| Research Article | ||
Open Vet. J.. 2025; 15(8): 3624-3630 Open Veterinary Journal, (2025), Vol. 15(8): 3624-3630 Research Article Experimental model of menopausal genitourinary syndrome: Ovariectomy and vaginectomy protocols in ratsAsih Anggraeni1,2*, Soetrisno Soetrisno1,2, Ida Nurwati1,3, Uki Retno Budihastuti1,2, Eti Poncorini Pamungkasari1,4, Brian Wasita1,5 and Paramasari Dirgahayu1,611Doctoral Program of Medical Sciences, Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia 2Department of Obstetric and Gynecology, Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia 3Department of Biochemistry, Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia 4Department of Public Health, Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia 5Department of Anatomical Pathology, Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia 6Department of Parasitology and Micology, Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia *Corresponding Author: Asih Anggraeni. Doctoral Program of Medical Sciences, Department of Obstetric and Gynecology, Faculty of Medicine, Universitas Sebelas Maret, Surakarta, Indonesia. Email: asihanggraeni [at] staff.uns.ac.id Submitted: 02/06/2025 Revised: 22/07/2025 Accepted: 27/07/2025 Published: 31/08/2025 © 2025 Open Veterinary Journal
ABSTRACTBackground: The synthesis of estrogen hormones decreases during menopause, leading to various physiological changes that might affect a woman’s quality of life. Genitourinary syndrome of menopause (GSM), which occurs because the vaginal and urethral epithelial layers contain a high density of estrogen receptors, is one of the most common clinical manifestations. These problems can be chronic and progressive, consequently affecting menopausal women’s sexual and quality of life. Therefore, innovation in novel therapeutic modalities or improvements to current therapies is necessary. Aim: This study aimed to provide a comprehensive and precise protocol guide for ovariectomy and vaginectomy to establish a suitable animal model for GSM cases. Method: Forty Wistar rats of Rattus norvegicus were used in the study and treated according to ethical principles. Results: The study recommends the double dorsolateral incision technique as the most effective method for ovariectomy. Vaginectomy is essential for the objective evaluation of the vaginal and urethral epithelium in GSM and for the histological assessment of therapeutic response. Conclusion: The therapeutic modalities that result from a suitable animal model protocol are expected to guarantee safety and efficacy. Keywords: Genitourinary, Menopause, Ovariectomy, Rats, Vaginectomy. IntroductionMenopause is a transitional phase between the reproductive and non-reproductive periods in women, usually occurring between the ages of 40 and 50, defined by 12 months of amenorrhea and LH levels >40 IU/l measured at least 4 weeks apart (Thangavel et al., 2019; Meeta et al., 2020; Khapre et al., 2022). Menopause decreases the levels of estrogen and other sex steroid hormones, triggering physiological changes that can affect a woman’s quality of life. This condition also negatively affects the clitoris, vaginal vestibule, labia majora and minora, urethra, and bladder. The vagina contains numerous estrogen receptors, particularly in the mucosal epithelium, so a decrease in estrogen hormone production causes the vaginal epithelium to be thinner due to a reduction in the number of epithelial cell layers and the degeneration of collagen and elastin fibers in the underlying connective tissue, causing the vagina to be less elastic and more fragile. This leads to clinical symptoms, including vaginal burning, dyspareunia, and post-coital bleeding. In addition to the vagina, the urethral epithelium contains various estrogen receptors; therefore, menopause commonly produces urogenital symptoms such as dysuria, urgency, and incontinence due to decreased collagen, urethra wrinkles, and sensitivity of the smooth muscle (Sarmento et al., 2021). The clinical condition described above is termed genitourinary syndrome of menopause (GSM), which can be chronic and progressive (Vodegel et al., 2023). The incidence of GSM was approximately 5 million in the 20th century, and its prevalence has risen to 13.5 million, constituting nearly one-third of the global female population (Ismail and Bibi, 2024). Consequently, constantly innovating the current therapy strategies is important. Evidence of safety and efficacy from preclinical trials utilizing suitable animal model protocols is necessary before the approval of the new therapeutic modality for human application.
