16. Dashora, A., Rathore, K., Raj, S. and Sharma, K. 2022. Synthesis of silver nanoparticles using polyalthia longifolia leaf extract and their in vitro antifungal activity against phytopathogen. Biochem. Biophys. Rep. 31, 101320. Dewi, M.K., Chaerunisaa, A.Y., Muhaimin, M. and Joni, I.M. 2022. Improved activity of herbal medicines through nanotechnology. Nanomaterials (Basel). 12(22), 4073. Elzein, B. 2024. Nano revolution: “Tiny tech, big impact: How nanotechnology is driving SDGs progress”. Heliyon 10(10), e31393. Hansen, U. and Thünemann, A.F. 2015. Characterization of silver nanoparticles in cell culture medium containing fetal bovine serum. Langmuir. 31(24), 6842–6852. Bautista-Guzman, J., Gomez-Morales, R., Asmat-Campos, D. and Checca, N.R. 2021. Influence of the alcoholic/ethanolic extract of Mangifera indica residues on the green synthesis of FeO nanoparticles and their application for the remediation of agricultural soils. Molecules 26(24), 7633; doi:10.1016/j.molecules.2012.09.016. Jun, S.H., Cha, S.H., Kim, J.H., Yoon, M., Cho, S. and Park, Y. 2015. Silver nanoparticles synthesized using Caesalpinia sappan extract as potential novel nanoantibiotics against methicillin-resistant Staphylococcus aureus. J. Nanosci. Nanotechnol. 15(8), 5543–5552. Kim, H.R., Kim, M.J., Lee, S.Y., and Oh, S.M. and Chung, K.H. 2011. Genotoxic effects of silver nanoparticles stimulated by oxidative stress in human normal bronchial epithelial (BEAS-2B) cells. Mutat. Res. 726(2), 129–135. Lagashetty, A., Ganiger, S.K. and Shashidhar. 2019. Synthesis, characterization, and antibacterial study of Ag–Au Bi-metallic nanocomposite by bioreduction using Piper betle leaf extract. J. Biol. Chem. Biol. Heliyon. 5(12), e02794. Lai, C.H., Fang, S.H., Rao, Y.K., Geethangili, M., Tang, C.H., Lin, Y.J., Hung, C.H., Wang, W.C. and Tzeng, Y.M. 2008. Inhibition of Helicobacter pylori-induced inflammation in human gastric epithelial AGS cells by Phyllanthus urinaria extracts. J. Ethnopharmacol. 118(3), 522–526. Lam Xuan Huong Nguyen, V.P.T., Hieu L.V., Nguyen, P.T.H. 2014. Synthesis of silver nanoparticles using tea leaf extract and antibacterial application (in Vietnamese). The 9th Scientific Conference of the University of Natural Sciences. 3–11. Li, S., Jiang, S., Jia, W., Guo, T., Wang, F., Li, J. and Yao, Z. 2024. Natural antimicrobials from plants: recent advances and future prospects. Food Chem. 432, 137231. Moteriya, P. and Chanda, S. 2018. Biosynthesis of silver nanoparticles formation from Caesalpinia pulcherrima stem metabolites and their broad spectrum biological activities. J. Genet. Eng. Biotechnol. 