| Research Article | ||
Open Vet. J.. 2025; 15(8): 3638-3644 Open Veterinary Journal, (2025), Vol. 15(8): 3638-3644 Research Article Designated primers targeted canine TP53 gene hotspot regionsFatimah Abdulmuttaleb Ali and Aoula Al-Zebeeby*Department of Pathology and Poultry Diseases, Faculty of Veterinary Medicine, University of Kufa, Al-Najaf Al-Ashraf, Iraq *Corresponding Author: Aoula Al-Zebeeby. Department of Pathology and Poultry Diseases, Faculty of Veterinary Medicine, University of Kufa, Al-Najaf Al-Ashraf, Iraq. Email: aoulae.alzebeeby [at] uokufa.edu.iq Submitted: 30/05/2025 Revised: 30/06/2025 Accepted: 09/07/2025 Published: 31/08/2025 © 2025 Open Veterinary Journal
ABSTRACTBackground: Tumor protein 53 gene (TP53) is a critical factor that controls different cell activities such as cell cycle, DNA repair mechanism, autophagy, apoptosis, and metabolism. The TP53 gene is the most commonly mutated gene, especially in the 4–8 exons region. This mutation enhances the development of many abnormalities, such as the initiation of different types of cancer. Aim: The main objective of this study was to design and evaluate the efficacy of three different primer sets that targeted the TP53 gene at the hotspot regions. Methods: To do that, twelve blood samples were collected from dogs belonging to the German Shepherd breed/K9 aged between 8–12 years. Then, the DNA extraction and polymerase chain reaction (PCR) took place by using the three primer sets, which were designed using SnapGene. The primer sets, namely, first primer, the second and the third targeted exons 5–9 located in the canine TP53 gene. In the following step, all the PCR products were sent for Sanger sequencing and then phylogenetic analysis. Results: Our findings indicated that the first primer set consistently showed higher amplification signal efficiency and reduced dimer formation compared with the second and third primer sets, respectively, with a 60ºC annealing temperature. In addition, all the sequenced samples aligned with the reference canine TP53 gene in the phylogenetic tree. Conclusion: This study offered the best TP53 primer design that targeted the hotspot regions of the canine TP53 gene for researchers who are interested in targeting such regions in this gene. Keywords: Canine, Exons 5-9, PCR, TP53 gene, TP53 hot spot regions. IntroductionTumor protein 53 gene (TP53) is a key factor that regulates different cell activities such as cell cycle, DNA repair mechanism, autophagy, apoptosis, and metabolism. In addition to its role in transcription and regulation, about 200 genes are related to different cell-signalling pathways. The TP53 gene is the most commonly mutated gene (Wang et al., 2023). The development of any abnormalities, such as the carcinogenesis process, is strongly associated with the dysfunction triggered by TP53 mutations (Lacroix et al., 2020). The first identification of p53 as an oncogene was in 1979 in a virally transformed cell due to Simian Vacuolating Virus infection (Lane and Crawford, 1979; Tornesello, 2025). Then, it has been found that p53 excessively segregates in both positive and negative viral-infected tumor cells compared with normal cells (Levine and Oren, 2009; Tornesello, 2025). In humans, the TP53 gene located on chromosome 17 at the short arm has 19,180 bp, while in the case of dogs, according to the National Centre for Biotechnology Information (NCBI) database, the TP53 gene located on chromosome 5 is made up of 17,325 bp (Saif et al., 2016; Foroutan, 2023). This gene consists of 11 exons plus two alternative exons in addition to 10 introns in humans (Foroutan, 2023). While in dogs it also