| Research Article | ||
Open Vet. J.. 2025; 15(8): 3468-3476 Open Veterinary Journal, (2025), Vol. 15(8): 3468-3476 Research Article Characterization of the immune response to recombinant GRA-4 protein (rGRA-4) of Toxoplasma gondii in Balb/c strain miceMuhammad Hanafiah1*, Nurliana2, Muhammad Bahi3, Fihiruddin4 and Febi Silvia Alfina51Parasitology Laboratory, Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia 2Laboratory of Veterinary public health, Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia 3Chemistry Department, Faculty of Mathematics and Natural Sciences, Universitas Syiah Kuala, Banda Aceh, Indonesia 4Politeknik Kesehatan, Mataram, Indonesia 5Work System Design and Ergonomics Laboratory, Faculty of Engineering, Industrial Engineering Study Program, Syiah Kuala University, Banda Aceh, Indonesia *Corresponding Author: Muhammad Hanafiah. Department of Parasitology, Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia. Email: hanafi_2015 [at] usk.ac.id Submitted: 29/05/2025 Revised: 20/07/2025 Accepted: 28/07/2025 Published: 31/08/2025 © 2025 Open Veterinary Journal
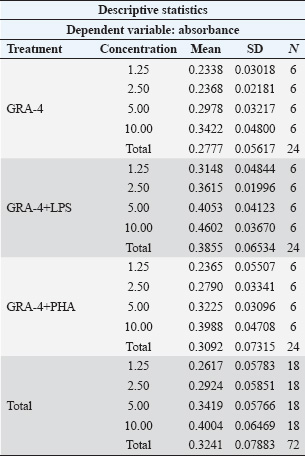
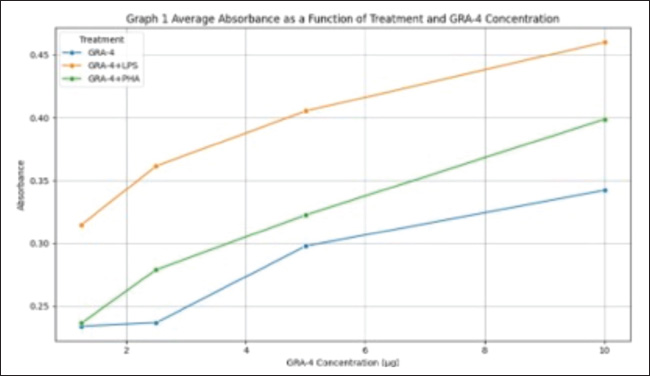
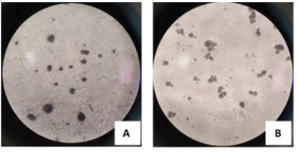
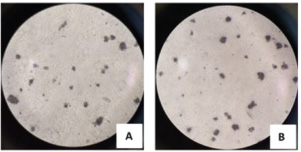
ABSTRACTBackground: Toxoplasma gondii is an obligate intracellular parasite capable of infecting several hosts, including humans and animals. Controlling toxoplasmosis remains challenging due to early diagnosis limitations and the lack of effective human vaccines. One strategic approach for diagnostic and vaccine development involves the use of recombinant proteins derived from T. gondii-specific antigens, such as dense granule (GRA) proteins. GRA-4, an excretion-secretion antigen, plays a critical role in the formation of parasitophorous vacuoles and modulation of host immune responses. This protein reportedly exhibits immunogenic potential, stimulating both humoral and cellular immune responses. Aim: This study aimed to characterize the immune response to recombinant GRA-4 protein (rGRA-4) of T. gondii in Balb/c strain mice. Methods: The samples used in this study included splenic organs from six 2-month-old male Balb/c strain mice to evaluate T and B cell proliferation. Recombinant GRA-4 protein was employed for transmembrane protein analysis using HMMTOP software, while SOPMA was used for protein structure analysis. Result: The results of the study using B cell proliferation analysis with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) showed that the optical density (OD) value obtained was different in each treatment. Treatment with a combination of LPS and GRA-4 protein showed the highest results at a concentration of 10 µg/ml. The results of microscopic examination with 400× magnification showed that the number of T cells stained with MTT was greater in treatment group 2 (added PHA and GRA-4 protein (T lymphocyte proliferation) and treatment group 3 [added lipopolysaccharide (LPS) and GRA-4 protein (B lymphocyte proliferation) compared to treatment group 1 added Phytoheamagglutinin (PHA) (Control). The results of the microscopic examination with 400× magnification of B lymphocyte proliferation showed that after being stained with MTT, the results were almost the same as those in treatment 2. Exposure to a mixture of LPS with GRA-4 protein in Roswell Park Memorial Institute media showed that the number of B lymphocytes stained with MTT was more than the media that were only exposed to LPS. Conclusion: The results of this study indicate that recombinant rGRA-4 protein has potential as a vaccine candidate to protect the host against T. gondii infection by inducing effective humoral and cellular immune responses. Keywords: Immune, Recombinant, rGRA4, Toxoplasma, Mice. IntroductionToxoplasma gondii is an opportunistic intracellular parasitic protozoan capable of infecting intermediate hosts, including humans, ruminants, rodents, and birds (Galeh et al., 2020; Rahmanian et al., 2019; Shariatzadeh et al., 2021; Maspi et al., 2021; Ahaduzzaman and Hasan, 2022; Hajimohammadi et al., 2022). This parasite has also been reported in cold-blooded animals, which may act as a source of infection for the hosts that consume them (Nayeri et al., 2021). Toxoplasma gondii infects approximately 30% of the global human population, causing diverse pathologies collectively termed toxoplasmosis. In addition to clinical manifestations, such as cerebral, ocular, and congenital toxoplasmosis, recent studies have associated this infection with behavioral changes and neuropsychiatric disorders (Akins et al., 2024). The severe damage caused by toxoplasmosis underscores the need for effective vaccines. GRA4 is secreted by both tachyzoites and bradyzoites