16(1), 105–113. Naghavi, M., Vollset, S.E., Ikuta, K.S., Swetschinski, L.R., Gray, A.P., Wool, E.E., Robles Aguilar, G., Mestrovic, T., Smith, G., Han, C., Hsu, R.L., Chalek, J., Araki, D.T., Chung, E., Raggi, C., Gershberg Hayoon, A., Davis Weaver, N., Lindstedt, P.A., Smith, A.E., Altay, U., Bhattacharjee, N.V., Giannakis, K., Fell, F., McManigal, B., Ekapirat, N., Mendes, J.A., Runghien, T., Srimokla, O., Abdelkader, A., Abd-Elsalam, S., Aboagye, R.G., Abolhassani, H., Abualruz, H., Abubakar, U., Abukhadijah, H.J., Aburuz, S., Abu-Zaid, A., Achalapong, S., Addo, I.Y., Adekanmbi, V., Adeyeoluwa, T.E., Adnani, Q.E.S., Adzigbli, L.A., Afzal, M.S., Afzal, S., Agodi, A., Ahlstrom, A.J., Ahmad, A., Ahmad, S., Ahmad, T., Ahmadi, A., Ahmed, A., Ahmed, H., Ahmed, I., Ahmed, M., Ahmed, S., Ahmed, S.A., Akkaif, M.A., Al Awaidy, S., Al Thaher, Y., Alalalmeh, S.O., AlBataineh, M.T., Aldhaleei, W.A., Al-Gheethi, A.A.S., Alhaji, N.B., Ali, A., Ali, L., Ali, S.S., Ali, W., Allel, K., Al-Marwani, S., Alrawashdeh, A., Altaf, A., Al-Tammemi, A.B., Al-Tawfiq, J.A., Alzoubi, K.H., Al-Zyoud, W.A., Amos, B., Amuasi, J.H., Ancuceanu, R., Andrews, J.R., Anil, A., Anuoluwa, I.A., Anvari, S., Anyasodor, A.E., Apostol, G.L.C., Arabloo, J., Arafat, M., Aravkin, A.Y., Areda, D., Aremu, A., Artamonov, A.A., Ashley, E.A., Asika, M.O., Athari, S.S., Atout, M.M.d.W., Awoke, T., Azadnajafabad, S., Azam, J.M., Aziz, S., Azzam, A.Y., Babaei, M., Babin, F.-X., Badar, M., Baig, A.A., Bajcetic, M., Baker, S., Bardhan, M., Barqawi, H.J., Basharat, Z., Basiru, A., Bastard, M., Basu, S., Bayleyegn, N.S., Belete, M.A., Bello, O.O., Beloukas, A., Berkley, J.A., Bhagavathula, A.S., Bhaskar, S., Bhuyan, S.S., Bielicki, J.A., Briko, N.I., Brown, C.S., Browne, A.J., Buonsenso, D., Bustanji, Y., Carvalheiro, C.G., Castañeda-Orjuela, C.A., Cenderadewi, M., Chadwick, J., Chakraborty, S., Chandika, R.M., Chandy, S., Chansamouth, V., Chattu, V.K., Chaudhary, A.A., Ching, P.R., Chopra, H., Chowdhury, F.R., Chu, D.-T., Chutiyami, M., Cruz-Martins, N., Da Silva, A.G., Dadras, O., Dai, X., Darcho, S.D., Das, S., De la Hoz, F.P., Dekker, D.M., Dhama, K., Diaz, D., Dickson, B.F.R., Djorie, S.G., Dodangeh, M., Dohare, S., Dokova, K.G., Doshi, O.P., Dowou, R.K., Dsouza, H.L., Dunachie, S.J., Dziedzic, A.M., Eckmanns, T., Ed-Dra, A., Eftekharimehrabad, A., Ekundayo, T.C., El, Sayed., Elhadi, M., El-Huneidi, W., Elias, C., Ellis, S.J., Elsheikh, R., Elsohaby, I., Eltaha, C., Eshrati, B., Eslami, M., Eyre, D.W., Fadaka, A.O., Fagbamigbe, A.F., Fahim, A., Fakhri-Demeshghieh, A., Fasina, F.O., Fasina, M.M., Fatehizadeh, A., Feasey, N.A., Feizkhah, A., Fekadu, G., Fischer, F., Fitriana, I., Forrest, K.M., Fortuna Rodrigues, C., Fuller, J.E., Gadanya, M.A., Gajdács, M., Gandhi, A.P., Garcia-Gallo, E.E., Garrett, D.O., Gautam, R.K., Gebregergis, M.W., Gebrehiwot, M., Gebremeskel, T.G., Geffers, C., Georgalis, L., Ghazy, R.M., Golechha, M., Golinelli, D., Gordon, M., Gulati, S., Gupta, R.D., Gupta, S., Gupta, V.K., Habteyohannes, A.D., Haller, S., Harapan, H., Harrison, M.L., Hasaballah, A.I., Hasan, I., Hasan, R.S., Hasani, H., Haselbeck, A.H., Hasnain, M.S., Hassan, I.I., Hassan, S., Hassan