| Research Article | ||
Open Vet. J.. 2025; 15(9): 4210-4218
Open Veterinary Journal, (2025), Vol. 15(9): 4210-4218 Research Article Changes in hepatic and renal Doppler ultrasonography: Current standard therapy in dogs with congestive heart failureÇağatay Esin and Murat GüzelDepartment of Veterinary Internal Medicine, Faculty of Veterinary Medicine, Ondokuz Mayis University, Samsun, Turkey *Corresponding Author: Çağatay Esin. Department of Veterinary Internal Medicine, Faculty of Veterinary Medicine, Ondokuz Mayis University, Samsun, Turkey. Email: cagatay.esin [at] omu.edu.tr Submitted: 08/06/2025 Revised: 01/08/2025 Accepted: 07/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
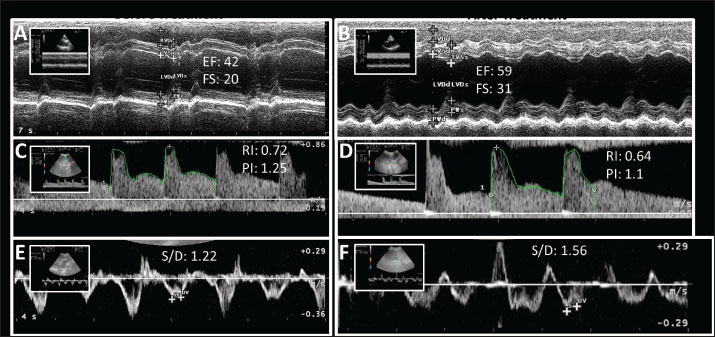
AbstractBackground: In congestive heart failure (CHF), the heart cannot deliver sufficient blood for the metabolic needs of the tissues, leading to vascular changes and organ dysfunction (e.g., kidney and liver). Aim: This study aimed to evaluate the effects of the current standard therapy on kidney and liver hemodynamic changes in dogs with congestive heart failure. Methods: Renal and hepatic Doppler ultrasonography was performed in 15 dogs diagnosed with stage C and D heart failure according to the American College of Veterinary Internal Medicine classification. As a current standard therapy, pimobendan, enalapril, and furosemide were administered to dogs for 4 weeks. Doppler ultrasound findings and serum biochemistry values of the patients were evaluated before and at weeks 1, 2, or 4 of treatment. The control group consisted of 10 healthy dogs. Results: Heart failure was diagnosed in the dogs by an echocardiographic evaluation. The values of hepatic and renal resistive index, renal pulsatility index, and hepatic vein systole–diastole ratio were deteriorated in the dogs with CHF compared with those in the healthy control group (p < 0.05). Hemodynamic abnormalities in the kidney and liver started to recover after a 2-week treatment period. Conclusion: Doppler ultrasonography findings demonstrated that heart failure worsens the vascular hemodynamics of the kidney and liver in dogs. Congestive heart failure treatment may improve the cardiorenal and cardiohepatic changes due to CHF. Keywords: Cardiohepatic syndrome, Cardiorenal syndrome, Dog, Doppler ultrasonography, Heart failure. IntroductionHeart failure is a syndrome characterized by insufficiency of blood pumped from the heart, improper distribution of blood, and inadequate tissue oxygenation. Hemodynamic changes occur as a result of a decrease in cardiac output, vasoconstriction, sodium and water retention, and activation of the sympathetic nervous system and renin-angiotensin-aldosterone system (Perkowski and Oyama, 2024). Cardiomyopathies, valve problems, and myocardial failure are the cardiac causes of heart failure (Saunders and Gordon, 2015). Diagnostic methods for HF include physical examination, radiographic, electrocardiographic, and echocardiographic examinations, and biochemical analyses (Hoque et al., 2019). Echocardiography is a highly reliable, non-invasive imaging modality that provides comprehensive information about cardiac anatomy, chamber dimensions, myocardial thickness, systolic and diastolic function, atrial performance, valvular integrity, blood flow patterns, and overall hemodynamic status, making it invaluable for diagnosing both congenital and acquired heart diseases (Bonagura and Blissitt, 1995; Mattoon et al., 2020). Heart failure is a progressive disease that is irreversible in some cases, except for surgical intervention (Summers, 2019). The aim of treatment is to improve cardiac output and eliminate clinical symptoms associated with increased venous pressure, particularly pulmonary dysfunction. Treatment aims to improve cardiac output, alleviate clinical symptoms associated with increased venous pressures (especially pulmonary congestion), and slow disease progression, and improve survival (Ware et al., 2021). Drugs used in heart failure include diuretics, positive inotrope, vasodilators, antiarrhythmics, and neuroendocrine activation regulators (Ware et al., 2021; Bagardi et al., 2022). The heart pumps oxygen-rich blood to all body parts, including the kidneys, through a network of vessels. Any problem in the circulatory system causes various disorders in the organs connected to each other by these vascular networks (Mingarelli, 2016). In heart failure, blood cannot be pumped sufficiently to the body, and kidney vessels become congested. As the pressure in the kidneys increases, oxygen-rich blood begins to fail to reach the kidneys (Gaze, 2023). Renal blood flow decreases, kidney autoregulation is impaired, and secondary kidney damage (cardiorenal syndrome) occurs in the case of heart failure (Tamayo-Gutierrez and Ibrahim, 2022). Serum biochemical analyzes are used in the diagnosis of CS. However, the most obvious change in kidney damage due to HF is the change in renal arterial resistance. The renal arterial resistive index (RI) and pulsatility index (PI) are noninvasive methods that allow the measurement of resistance in renal arteries