consists of 11 exons, but the first one is a noncoding exon (Cho et al., 2020). In both species, human and dog share 87% DNA sequence similarity at the gene level (Zhang et al., 2009). In the context of protein level, they share 81% identity; in humans p53 is made up of 393 amino acids, and 381 amino acids in dogs (York et al., 2012; Shin, 2023). Generally, the p53 structure is composed of several domains, namely the Tetramerization Domain and the Regulatory Domain (both located at the C-terminus), then the DNA Binding Domain (DBD) (considered the most important domain), followed by Transcriptional-Activation Domains and the Proline Rich Domain (both located at the N-terminus). The most mutated exons are those that translate the DNA binding domain at the protein level. The DBD represents the active site of the p53 (Babamohamadi et al., 2022). Over the years, a rising number of studies that heavily researched the TP53 pathway and its relation with different diseases, especially cancer development in human (Levine and Oren, 2009; Olivier et al., 2010; Monti et al., 2011; Shin, 2023). However, research that is interested in TP53 alterations in dogs and at the molecular level is limited. Therefore, this study presents three different PCR-designated primers that target the same hotspot regions (exons 5–9). These hotspot regions are encoding the DBD domain of dog TP53 to evaluate the efficacy. MethodsSamplesTwelve blood samples were collected from dogs belonging to the German Shepherd breed/K9 at the Department of K9 Unit/Al-Najaf Police Headquarters/Ministry of Internal Affairs, aged 8–12 years, under routine care examination. The samples were placed in an ethylenediaminetetraacetic acid tube and kept at −20ºC for the next step. Primer designThe primer’s design was conducted using SnapGene software as follows: The first primer set targeted the exons 5–9. The forward sequence primer was (5’ CTGCTCTGACAGTAGTGACGGT 3’), while the reverse sequence primer was (5’GGGCAAGAGCCTGCCTTAT3’). The second primer set targeted the exons 5–9; the forward sequence primer was (5’TTCCTCCCCGATGGCTCTTA3’) and the reverse sequence primer was (5’ GCCTTGGTACCTGAAGGGTG3’). The third primer set targeted the exons 5–9; the forward sequence primer was (5’ CGAGGTCTGGTTTGGCATCT3’) and the reverse sequence primer was (5’ TGTTCCTCCCAGCCTTGGTA3’). The accession number of the TP53 reference gene (NC_051809), which was used to design these primers. DNA extraction and PCR examinationThe DNA extraction process was carried out according to the manufacturer’s protocol of the DNA extraction kit (ADDBio, Korea) using 12 whole blood samples. Then the final eluted DNA concentration was measured using a Quantus™ Fluorometer (Promega, USA) and kept at –20ºC. The thermocycler conditions for DNA amplification were set as follows: Initial denaturation at 95ºC/1 cycle for 3 minutes, then the next steps repeated 39 cycles, namely denaturation at 95ºC for 30 seconds, annealing at 60ºC for 35 seconds, extension at 72ºC, and the final extension at 72ºC for 5 minutes/1 cycle. DNA products were then subjected to electrophoresis in a 1.5% agarose gel for 1 hour at 100 volts. Sanger sequencing and the phylogenetic analysisThe eluted PCR outputs were directed into MACROGEN® for Sanger sequencing. The following step, the sequence was scrutinised and trimmed using SnapGene 5.2.4 software. Then, the final sequences were submitted to the NCBI and the BLASTn search against the database. The BLASTn results of all samples were aligned with the reference sequence and used for the construction of the phylogenetic tree by using MEGA X software version 10 (Kumar et al., 2018).