among the dense granule antigens. The GRA4 gene is intron-free and encodes one of the major T. gondii immunogenic proteins. Current preventive measures against Toxoplasma remain limited to vaccination. Vaccination is widely regarded as the most cost-effective disease prevention method, with a significant socioeconomic impact (Rémy et al., 2015). In this context, an effective vaccine to prevent toxoplasmosis would represent a major medical advancement with global benefits. Toxovax®, a live-attenuated vaccine based on the S48 strain of T. gondii tachyzoite, is the only commercially available toxoplasmosis vaccine. However, it has critical limitations, including restricted veterinary use, a short shelf life of 10 days (Dubey, 2009), and safety concerns associated with live vaccines. Thus, novel and affordable recombinant vaccines that stimulate protective T-cell-mediated immunity are urgently needed. The application of recombinant antigens as potential vaccine candidates against toxoplasmosis has been explored since the 1990s (Gedik et al., 2016). Over 10 genes, including GRA4, have been successfully cloned and expressed in both eukaryotic and bacterial systems in recent years (Martin et al., 2004; Liu et al., 2010; Dziadek et al., 2012; Gedik and Can, 2016). These antigens have been used to detect specific antibodies in the serum of mice, pigs, and cats, as well as to evaluate host immune responses. Findings from various studies indicate that immunization with these recombinant antigens can effectively elicit strong immune responses. GRA proteins are localized in the parasitophorous vacuole (PV) and cyst walls. GRA gene expression studies indicate that these proteins play essential roles in PV maturation and cyst formation (Mercier et al., 2005; Cesbron-Delauw et al., 2008). GRA4, a key protein secreted by both active (tachyzoite) and latent (bradyzoite) forms, is encoded by an intron-free gene (Cornelissen et al., 1984). Martin et al. (2006) demonstrated that a combined GRA4-ROP2 recombinant vaccine with alum adjuvant elicited higher IgG1 responses to ROP2 than GRA4 alone. Hanafiah et al. (2018) successfully cloned the GRA4 gene into an E. coli plasmid, achieving 99.9% sequence similarity to tachyzoite DNA. However, functional testing of the rGRA4 protein was not performed. This study aims to advance immunological response testing to evaluate rGRA4’s potential as a diagnostic kit component or vaccine candidate. To address these challenges, this study focuses on developing a toxoplasmosis diagnostic and vaccine candidate using purified rGRA4 protein. This study aims to characterize the immune response to the recombinant GRA-4 protein (rGRA-4) of T. gondii in Balb/c strain mice. Materials and MethodsThe following materials were used for immunomodulatory activity testing: splenic organs from 2-month-old male Balb/c strain mice, 70% ethanol (Merck), Roswell Park Memorial Institute (RPMI) 1640 medium (Gibco), complete RPMI 1640 medium containing 10% (v/v) fetal bovine serum (Caisson), phosphate-buffered saline (Gibco), hepatitis B vaccine (Engerix®), phytohemagglutinin (PHA, Gibco), MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, Sigma), 10% SDS stopper (sodium dodecyl sulfate, Merck), 0.01 N HCl (Merck), penicillin-streptomycin (Gibco), and Fungizone/amphotericin B (Gibco). T- and B-cell proliferation analysis Lymphocyte isolation from Balb/c strain miceLymphocyte isolation was carried out from the spleen organs of Balb/c strain mice. The first step involved anesthetizing the strain mice with ketamine (1:5) at a dose of 0.2 ml intracutaneously, followed by a 10–20 minute waiting period. The strain mice were then sacrificed via cervical dislocation, dissected, and their splenic tissues were collected. Splenic tissue was aseptically isolated and placed in a Petri dish with a diameter of 50 mm containing 10 ml of RPMI medium. Tissue lysis was performed by flushing the spleen with RPMI medium to release lymphocytes into the medium. The cell suspension was transferred to a 10 ml centrifuge tube and centrifuged at 6,000 rpm for 5 minutes. The resulting pellet was resuspended in 1 ml of tris-ammonium chloride (NH₄Cl) buffer for erythrocyte lysis for 5 minutes. After homogenization, the sample was centrifuged again at 6,000 rpm for 5 minutes, and the supernatant was discarded. The pellet was resuspended in 10 mL of RPMI medium, and cells were counted using a hemocytometer. The lymphocytes were then ready for culture in a 5% CO₂ incubator at 37°C and subjected to subsequent activity testing (Hay and Westwood, 2002). MTT analysisMTT (3-[4,5-dimethylthiazol-2-il]-2,5-diphenyltetrazolium bromide) analysis is a standard method for evaluating cell cytotoxicity, proliferation, viability, and