by Doppler ultrasonographic analysis (Priyanka et al., 2018). One of the most important early findings in patients with kidney disease is the increase in renal arterial resistance and the detection of the severity of renal cortical vasoconstriction by Doppler ultrasound (Novellas et al., 2008). Although the liver is a highly vascular organ and is sensitive to hemodynamic changes, it is resistant to ischemic damage due to its strong vascular defense mechanisms (Ford et al., 2015). Studies have shown that there is a similar interaction between the heart and liver, just like in cardiorenal syndrome. Cardiohepatic syndrome may occur in heart failure (Popov et al., 2012; Aspromonte et al., 2023). Blood fills the hepatic and portal systems in heart failure due to increased central venous pressure. Hepatic edema, hepatocyte atrophy, hepatic venous congestion, and portal hypertension occur. In addition, decreased cardiac output results in hepatocellular necrosis and perfusion disorder in the liver (Novellas et al., 2008). This condition is called cardiac hepatopathy or cardiohepatic syndrome (Aspromonte et al., 2023). To determine liver damage, portal vein, hepatic vein, and hepatic arterial hemodynamic changes are evaluated using Doppler ultrasonography (Silva et al., 2020). Therefore, this study aimed to evaluate the effects of the current standard therapy on kidney and liver hemodynamic changes in dogs with congestive heart failure. Materials and MethodsThe study group consisted of 15 dogs with heart failure of C and D, according to the American College of Veterinary Internal Medicine classification, with complaints of exercise intolerance, breathing difficulties, and cough. The congestive heart failure (CHF) group consisted of 8 males and 7 females aged between 8 and 14 years. This group included dogs of various breeds, such as Kangal Turkish Shepherd (n=4), Terrier (n=4), Siberian Husky, Pekingese, Beagle, Golden Retriever, Welsh Corgi, German Shepherd, and one mixed-breed dog. The control group consisted of 10 healthy dogs brought for vaccination, parasitic application, or general examination. The control group comprised 5 males and 5 females, aged between 8 and 13 years, with the following breed distribution: mixed-breed (n=3), Terrier (n=2), Golden Retriever, French Bulldog, Pointer, German Shepherd, and Kangal Turkish Shepherd. All dogs in the CHF group received the current standard therapy protocol consisting of pimobendan (0.3 mg/kg PO q12 hours), furosemide (2 mg/kg PO q12 hours), and enalapril (0.5 mg/kg PO q12 hours). The therapeutic combination remained consistent across all animals throughout the study period. For radiographic examination, right lateral and dorsoventral chest radiographs were obtained. In radiography, VHS, lung density (edema), bronchovascular structures, tracheal course, and presence of pericardial and pleural effusion were evaluated. Electrocardiography (ECG) recordings of the dogs were recorded at a speed of 50 mm/second and an amplitude of 1 mV=10 mm in automatic mode without a filter. Dogs that could not tolerate the right lateral position were taken standing on all 4 legs or in the sternal position. ECG evaluations were performed in all leads. Echocardiographic examinations were conducted using a phased array cardiac probe with a frequency of 4.0–7.5 MHz. Dogs were positioned in both right and left lateral recumbency, and parasternal and apical views were evaluated in both short and long axes. No sedation or anesthesia was administered during the procedure. Standard two-dimensional and M-mode echocardiography was used to assess the left atrial and ventricular dimensions, interventricular septum and left ventricular free wall thickness, and systolic and diastolic function. Color and spectral Doppler modalities were employed to detect mitral, tricuspid, aortic, and pulmonary valvular leakage flows and to evaluate pulmonary arterial flow. M-mode measurements included right ventricular diastolic diameter (RVDd), interventricular septal thickness in diastole and systole (IVSd, IVSs), left ventricular diastolic and systolic diameter (LVDd, LVDs), and posterior wall thickness in diastole and systole (PWd, PWs). The left ventricular function was assessed using ejection fraction (EF%) and fractional shortening (FS%). The left atrium and aortic root diameters were measured from a right parasternal short-axis view to calculate the LA/Ao ratio. Echocardiographic assessments were performed once in control dogs and four times in CHF dogs: day 0 (pre-treatment) and at weeks 1, 2, and 4 of treatment. The first author performed all echocardiographic evaluations. Abdominal Doppler ultrasonography was performed using a 5–8 MHz microconvex probe. As with echocardiography, the examination was conducted once in the control group and repeated at four time points in the CHF group (day 0 and weeks 1, 2, and 4). For renal Doppler evaluation, dogs were positioned in lateral recumbency, and color Doppler was used to locate the interlobar artery within the renal medulla. The probe was angled at approximately 45º relative to the long axis of