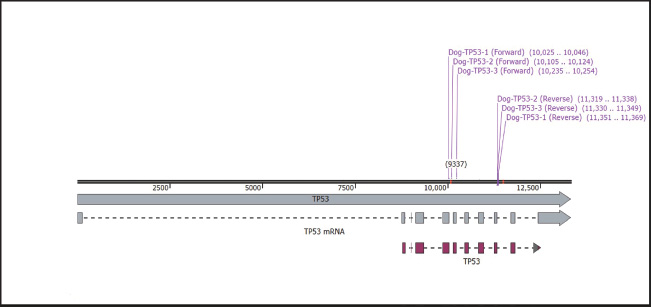
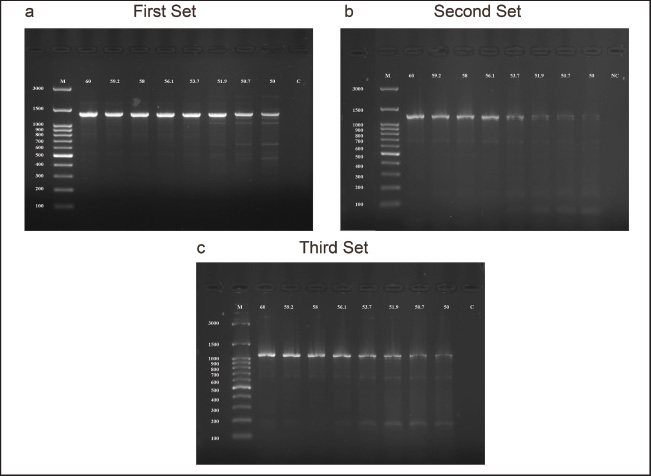
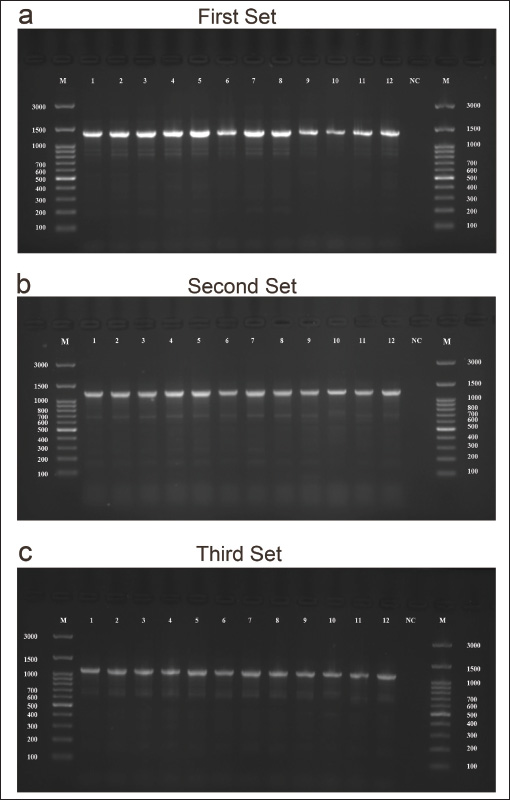
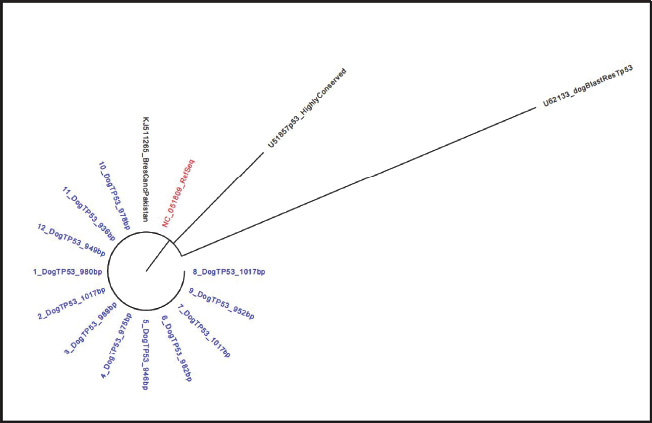
Fig. 1. The location of the designed primers of TP53 in dog. All three primer sets targeted the same region, which is located on chromosome 5 and includes the 4–8 exons for the first primer set exons. While the 5–8 exons for the second and the third primer sets. Ethical approvalAccording to the animal welfare and ethical instructions, this research was officially permitted by the Ethical Committee of Animal Care and Use/University of Kufa, as chronicled through the formal document No. 2111 on the 23rd of January 2025. ResultsGradients protocol for optimal annealing temperatureIn order to investigate the best annealing temperature range between 60ºC–50ºC. The electrophoresis of agarose gel took place, and the serial temperatures were as follows: 60ºC, 59.2ºC, 58ºC, 56.1ºC, 53.7ºC, 50.7ºC, and 50ºC with fixing other reaction conditions for all three designated set primers after checking them in silico (Fig. 1). The results indicated that 60ºC is the optimal annealing temperature for all three primer sets compared with other temperatures (Fig. 2a–c). The superior performance of the first primer set compared with the other two primersIn order to investigate the best primer set amplification, the PCR reaction was carried out for all three primer sets, namely the first, the second and third, which targeted the same region (5–9 exons). The results indicated that the first primer set consistently exhibited higher amplification signal efficiency, reduced dimer formation, and a product size of 1,345 bp compared with the second and third primer sets, where the product sizes were 1,234 and 1,115 bp, respectively (Fig. 3a–c). Taken together, these results indicated that the first primer set was the best primer design for targeting canine TP53. The phylogenetic analysisThe phylogenetic tree of the canine partial TP53 gene indicated that the alignment of the sequence’s samples presented in this study is identical to the reference of the TP53 gene in the database at NCBI (Fig. 4).