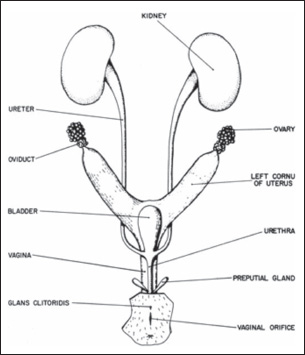
Fig. 1. Female rat urogenital system anatomy (Suckow et al., 2019). Rabbits, rats, mice, guinea pigs, dogs, fish, and non-human primates are all acceptable for use in preclinical studies. Nevertheless, rats (Rattus norvegicus) and mice (Mus musculus) are the most frequently utilized species due to their numerous morphological, physiological, genetic, and biochemical similarities to humans. Furthermore, these mammals are small, inexpensive to house, easy to obtain, capable of rapid growth, and can be modified at a minimal cost (Nascimento-Gonçalves et al., 2024). Rats undergo natural menopause between 18 and 24 months of age; therefore, ovariectomy is frequently used to induce menopause in experimental animals due to its advantages, including ease of application, reduced cost, and rapid onset of hypoestrogenism. Moreover, ovariectomy-induced menopause shows identical key characteristics to human menopause, including a similar hormonal profile, hypothalamic-pituitary-gonadal (HPG) axis dysregulation, and cognitive and cardiometabolic changes similar to those observed in menopausal women. The predominant rat strains utilized are Sprague-Dawley and Wistar rats, which show comparable responses to ovariectomy (Medina-Contreras et al., 2020; Yousefzadeh et al., 2020). Furthermore, vaginal and urethral tissue samples must be objectively analyzed for the histopathological alterations and therapeutic response in rats with GSM (Ho et al., 2009). This study aimed to provide comprehensive and precise guidance on how to perform ovariectomy and vaginectomy in rats to obtain ovarian, vaginal, and urethral tissue simultaneously while developing suitable animal model protocols. Materials and MethodsThis study used 40 female Wistar rats of R. norvegicus, each over 200 g, ethically sourced. An anesthetic protocol was administered using ketamine-xylazine at a dose of 40 mg/kg body weight by intraperitoneal injection before the ovariectomy. Subsequently, euthanasia was conducted at the conclusion of the protocol prior to the collection of vaginal and urethral tissue from the rats, utilizing ketamine-xylazine at five times the anesthetic dose (Soewondo et al., 2023). Ethical approvalThe research protocol was approved by the research ethics committee of the Faculty of Medicine, Sebelas Maret University, under the number 207/UN27.06.11/KEP/EC/2024. ResultsUrogenital system anatomy in female ratsIn female rats, the ovaries are located within the adipose tissue at the posterolateral pole of the kidneys and connected by the mesovarium to the abdominal cavity’s posterior wall. In mature ovaries, they form as a mass of follicles. The open end of the oviduct attaches to the ovary, while the distal end connects to the uterus. Despite the visible fusion of the uterine horns at the distal end, two different uterine ossa exist that lead into the vagina. Each uterus has an internum and an eksternum ostium and a cervical canal. The bladder empties into the urethra, which opens into the perineum at the base of the clitoris through the urethral orifice. The vaginal orifice is on the dorsal aspect of the urethra (Suckow et al., 2019; Herrera-Pérez et al., 2024). Ovariectomy techniquesThere are three ovariectomies techniques (Rejeki et al., 2018; Yousefzadeh et al., 2020; Herrera-Pérez et al., 2024):
A. Pre-operative Stage
B. Double dorsolateral incision ovariectomy
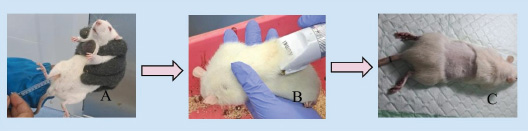
Fig. 2. Pre-operative stage (A) intraperitoneal anesthesia was administered to rats; (B) shaving the fur of the rat; (C) rats disinfected and ready for surgery.