the vessel. The RI was automatically calculated using peak systolic and end-diastolic velocities, while PI was derived from three consecutive waveform cycles. Each measurement was repeated three times, and the mean values were recorded. The portal vein diameter and peak flow velocity, hepatic vein systole-to-diastole (S/D) ratio, and hepatic arterial RI were measured for hepatic Doppler assessment. The portal vein was visualized from the right 9th to 11th intercostal space with the dog in dorsal recumbency. Its hyperechoic wall and position caudal to the liver’s right lobe and left of the gallbladder aided identification. The hepatic artery RI was measured from a tortuous point near its hepatic entry, adjacent to the caudal vena cava. The hepatic vein S/D ratio was assessed from the right liver lobe, dorsal to the caudal vena cava, and ventral to the gallbladder. Blood samples were collected from the vena cephalica antebrachii into yellow gel blood tubes and centrifuged at 3,000 rpm for 5 minutes to obtain serum samples. Biochemical analyses were performed to confirm cardiac, renal, and hepatic damage, such as alanine aminotransferase (ALT), alkaline phosphatase (ALP), gamma-glutamyl transferase (GGT), total bilirubin, urea, and creatinine. symmetric dimethylarginine (SDMA) (SunRedbio®) and cardiac troponin I (cTn-I) values (SunRedbio®) were measured by ELISA according to the manufacturer’s instructions. Serum biochemistry analyses were repeated once in the control group and on day 0 and weeks 1, 2, and 4 in the CHF group. Certain exclusion criteria were used to ensure a homogeneous population and to evaluate the main Doppler ultrasonography effect of CHF on hepatic and renal hemodynamics. Dogs presenting with severe ascites, systemic illnesses, infection, and abnormal leukogram (e.g., leukocytosis, neutrophilia), as well as those receiving pharmacological treatment, were excluded from the study. Dogs diagnosed with primary hepatic or renal disease—determined by clinical, ultrasonography, or laboratory findings—were also excluded from the study. Statistical analysisStatistical analyses were performed using the SPSS 21 analysis program. Whether the data had a normal distribution was evaluated using the Kolmogorov-Smirnov test. The Student’s t-test was applied to values showing normal distribution between control and CHF dogs, and Mann–Whitney U test was applied to parameters that did not show normal distribution. To evaluate the effectiveness of treatment in the CHF group, repeated measures ANOVA post-hoc Duncant test was used for statistical evaluations among measurements at different weeks (day 0 and weeks 1, 2, and 4 treatment), and the Friedman test was used for values that did not show normal distribution. While IVSd, PWd, IVSs, PWs, EF, FS, Left renal RI, Left renal PI, Right renal RI, Right renal PI, portal vein flow rate, hepatic vein S/D ratio, and hepatic arterial RI exhibited a normal distribution, RVDd, LVDd, LVDs, LA/Ao, portal vein diameter, CTn-I, urea, creatinine, SDMA, ALT, ALP, GGT, and TBIL did not follow a normal distribution. In the tables, the capital letters (A, B, C) in the same row indicate statistical differences (p < 0.05) between the control and CHF groups (between groups). Lowercase letters (a, b, and c) in the same row indicate statistically significant differences (p < 0.05) between dogs in the CHF group on a weekly basis (within the group of dogs with CHF). Descriptive statistics are presented in the tables as mean ± standard deviation for the parameters. A p value of 0.05 was considered statistically significant. Ethical approvalThis study was conducted at the Faculty of Veterinary Medicine, Animal Hospital of Ondokuz Mayis University, with the approval of the Local Ethics Committee for Animal Experiments (Approval no: 68489742-604.01.03-E.12337). ResultsFigure 1 shows an illustrative image of echocardiographic and Doppler ultrasonographic evaluation of the heart, kidneys, and liver.
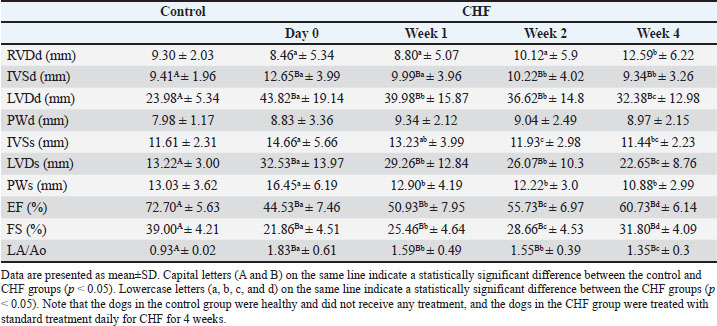
Fig. 1. An illustrative appearance of echocardiography and Doppler ultrasonography evaluation of the heart, kidney, and liver before (A, C, and E) and after (B, D, and F) treatment in a dog with CHF. A and B: M-mode left ventricle echocardiographic appearance. EF increases from 42% to 59% and FS increases from 20% to 31% with treatment. C and D: Doppler ultrasonography of the renal interlobar artery. The renal RI decreased from 0.72 to 0.64 and the renal PI decreased from 1.25 to 1.1 with treatment. E and F: Doppler ultrasonography of the hepatic vein. The hepatic vein S/D increased from 1.22 to 1.56 with treatment. When the echocardiographic findings of the dogs were evaluated, a significant difference was found between the healthy and CHF groups’ %EF values (between the healthy group and CHF group day 0, p=0.0002). In the CHF group, the regular increase in %EF values in weeks 1, 2, and 4 of treatment showed a significant difference compared with that before treatment (p=0.0015, p=0.0007, and p=0.0006, respectively) (Table 1). Table 1. Echocardiography findings in the control and CHF groups.