Fig. 2. The optimal annealing temperature was 60ºC for all primer genes designated for dog’s TP53 gene. (a) Agarose gel electrophoresis image showed a serial annealing temperatures were ranged as follows: 60ºC, 59.2ºC, 58ºC, 56.1ºC, 53.7ºC, 50.7ºC, and 50ºC for the first set primer with fixed for other reaction conditions. (b) The second primer set with similar condition reactions and ranged annealing temperature 60ºC–50ºC were placed for the first primer set. (c) Third primer set, similar reactions were mentioned in a and b. M was the molecular ladder (3,000–100 bp). (c) The control negative H2O was added, n=12.
Fig. 3. The first primer set was the best primer, targeting the 5–9 exon region of TP53 in dogs. (a), (b), and (c) Agarose gel electrophoresis images of the first primer set (targeted 4–8 exon and the product size was 1,345 bp), second and third primer sets (targeted the 5–8 exons and the products size were 1,234 and 1,115 bp, respectively) set primers showed the amplicons of TP53 in dog. M was the molecular ladder (3,000–100 bp). c the control negative H2O was added, n=12.
Fig. 4. Phylogenetic tree of canine partial TP53 gene sequence. The sequences presented in this study (blue coloured shade) aligned with the reference sequence (red coloured shade). The tree was conducted using MEGA X software with the neighbour-joining method. DiscussionThe TP53 gene is the key regulator for several cellular signalling pathways and has a critical role as a transcriptional factor for many important genes (Wang et al., 2023). This gene is the most frequently mutated gene in various canine tumors, such as mammary adenocarcinoma, osteosarcoma, histiocytic sarcoma, and different types of haematological cancer (Zhang et al., 2009; Alsaihati et al., 2021). Therefore, this gene presented a very attractive target for study and investigation in canine cancers. Given the high percentage of identity between human and canine TP53, which reached 87% at the gene level, this indicates that dogs are a very useful animal model to study this gene and highlight the importance of this gene in the context of human and canine cancer research (Alsaihati et al., 2021). However, the studies regarding the importance of the canine TP53 gene are generally limited. Therefore, the presented study illustrated a comparative investigation of three primer sets that were designed to target exons 5–9 of the canine TP53 gene. Firstly, the designed primers were tested in silico to check the best design that gave a product without any artefact. In addition, checking the annealing temperature, which ranged between 60ºC and 50ºC in the dry lab (Fig. 1). The observation indicated that canine TP53 has GC-rich content, and this determined the annealing temperature (Krypuy et al., 2007; Kehl et al., 2017). Then, the gradient protocol was used to emphasise the best annealing temperature, which was 60ºC (Fig. 2a–c). This was quite important and aligned with different studies that used a similar method to emphasise the temperature in both in silico analysis and in wet lab (Naqib et al., 2019; Chukwuemeka et al., 2020; Kalendar et al., 2024). Some studies revealed using different primers targeted to canine TP53 exons separately with short amplicons (York et