| Research Article | ||
Open Vet. J.. 2025; 15(9): 4181-4189 Open Veterinary Journal, (2025), Vol. 15(9): 4181-4189 Research Article Therapeutic effect of potassium permanganate and ethanolic extract of Saussurea costus roots on Leghorn chicken coccidiosisNagia A.S. Abdalsalam1*, Hanan A. Alkallani2, Ibrahim Saleh Milad3 and Somia A. Alsanousi41Department of Pathology and Clinical Pathology, Faculty of Veterinary Medicine, Omar Al-Mukhtar University, Al-Bayda, Libya 2Department of Pharmacology, Faculty of Veterinary Medicine, Omar Al-Mukhtar University, Al-Bayda, Libya 3Department of Animal Production, Faculty of Agriculture, Omar Al-Mukhtar University, Al-Bayda, Libya 4Department of Microbiology and Parasitology, Faculty of Veterinary Medicine, Omar Al-Mukhtar University, Al-Bayda, Libya *Corresponding Author: Nagia A.S. Abdalsalam. Department of Pathology and Clinical Pathology, Faculty of Veterinary Medicine, Omar Al-Mukhtar University, Al-Bayda, Libya. Email: nagia.abdalsalam [at] omu.edu.ly Submitted: 28/05/2025 Revised: 12/08/2025 Accepted: 20/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
ABSTRACTBackground: The roots of Saussurea costus have been used in traditional medicine for their effectiveness in treating various ailments. Potassium permanganate (KMnO4) is an effective oxidizing agent commonly used in poultry farms to sterilize water distribution systems by decomposing organic materials in water. Aim: This study aimed to compare the effects of a mixture of ethanolic extract of S. costus and potassium permanganate on Eimeria infection in chickens in mitigating their negative effects. Methods: A total of 24 Leghorn chickens naturally infected with coccidia were divided into four groups of six birds each. One group served as a positive control and received only the standard ration, while two groups were evaluated for both KMnO4 (0.01 mg/l) and the ethanolic extract of S. costus (0.5 and 1 ml) (0.25 g/ml). One group received only KMnO4 (0.01 mg/l) once a day for 4 weeks. Before treatment, Eimeria oocysts researchers detected by direct and flotation tests as well as physiological parameters. Results: The findings showed a reduction in the number of Eimeria oocysts along with the blood parameters examined (including red blood cells, white blood cells, monocytes, neutrophils, and lymphocytes) and biochemical markers such as amylase and lactate dehydrogenase, revealing significant differences between the measured values across the experimental groups (p < 0.05). Conclusion: The results of the current study recommend that a combination of an ethanolic extract of S. costus roots and potassium permanganate is effective in treating parasitic infections. This study offers a potential therapeutic approach and control method for coccidiosis in industrial poultry farms. Keywords: Blood biochemical parameters, Coccidia, Potassium permanganate, Saussurea costus roots, Whie Leghorn. IntroductionCoccidiosis is a widespread disease caused by protozoa belonging to the Apicomplexa phylum, specifically from the Eimeriidae family (Cynthia and Scottl, 2000). Among poultry, the genus Eimeria is the primary culprit, causing extensive damage to the intestinal lining, leading to diarrhea, dehydration, and, in severe cases, death, particularly in younger animals (Levine, 1985). In many parts of the world, it accounts for a significant proportion of cases handled by poultry disease diagnostic laboratories (Abadi et al., 2012) and posing significant economic losses (Vermeulen et al., 2001). Strategies have focused toward effective management and control of the parasite rather than its total elimination (Zhang et al., 2013). The longstanding use of preventive and therapeutic medications has been successful in managing the disease; however, a significant challenge persists due to the continual development of drug resistance. This issue has motivated researchers to explore innovative strategies to address the problem (Williams and Catchpole, 2000). Some researchers have focused on educational campaigns, whereas others have focused on therapeutic methods involving anticoccidial compounds. Additionally, immunological approaches for disease management have received considerable attention. Various immunization strategies have been employed, such as combining natural exposure with medications, utilizing inactivated materials derived from the parasite, and using attenuated strains or controlled natural exposure to the parasite (Anderson and Jorgensen, 2004). Moreover, concerns have arisen over the potential side effects of the consumption of chicken meat treated using these remedies on human health (Allen and Maizels, 2011). Nonetheless, studies have confirmed that coccidiosis can be effectively controlled and managed using anticoccidial chemical compounds (El-Shall et al., 2022). Potassium permanganate solution is a highly effective disinfectant that is often employed against a broad spectrum of microorganisms, as demonstrated in various studies. It has also been evaluated for its impact on specific physiological factors in chickens. Potassium permanganate is commonly used as a wet dressing for surface wounds, particularly those that are blistered or oozing pus. It is beneficial in managing skin conditions caused by bacterial or fungal infections, such as athlete’s foot and impetigo. Moreover, it has been used to treat diarrhea. This inorganic compound, (KMnO4), historically known as “permanganate of potash” or “Condy’s crystals,” it