Zadeh Tabatabaei, M.S., Hayat, K., He, J., Hegazi, O.E., Heidari, M., Hezam, K., Holla, R., Holm, M., Hopkins, H., Hossain, M.M., Hosseinzadeh, M., Hostiuc, S., Hussein, N.R., Huy, L.D., Ibáñez-Prada, E.D., Ikiroma, A., Ilic, I.M., Islam, S.M.S., Ismail, F., Ismail, N.E., Iwu, C.D., Iwu-Jaja, C.J., Jafarzadeh, A., Jaiteh, F., Jalilzadeh Yengejeh, R., Jamora, R.D.G., Javidnia, J., Jawaid, T., Jenney, A.W.J., Jeon, H.J., Jokar, M., Jomehzadeh, N., Joo, T., Joseph, N., Kamal, Z., Kanmodi, K.K., Kantar, R.S., Kapisi, J.A., Karaye, I.M., Khader, Y.S., Khajuria, H., Khalid, N., Khamesipour, F., Khan, A., Khan, M.J., Khan, M.T., Khanal, V., Khidri, F.F., Khubchandani, J., Khusuwan, S., Kim, M.S., Kisa, A., Korshunov, V.A., Krapp, F., Krumkamp, R., Kuddus, M., Kulimbet, M., Kumar, D., Kumaran, E.A.P., Kuttikkattu, A., Kyu, H.H., Landires, I., Lawal, B.K., Le, T.T.T., Lederer, I.M., Lee, M., Lee, S.W., Lepape, A., Lerango, T.L., Ligade, V.S., Lim, C., Lim, S.S., Limenh, L.W., Liu, C., Liu, X., Liu, X., Loftus, M.J., M Amin, H.I., Maass, K.L., Maharaj, S.B., Mahmoud, M.A., Maikanti-Charalampous, P., Makram, O.M., Malhotra, K., Malik, A.A., Mandilara, G.D., Marks, F., Martinez-Guerra, B.A., Martorell, M., Masoumi-Asl, H., Mathioudakis, A.G., May, J., McHugh, T.A., Meiring, J., Meles, H.N., Melese, A., Melese, E.B., Minervini, G., Mohamed, N.S., Mohammed, S., Mohan, S., Mokdad, A.H., Monasta, L., Moodi Ghalibaf, A., Moore, C.E., Moradi, Y., Mossialos, E., Mougin, V., Mukoro, G.D., Mulita, F., Muller-Pebody, B., Murillo-Zamora, E., Musa, S., Musicha, P., Musila, L.A., Muthupandian, S., Nagarajan, A.J., Naghavi, P., Nainu, F., Nair, T.S., Najmuldeen, H.H.R., Natto, Z.S., Nauman, J., Nayak, B.P., Nchanji, G.T., Ndishimye, P., Negoi, I., Negoi, R.I., Nejadghaderi, S.A., Nguyen, Q.P., Noman, E.A., Nwakanma, D.C., O’Brien, S., Ochoa, T.J., Odetokun, I.A., Ogundijo, O.A., Ojo-Akosile, T.R., Okeke, S.R., Okonji, O.C., Olagunju, A.T., Olivas-Martinez, A., Olorukooba, A.A., Olwoch, P., Onyedibe, K.I., Ortiz-Brizuela, E., Osuolale, O., Ounchanum, P., Oyeyemi, O.T., Paredes, J.L., Parikh, R.R., Patel, J., Patil, S., Pawar, S., Peleg, A.Y., Peprah, P., Perdigão, J., Perrone, C., Petcu, I.-R., Phommasone, K., Piracha, Z.Z., Poddighe, D., Pollard, A.J., Poluru, R., Ponce-De-Leon, A., Puvvula, J., Qamar, F.N., Qasim, N.H., Rafai, C.D., Raghav, P., Rahbarnia, L., Rahim, F., Rahimi-Movaghar, V., Rahman, M., Rahman, M.A., Ramadan, H., Ramasamy, S.K., Ramesh, P.S., Ramteke, P.W., Rana, R.K., Rani, U., Rashidi, M.