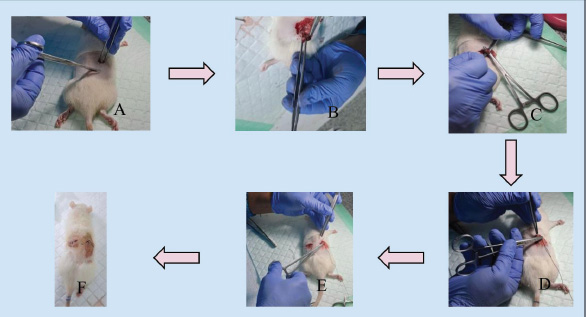
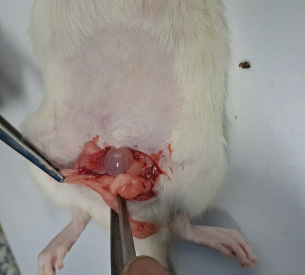
Fig. 3. Double dorsolateral incision ovariectomy (A) incision on the right dorsolateral; (B) visualization of the fat pad and ovary; (C) ligation and ovary removal; (D) suturing the muscle and skin of the rat; (E) the same procedure was performed on the contralateral ovary; (F) ovariectomy completed. C. Single midline dorsal incision ovariectomy
Fig. 4. Single midline dorsal incision ovariectomy (A) incision along the midline in the mid-dorsum area; (B) visualizing and removing the fat pad and ovaries using the previous method without creating additional incisions. D. Ventral or transverse incision ovariectomy
Fig. 5. Transverse incision slightly to the right in the central abdomen, followed by alternating ovaries removal using the same incision site as before.
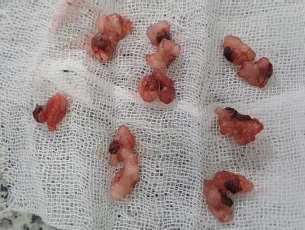
Fig. 6. Rat’s ovary. E. Post-operative Stage
Technique for collecting vaginal and urethral tissue (Ho et al., 2009; Koebele et al., 2023; Soewondo et al., 2023)
DiscussionDuring menopause, estrogen hormone synthesis decreases, leading to various physiological changes that might affect a woman’s quality of life. GSM is one of the most common clinical manifestations. The vaginal and urethral epithelial layers contain numerous estrogen receptors; therefore, a decrease in estrogen hormones results in a variety of symptoms of the genitourinary system. These problems may be chronic and progressive, consequently affecting menopausal women’s sexual and quality of life (Sarmento et al., 2021; Vodegel et al., 2023).
Fig. 7. Post-operative stage.
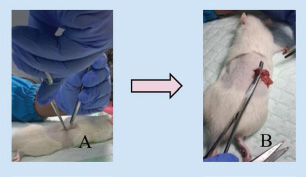
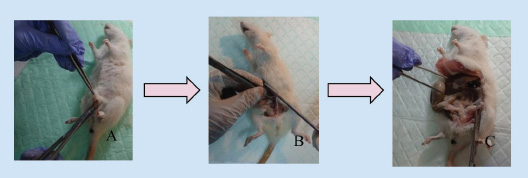
Fig. 8. Vaginectomy in rats (A) rat after anesthesia and disinfection; (B) incision on the midline of the ventral abdomen; (C) separation of the genitourinary tissue from the abdomen. Natural menopause (estropause), ovariectomy, and ovotoxins, which can accelerate ovarian failure, are all approaches for inducing menopause in rats. Estropause in rats shares similarities to human menopause, despite only 25% of estrouse rats demonstrating the same hormonal profile, and this procedure takes a long time to apply. Rats induced into menopause with the ovotoxin 4-vinylcyclohexene diepoxide (VCD) to accelerate ovarian failure have ovarian and hormonal profiles similar to most menopausal women. However, this method has limitations because VCD is toxic at high doses and requires 15 intraperitoneal injections, increasing concerns about stress on the animals. Consequently, ovariectomy is frequently considered the gold standard procedure due to its numerous advantages over the other two approaches (Medina-Contreras et al., 2020). Ovariectomy has numerous advantages, including ease of application, reduced cost, and a quick onset of hypoestrogenism. Furthermore, ovariectomy-induced menopause shows comparable main characteristics to human menopause, including a similar hormonal profile, HPG axis dysregulation, and cognitive and cardiometabolic alterations identical to those observed in menopausal women. Three ovariectomy techniques in rats include the double dorsolateral incision, single midline dorsal incision, and ventral or transverse incision. In our study, ventral incision ovariectomy had a shorter surgical time (<10 minutes), but wound healing took longer (10–15 days), due to the rat’s anatomical position, which causes the incision wound on the abdomen to be pressed and constantly in contact with the bedding, making it more vulnerable to breaking and infection (Rejeki et al., 2018; Yousefzadeh et al., 2020; Herrera-Pérez et al., 2024). This contradicts other studies that have reported that ventral incision ovariectomy wounds heal faster, in less than 9 days, and is a painless surgical technique. This technique also involves manipulation of the abdominal organs and is associated with a greater mortality rate of 30% within the first 24 hour, making it less recommended (Khajuria et al., 2012; Yousefzadeh et al., 2020; Herrera-Pérez et al., 2024). The single midline dorsal incision technique requires a larger incision to access the right and left ovary from the dorsal midline, resulting in a longer surgery and wound healing time. In this study, the surgery time for the single midline dorsal incision technique was more than 15 minutes, with a wound healing time of 10–15 days. The double dorsolateral incision has the advantages of accelerated wound healing, a shorter recovery time, and a lower mortality rate with a smaller incision wound. This ovariectomy technique has a surgical time of less than 10 minutes, with a wound healing time of less than 10 days. This incision technique is the most recommended (Yousefzadeh et al., 2020).
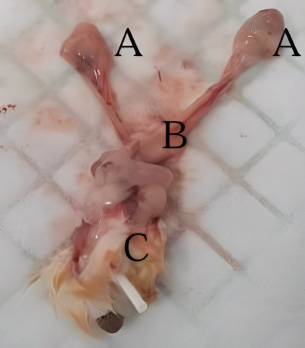
Fig. 9. (A) Oviduct; (B) uterus; (C) vagina (dorsal) and urethra (ventral). Subsequently, to find objective evidence of GSM, vaginal and urethral tissues were collected through vaginectomy when the rats reached menopause after day 21 post-ovariectomy, and histological analysis was performed. At the conclusion of the study, tissue collection through vaginectomy is essential to evaluate the response of vaginal and urethral tissues to a treatment intervention (Rejeki et al., 2018; Ye et al., 2018). ConclusionGSM is a condition that significantly impacts the quality of life of menopausal women. Consequently, the development of novel therapeutic modalities or the improvement of current therapies remains essential. Therefore, comprehensive and precise protocols for the development of suitable animal models are required to guarantee the safety and efficacy of the therapeutic modalities. AcknowledgmentsThe authors would like to express their gratitude to the Animal Testing Laboratory at the Faculty of Medicine, Sebelas Maret University, for providing research facilities and animal care services. The authors would also like to thank Hanifah Karim, MD, for her technical support as a research assistant and contributions to this manuscript’s editing. Conflict of interestThe authors declare no conflict of interest. FundingThis study received no funding or external assistance. Authors’ contributionsConception and design: A.A; administrative support: A.A and S; collection and assembly of data: A.A, S, I.N, and U.R.B; data analysis and interpretation: A.A, P.D, E.P.P, and B.W; manuscript writing: all authors; and final approval of the manuscript: all authors. ReferencesHerrera-Pérez, J.J., Hernández-Hernández, O.T., Flores-Ramos, M., Cueto-Escobedo, J., Rodríguez-Landa, J.F. and Martínez-Mota, L. 2024. The intersection between menopause and depression: overview of research using animal models. Front. Psychiatry 15, 1408878; doi:10.3389/fpsyt.2024.1408878 Ho, M.H., Heydarkhan, S., Vernet, D., Kovanecz, I., Ferrini, M.G., Bhatia, N.N. and Gonzalez-Cadavid, N.F. 2009. Stimulating vaginal repair in rats through skeletal muscle–derived stem cells seeded on small intestinal submucosal scaffolds. Obstet. Gynecol. 