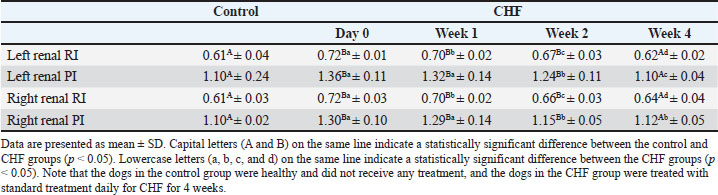
There was a significant difference between the %FS values in the control and CHF groups (between the healthy group and CHF group day 0, p=0.0003). The increases in %FS values of the CHF group in weeks 1, 2, and 4 of treatment were found to be significant (p=0.0014, p=0.0006, and p=0.0005, respectively) compared with those before treatment. Considering the LA/Ao, a significant difference was determined between day 0 LA/Ao in the CHF group and the healthy group (p=0.0002). In weeks 1, 2, and 4 of treatment, the LA/Ao ratio decreased steadily and significantly (p=0.0014, p=0.0006, and p=0.0003, respectively). According to renal Doppler ultrasound findings, RI and PI values in both kidneys in the CHF group were significantly (between healty group and CHF group day 0 for left renal RI p=0.0004, left renal PI p=0.0006, right renal RI p=0.0005, and right renal PI p=0.0005) higher than those in healthy dogs. RI and PI values decreased significantly with treatment (for left renal RI p=0.0315, p=0.0014, and p=0.0006, respectively; for left renal PI p=0.348, p=0.0021, and p=0.0006, respectively; for right renal RI p=0.0092, p=0.0009, and p=0,0006, respectively; for right renal PI p=0.551, p=0.0009, and p=0.0006, respectively) decreased to the control group level at the end of 4 weeks of treatment (Table 2). Table 2. Doppler ultrasonography findings of the kidneys.
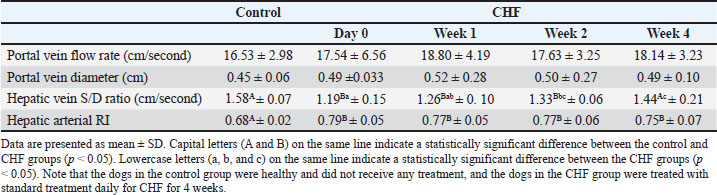
When Doppler ultrasound findings of the liver were evaluated, there was no significant difference in the portal vein flow rate between the control and CHF groups, including treatment (p > 0.05). No significant difference in portal vein diameter was observed between the control group and the CHF group (p > 0.05). The hepatic vein ratio S/D values in the CHF group before treatment were significantly different from those in the healthy group (p=0.006). Hepatic vein S/D increased significantly in weeks 1 (p=0.0554), weeks 2 (p=0.0123), and weeks 4 (p=0.0100) of treatment, and at the end of the 4-week treatment, the significant difference with the control group disappeared (p > 0.05). A statistically significant difference was found between hepatic arterial RI values in the healthy group and CHF group before treatment (p=0.0364). Although there was a decrease in hepatic artery RI values in CHF group before treatment, it was not statistically significant among weeks 1, 2, and 4 (p > 0.05) (Table 3). Table 3. Doppler ultrasonography findings of the liver.
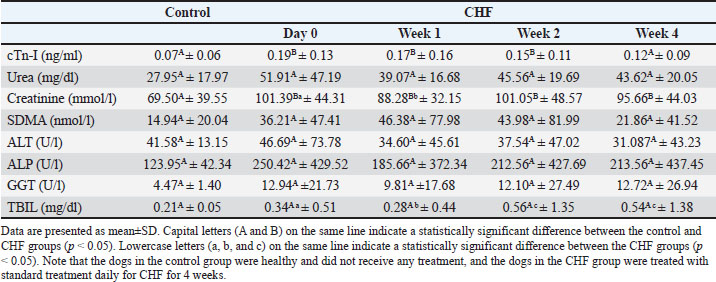
Table 4 summarizes the serum biochemical parameters in the groups. The cTn-I, urea, creatinine, SDMA, ALT, ALP, GGT, and TBIL values in the control group were within physiological ranges on day 0 and week 4. Serum cTn-I and creatinine levels were higher in the CHF group than in the control group on day 0 (p=0.005 and p=0.0069, respectively). Creatinine levels varied during the 4 weeks (between day 0 and week 1, p=0.0496), while urea showed a non-significant decreasing trend (p > 0.05). SDMA peaked at week 1 but declined by week 4 without reaching statistical significance (p > 0.05). Throughout the study, ALT and ALP levels were higher in the CHF group but showed no significant intra- or intergroup differences (p > 0.05). Table 4. Serum biochemical parameters of the control and CHF groups.
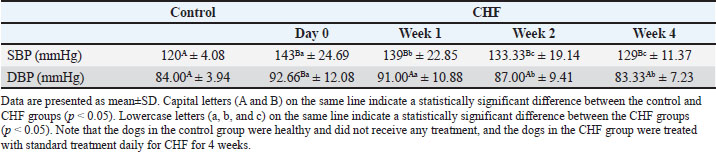
Doppler-derived blood pressure measurements are summarized in Table 5. On day 0, both systolic (SBP) and diastolic (DBP) blood pressures were significantly higher in the dogs with CHF than in the healthy group (p=0.007 and p=0.001, respectively). When evaluating the treatment effect in the dogs within the CHF group, the values for SBP and DBP were found to be decreased in week 1, 2 and 4 by the standard treatment (for SBP p=0.0141, p=0.0017, and p=0.008, respectively; for DBP p=0.2482, p=0.0171, and p=0.0104, respectively). Table 5. Systemic blood pressure findings in the control and CHF groups.