-M., Rathish, D., Rattanavong, S., Rawaf, S., Redwan, E.M.M., Reyes, L.F., Roberts, T., Robotham, J.V., Rosenthal, V.D., Ross, A.G., Roy, N., Rudd, K.E., Sabet, C.J., Saddik, B.A., Saeb, M.R., Saeed, U., Saeedi, Moghaddam., Saengchan, W., Safaei, M., Saghazadeh, A., Saheb Sharif-Askari, N., Sahebkar, A., Sahoo, S.S., Sahu, M., Saki, M., Salam, N., Saleem, Z., Saleh, M.A., Samodra, Y.L., Samy, A.M., Saravanan, A., Satpathy, M., Schumacher, A.E., Sedighi, M., Seekaew, S., Shafie, M., Shah, P.A., Shahid, S., Shahwan, M.J., Shakoor, S., Shalev, N., Shamim, M.A., Shamshirgaran, M.A., Shamsi, A., Sharifan, A., Shastry, R.P., Shetty, M., Shittu, A., Shrestha, S., Siddig, E.E., Sideroglou, T., Sifuentes-Osornio, J., Silva, L.M.L.R., Simões, E.A.F., Simpson, A.J.H., Singh, A., Singh, S., Sinto, R., Soliman, S.S.M., Soraneh, S., Stoesser, N., Stoeva, T.Z., Swain, C.K., Szarpak, L., Tabatabai, S., Tabche, C., Taha, Z.M.-A., Tan, K.-K., Tasak, N., Tat, N.Y., Thaiprakong, A., Thangaraju, P., Tigoi, C.C., Tiwari, K., Tovani-Palone, M.R., Tran, T.H., Tumurkhuu, M., Turner, P., Udoakang, A.J., Udoh, A., Ullah, N., Ullah, S., Vaithinathan, A.G., Valenti, M., Vos, T., Vu, H.T.L., Waheed, Y., Walker, A.S., Walson, J.L., Wangrangsimakul, T., Weerakoon, K.G., Wertheim, H.F.L., Williams, P.C.M., Wolde, A.A., Wozniak, T.M., Wu, F., Wu, Z., Yadav, M.K.K., Yaghoubi, S., Yahaya, Z.S., Yarahmadi, A., Yezli, S., Yismaw, Y.E., Yon, D.K., Yuan, C.-W., Yusuf, H., Zakham, F., Zamagni, G., Zhang, H., Zhang, Z.-J., Zielińska, M., Zumla, A., Zyoud, S.e.H.H., Zyoud, S.H., Hay, S.I., Stergachis, A., Sartorius, B., Cooper, B.S., Dolecek, C. and Murray, C.J.L. 2024. Global burden of bacterial antimicrobial resistance 1990–2021: a systematic analysis with forecasts to 2050. Lancet 404(10459), 1199–1226. Nguyen, T.H. and Nguyen, M.D. 2013. Antimicrobial activities of phyllanthus urinaria extracts before and after combined with Pandanus tectorius and Lactobacillus rhamnosus PN04. Biomed. pharmacol. J. 6(2), 241–247. O’Neill, J. 2014. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. Available via https://www.who.int/news/item/29-04-2019-new-report-calls-for-urgent-action-to-avert-antimicrobial-resistance-crisis Patra, J.K. and Baek, K.H. 2017. Antibacterial activity and synergistic antibacterial potential of biosynthesized silver nanoparticles against foodborne pathogenic bacteria along with their anticandidal and antioxidant effects. Front. Microbiol. 8, 167. Paul Das, M., Rebecca Livingstone, J., Veluswamy, P. and Das, J. 2018. Exploration of Wedelia chinensis leaf-assisted silver nanoparticles for antioxidant, antibacterial, and in vitro cytotoxic applications. J. Food Drug Anal. 26(2), 917–925. Sahoo, R.K., Tamuli, K.J., Narzary, B., Bordoloi, M., Sharma, H.K., Gogoi, K. and Bhattacharyya, D.R. 2020. Clerodendrum viscosum vent leaf extract-supported nanosilver particles: characterization, antiplasmodial and anticancer activity. Chem. Phys. Lett. 738, 136893. Shao, Y., Wang, Y., Yuan, Y. and Xie, Y. 2021. A systematic review of the misuse of antibiotics in livestock and aquaculture and its regulation implications in China. Sci. Total Environ. 