| Research Article | ||
Open Vet. J.. 2025; 15(9): 4199-4203
Open Veterinary Journal, (2025), Vol. 15(9): 4199-4203 Research Article Impact of varying L-carnitine doses on the motility and activation parameters of bull sperm in vitro during low-motile sperm cryopreservationAhmed Saed Al-Ebady*Department of Surgery and Obstetrics, College of Veterinary Medicine, University of Al-Qadisiyah, Al Diwaniyah, Iraq *Corresponding Author: Ahmed Saed Al-Ebady. Department of Surgery and Obstetric-College of Veterinary Medicine, University of Al-Qadisiyah, Iraq. Email: ahmed.abdulshaheed [at] qu.edu.iq Submitted: 01/06/2025 Revised: 01/08/2025 Accepted: 12/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
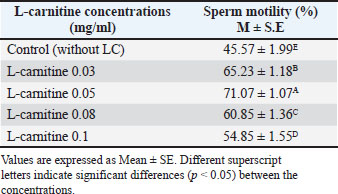
AbstractBackground: L-carnitine is an important amino acid in sperm metabolism and possesses antioxidant properties. It makes energy easy for spermatozoa to access, which helps in sperm movement, and has the effect of decreasing reactive oxygen species (ROS) production, which helps in lessening their harmful effects. Aim: To investigate the optimal dose of L-carnitine to be used in bull semen extenders for mitigating the harmful effects of ROS during semen cryopreservation. Methods: A total of 38 ejaculates (30%–55% motility) were collected and extended using a Tris-yolk-fructose extender. Each sample was divided into five groups: a control and four groups supplemented with L-carnitine (0.03, 0.05, 0.08, and 0.1 mg/ml). Sperm motility, viability, and abnormalities were assessed before and after cryopreservation. Results: Supplementation of the semen extender with L-carnitine for low-motile bull sperm significantly improved sperm motility, with the optimal concentration being 0.05 mg/ml (p < 0.05). Additionally, L-carnitine reduced sperm abnormalities and mortality post-thaw. These findings indicate that L-carnitine can be a valuable semen extender supplement to enhance sperm preservation during cryopreservation. Conclusion: Both before and after cryopreservation, certain sperm functional parameters improved following in vitro bull sperm activation with L-carnitine-enriched media. Keywords: L-carnitine, Poor motile bull sperm, Cryopreservation, Sperm activation. IntroductionL-carnitine, a quaternary ammonium compound, plays two essential physiological roles. First, it facilitates the transport of fatty acids from the cytosol to the mitochondria, where they undergo β-oxidation, leading to energy production (Rebouche and Seim, 1998). Second, it serves as a potent antioxidant (Vaz and Wanders, 2002), reducing the production of reactive oxygen species (ROS) and mitigating their harmful effects (Sarica et al., 2007). The primary function of L-carnitine is to shuttle fatty acids into mitochondria, enabling their breakdown for energy generation (Stradaioli et al., 2004). Sperm utilize fatty acids as a metabolic energy source in the epididymis. Mature sperm convert some L-carnitine into acetyl L-carnitine, which supports sustained energy production. Additionally, the newly formed acetyl L-carnitine provides readily available acetyl groups for metabolic processes in sperm Kerner and Hoppel, 2000). Several studies have shown that the amount of energy produced directly influences sperm motility Kerner and Hoppel, 2000; Agarwal and Said, 2004; Stradaioli et al., 2004). To meet their energy demands, mammalian sperm rely on multiple metabolic pathways, including anaerobic glycolysis, aerobic glycolysis, and β-oxidation of endogenous substrates such as fatty acids (Kerner and Hoppel, 2000). L-carnitine acts as a co-factor in β-oxidation, ensuring efficient energy production in the presence of oxygen (Agarwal and Said, 2004). Furthermore, ROS negatively impacts the lipid, protein, and carbohydrate components of the cell membrane (Agarwal et al., 2006). Excessive ROS levels can impair sperm motility by inhibiting adenosine triphosphate (ATP) synthesis and disrupting flagellar phosphorylation (Perchec et al., 1995; Parikh et al., 2009). As a crucial sperm stimulant, L-carnitine exhibits antioxidant and ROS-scavenging properties, protecting sperm cells from oxidative stress and preserving their