DiscussionEF is a key indicator of myocardial contractile function, playing a vital role in heart failure diagnosis, prognosis assessment, and treatment guiding (Njoroge and Teerlink, 2020). In healthy dogs, EF ranges from 60% to 82% (De Madron et al., 2015). A reduced EF, especially below 40%, indicates systolic dysfunction, while values ≥50% are considered preserved (Keene et al., 2019). In this study, EF significantly increased in the first and second weeks of pimobendan treatment (50.93% ± 7.95% and 55.73% ± 6.97%, respectively), reaching the physiological range by the 4th week (60.73% ± 6.14%). FS also reflects ventricular contractility, particularly in symmetrically contracting myocardium. The normal FS range in dogs is 28%–45% (Caivano et al., 2018). Reduced FS due to impaired contraction is common in heart failure, dilated cardiomyopathy, myxomatous mitral valve disease, and pulmonary hypertension (Morgan et al., 2020). In this study, at day 0, FS in dogs with CHF (21.86% ± 4.56%) was below both the physiological range and control group values. However, in week 2, the FS increased to 28.66% ± 4%, reaching normal limits. LA enlargement, often seen in valve disorders, systemic hypertension, aortic stenosis, and cardiomyopathy, is quantified by the LA/Ao ratio, with values <1.5 considered normal (Ljungvall et al., 2013; Höllmer et al., 2017). In our study, at day 0, LA/Ao was elevated (1.83 ± 0.61), but significantly decreased by weeks 1 and 2 (1.59 ± 0.49 and 1.55 ± 0.39, respectively), reaching the normal range by week 4 (1.35 ± 0.30). The heart and kidneys play a critical role in maintaining hemodynamic stability, which is essential for life. Acute or chronic damage to the heart or kidneys affects other organs, causing dysfunction and cardiorenal syndrome (Szczepankiewicz et al., 2019). In heart failure, reduced cardiac output impairs glomerular filtration, increases tubular reabsorption of urea and toxins, and activates the renin-angiotensin-aldosterone system, leading to altered renal perfusion and hemodynamics (Ferreira et al., 2019). Studies have shown that the renal resistive RI rises earlier than blood biomarkers such as SDMA, urea, creatinine, and cystatin-C in both dogs and humans with cardiorenal syndrome (Choi et al., 2017). This is because blood biomarkers increase only after significant renal damage—typically when 50%–75% of nephrons are affected (Kopitkó et al., 2020). The normal renal RI in dogs is between 0.56 and 0.67 (Agut et al., 2020). In a 2012 study, dogs with myxomatous mitral valve disease had RI values between 0.67 and 0.77 (Chetboul et al., 2012). In our study, dogs with CHF exhibited elevated RI (0.72 ± 0.03) compared with the control and reference ranges. This increase likely reflects reduced renal blood flow, congestion-induced pressure, and parenchymal resistance, supporting the presence of CR syndrome. Previous findings show that diuretics and ACE inhibitors reduce RI by lowering renal pressure and inducing vasodilation (Szczepankiewicz et al., 2019). Similarly, in our study, RI values decreased significantly with pimobendan, enalapril, and furosemide, reaching normal ranges from week 2. PI also reflects renal vascular resistance during the cardiac cycle. RAAS activation and increased angiotensin-II increase PI by elevating vascular tone (Manukyan et al., 2022). The upper PI limit in healthy dogs is 1.27 (Constantinescu et al., 2015). In this study, PI in the CHF group was elevated (1.36 ± 0.11) but significantly decreased with standard therapy, mirroring RI changes. Furosemide reduces intravascular pressure, while ACE inhibitors improve renal perfusion through vasodilation (Kato et al., 2017), contributing to normalized PI in week 2. Interactions between the heart and liver—termed cardiohepatic syndrome—are well documented in human medicine (Xanthopoulos et al., 2019; Gonul et al., 2020). In veterinary medicine, this relationship has been less explored. The normal portal vein flow velocity in healthy dogs is 18 ± 7.6 cm/second (Sartor et al., 2010); in this study, it was 16.53 ± 2.98 cm/second in the control group. Increased atrial pressure and impaired systolic function may cause portal congestion and reduce flow velocity in patients with heart failure (Ikeda et al., 2018; Zhang et al., 2021). However, in this study, the flow velocity in the CHF group remained within normal limits. Similarly, the portal vein diameter remained within the reference values (0.45 ± 0.06 cm), despite the expected alterations in heart failure. This may be explained by hepatic compensatory mechanisms: the hepatic arterial buffer response and sinusoidal permeability, which maintain hepatic blood flow even with a portal flow reduction of up to 60% (Poelzl and Auer, 2015). These mechanisms may account for the preservation of portal flow and diameter despite the elevated hepatic arterial RI values in the study group. The hepatic vein S/D ratio is typically 1.41–1.97 in healthy dogs (Kim et al., 2017); in this study, it was 1.58 ± 0.07 in the control group. In patients with heart failure, venous