798, 149205. Slavin, Y.N., Asnis, J., Häfeli, U.O. and Bach, H. 2017. Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 15(1), 65. Thi Kim Cuc Nguyen, T.K.D.N., Nguyen, M.A., Nguyen, T.N. and Pham, V.C. 2014. Evaluation of antibacterial activity of nanochitosan complex - turmeric essential oil and nano silver (in vietnamese). J. Sci. Technol. 52(2), 177–184. Trinh, B.T.D., Staerk, D. and Jäger, A.K. 2016. Screening for potential α-glucosidase and α-amylase inhibitory constituents from selected Vietnamese plants used to treat type 2 diabetes. J. Ethnopharmacol. 186, 189–195. Tshibangu-Kabamba, E. and Yamaoka, Y. 2021. Helicobacter pylori infection and antibiotic resistance: from biology to clinical implications. Nat. Rev. Gastroenterol. Hepatol. 18(9), 613–629. Usha Rani, P. and Rajasekharreddy, P. 2011. Green synthesis of silver-protein (core–shell) nanoparticles using Piper betle L. leaf extract and its ecotoxicological studies on Daphnia magna. Colloids Surf. A Physicochem. Eng. Aspects. 389(1), 188–194. Vanlalveni, C., Lallianrawna, S., Biswas, A., Selvaraj, M., Changmai, B. and Rokhum, S.L. 2021. Green synthesis of silver nanoparticles using plant extracts and their antimicrobial activities: a review of recent literature. RSC Adv. 11(5), 2804–2837. Yamaguchi, T., Okihashi, M., Harada, K., Konishi, Y., Uchida, K., Do, M.H., Bui, H.D., Nguyen, T.D., Nguyen, P.D., Chau, V.V., Dao, K.T., Nguyen, H.T., Kajimura, K., Kumeda, Y., Bui, C.T., Vien, M.Q., Le, N.H., Hirata, K. and Yamamoto, Y. 2015. Antibiotic residue monitoring results for pork, chicken, and beef samples in Vietnam in 2012-2013. J. Agric. Food Chem. 63(21), 5141–5145. Yaseen, M.W., Sufyan, M., Nazir, R., Naseem, A., Shah, R., Sheikh, A.A. and Iqbal, M. 2021. A simple and cost-effective approach to the synthesis of iron–magnesium oxide nanoparticles using Alstonia scholaris and Polyalthia longifolia leaf extracts and their antimicrobial, antioxidant and larvicidal activities. Appl. Nanosci. 11(9), 2479–2488. Yehia, N., Salem, H.M., Mahmmod, Y., Said, D., Samir, M., Mawgod, S.A., Sorour, H.K., AbdelRahman, M.A.A., Selim, S., Saad, A.M., El-Saadony, M.T., El-Meihy, R.M., Abd El-Hack, M.E. and El-Tarabily, K.A. and Zanaty, A.M. 2023. Common viral and bacterial avian respiratory infections: an updated review. Poult. Sci. 102(5), 102553. Zhang, X.F., Liu, Z.G. and Shen, W. and Gurunathan, S. 2016. Silver nanoparticles: synthesis, characterization, properties, applications, and therapeutic approaches. Int. J. Mol. Sci. 17(9), 1534. | ||
| How to Cite this Article |