al., 2012; Asada et al., 2019). However, in the presented study, the first primer was confirmed to have the best amplification proficiency and specificity across all samples under study, with a product size 1,345 pb. According to the results that indicated optimal design, which minimised the mismatches, in addition to the nonspecific band formation that caused decreased PCR efficiency (Fig. 3a). In the same context, phylogenetic tree analysis of the partial TP53 gene of the sequenced samples confirmed the identity with the reference gene in the data base (Fig. 4). This confirmation is quite important for giving a more meaningful visualisation of the in silico results. Moreover, increase the efficacy of the primer and reduce the possibility of dimer artefact formation, which increases the probability of single -nucleotide polymorphisms (SNPs) detection (Yang et al., 2020). Given the fact that the canine TP53 gene mutations mostly occur at hotspot regions (Babamohamadi et al., 2022). Therefore, the precise amplification of these exons (5–9) was critical for the accurate detection of possible mutations. It has been reported that two base insertions in exon 5 in the case of canine histiocytic sarcoma (Asada et al., 2019). Furthermore, other studies revealed that canine TP53 mutations targeted hotspot regions in different types of canine cancer, such as osteosarcoma, mammary adenocarcinoma, intestinal adenocarcinoma, and haematological cancer (Kirpensteijn et al., 2008; Souza et al., 2012; Alsaihati et al., 2021; Das et al., 2021). Therefore, this study offered the best TP53 primer design (first primer set) that targeted the hotspot regions of the canine TP53 gene for another researcher who is interested in these regions. AcknowledgmentsWe would like to acknowledge Asst. Prof. Dr. Ali Hadi Abbas Department of Microbiology and Faculty of Veterinary Medicine, University of Kufa, for his help in primer design. Conflicts of interestThe authors state no conflicts of interest about this publication. FundingThis research received no specific funding. Author’s contributionF.A.A. and A.A. designed the study and performed the experiment. A.A. analysed the data, wrote the manuscript, and prepared the figures. ReferencesAlsaihati, B.A., Ho, K.L., Watson, J., Feng, Y., Wang, T., Dobbin, K.K. and Zhao, S. 2021. Canine tumor mutational burden is correlated with TP53 mutation across tumor types and breeds. Nat. Commun. 12(1), 4670. doi:10.1038/s41467-021-24836-9. Asada, H., Ichii, O., Tomiyasu, H., Uchida, K., Chambers, J.K., Goto-Koshino, Y., Ohno, K., Kon, Y. and Tsujimoto, H. 2019. The intratumor heterogeneity of TP53 gene mutations in canine histiocytic sarcoma. J. Vet. Med. Sci. 81(3), 353–356. doi:10.1292/jvms.18-0419. Babamohamadi, M., Babaei, E., Ahmed Salih, B., Babamohammadi, M., Jalal Azeez, H. and Othman, G. 2022. Recent findings on the role of wild-type and mutant p53 in cancer development and therapy. Front. Mol. Bio. 9, 1–11. doi:10.3389/fmolb.2022.903075. Cho, S.H., Seung, B.J., Kim, S.H., Lim, H.Y. and Sur, J.H. 2020. Over expression and mutation of p53 exons 4–8 in canine Intestinal adenocarcinoma. J. Com. Patho. 