congestion impairs normal tetrabasic flow, reducing the S/D ratio (Kim et al., 2017; Silva et al., 2022). In our study, this ratio was 1.19 ± 0.15 at day 0 in the CHF group but improved significantly during therapy, reaching 1.44 ± 0.00 by week 4. Diuretics, such as furosemide, and inotropes, such as pimobendan, may enhance hepatic venous drainage and improve this ratio (Dupont et al., 2011; Guinot et al., 2022). Hepatic blood reaches the sinusoids via the portal vein and hepatic artery and exits through the hepatic vein (Quaia, 2020). Two key compensatory mechanisms—the hepatic arterial buffer response and high sinusoidal permeability—protect hepatic perfusion. Normal hepatic artery RI ranges from 0.64 to 0.70 in healthy dogs (Le Roy et al., 2018); our control group averaged 0.68 ± 0.02. In contrast, HF induces hepatic hypoperfusion, venous congestion, and hypoxia (Kavoliuniene et al., 2013), impairing compensation. Hepatic arterial RI was elevated (0.79 ± 0.05) in the CHF group and remained above normal in all weekly measurements, confirming impaired hepatic perfusion. The improvements observed in hepatic and renal Doppler parameters following treatment indicate that organ perfusion is particularly related to dysregulated hemodynamics caused by heart failure affecting the organs (e.g., liver and kidney). Furthermore, it is important to note herein that the compensation mechanisms may complicate the hemodynamics in the body, and it needs to be evaluated for a longer duration to make a certain conclusion in such cases with respect to QoL, exercise tolerance, and survival. Future studies incorporating long-term clinical follow-up, objective functional assessments, or survival analysis are necessary to determine the beneficial effects of Doppler-based improvements in dogs with CHF. This study has some limitations. First, the relatively small sample size may limit the statistical power and generalizability of the findings. Second, while efforts were made to standardize treatment protocols, individual variations in response to medications and owner compliance may have influenced the outcomes of this study. In addition, the exclusion of dogs with advanced ascites or concurrent systemic illnesses, although necessary for data clarity, may limit the applicability of this study to more severely affected clinical populations. In this study, the day 0 renal RI and PI values in dogs with CHF were higher than those in healthy animals. Doppler measurements of the liver revealed that the hepatic vein S/D ratio was lower than that of healthy animals and reference values. Vascular hemodynamic changes can occur before abnormal enzyme levels are detected in biochemical analyses. Treatment consisting of pimobendan, enalapril, and furosemide used in CHF had positive effects on the disease. In this study, renal RI and PI values decreased steadily and reached the reference range with current standard therapy. Therefore, this study concluded that the treatment has a curative effect on cardiorenal syndrome. The hepatic vein S/D ratio increased weekly with treatment and reached the reference value. In conclusion, cardiorenal and cardiogenic hepatorenal syndromes are observed in dogs with CHF, similar to those in humans. Renal and hepatic hemodynamic changes appear to improve after the 2nd week of CHF treatment in dogs. AcknowledgmentsNone. Conflict of interestThe authors declare no conflicts of interest. FundingThis work was supported by the Scientific Research Projects Unit of Ondokuz Mayis University. Project number: PYO.VET.1904.19.019. Authors’ contributionsCE: Writing–original draft, project administration, Doppler ultrasonography methodology, data curation, conceptualization. MG: Methodology, Conceptualization, and Review and Editing. Data availabilityThe datasets used and analyzed for this research are available upon reasonable request from the corresponding author (CE). ReferencesAgut, A., Soler, M. and Fernández-del Palacio, M.J. 2020. Changes in renal resistive index values in healthy puppies during the first months of life. Animals 10(8), 1338. Aspromonte, N., Fumarulo, I., Petrucci, L, Biferali, B., Liguori, A., Gasbarrini, A., Massetti, M. and Miele, L. 2023. The liver in heart failure: biomarkers to clinical risk. Int. J. Mol. Sci. 24(21), 15665. Bagardi, M., Zamboni, V., Locatelli, C., Galizzi, A., Ghilardi, S. and Brambilla, P.G. 2022. Chronic congestive heart failure caused by myxomatous mitral valve disease in dogs: a narrative review from 1970 to 2020. Animals 12(2), 209. Bonagura, J.D. and Blissitt, K.J. 1995. Review article: echocardiography. Equine Vet. J. 27(S19), 5–17. Caivano, D., Rishniw, M., Birettoni, F., Patata, V., Giorgi, M. E., Dei, K. and Porciello, F. 2018. Right ventricular outflow tract fractional shortening: an echocardiographic index of right ventricular systolic function in dogs with pulmonary hypertension. J. Vet. Cardiol. 