motility (Banihani et al., 2014). Materials and MethodsThis study was conducted in the College of Veterinary Medicine, University of Baghdad, during the winter months of November to February. A total of 38 ejaculates with poor motile sperm (mass motility 30%–55% and a concentration of about 5×108 sperm/ml) were collected by artificial vagina and extended (in a ratio of 1:5) with Tris-yolk-fructose extender. Each extended ejaculate was divided into five portions (one used as control, and the others were supplemented with different concentrations of L-carnitine (0.03, 0.05, 0.08, and 0.1 mg/ml). Sperm parameters (motility, dead, and abnormality percentages) were assessed and calculated before and after cryopreservation. Sperm mass motility assessmentEstimated under a light microscope by placing one drop (10 µl) of raw semen on a slide, using a 10× objective lens. Sperm morphology assessmentHematoxylin-eosin staining was used to examine sperm with aberrant morphology. Hematoxylin-eosin stain was used to assess the semen samples after 5 µl was placed on a slide, a thin smear was created, allowed to dry at room temperature, and then fixed. Using a high-resolution (100×) objective lens and oil immersion, 200 sperm were scored in randomly selected fields. Sperm vitality assessmentThe eosin-nigrosin stain was used to distinguish live and dead sperm percentages by calculating 200 white and red sperm in randomly chosen fields under a 40× objective lens. Preparation for in vitro sperm activationFollowing collection, ejaculates were maintained in a water bath at 37°C–38°C. Motility was assessed before semen was extended at a 1:5 dilution ratio with Tris-yolk-fructose-glycerol. The extended semen was divided into five equal sections in test tubes. The control group contained only diluted semen, whereas the other four groups were supplemented with L-carnitine at concentrations of 0.03, 0.05, 0.08, and 0.1 mg/ml. All test tubes containing L-carnitine and diluted semen were kept at room temperature for 15 minutes. Cryopreservation processSemen samples were packed into 0.5 ml straws and pre-cooled for 2 hours in a refrigerator at 5°C. The straws were then exposed to liquid nitrogen vapor for 9 minutes at a fixed height above the liquid phase before being fully immersed in a liquid nitrogen tank at −196°C for long-term storage. Thawing processAfter 48 hours of storage, the straws were thawed for 30 seconds in a water bath at 37°C for 30 seconds. Post-thaw sperm quality assessments, including motility, viability, and morphological integrity, were conducted immediately after thawing. Statistical analysisData were analyzed using the Statistical Analysis System (SAS Institute, 2016). The least significant difference test was used to compare means, and differences were considered statistically significant at p < 0.05. The normality of data distribution and homogeneity of variance were tested before statistical comparisons to ensure validity. Ethical approvalNot needed for this study as samples of semen in this study were collected from the bulls of the artificial insemination center of Abu Ghareeb-ministry of Agriculture, as a routine daily work of the center. ResultsSperm motility before freezingThe effect of L-carnitine on sperm motility is presented in Table 1. Sperm motility increased with L-carnitine concentration, showing significant differences (p < 0.05) compared with the control. The highest motility was observed at 0.05 mg/ml L-carnitine concentration. Table 1. Effect of L-carnitine on sperm motility before freezing (%).
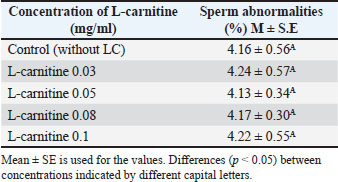
Sperm abnormalities before freezingSperm abnormalities across different L-carnitine concentrations are shown in Table 2: No significant differences (p < 0.05) were observed between the groups. Table 2. Effect of L-carnitine on sperm abnormalities before freezing (%).
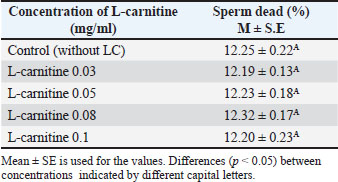
Sperm viability before freezingSperm viability across different L-carnitine concentrations is shown in Table 3. No significant differences (p < 0.05) were observed between the groups. Table 3. Effect of L-carnitine on the percentage of dead sperm before freezing.