114(2), 300–309; doi:10.1097/aog.0b013e3181af6abd Ismail, A. and Bibi, I. 2024. Exploring genitourinary syndrome of menopause: analysis of prevalence, determinants, and health impacts in Pakistani women. Pak. Biomed. J. 7, 16–20; doi:10.54393/pbmj.v7i02.1035 Khajuria, D.K., Razdan, R. and Mahapatra, D.R. 2012. Description of a new method of ovariectomy in female rats. Rev. Bras. Reumatol. 52(3), 462–470. Khapre, S., Deshmukh, U. and Jain, S. 2022. The impact of soy isoflavone supplementation on the menopausal symptoms in perimenopausal and postmenopausal women. J. Midlife Health 13(2), 175–184; doi:10.4103/jmh.jmh_190_21 Koebele, S.V., Bernaud, V.E., Northup-Smith, S.N., Willeman, M.N., Strouse, I.M., Bulen, H.L., Schrier, A.R., Newbern, J.M., DeNardo, D.F., Mayer, L.P., Dyer, C.A. and Bimonte-Nelson, H.A. 2023. Gynecological surgery in adulthood imparts cognitive and brain changes in rats: a focus on hysterectomy at short-, moderate-, and long-term intervals after surgery. Horm. Behav. 155, 105411; doi:10.1016/j.yhbeh.2023.105411 Medina-Contreras, J., Villalobos-Molina, R., Zarain-Herzberg, A. and Balderas-Villalobos, J. 2020. Ovariectomized rodents as a menopausal metabolic syndrome model. A minireview. Mol. Cell Biochem. 475(1–2), 261–276; doi:10.1007/s11010-020-03879-4 Meeta, M., Digumarti, L., Agarwal, N., Vaze, N., Shah, R. and Malik, S. 2020. Clinical practice guidelines on menopause: an executive summary and recommendations: Indian menopause society 2019–2020. J. Midlife Health 11(2), 55; doi:10.4103/jmh.jmh_137_20 Nascimento-Gonçalves, E., Faustino-Rocha, A.I., Seixas, F., Colaço, B., Ferreira, R. and Oliveira, P.A. 2024. Successful hormonal and chemical induction of prostate cancer in a rat model: practical guidelines. Vet. Res. Forum 15(9), 445–453; doi:10.30466/vrf.2023.1999343.3838 Rejeki, P.S., Putri, E.A.C. and Prasetya, R.E. 2018. Ovariectomy in rats and mice. Surabaya, Indonesia: Airlangga University Press. Sarmento, A.C.A., Costa, A.P.F., Vieira-Baptista, P., Giraldo, P.C., Eleutério, Jr., J. and Gonçalves, A.K. 2021. Genitourinary syndrome of menopause: epidemiology, physiopathology, clinical manifestation and diagnostic. Front. Reprod. Health 3, 779398; doi:10.3389/frph.2021.779398 Soewondo, S.S., Parawansa, S.S.R. and Amri, U. 2023. The concept of euthanasia in various countries and its reforms in Indonesia. Media Iuris 6(2), 231–254; doi:10.20473/mi.v6i2.43841 Suckow, M.A., Hankenson, F.C., Wilson, R.P. and Foley, P.L. 2019. The laboratory rat. Cambridge, MA: Academic Press. Thangavel, P., Puga-Olguín, A., Rodríguez-Landa, J.F. and Zepeda, R.C. 2019. Genistein as potential therapeutic candidate for menopausal symptoms and other related diseases. Molecules 24(21), 3892; doi:10.3390/molecules24213892 Vodegel, E.V., Guler, Z., Ras, L., Mackova, K., Groeneveld, A.C.H.M., Bezuidenhout, D., Deprest, J., Jeffery, S.T. and Roovers, J.W.R. 2023. Vaginal changes after ovariectomy in ewes: a large animal model for genitourinary syndrome of menopause. Int. J. Gynaecol. Obstet. 162(3), 1042–1049; doi:10.1002/ijgo.14816 Ye, Y., Liu, C.-Z., Wang, R., Zhang, Y.-W., Zhang, B., Cui, Y., Liu, X.-W. and Huang, S.-M. 2018. A new animal model for menopausal transition: combination of ovariectomy and empty bottle stimulation. Gynecol. Endocrinol. 34(10), 840–844; doi:10.1080/09513590.2018.1451835 Yousefzadeh, N., Kashfi, K., Jeddi, S. and Ghasemi, A. 2020. Ovariectomized rat model of osteoporosis: a practical guide. EXCLI J. 19, 89–107; doi:10.17179/excli2019-1990 | ||