toxicity. A white blood cell suspension was prepared at a concentration of 10⁵ cells/100 μl. For each plate, 10 ml of medium containing either 0.2 ml PHA (for T-cell proliferation) or 0.2 ml LPS (for B-cell proliferation) was prepared. The PHA-containing cell suspension (100 μl) was pipetted into each well. Plates were incubated at 37°C for 24 hours in a 5% CO₂ incubator. Microscopic observation was used to assess cell viability, where live cells appeared in grape-like clusters while dead cells caused medium turbidity. Following the initial incubation, 100 ml of GRA-4 protein at various concentrations was added to the designated wells. Additional incubation periods were conducted for 24, 48, and 72 hours under identical conditions (37°C, 5% CO₂). For MTT staining, 10 µl of MTT reagent was added to each well and mixed by gentle plate tapping. Plates were incubated for 4 hours at 37°C in 5% CO₂. The reaction was terminated by the addition of 100 µl of stop solution to each well, followed by a 24-hour incubation at room temperature. Subsequently, the optical density was measured at a wavelength of 546 nm. Finally, optical density measurements were taken at 546 nm wavelength. Protein structureThe secondary protein structure was predicted using the SOPMA secondary structure prediction method, available at (https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=npsa_sopma.html). Data analysisThe data obtained from formazan compound measurements using an ELISA reader consisted of absorbance or Optical Density (OD) values. These OD values reflect the lymphocyte proliferation activity. The acquired OD values were analyzed using SPSS 16 for Windows, employing the non-parametric Friedman test followed by the Mann–Whitney test at a 95% confidence level. T-cell and B-cell proliferation data were analyzed descriptively. In silico prediction of antigenic epitopes of the GRA-4 protein for T-cell recognition was performed using GENETIX version 8.0, while B-cell epitope prediction was conducted with the ABCPrep software. The secondary structure of the GRA-4 protein was analyzed using the SOPMA program. Ethical approvalAll procedures in this study were approved by the Animal Ethics Commission on the Faculty of Veterinary Medicine Universitas Syiah Kuala certificate number Ref: 410/KEPH/VII/2025, Banda Aceh, July 17, 2025. ResultsImmune response of Balb/c mice to immunization with recombinant rGRA-4 proteinDescriptive analysis revealed that immunization of strain mice with recombinant rGRA-4 protein combined with adjuvant elicited a significant immune response compared with the control group. Immune response evaluation was performed through T-cell and B-cell proliferation analysis using the MTT method, and the results are shown in Table 1. As shown in Graph 1, all treatment groups exhibited a consistent trend of increasing absorbance with increasing GRA-4 concentrations (1.25–10 µg). The GRA-4 + LPS combination demonstrated the highest absorbance values across all concentrations compared to other treatments. GRA-4 + PHA showed an intermediate response, exceeding GRA-4 alone but remaining lower than GRA-4 + LPS. The lowest absorbance values were observed with GRA-4 alone. These findings support the ANOVA results, indicating that both the treatment type and concentration significantly influenced the absorbance measurements. The combination of GRA-4 and LPS exhibited the strongest stimulatory effect on the absorbance response. T-cell proliferation analysisThe OD values from the MTT-based T-cell proliferation analysis in response to GRA-4 protein are presented in Table 1. The results demonstrated that the highest OD value (0.398) was achieved with the combined treatment of PHA and 10 µg/ml GRA-4 protein. Lower concentrations of 5, 2.5, and 1.25 µg/ml yielded progressively decreasing OD values of 0.322, 0.279, and 0.236, respectively. Microscopic examination at 400× magnification (Fig. 1) revealed significantly more MTT-stained T cells in Treatment B (PHA + GRA-4 protein of T. gondii) compared to Treatment A (PHA alone). Similarly, microscopic analysis of B-lymphocyte proliferation at 400× magnification (Fig. 2) showed staining intensity comparable to that shown in Figure 1. RPMI media containing the LPS + T. gondii GRA-4 combination exhibited a greater number of MTT-stained B-lymphocytes than media treated with LPS alone. Table 1. Immune response evaluation results from T-cell and B-cell proliferation analysis using the MTT method.
Graph 1. Mean absorbance values according to treatment group and GRA-4 concentration.
Fig. 1. Microscopic examination results (400× magnification) of MTT-stained T lymphocytes. Lymphocyte proliferation induced by (A) PHA alone; (B) PHA combined with GRA-4 protein.