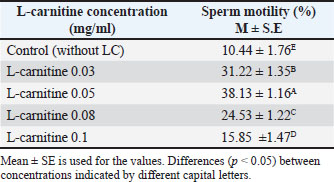
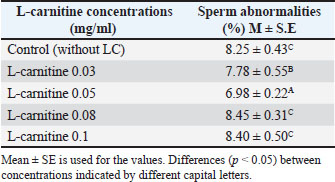
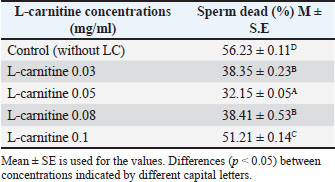
Cryopreservation and thawingFollowing freezing and thawing, sperm motility declined significantly across all treatments. However, the 0.05 mg/ml concentration retained the highest motility (p < 0.05) compared to other concentrations and the control as shown in Table 4, also Table 5 shows that sperm abnormalities were significantly lower (p < 0.05) at 0.05 mg/ml compared to other concentrations and the control, as well as Table 6 indicates that sperm viability was best preserved at 0.05 mg/ml, showing the lowest percentage of dead sperm after freezing and thawing (p < 0.05). Table 4. Effect of L-carnitine on sperm motility (%) after freezing and thawing.
Table 5. Effect of L-carnitine on sperm abnormalities (%) after freezing and thawing.
Table 6. Effect of L-carnitine on the percentage of dead sperm after freezing and thawing.
DiscussionThe results presented in Table 1 indicate that adding L-carnitine to low-motility bull sperm increases their motility in a concentration-dependent manner. This effect is attributed to the role of L-carnitine in facilitating fatty acid transport to the mitochondrial membrane, enhancing mitochondrial activity, and producing ATP through β-oxidation. Increased ATP production subsequently improves sperm motility, as ATP is the primary energy source for sperm movement (Agarwal and Said, 2004; Fattah et al., 2017a). Additionally, previous studies have demonstrated that supplementing liquid-stored diluents with L-carnitine can double ATP production compared with controls (Longobardi et al., 2017). However, the findings also show that L-carnitine reduces sperm motility at higher concentrations. This may be due to its strong osmotic properties, which could disrupt the sperm plasma membrane stability, ultimately impairing motility (Fattah et al., 2017b). Thus, while L-carnitine offers beneficial effects at optimal concentrations, excessive amounts may negatively impact sperm function. Table 4 demonstrates a significant decline in sperm motility after freezing and thawing in all five diluted portions compared with pre-freezing motility. The plasma membrane of mammalian sperm contains high levels of polyunsaturated fatty acids, making it highly susceptible to oxidative stress. During semen processing, including dilution, cooling, freezing, and thawing, ROS, such as H2O2, are generated. Additionally, during these processes, changes in mitochondrial membrane fluidity can increase mitochondrial membrane potential, leading to elevated ROS production in diluted semen. ROS accumulation results in lipid peroxidation, sperm membrane damage, and DNA fragmentation, significantly reducing sperm motility, viability, and DNA integrity after thawing (Sariozkan et al., 2012; Perumal et al., 2013). Oxidative stress during cryopreservation can occur due to increased ROS production and/or decreased antioxidant defense mechanisms (Bilodeau et al., 2000; Chatterjee and Gagnon, 2001). Therefore, incorporating antioxidants into the dilution medium may help mitigate cryoinjury. L-carnitine is thought to stabilize the mitochondrial membrane and protect DNA from oxidative damage (Qi et al., 2006; Deon et al., 2015). According to Longobardi et al. (2017), L-carnitine supplementation in semen diluents reduces ROS production while enhancing total antioxidant capacity. Lower ROS levels contribute to maintaining sperm membrane integrity and improving sperm viability, supporting the results shown in Tables 5 and 6. However, the impact of ROS on sperm function varies depending on factors such as ROS