| How to Cite this Article |
| Pubmed Style Anggraeni A, Soetrisno S, Nurwati I, Budihastuti UR, Pamungkasari EP, Wasita B, Dirgahayu P. Experimental model of menopausal genitourinary syndrome: Ovariectomy and vaginectomy protocols in rats. Open Vet. J.. 2025; 15(8): 3624-3630. doi:10.5455/OVJ.2025.v15.i8.25 Web Style Anggraeni A, Soetrisno S, Nurwati I, Budihastuti UR, Pamungkasari EP, Wasita B, Dirgahayu P. Experimental model of menopausal genitourinary syndrome: Ovariectomy and vaginectomy protocols in rats. https://www.openveterinaryjournal.com/?mno=262391 [Access: January 26, 2026]. doi:10.5455/OVJ.2025.v15.i8.25 AMA (American Medical Association) Style Anggraeni A, Soetrisno S, Nurwati I, Budihastuti UR, Pamungkasari EP, Wasita B, Dirgahayu P. Experimental model of menopausal genitourinary syndrome: Ovariectomy and vaginectomy protocols in rats. Open Vet. J.. 2025; 15(8): 3624-3630. doi:10.5455/OVJ.2025.v15.i8.25 Vancouver/ICMJE Style Anggraeni A, Soetrisno S, Nurwati I, Budihastuti UR, Pamungkasari EP, Wasita B, Dirgahayu P. Experimental model of menopausal genitourinary syndrome: Ovariectomy and vaginectomy protocols in rats. Open Vet. J.. (2025), [cited January 26, 2026]; 15(8): 3624-3630. doi:10.5455/OVJ.2025.v15.i8.25 Harvard Style Anggraeni, A., Soetrisno, . S., Nurwati, . I., Budihastuti, . U. R., Pamungkasari, . E. P., Wasita, . B. & Dirgahayu, . P. (2025) Experimental model of menopausal genitourinary syndrome: Ovariectomy and vaginectomy protocols in rats. Open Vet. J., 15 (8), 3624-3630. doi:10.5455/OVJ.2025.v15.i8.25 Turabian Style Anggraeni, Asih, Soetrisno Soetrisno, Ida Nurwati, Uki Retno Budihastuti, Eti Poncorini Pamungkasari, Brian Wasita, and Paramasari Dirgahayu. 2025. Experimental model of menopausal genitourinary syndrome: Ovariectomy and vaginectomy protocols in rats. Open Veterinary Journal, 15 (8), 3624-3630. doi:10.5455/OVJ.2025.v15.i8.25 Chicago Style Anggraeni, Asih, Soetrisno Soetrisno, Ida Nurwati, Uki Retno Budihastuti, Eti Poncorini Pamungkasari, Brian Wasita, and Paramasari Dirgahayu. "Experimental model of menopausal genitourinary syndrome: Ovariectomy and vaginectomy protocols in rats." Open Veterinary Journal 15 (2025), 3624-3630. doi:10.5455/OVJ.2025.v15.i8.25 MLA (The Modern Language Association) Style Anggraeni, Asih, Soetrisno Soetrisno, Ida Nurwati, Uki Retno Budihastuti, Eti Poncorini Pamungkasari, Brian Wasita, and Paramasari Dirgahayu. "Experimental model of menopausal genitourinary syndrome: Ovariectomy and vaginectomy protocols in rats." Open Veterinary Journal 15.8 (2025), 3624-3630. Print. doi:10.5455/OVJ.2025.v15.i8.25 APA (American Psychological Association) Style Anggraeni, A., Soetrisno, . S., Nurwati, . I., Budihastuti, . U. R., Pamungkasari, . E. P., Wasita, . B. & Dirgahayu, . P. (2025) Experimental model of menopausal genitourinary syndrome: Ovariectomy and vaginectomy protocols in rats. Open Veterinary Journal, 15 (8), 3624-3630. doi:10.5455/OVJ.2025.v15.i8.25 |