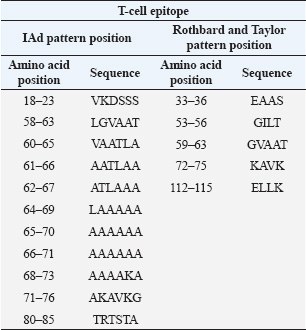
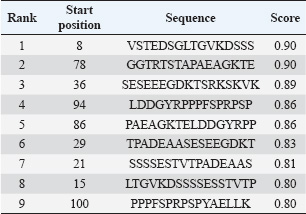
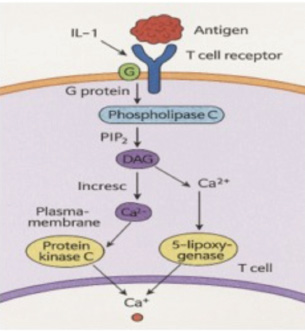
Fig. 2. Microscopic examination results (400× magnification) of MTT-stained B lymphocytes. Lymphocyte proliferation induced by (A) LPS alone; (B) LPS combined with GRA-4 protein. B-cell proliferation analysisThe results of B-cell proliferation analysis using MTT (Fig. 2) showed different OD values for each treatment. The combination treatment of LPS and GRA-4 protein at concentrations of 1.25, 2.5, 5, and 10 µg/ml showed OD values of 0.314, 0.361, 0.405, and 0.461, respectively. Antigenic epitope analysis of the GRA-4 protein against T-cells was performed using GENETIX version 8.0 software. The analysis results showed that 11 epitopes were based on the IAD pattern position and 5 epitopes were based on the Rothbard/Taylor pattern position. Based on the IAD pattern position, epitopes were predominantly found in amino acid sequences 50–100 (10 epitopes), while only 1 epitope was found below position 50. Based on the Rothbard/Taylor pattern position, 4 epitopes were found in amino acid sequences above 50 to 100, one epitope below position 50, and one epitope above position 100 (Table 2). Prediction of antigenic epitopes of T. gondii GRA-4 against B cells using ABCPrep prediction software showed 9 epitopes with scores above 0.80, with the majority located in amino acid sequences below 50. The highest number of GRA-4 protein epitope positions against B cells was found in amino acid sequences below 50 (5 epitopes, 55.6%), specifically at amino acid positions 8–23, 15–31, 21–36, 29–44, and 36–52. Four epitopes (44.4%) were located in amino acid sequences 50–100, at positions 78–93, 86–101, 94–109, and 100–115, as shown in Table 3. Figure 3 illustrates the proposed mechanism of T-cell activation involving G protein signaling pathways. Upon stimulation by the rGRA4 antigen and IL-1, T-cell receptors engage downstream signaling cascades, including G-protein coupled receptor (GPCR)-mediated pathways, leading to T-cell proliferation and cytokine production. Table 2. T-cell epitope prediction using T. gondii recombinant GRA-4 protein.
Table 3. B-cell epitope prediction of T. gondii GRA-4 antigen using ABCPrep prediction software (score ≥ 0.80) was performed.
Fig. 3. T-cell activation mechanism through the G-protein signaling pathway mediated by antigen and IL-1 (generated by AI).
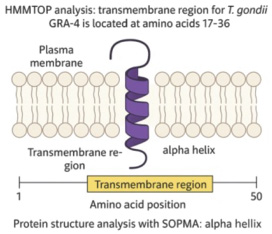
Fig. 4. Protein structure analysis of the T. gondii GRA-4 gene using SOPMA 3 (generated by AI). Transmembrane protein analysis using HMMTOP software showed that the transmembrane protein for T. gondii GRA-4 is located at amino acid positions 17–36, while protein structure analysis using SOPMA is shown in Figure 4. DiscussionAs shown in Table 1, the immunization of Balb/c mice with recombinant GRA-4 protein combined with an adjuvant significantly enhanced the immune response compared with that of controls. This enhancement is likely due to three factors: (1) the immunogenic nature of GRA-4, which activates T cells and promotes antibody production; (2) the adjuvant’s role in enhancing antigen presentation and immune cell activation; and (3) a synergistic effect between GRA-4 and the adjuvant, which promotes Th1 pathway activation, elevated IgG levels, and lymphocyte proliferation as evidenced by increased OD values in the MTT assay (Chyb et al., 2023). Graph 1 shows a clear increase in absorbance with higher GRA-4 concentrations (1.25–10 µg) across all treatments. The GRA-4 + LPS group showed the highest response (1.263 at 10 µg), followed by GRA-4 + PHA, while GRA-4 alone had the lowest (0.683 at 10 µg). This pattern suggests enhanced immune activation, with LPS providing a strong synergistic effect via TLR4 signaling (Akira and Takeda, 2004). PHA moderately boosted T-cell proliferation, and GRA-4 alone showed dose-dependent immunogenicity (Zhu et al., 2012). ANOVA confirmed that both treatment and GRA-4 concentration significantly affected absorbance (p < 0.05) (Janeway et al., 2001). The MTT assay is a widely used colorimetric method for assessing cell viability and proliferation by measuring mitochondrial metabolic activity. Viable cells convert yellow tetrazolium salt (MTT) into insoluble purple formazan crystals through the action of succinate dehydrogenase, a mitochondrial enzyme. The amount of formazan produced, quantified via absorbance, directly reflects the number and metabolic status of living cells (Mosmann, 1983). Although the mechanism of MTT staining is well understood, it is important to emphasize how this method specifically supports the evaluation of mitogen-induced effects on particular cell populations. This assay is particularly effective in evaluating lymphocyte responses when combined with specific mitogens such as phytohemagglutinin (PHA) for T cells and lipopolysaccharide (LPS) for B