20(5), 354–363; doi: 10.1016/j.jvc.2005.05.0 Chetboul, V., Daste, T., Gouni, V., Concordet, D., Trehiou-Sechi, E., Serres, F., Pouchelon, J.L., Germain, C.A., Layssol-Lamour, C. and Lefebvre, H.P. 2012. Renal resistive index in 55 dogs with degenerative mitral valve disease. J. Vet. Int. Med. 26(1), 101–108. Choi, B.S., Moon, H.S., Seo, S.H. and Hyun, C. 2017. Serum cystatin-C and symmetric dimethylarginine concentrations in dogs with heart failure from chronic mitral valvular insufficiency. J. Vet. Med. Sci. 79(1), 41–46. Constantinescu, R., Crivineanu, V., Goran, G., Nae, R.T. and Codreanu, M.D. 2015. Evaluation of renal vascular resistance and blood pressure in dogs with different renal diseases. Sci. Works, Ser. C Vet. Med. LXI, 178–183. De Madron, E., Chetboul, V. and Bussadori, C. 2015. Clinical echocardiography of dogs and Cats. St. Louis, MO: Elsevier Health Sciences. Dupont, M., Mullens, W. and Tang, W.H.W. 2011. Impact of systemic venous congestion in HF. Curr. Heart Fail. Rep. 8, 233–241 Ferreira, J.P., Rossignol, P. and Zannad, F. 2019. Renin–angiotensin–aldosterone system and kidney interactions in HF. J. Renin–Angiotensin–Aldosterone Syst. 20(4), 1470320319889415. Ford, R.M., Book, W. and Spivey, J.R. 2015. Liver disease related to the heart. Transplant. Rev. 29(1), 33–37. Gaze, D.C. Ed. 2023. Novel pathogenesis and treatments for cardiovascular sisease. IntechOpen; doi:10.5772/intechopen.111799 Gonul, R., Koenhemsi, L., Bayrakal, A., Yildiz, K., Bahceci, T., Or, M.E. and Uysal, A. 2020. Hepatorenal arterial resistive and pulsatility indexes in dogs with ascites: a retrospective study. Indian J. Anim. Res. 54(3), 359–362. Guinot, P.G., Bahr, P.A., Andrei, S., Popescu, B.A., Caruso, V., Mertes, P.M., Berthoud, V., Nguyen, M. and Bouhemad, B. 2022. Doppler study of portal vein and renal venous velocity predict the appropriate fluid response to diuretic in intensive care unit: a prospective observational echocardiographic evaluation Crit. Care 26(1), 305. Höllmer, M., Willesen, J.L., Tolver, A. and Koch, J. 2017. Volume and function of the left atrium in dogs with naturally occurring myxomatous mitral valve disease. J. Vet. Cardiol. 19(1), 24–34. Hoque, M., Saxena, A.C., Gugjoo, M.B. and Bodh, D. 2019. Cardiac diseases in dogs. Indian J. Anim. Health 58(1), 1–20. Ikeda, Y., Ishii, S., Yazaki, M., Fujita, T., Iida, Y., Kaida, T., Nabeta, T., Nakatani, E., Maekawa, E., Yanagisawa, T. 2018. Portal congestion and intestinal edema in patients with heart failure who were hospitalized. Heart Vessels 33, 740–751 Kato, M., Tohyama, K., Ohya, T. and Hirayama, A. 2017. Clinical effects of tolvaptan addition to intravenous furosemide in patients with congestive heart failure. Res. Cardiovas. Med. 6(4), 14–19. Kavoliuniene, A., Vaitiekiene, A. and Cesnaite, G. 2013. Congestive hepatopathy and hypoxic hepatitis in patients with heart failure: a cardiologist’s point of view. Int. J. Cardiol. 166(3), 554–558; doi: 10.1016/j.ijc.2015.03.005 Keene, B., Atkins, C., Bonagura, J., Fox, P., Häggström, J., Fuentes, V.L. and Uechi, M. 2019. ACVIM consensus guidelines for the diagnosis and treatment of myxomatous mitral valve disease in dogs. J. Vet. Intern. Med. 33, 1127–1140. Kim, J., Kim, S. and Eom, K. 2017. Pulsed-wave Doppler ultrasonographic evaluation of the hepatic vein in dogs with tricuspid regurgitation: a case report. J. Vet. Sci. 18, 73–79. Kopitkó, C., Gondos, T., Fülöp, T. and Medve, L. 2020. Reinterpreting renal hemodynamics: the importance of venous congestion and effective organ perfusion in acute kidney injury: a retrospective study. Am. J. Med. Sci. 359(4), 193–205. Le Roy, B., Dupré, A., Gallon, A., Chabrot, P., Gagnière, J. and Buc, E. 2018. Liver hypertrophy: underlying mechanisms and promoting procedures before major hepatectomy: a systematic review. J. Visceral Surg. 155(5), 393–401. Ljungvall, I., Höglund, K., Lilliehöök, I., Oyama, M.A., Tidholm, A., Tvedten, H. and Häggström, J. 2013. Serum serotonin concentration is associated with myxomatous mitral valve disease severity in dogs. J. Vet. Intern. Med. 27(5), 1105–1112. Manukyan, M., Falkovskaya, A., Mordovin, V., Pekarskiy, S., Zyubanova, I., Solonskaya, E., Ryabova, T., Khunkhinova, S., Vtorushina, A. and Popov, S. 2022. Renal denervation has a favorable effect on elevated renal vascular resistance in patients with resistant hypertension and type 2 diabetes mellitus. Front. Cardiovasc. Med. 9, 1010546. Mattoon, J.S., Sellon, R.K. and Berry, C.R. 2020. Small animal diagnostic ultrasound (e-book). St. Louis, MO: Elsevier Health Sciences. Mingarelli, M. 2016. The cardiovascular system renal regulation. Nephrology 2(1), pocj-5000201. Morgan, K.R.S., Monteith, G., Raheb, S., Colpitts, M. and Fonfara, S. 2020. Echocardiographic parameters for congestive heart failure assessment in dogs with myxomatous mitral valve disease and moderate to severe mitral regurgitation. Vet. J. 263, 105518. Njoroge, J.N. and Teerlink, J.R. 2020. Systolic time intervals in patients with heart failure: time to teach old tricks to new dogs. European J. Heart Fail. 22(7), 1183–1184. Novellas, R., Ruiz de Gopegui, R. and Espada, Y. 2008. Determination of renal vascular resistance in dogs with DM and hyperadrenocorticism. Vet. Record 163(20), 592–595. Perkowski, S.Z. and Oyama, M.A. 2024. Pathophysiology and anesthetic management of patients with cardiovascular disease: a retrospective study. In Veterinary anesthesia and analgesia: the sixth edition of Lumb and Jones. Hoboken, NJ: John Wiley & Sons, pp: 680–696. Poelzl, G. and Auer, J. 2015. Cardiohepatic syndrome. Curr. Heart Fail. Rep. 12(1), 68–78. Popov, D., Krasteva, R., Ivanova, R., Mateva, L.and Krastev, Z. 2012. Doppler parameters of hepatic and renal hemodynamics in patients with liver cirrhosis: a retrospective study. Int. J. Nephrol. 