| Pubmed Style Nguyen MT, Nguyen HTT, Nguyen TT. Effects of nanoparticle antibiotics on cytotoxicity and in vitro antibacterial properties of Escherichia coli isolated from respiratory tract infection in chickens. Open Vet. J.. 2025; 15(8): 3770-3779. doi:10.5455/OVJ.2025.v15.i8.41 Web Style Nguyen MT, Nguyen HTT, Nguyen TT. Effects of nanoparticle antibiotics on cytotoxicity and in vitro antibacterial properties of Escherichia coli isolated from respiratory tract infection in chickens. https://www.openveterinaryjournal.com/?mno=264382 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i8.41 AMA (American Medical Association) Style Nguyen MT, Nguyen HTT, Nguyen TT. Effects of nanoparticle antibiotics on cytotoxicity and in vitro antibacterial properties of Escherichia coli isolated from respiratory tract infection in chickens. Open Vet. J.. 2025; 15(8): 3770-3779. doi:10.5455/OVJ.2025.v15.i8.41 Vancouver/ICMJE Style Nguyen MT, Nguyen HTT, Nguyen TT. Effects of nanoparticle antibiotics on cytotoxicity and in vitro antibacterial properties of Escherichia coli isolated from respiratory tract infection in chickens. Open Vet. J.. (2025), [cited January 25, 2026]; 15(8): 3770-3779. doi:10.5455/OVJ.2025.v15.i8.41 Harvard Style Nguyen, M. T., Nguyen, . H. T. T. & Nguyen, . T. T. (2025) Effects of nanoparticle antibiotics on cytotoxicity and in vitro antibacterial properties of Escherichia coli isolated from respiratory tract infection in chickens. Open Vet. J., 15 (8), 3770-3779. doi:10.5455/OVJ.2025.v15.i8.41 Turabian Style Nguyen, Manh Tuong, Ha Thi Thanh Nguyen, and Thanh Trung Nguyen. 2025. Effects of nanoparticle antibiotics on cytotoxicity and in vitro antibacterial properties of Escherichia coli isolated from respiratory tract infection in chickens. Open Veterinary Journal, 15 (8), 3770-3779. doi:10.5455/OVJ.2025.v15.i8.41 Chicago Style Nguyen, Manh Tuong, Ha Thi Thanh Nguyen, and Thanh Trung Nguyen. "Effects of nanoparticle antibiotics on cytotoxicity and in vitro antibacterial properties of Escherichia coli isolated from respiratory tract infection in chickens." Open Veterinary Journal 15 (2025), 3770-3779. doi:10.5455/OVJ.2025.v15.i8.41 MLA (The Modern Language Association) Style Nguyen, Manh Tuong, Ha Thi Thanh Nguyen, and Thanh Trung Nguyen. "Effects of nanoparticle antibiotics on cytotoxicity and in vitro antibacterial properties of Escherichia coli isolated from respiratory tract infection in chickens." Open Veterinary Journal 15.8 (2025), 3770-3779. Print. doi:10.5455/OVJ.2025.v15.i8.41 APA (American Psychological Association) Style Nguyen, M. T., Nguyen, . H. T. T. & Nguyen, . T. T. (2025) Effects of nanoparticle antibiotics on cytotoxicity and in vitro antibacterial properties of Escherichia coli isolated from respiratory tract infection in chickens. Open Veterinary Journal, 15 (8), 3770-3779. doi:10.5455/OVJ.2025.v15.i8.41 |