concentration, type, localization, and exposure duration, which may explain discrepancies in findings across different studies (Agarwal and Saleh, 2002). Further research is needed to optimize L-carnitine concentrations for semen preservation and to explore additional antioxidant strategies that could enhance post-thaw sperm viability and function. ConclusionThere is a favorable impact and improvement in certain sperm functional parameters following in vitro bull sperm activation with L-carnitine-enriched media, especially at 0.05 mg/ml. AcknowledgmentsI would like to express my gratitude to the Department of Surgery and Obstetrics, College of Veterinary Medicine, University of Baghdad, and the staff of the Artificial Insemination Center of Abu-Ghareeb for their facilities and support. Conflict of interestThe author declares no conflict of interest. FundingThis study did not receive any funding support. Authors’ contributionsThis study was designed and written by the author. Data availabilityAll data supporting this study’s findings are available in the manuscript. ReferencesAgarwal, A. and Said, T.M. 2004. Carnitines and male infertility. Reprod. Biomed. Online 8(4), 376–384. Agarwal, A. and Saleh, R.A. 2002. Role of oxidants in male infertility: rationale, significance, and treatment. Urol. Clin. North Am. 29(4), 817–827. Agarwal, A., Said, T.M., Bedaiwy, M.A., Banerjee, J. and Alvarez, J.G. 2006. Oxidative stress in an assisted reproductive techniques setting. Fert. Ster. 86(3), 503–512. Banihani, S., Agarwal, A., Sharma, R. and Bayachou, M. 2014. Cryoprotective effect of L-carnitine on human spermatozoa motility, vitality, and DNA oxidation. Andrology 46(6), 637–641. Bilodeau, J.F., Chatterjee, S., Sirard, M.A. and Gagnon, C. 2000. Levels of antioxidant defenses are decreased in bovine spermatozoa after a cycle of freezing and thawing. Mol. Reprod. Develop. 55(3), 282–288. Chatterjee, S. and Gagnon, C. 2001. Production of reactive oxygen species by spermatozoa undergoing cooling, freezing, and thawing. Mol. Reprod. Develop. 59(4), 451–458. Deon, M., Landgraf, S.S., Lamberty, J.F., Moura, D.J., Saffi, J., Wajner, M. and Vargas, C.R. 2015. Protective effect of L-carnitine on Phenylalanine-induced DNA damage. Metabolic Brain Dis. 30(4), 925–933. Fattah, A., Sharafi, M., Masoudi, R., Shahverdi, A. and Esmaeili, V. 2017a. L-carnitine is a survival factor for chilled storage of rooster semen for a long time. Cryobiology 74, 13–18. Fattah, A., Sharafi, M., Masoudi, R., Shahverdi, A., Esmaeili, V. and Najafi, A. 2017b. L -Carnitine in rooster semen cryopreservation: flow cytometric, biochemical and motion findings for frozen-thawed sperm. Cryobiology 74, 148–153. Kerner, J. and Hoppel, C. 2000. Fatty acid import into mitochondria. Biochim. Biophys. Acta. 1486(1), 1–17. Longobardi, V., Salzano, A., Campanile, G., Marrone, R., Palumbo, F., Vitiello, M., Zullo, G. and Gasparrini, B. 2017. Carnitine supplementation decreases capacitation-like changes of frozen-thawed buffalo spermatozoa. Theriogenology 88, 236–243. Parikh, S., Saneto, R., Falk, M.J., Anselm, I., Cohen, B.H. and Haas, R. 2009. A modern approach to the treatment of mitochondrial disease. Curr. Treat. Opt. Neurol. 11(6), 414–430. Perchec, G., Jeulin, C., Cosson, J., André, F. and Billard, R. 1995. Relationship between sperm ATP content and motility of carp spermatozoa. J. Cell Sci. 108(2), 747–753. Perumal, P., Vupru, K. and Rajkhowa, C. 2013. Effect of addition of taurine on the liquid storage (5°C) of mithun (Bos frontalis) semen. Vet. Med. Inter. 