2012, 961654. Priyanka, M., Jeyaraja, K. and Thirunavakkarasu, P.S. 2018. Abnormal renovascular resistance in dogs with DM: correlation with glycemic status and proteinuria. Iranian J. Vet. Res. 19(4), 304. Quaia, E., Ed. 2020. Imaging of the liver and intra-hepatic biliary tract: volume 1 – imaging techniques and non-tumoral pathologies (medical radiology, diagnostic imaging series). Cham, Switzerland: Springer. Sartor, R., Maprim, M. and Takahira, R. 2010. Morphometric evaluation, by ultrasonographic exam, of the portal vein, caudal vena cava, and abdominal aorta by ultrasonography in healthy dogs of different body weights. Arch. Vet. Sci. 15, 143–148. Saunders, A.B. and Gordon, S.G. 2015. Heart failure in dogs. Today’s Vet. Prac. 5(4), 23–28. Silva, V.B.C., Froes, T.R., Wolf, M., Lucina, S.B. and Sousa, M.G. 2022. Doppler spectrum characterization of hepatic veins in dogs with pulmonary hypertension. Res. Vet. Sci. 150, 131–136. Silva, V.B.C., Froes, T., Gil, E.M.U., Wolf, M., Lucina, S.B. and Sousa, M.G. 2020. Characterization of the Doppler spectrum of hepatic veins and correlation with the structural and functional variables of the right ventricle in healthy dogs. J. Vet. Intern. Med. 34(1), 45–52. Summers, A. 2019. Common diseases of companion animals (e-book). St. Louis, MO: Elsevier Health Sciences. Szczepankiewicz B, Paslawska U, Paslawski R, Gebarowski T, Zasada W, Michalek M, Noszczyk-Nowak A. 2019. Urine podocin/creatinine ratio as a novel biomarker of cardiorenal syndrome in dogs with degenerative mitral valve disease. J. Physiol. Pharmacol. 70(2), 57–76. Tamayo-Gutierrez, A. and Ibrahim, H.N. 2022. The kidney in heart failure: role of venous congestion. Methodist DeBakey Cardiovasc. J. 18(4), 4. Ware, W.A., Bonagura, J.D. and Scansen, B.A. 2021. Management of heart failure. In Cardiovascular disease in companion animals. Boca Raton, FL: CRC Press, pp: 337–360. Xanthopoulos, A., Starling, R. C., Kitai, T. and Triposkiadis, F. 2019. Heart failure and liver disease: cardiohepatic interactions. JACC: Heart Fail. 7(2), 87–97. Zhang, J., Zhang, M., Tang, J., Yin, G., Long, Z., He, L., Zhou, C., Luo, L., Qi, L. and Wang, L. 2021. Animal models of BPH. Prostate Cancer Prostatic Dis. 24(1), 49–57. | ||
| How to Cite this Article |
| Pubmed Style Esin C, Guzel M. Changes in hepatic and renal Doppler ultrasonography: Current standard therapy in dogs with congestive heart failure. Open Vet. J.. 2025; 15(9): 4210-4218. doi:10.5455/OVJ.2025.v15.i9.27 Web Style Esin C, Guzel M. Changes in hepatic and renal Doppler ultrasonography: Current standard therapy in dogs with congestive heart failure. https://www.openveterinaryjournal.com/?mno=263364 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i9.27 AMA (American Medical Association) Style Esin C, Guzel M. Changes in hepatic and renal Doppler ultrasonography: Current standard therapy in dogs with congestive heart failure. Open Vet. J.. 2025; 15(9): 4210-4218. doi:10.5455/OVJ.2025.v15.i9.27 Vancouver/ICMJE Style Esin C, Guzel M. Changes in hepatic and renal Doppler ultrasonography: Current standard therapy in dogs with congestive heart failure. Open Vet. J.. (2025), [cited January 25, 2026]; 15(9): 4210-4218. doi:10.5455/OVJ.2025.v15.i9.27 Harvard Style Esin, C. & Guzel, . M. (2025) Changes in hepatic and renal Doppler ultrasonography: Current standard therapy in dogs with congestive heart failure. Open Vet. J., 15 (9), 4210-4218. doi:10.5455/OVJ.2025.v15.i9.27 Turabian Style Esin, Cağatay, and Murat Guzel. 2025. Changes in hepatic and renal Doppler ultrasonography: Current standard therapy in dogs with congestive heart failure. Open Veterinary Journal, 15 (9), 4210-4218. doi:10.5455/OVJ.2025.v15.i9.27 Chicago Style Esin, Cağatay, and Murat Guzel. "Changes in hepatic and renal Doppler ultrasonography: Current standard therapy in dogs with congestive heart failure." Open Veterinary Journal 15 (2025), 4210-4218. doi:10.5455/OVJ.2025.v15.i9.27 MLA (The Modern Language Association) Style Esin, Cağatay, and Murat Guzel. "Changes in hepatic and renal Doppler ultrasonography: Current standard therapy in dogs with congestive heart failure." Open Veterinary Journal 15.9 (2025), 4210-4218. Print. doi:10.5455/OVJ.2025.v15.i9.27 APA (American Psychological Association) Style Esin, C. & Guzel, . M. (2025) Changes in hepatic and renal Doppler ultrasonography: Current standard therapy in dogs with congestive heart failure. Open Veterinary Journal, 15 (9), 4210-4218. doi:10.5455/OVJ.2025.v15.i9.27 |