cells due to their ability to trigger cell-specific activation pathways (Janeway et al., 2005; Abbas and Pillai, 2017). Thus, MTT analysis serves as a reliable indicator for assessing antigen-induced immune responses and immunomodulatory agent efficacy. Microscopic examination (400×; Figs. 1 and 2) revealed more MTT-stained T cells in treatment B than in treatment A, possibly due to differences in media nutrient content. T-cell proliferation was stimulated using phytohemagglutinin (PHA), whereas B-cell proliferation was induced with lipopolysaccharide (LPS). The cell cultures were incubated in RPMI medium for 48 hours at 37°C in a humidified atmosphere containing 10% CO₂. (Abbas et al., 2007). Although the microscopic findings support the MTT results, a deeper understanding of mitogen mechanisms is essential for interpreting these immunological data. According to Abbas and Pillai (2017), PHA specifically stimulates CD4⁺ T cells through signaling pathways that trigger proliferation and cytokine release. LPS activates B cells via TLR4, promoting antibody production. MTT staining differences reflect these specific mitogen actions, with more intense staining in T cells treated with PHA and in B cells treated with LPS. Thus, the MTT assay effectively quantifies the proliferation of T and B lymphocytes when paired with appropriate mitogens (Janeway et al., 2005; Abbas and Pillai, 2017). To reinforce this immunological analysis, understanding the biological origin and characteristics of the mitogens used is also important. In immunological studies, PHA and LPS are commonly used mitogens to selectively stimulate T and B lymphocyte proliferation, respectively. PHA, derived from Phaseolus vulgaris, activates T cells via glycoprotein binding and intracellular signaling (Abbas and Pillai, 2017), while LPS, a component of Gram-negative bacterial walls, stimulates B cells through TLR4-mediated pathways, enhancing antibody production (Janeway et al., 2005). Lymphocyte proliferation is initiated when antigens engage cell surface receptors, triggering a signaling cascade involving G protein activation, PIP₂ hydrolysis, and Ca²⁺ release, leading to IL-2 production that drives T and B cell expansion (Roitt and Delves, 2001). In this study, GRA-4, a dense granule protein from T. gondii, was evaluated for its immunogenic potential. Recombinant GRA-4 induced strong humoral and cellular responses in Balb/c mice, evidenced by IgG recognition of the C-terminal region (aa 297–345) and intestinal IgA detection. Major B-cell epitopes were localized to the C-terminal end, whereas the N-terminal region exhibited low immunoreactivity. Oral immunization with cholera toxin-conjugated GRA-4 fragments in C57BL/6 mice triggered serum IgG responses and conferred partial protection against T. gondii infection (Carruthers and Suzuki, 2007; Zhou et al., 2020). GRA-4 is a strong candidate for oral vaccination against T. gondii because of its ability to induce both humoral and cellular immunity (Mévélec, 1998; Wang et al., 2022). In this study, bioinformatics analysis identified 11 T-cell epitopes (via IAD pattern) and 9 B-cell epitopes of the GRA-4 protein (Table 2). These results partially differ from those of Wang and Yin (2014), who reported six key B-cell epitopes, with peptides at positions 62–77, 233–252, and 314–333 recognized consistently by infected pig sera. Table 3 shows that nine linear B-cell epitopes of T. gondii GRA-4 were predicted using ABCPred with scores above 0.80. Most epitopes (55.6%) were located in the N-terminal region (aa 8–52), while the rest (44.4%) were in the central region (aa 78–115). This suggests that the N-terminal domain is more antigenic and likely surface-exposed, making it a promising target for antibody recognition. Epitope clustering in these regions may enhance humoral responses, supporting their potential for diagnostic or vaccine development (Jespersen et al., 2017). Variations in predicted epitopes may reflect differences in T. gondii strain genetics, post-translational modifications, prediction algorithms, or host-specific major histocompatibility complex (MHC) profiles (Jespersen et al., 2017; Vita et al., 2019). Despite these differences, multiple studies confirm GRA-4’s immunodominant nature and its capacity to trigger protective responses across host species. Recombinant GRA-4 protein has demonstrated a significant reduction in parasite load in key organs (e.g., brain and liver), as confirmed by PCR (Xiao et al., 2018). Compared with other antigens like SAG1 or ROP18, GRA-4 exhibits a more balanced immune response, effectively stimulating both antibody production and T-cell proliferation (Cong et al., 2019; Zhang et al., 2021). The conserved structure and membrane-associated nature of GRA-4 further enhance its vaccine potential. Collectively, these findings support GRA-4 as a robust and versatile antigen for future