2013, 165348. Qi, S.N., Zhang, Z.F., Wang, Z.Y., Yoshida, A. and Ueda, T. 2006. L-carnitine inhibits apoptotic DNA fragmentation induced by a new spin-labeled derivative of podophyllotoxin via caspase-3 in Raji cells. Oncol. Rep. 15(1), 119–122. Rebouche, C.J. and Seim, H. 1998. Carnitine metabolism and its regulation in microorganisms and mammals. Annu. Rev. Nutr. 18(1), 39–61. Sarica, S., Corduk, M., Suicmez, M., Cedden, F., Yildirim, M. and Kilinc, K. 2007. The effects of dietary l-carnitine supplementation on semen traits, reproductive parameters, and testicular histology of Japanese Quail breeders. J. Appl. Poultry Res. 16(2), 178–186. Sariozkan, S., Bucak, M.N., Canturk, F., Ozdamar, S., Yay, A.P., Tuncer, P.B., Ozcan, S., Sorgucu, N. and Caner, Y. 2012. The effect of different sugars on motility, morphology and DNA damage during the liquid storage of rat epididymal sperm 4°C. Cryobiology 65(2), 93–97. SAS Institute. 2016. Statistical analysis software (SAS) User’s guide version 9.4. Cary, NC: SAS Institute, Stradaioli, G., Sylla, L., Zelli, R., Chiodi, P. and Monaci, M. 2004. Effect of L-carnitine administration on the seminal characteristics of oligoasthenospermic stallions. Theriogenology 62(3-4), 761–777. Vaz, F.M. and Wanders, R.J.A. 2002. Carnitine biosynthesis in mammals. Biochem. J. 361(3), 417–429. | ||
| How to Cite this Article |
| Pubmed Style Ahmed Saed Al-Ebady. Impact of varying L-carnitine doses on the motility and activation parameters of bull sperm in vitro during low-motile sperm cryopreservation. Open Vet. J.. 2025; 15(9): 4199-4203. doi:10.5455/OVJ.2025.v15.i9.25 Web Style Ahmed Saed Al-Ebady. Impact of varying L-carnitine doses on the motility and activation parameters of bull sperm in vitro during low-motile sperm cryopreservation. https://www.openveterinaryjournal.com/?mno=262138 [Access: January 26, 2026]. doi:10.5455/OVJ.2025.v15.i9.25 AMA (American Medical Association) Style Ahmed Saed Al-Ebady. Impact of varying L-carnitine doses on the motility and activation parameters of bull sperm in vitro during low-motile sperm cryopreservation. Open Vet. J.. 2025; 15(9): 4199-4203. doi:10.5455/OVJ.2025.v15.i9.25 Vancouver/ICMJE Style Ahmed Saed Al-Ebady. Impact of varying L-carnitine doses on the motility and activation parameters of bull sperm in vitro during low-motile sperm cryopreservation. Open Vet. J.. (2025), [cited January 26, 2026]; 15(9): 4199-4203. doi:10.5455/OVJ.2025.v15.i9.25 Harvard Style Ahmed Saed Al-Ebady (2025) Impact of varying L-carnitine doses on the motility and activation parameters of bull sperm in vitro during low-motile sperm cryopreservation. Open Vet. J., 15 (9), 4199-4203. doi:10.5455/OVJ.2025.v15.i9.25 Turabian Style Ahmed Saed Al-Ebady. 2025. Impact of varying L-carnitine doses on the motility and activation parameters of bull sperm in vitro during low-motile sperm cryopreservation. Open Veterinary Journal, 15 (9), 4199-4203. doi:10.5455/OVJ.2025.v15.i9.25 Chicago Style Ahmed Saed Al-Ebady. "Impact of varying L-carnitine doses on the motility and activation parameters of bull sperm in vitro during low-motile sperm cryopreservation." Open Veterinary Journal 15 (2025), 4199-4203. doi:10.5455/OVJ.2025.v15.i9.25 MLA (The Modern Language Association) Style Ahmed Saed Al-Ebady. "Impact of varying L-carnitine doses on the motility and activation parameters of bull sperm in vitro during low-motile sperm cryopreservation." Open Veterinary Journal 15.9 (2025), 4199-4203. Print. doi:10.5455/OVJ.2025.v15.i9.25 APA (American Psychological Association) Style Ahmed Saed Al-Ebady (2025) Impact of varying L-carnitine doses on the motility and activation parameters of bull sperm in vitro during low-motile sperm cryopreservation. Open Veterinary Journal, 15 (9), 4199-4203. doi:10.5455/OVJ.2025.v15.i9.25 |