175, 79–84. doi:10.1016/j.jcpa.2019.12.008. Chukwuemeka, P.O., Umar, H.I., Olukunle, O.F., Oretade, O.M., Olowosoke, C.B., Akinsola, E.O., Elabiyi, M.O., Kurmi, U.G., Eigbe, J.O., Oyelere, B.R. and Isunu, L.E. 2020. In silico design and validation of a highly degenerate primer pair: a systematic approach. J. Gene Eng. Biotech. Acad. Sci. Res. Tech. 18(1), 72. doi:10.1186/s43141-020-00086-y. Das, S., Idate, R., Regan, D.P., Fowles, J.S., Lana, S.E., Thamm, D.H., Gustafson, D.L. and Duval, D.L. 2021. Immune pathways and TP53 missense mutations are associated with longer survival in canine osteosarcoma. Commun. Bio. 4(1), 1–15. doi:10.1038/s42003-021-02683-0. Foroutan, B. 2023. A narrative review of the TP53 and its product the p53 protein. OBM Gene 7(3), 1–53. doi:10.21926/obm.genet.2302185. Kalendar, R., Shevtsov, A., Otarbay, Z. and Ismailova, A. 2024. In silico PCR analysis: a comprehensive bioinformatics tool for enhancing nucleic acid amplification assays. Front. Bioinform. 4,1–12. doi:10.3389/fbinf.2024.1464197. Kehl, A., Aupperle-Lellbach, H., de Brot, S. and van der Weyden, L. 2017. Review of molecular technologies for investigating canine cancer. Canine Can. 14(5), 769. doi:10.59317/9789389130539. Kirpensteijn, J., Kik, M., Teske, E. and Rutteman, G.R. 2008. TP53 gene mutations in canine osteosarcoma. Vet. Surg. 37(5), 454–460. doi:10.1111/j.1532-950X.2008.00407.x. Krypuy, M., Ahmed, A.A., Etemadmoghadam, D., Hyland, S.J. 2007. High resolution melting for mutation scanning of TP53 exons 5-8. BMC Cancer 7, 1–13. doi:10.1186/1471-2407-7-168. Kumar, S., Stecher, G., Li, M., Knyaz, C. and Tamura, K. 2018. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol. Bio. Evol. 35(6), 1547–1549. doi:10.1093/molbev/msy096. Lacroix, M., Riscal, R., Arena, G., Linares, L.K. and Le Cam, L. 2020. Metabolic functions of the tumor suppressor p53: implications in normal physiology, metabolic disorders, and cancer. Mol. Metabo. 33, 2–22. doi:10.1016/j.molmet.2019.10.002. Lane, D.P. and Crawford, L.V. 1979. T antigen is bound to a host protein in SY40-transformed cells. Nature 278(5701), 261–263. doi:10.1038/278261a0. Levine, A.J. and Oren, M. 2009. The first 30 years of p53: growing ever more complex. Nature Rev. Can. 9(10), 749–758. doi:10.1038/nrc2723. Monti, P., Perfumo, C., Bisio, A., Ciribilli, Y., Menichini, P., Russo, D., Umbach, D.M., Resnick, M.A., Inga, A. and Fronza, G. 2011. Dominant-negative features of mutant TP53 in germline carriers have limited impact on cancer outcomes. Mol. Can. Res. 9(3), 271–279. doi:10.1158/1541-7786.MCR-10-0496. Naqib, A., Jeon, T., Kunstman, K., Wang, W., Shen, Y., Sweeney, D., Hyde, M. and Green, S.J. 2019. PCR effects of melting temperature adjustment of individual primers in degenerate primer pools. PeerJ. 2019(3), e6570. doi:10.7717/peerj.6570. Olivier, M., Hollstein, M. and Hainaut, P. 2010. TP53 mutations in human cancers: origins, consequences, and clinical use. Cold Spring Harb. Perspec. Bio. 2(1), 1–18. doi:10.1101/cshperspect.a001008. Saif, R., Khan, E., Azhar, A., Choudhary, S., Hussain, T., Babar, M.E., Awan, A.R., Tayyab, M., Zia, S. and Wasim, M. 2016. Insight of Tp53 Mutations and their effect on protein in different Feline and Canine Neoplasms. Adv. Life Sci. 3(2), 42–50. Shin, D.Y. 2023. TP53 mutation in acute myeloid leukemia: an old foe revisited. Cancers 15(19), 1–16. doi:10.3390/cancers15194816. Souza, D.M.B., Barros, M.G.O., Silva, J.S.C., Coleto, Z.F., Jimenez, G.C., Adrião, M. and Wischral, A. 2012. Detection of mutations within exons 4 to 8 of the p53 tumor suppressor gene in canine mammary glands. Arq. Bras. Med. Vet. Zootec. 