toxoplasmosis vaccine development, with potential applications in both human and veterinary medicine. The recombinant GRA4 (rGRA4) protein from T. gondii acts as an antigen recognized by the host immune system. Upon presentation by antigen-presenting cells, rGRA4 is detected by T-cell receptors, initiating T-cell activation. This process is further enhanced by pro-inflammatory cytokines such as interleukin-1 (IL-1), which serve as essential co-stimulatory signals during immune responses. IL-1 can activate GPCRs on the surface of T cells, triggering downstream intracellular signaling cascades. These cascades lead to the activation of transcription factors such as NF-κB and AP-1, which are critical for promoting T-cell proliferation, differentiation, and cytokine production. Therefore, the combined stimulation from antigen presentation and IL-1-mediated GPCR signaling contributes to a robust adaptive immune response to rGRA4, as illustrated in Figure 3 (Liu et al., 2010). Furthermore, Hanafiah et al. (2018) successfully cloned and expressed the recombinant T. gondii GRA-4 protein. However, bioinformatics analysis using I-Mutant 3.0 and SIFT Program online servers revealed a critical N (Asparagine) to S (Serine) mutation at position 104 of the GRA4 fragment. Bioinformatics analysis revealed a transmembrane domain at positions 17–36 of the GRA-4 protein, as predicted by HMMTOP (Fig. 4) and supported by the identification of an alpha-helical segment by SOPMA (Tusnády and Simon, 2001). This region likely binds GRA-4 to the parasite membrane, explaining its potential role in antigen presentation and immune activation. In addition, a point mutation (N→S at position 104), predicted by I-Mutant 3.0 and SIFT (Capriotti et al., 2005; Sim et al., 2012), may alter protein stability or MHC binding. These findings are in agreement with the observed immune responses, indicating that the membrane-associated nature and the C-terminal epitope of GRA-4 are functionally immunodominant. ConclusionIn conclusion, this study shows that recombinant rGRA-4 protein shows promising results for further evaluation as a vaccine candidate. AcknowledgmentsThe authors would like to thank Mrs. Arsiah from PAU UGM Yogyakarta and Fihiruddin from Mataram Health Polytechnic for their assistance during the process of Characterization of Immune Response to Recombinant GRA4 Protein (rGRA4) Toxoplasma gondii in Balb/c Strain Mice. Conflicts of interestThe authors declare no conflict of interest. FundingThis research was funded by the Ministry of Research Technology and Higher Education of the Republic of Indonesia through Grant No. 268/UN11/SPK/PNBP/2020 Dated Maret 17, 2020. Authors’ contributionsMHF and NUR: Conceptualized and conducted the research and prepared the manuscript. FIH: Performed immunological analysis to evaluate immune responses induced by recombinant GRA-4 (gRGA-4) protein in Balb/c strain mice, specifically analyzing rGRA4 protein effects on T and B lymphocytes. FSA: Conducted data analysis. MBH: Collected samples and drafted the article. All authors have read, reviewed, and approved the final version of the manuscript. Data availabilityAll data supporting the findings of this study are available within the manuscript, and no additional data sources are required. ReferencesAbbas A., Lichtman A. H., & Pillai S., 2007, Cellular and molecular immunology, 6th Ed, WB. Abbas., A. K.., Pillai. and S.. 2017. Cellular and molecular immunology. 9th ed., Elsevier. Ahaduzzaman, M. and Hasan, T. 2022. Seroprevalence of Toxoplasma gondii infection in sheep and goats from different geographical regions of the world: systematic review and meta-analysis. Transbound. Emerg. Dis. 69(6), 3790–3822. Akins, G.K.H., Furtado, J.M. and Smith, J.R. 2024. Diseases caused by and behaviors associated with Toxoplasma gondii infection. Pathogens 13, 968; doi:10.3390/pathogens13110968 Capriotti, E., Fariselli, P. and Casadio, R. 2005. Predicting stability changes from the protein sequence or structure upon mutation. Nucleic Acids Res. 33, W306–W310. Carruthers, V.B. and Suzuki, Y. 2007. Effects of Toxoplasma gondii infection on the brain. Schizophrenia Bull. 33(3), 745–751. Cesbron-Delauw, M.F., Gendrin, C., Travier, L., Ruffiot, P. and Mercier, C. 2008. Toxoplasma gondii uses unusual sorting mechanisms to deliver proteins into the host cell. Traffic 9(9), 1331–1345. Chyb, M., Dziadek, B., Dzitko, K., Ferra, B.T., Kawka, M., Holec-Gąsior, L. and Gatkowska, J. 2023. Evaluation of long-term immunity and protection against T. gondii after immunization with multivalent recombinant chimeric T. gondii proteins. Sci. Rep. 13, 1–12. Cong, W., Zhang, N.Z., Yuan, D.Q., Zou, Y., Li, S. and Liang, Z.L. 2019. Detection and genetic characterization of Toxoplasma gondii in market-sold mussels (Mytilus edulis) in certain provinces of China. Microb. Pathog. 136, 103687–103686; doi:10.1016/j.micpath.2019.103687 Cornelissen, A.W.C.A., Overdulve, J.P. and van der Ploeg, M. 1984. Determination of nuclear DNA of five eucoccidian parasites, Isospora (Toxoplasma) gondii, Sarcocystis cruzi, Eimeria tenella, E. acervulina and Plasmodium berghei, with special reference to gametogenesis and meiosis in I. (T.) gondii. Parasitology 88(3), 531–553. Dubey, J.P. 2009. History of the discovery of the life cycle of Toxoplasma gondii. Int. J. Parasitol. 39(8), 877–882; doi:10.1016/j.ijpara.2009.01.005 Dziadek, B., Gatkowska, J. and Grzybowskim, M. 2012. Toxoplasma gondii: the vaccine potential of three trivalent antigen-cocktails composed of recombinant ROP2, ROP4, GRA4 and SAG1 proteins against chronic toxoplasmosis in BALB/c mice. J. Biol. Chem. Biotechnol. Parasitol. 131(1), 133–138. Galeh, T.M., Sarvi, S., Montazeri, M., Moosazadeh, M., Nakhaei, M., Shariatzadeh, S.A. and Daryani, A. 2020. Global status of Toxoplasma gondii seroprevalence in rodents: a systematic review and meta-analysis. Front. Vet. Sci. 7, 461; doi:10.3389/fvets.2020.00461 Gedik, Y., Gülçe Iz, S., Can, H., Değirmenci Döşkaya, A., Ismet Deliloğlu Gürhan, S., Gürüz, Y. and Döşkaya, M. 2016. Immunogenic multistage recombinant protein vaccine confers partial protection against experimental toxoplasmosis mimicking natural infection in murine model. Trials Vaccinol. 5, 15–23. Hajimohammadi, B., Ahmadian, S., Firoozi, Z., Askari, M., Mohammadi, M., Eslami, G., Askari, V., Loni, E., Barzegar-Bafrouei, R. and Boozhmehrani, M.J. 2022. A meta-analysis of the prevalence of toxoplasmosis in livestock and poultry worldwide. EcoHealth 19(1), 55–74. Hanafiah, M., Prastowo, J., Hartati, S., Aliza, D. and Nurcahyo, R.W. 2018. Detection of Toxoplasma gondii copro-prevalence by polymerase chain reaction using repetitive 529 bp gene in feces of pet cats (Felis catus) in Yogyakarta, Indonesia. Vet. World 11, 1338–1343. Janeway, C. A. Jr., Travers, P., Walport, M. and Shlomchik, M. 2001. Immunobiology: the immune system in health and disease, 5th ed. New York: Garland Science. Janeway, C.A., Travers, P., Walport, M. and Shlomchik, M.J. 2005. Immunobiology: the immune system in health and disease, 6th ed. Garland Science. Jespersen, M.C., Peters, B., Nielsen, M. and Marcatili, P. 2017. BepiPred-2.0: improving sequence-based B-cell epitope prediction using conformational epitopes. Nucleic Acids Res. 45(1), W24–W29; doi:10.1093/nar/gkx346 Liu, M., Yuan, Z. and Peng, G. 2010. Toxoplasma gondii micro neme protein 8 (MIC8) is a potential vaccine candidate against toxoplasmosis. Parasitol. Res. 106(5), 1079–1084. Martin, V., Supanitsky, A. and Echeverria, P.C. 2004. Recombinant GRA4 or ROP2 protein combined with alum or the gra4 gene provides partial protection in chronic murine models of toxoplasmosis. J. Biol. Chem. Phys. Clin. Vaccine Immunol. 11(4), 704–710. Martin, V., Supanitsky, S., Anguissola, S., Di Girolamo, C. and Angel, S.O. 2006. Kinetic analysis of the humoral immune response to 3 recombinant Toxoplasma gondii antigens in experimentally infected mice. Clin. Diagn. Lab. Immunol. 13(1), 123–127. Maspi, N., Nayeri, T., Moosazadeh, M., Sarvi, S., Sharif, M. and Daryani, A. 2021. Global seroprevalence of Toxoplasma gondii in Camelidae: a systematic review and meta-analysis. Acta Parasitol. 66, 733–744. Mercier, C., Adjogble, K.D., Däubener, W. and Delauw, M.F. 2005. Dense granules: are they key organelles to help understand the parasitophorous vacuole of all apicomplexa parasites?. Int. J. Parasitol. 35, 829–849. Mévélec, M.N., Mercereau-Puijalon, O., Buzoni-Gatel, D., Bourguin, I., Chardès, T., Dubremetz, J.F. and Bout, D. 1998. Mapping of B epitopes in GRA4, a dense granule antigen of Toxoplasma gondii and protection studies using recombinant proteins administered by the oral route. Parasite Immunol. 20(4), 183–195. Mosmann, T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65(1-2), 55–63; doi:10.1016/0022-1759(83)90303-4 Nayeri, T., Sarvi, S. and Daryani, A. 2021. Toxoplasma gondii in mollusks and cold-blooded animals: a systematic review. Parasitology 148(8), 895–903. Rahmanian, V., Rahmanian, K., Jahromi, A.S. and Bokaie, S. 2019. Seroprevalence of Toxoplasma gondii infection: an umbrella review of updated systematic reviews and meta-analyses. Fam. Med. Prim. 57(8), 59–69. Rémy, V., Zöllner, Y. and Heckmann, U. 2015. Vaccination: the cornerstone of an efficient healthcare system. J. Market. Access Health Policy 3, 27041–27046; doi:10.3402/jmahp.v3.27041 Roitt, I.M. and Delves, P.J. 2001. Roitt’s essential immunology, 10th ed. Blackwell Publishing. Shariatzadeh, S.A., Sarvi, S., Hosseini, S.A., Sharif, M., Gholami, S., Pagheh, A.S., Montazeri, F., Nayeri, T., Nakhaei, M., Mikaeili, T., Galeh. and Daryani, A. 2021. Global seroprevalence of Toxoplasma gondii infection in bovines: a systematic review and meta-analysis. Parasitology 148(12), 1417–1433. Sim, N.L., Kumar, P., Hu, J., Henikoff, S., Schneider, G. and