64(2), 341–348. Tornesello, M.L. 2025. TP53 mutations in cancer: molecular features and therapeutic opportunities (review). Intern. J. Mol. Med. 55(1), 7. doi:10.3892/ijmm.2024.5448. Wang, H., Guo, M., Wei, H. and Chen, Y. 2023. Targeting p53 pathways: mechanisms, structures, and advances in therapy. Signal Transd. Target Therapy 8(1), 1–35. doi:10.1038/s41392-023-01347-1. Yang, Z., Le, J.T., Hutter, D., Bradley, K.M., Overton, B.R., McLendon, C. and Benner, S.A. 2020. Eliminating primer dimers and improving SNP detection using self-avoiding molecular recognition systems. Bio. Methods Prot. 5(1), 1–13. doi:10.1093/biomethods/bpaa004. York, D., Higgins, R.J., LeCouteur, R.A., Wolfe, A.N., Grahn, R., Olby, N., Campbell, M. and Dickinson, P.J. 2012. TP53 mutations in canine brain tumors. Vet. Path. 49(5), 796–801. doi:10.1177/0300985811424734. Zhang, J., Chen, X., Kent, M.S., Rodriguez, C.O. and Chen, X. 2009. Establishment of a dog model for the p53 family pathway and identification of a novel isoform of p21 cyclin-dependent kinase inhibitor. Mol. Cancer Res. 7(1), 67–78. doi:10.1158/1541-7786.MCR-08-0347. | ||
| How to Cite this Article |
| Pubmed Style Ali FA, Al-zebeeby A. Designated primers targeted canine TP53 gene hotspot regions. Open Vet. J.. 2025; 15(8): 3638-3644. doi:10.5455/OVJ.2025.v15.i8.27 Web Style Ali FA, Al-zebeeby A. Designated primers targeted canine TP53 gene hotspot regions. https://www.openveterinaryjournal.com/?mno=261740 [Access: January 24, 2026]. doi:10.5455/OVJ.2025.v15.i8.27 AMA (American Medical Association) Style Ali FA, Al-zebeeby A. Designated primers targeted canine TP53 gene hotspot regions. Open Vet. J.. 2025; 15(8): 3638-3644. doi:10.5455/OVJ.2025.v15.i8.27 Vancouver/ICMJE Style Ali FA, Al-zebeeby A. Designated primers targeted canine TP53 gene hotspot regions. Open Vet. J.. (2025), [cited January 24, 2026]; 15(8): 3638-3644. doi:10.5455/OVJ.2025.v15.i8.27 Harvard Style Ali, F. A. & Al-zebeeby, . A. (2025) Designated primers targeted canine TP53 gene hotspot regions. Open Vet. J., 15 (8), 3638-3644. doi:10.5455/OVJ.2025.v15.i8.27 Turabian Style Ali, Fatimah Abdulmuttaleb, and Aoula Al-zebeeby. 2025. Designated primers targeted canine TP53 gene hotspot regions. Open Veterinary Journal, 15 (8), 3638-3644. doi:10.5455/OVJ.2025.v15.i8.27 Chicago Style Ali, Fatimah Abdulmuttaleb, and Aoula Al-zebeeby. "Designated primers targeted canine TP53 gene hotspot regions." Open Veterinary Journal 15 (2025), 3638-3644. doi:10.5455/OVJ.2025.v15.i8.27 MLA (The Modern Language Association) Style Ali, Fatimah Abdulmuttaleb, and Aoula Al-zebeeby. "Designated primers targeted canine TP53 gene hotspot regions." Open Veterinary Journal 15.8 (2025), 3638-3644. Print. doi:10.5455/OVJ.2025.v15.i8.27 APA (American Psychological Association) Style Ali, F. A. & Al-zebeeby, . A. (2025) Designated primers targeted canine TP53 gene hotspot regions. Open Veterinary Journal, 15 (8), 3638-3644. doi:10.5455/OVJ.2025.v15.i8.27 |