Ng, P.C. 2012. SIFT web server: predicting effects of amino acid substitutions on proteins. Nucleic Acids Res. 40(W1), W452–W457; doi:10.1016/j.nar.2013.09.010 Tusnády, G.E. and Simon, I. 2001. The HMMTOP transmembrane topology prediction server. Bioinformatics 17(9), 849–850; doi:10.1093/bioinformatics/17.9.849 Vita, R., Mahajan, S., Overton, J.A., Dhanda, S.K., Martini, S., Cantrell, J.R., Wheeler, D.K., Sette, A. and Peters, B. 2019. The immune epitope database (IEDB): 2018 update. Nucleic Acids Res. 47, D339–D343; doi:10.1093/nar/gky1006 Wang, Y. and Yin, H. 2014. Research progress on surface antigen 1 (SAG1) of Toxoplasma gondii. Parasites Vectors 7(180), G1–G8. Wang, Z., Qu, T., Qi, H., Zhao, S., Shi, H., Bai, W., Yu, Y., Wu, X. and Zhao, P. 2022. Seroprevalence of Toxoplasma gondii infection in women with a gynecological tumor living in eastern China. PeerJ 10, e14569; doi:10.7717/peerj.14569 Xiao, J., Prandovszky, E., Kannan, G., Pletnikov, M.V., Dickerson, F., Severance, E.G. and Yolken, R.H. 2018. Toxoplasma gondii: biological parameters of the connection to schizophrenia. Schizophr. Bull. 44(5), 983–992; doi:10.1093/schbul/sby082 Zhang, J., Chen, J., Lv, K., Li, B., Yan, B., Gai, L., Shi, C., Wang, X., Si, H. and Zhang, J. 2021. Myrislignan induces redox imbalance and activates autophagy in Toxoplasma gondii. Front. Cellular Infect Microbiol. 11, 1–10; doi:10.3389/fcimb.2021.730222 Zhou, C.X., Ai, K., Huang, C.Q., Guo, J.J., Cong, H. and He, S.Y. 2020. miRNA and circRNA expression patterns in mouse brain during toxoplasmosis development. BMC Genom. 21, 46; doi:10.1186/s12864-020-6464-9 Zhu, X., Wang, F., Liang, C., Li, J. and Sun, X. 2012. Quality credit evaluation based on TOPSIS: evidence from air-conditioning market in China. Procedia Comput. Sci., 9, 1256-1262. Zou, Y., Meng, J.X., Wei, X.Y., Gu, X.Y., Chen, C., Geng, H.L., Yang, L.H., Zhang, X.X. and Cao, H.W. 2022. CircRNA and miRNA expression analysis in livers of mice with Toxoplasma gondii infection. J. Microbiol. Biotechnol. Biotechnol. Front. Cell. Infect. Microbiol. 12, 1–11; doi: 10.3389/fcimb.2022.1037586 | ||
| How to Cite this Article |
| Pubmed Style Hanafiah M, Nurliana N, Bahi M, Fihiruddin F, Alfina FS. Characterization of the immune response to recombinant GRA-4 protein (rGRA-4) of Toxoplasma gondii in Balb/c strain mice. Open Vet. J.. 2025; 15(8): 3468-3476. doi:10.5455/OVJ.2025.v15.i8.10 Web Style Hanafiah M, Nurliana N, Bahi M, Fihiruddin F, Alfina FS. Characterization of the immune response to recombinant GRA-4 protein (rGRA-4) of Toxoplasma gondii in Balb/c strain mice. https://www.openveterinaryjournal.com/?mno=261367 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i8.10 AMA (American Medical Association) Style Hanafiah M, Nurliana N, Bahi M, Fihiruddin F, Alfina FS. Characterization of the immune response to recombinant GRA-4 protein (rGRA-4) of Toxoplasma gondii in Balb/c strain mice. Open Vet. J.. 2025; 15(8): 3468-3476. doi:10.5455/OVJ.2025.v15.i8.10 Vancouver/ICMJE Style Hanafiah M, Nurliana N, Bahi M, Fihiruddin F, Alfina FS. Characterization of the immune response to recombinant GRA-4 protein (rGRA-4) of Toxoplasma gondii in Balb/c strain mice. Open Vet. J.. (2025), [cited January 25, 2026]; 15(8): 3468-3476. doi:10.5455/OVJ.2025.v15.i8.10 Harvard Style Hanafiah, M., Nurliana, . N., Bahi, . M., Fihiruddin, . F. & Alfina, . F. S. (2025) Characterization of the immune response to recombinant GRA-4 protein (rGRA-4) of Toxoplasma gondii in Balb/c strain mice. Open Vet. J., 15 (8), 3468-3476. doi:10.5455/OVJ.2025.v15.i8.10 Turabian Style Hanafiah, Muhammad, Nurliana Nurliana, Muhammad Bahi, Fihiruddin Fihiruddin, and Febi Silvia Alfina. 2025. Characterization of the immune response to recombinant GRA-4 protein (rGRA-4) of Toxoplasma gondii in Balb/c strain mice. Open Veterinary Journal, 15 (8), 3468-3476. doi:10.5455/OVJ.2025.v15.i8.10 Chicago Style Hanafiah, Muhammad, Nurliana Nurliana, Muhammad Bahi, Fihiruddin Fihiruddin, and Febi Silvia Alfina. "Characterization of the immune response to recombinant GRA-4 protein (rGRA-4) of Toxoplasma gondii in Balb/c strain mice." Open Veterinary Journal 15 (2025), 3468-3476. doi:10.5455/OVJ.2025.v15.i8.10 MLA (The Modern Language Association) Style Hanafiah, Muhammad, Nurliana Nurliana, Muhammad Bahi, Fihiruddin Fihiruddin, and Febi Silvia Alfina. "Characterization of the immune response to recombinant GRA-4 protein (rGRA-4) of Toxoplasma gondii in Balb/c strain mice." Open Veterinary Journal 15.8 (2025), 3468-3476. Print. doi:10.5455/OVJ.2025.v15.i8.10 APA (American Psychological Association) Style Hanafiah, M., Nurliana, . N., Bahi, . M., Fihiruddin, . F. & Alfina, . F. S. (2025) Characterization of the immune response to recombinant GRA-4 protein (rGRA-4) of Toxoplasma gondii in Balb/c strain mice. Open Veterinary Journal, 15 (8), 3468-3476. doi:10.5455/OVJ.2025.v15.i8.10 |