also plays a significant role as a disinfectant for purifying drinking water, making it an essential disinfectant, particularly in safeguarding poultry during water contamination or when microbial loads are high. Its application helps prevent the transmission of bacterial diseases to poultry through water, offering protection against infections such as coryza, salmonella, Escherichia coli, and cholera. Parasitic diseases continue to be a major challenge hindering the growth and advancement of agricultural economies in developing countries. This issue persists largely because skilled husbandry practices cannot keep pace with the rapid expansion and intensification of poultry farming operations. Saussurea costus has been noted in traditional medicinal practices, particularly those linked to Prophets, for its effectiveness in treating a range of ailments, such as anemia, asthma, bronchitis, leprosy, jaundice, and various skin and urinary disorders (Sivarajan and Balachandran, 1994). This study aimed to evaluate the impact of potassium permanganate on coccidiosis infection rates and assess the protective effects of potassium permanganate on S. costus. Materials and MethodsExperimental protocolThis study was conducted in January and February 2023 at the research units of the Department of Animal Production, Faculty of Agriculture, Omar Al-Mukhtar University, Al-Beyda, Libya. A total of 24 Leghorn chickens aged between 22 and 24 months were purchased from the local market in Al-Beyda city. Potassium permanganate powder, manufactured by Tome Peaker Company Ltd, India, was also obtained from the local market and stored in the laboratory for use in the experiments. A potassium permanganate solution was prepared by dissolving 300 mg of the powder in 30 l of distilled water at a concentration of 0.01 mg/ml (as described by Mustafa, 1996); (Mustafa, 2008). The roots of S. costus were collected from different herb shops in Al-Beyda, Libya. After cleaning and shade drying, the roots were ground using a mechanical grinder. A mixture of 50 g of this powder and 200 ml of 70% ethanol was extracted in a round-bottom flask at 70°C for 48 hour. The resulting extract was filtered using Whatman filter paper and dried on a shaded area on a Petri dish at room temperature. The final extracts were collected and stored in tightly sealed containers until further use. The substance was assessed using 0.25 g/ml for treatment groups (Herborn, 1973). Following the confirmation of coccidiosis, the chickens were randomly divided into four groups of six birds each. Group 1, serving as the positive control, was administered a standard ration only. Group 2 received drinking water containing potassium permanganate. Group 3 was administered potassium permanganate, followed by 0.5 ml of ethanolic extract from S. costus an hour later. Similarly, Group 4 was administered potassium permanganate, followed by 1.0 ml of the ethanolic extract of S. costus an hour later. The birds were administered the compounds once a day for 4 weeks. Subsequently, the chickens were slaughtered, and blood samples were drawn from the jugular vein. The blood was collected into clearly labeled test tubes—some containing the anticoagulant Ethylene Diamine Tetra Acetic Acid for blood smear preparation and complete blood count (CBC) analysis, including red blood cells (RBCs), white blood cells (WBCs), monocytes (MO), neutrophils (NET), and lymphocytes (LYs). Other samples were collected in empty tubes for the biochemical analysis of amylase and lactate dehydrogenase (LDH). Laboratory procedures, including parasite examination, blood smears, and sample centrifugation (at 4,000 rpm at 4°C for 15 minute to obtain serum), were performed in the laboratory diagnostics unit at the College of Veterinary Medicine, Omar Al-Mukhtar University. Blood analysis was performed at the Safwa Laboratory in Al-Beyda, Libya. Statistical analysisAll quantitative data were analyzed using analysis of variance in SPSS 26.0 (SPSS Inc., USA) and reported as mean ± SEM. A p-value of < 0.05 was deemed statistically significant. The statistical analysis for this experiment involved the least significant difference method at a probability level of p < 0.05 to determine significant differences between treatments. Clinical signsThe characteristic indicators of the chicken were documented by observing lethargy, bloody diarrhea, loss of appetite, and disheveled feathers, along with recording the mortality rate in each group throughout the experimental period. Dissection of the deceased birds was performed to confirm the causes of their deaths (McDonald, 1999). Direct examination of the fecal samplesPlace a drop of physiological salt solution on a microscope slide. Using a toothpick tip, collect a small amount of excreta and mix it thoroughly with the salt solution. Avoid creating a suspension that is too dense, as it will hinder clear observation under the microscope. Aim for an emulsion light enough to read newsprint through it—this consistency is approximately correct. Carefully place a coverslip over the drop and examine it under a microscope using low and high dry power settings. Iodine staining can be used to highlight specific features for wet smears. Prepare a slightly denser fecal suspension than the earlier one and mix it with an equal volume of Lugol’s solution, diluted with four parts of distilled water. Flotation techniqueVarious techniques have been employed, and numerous adaptations of these methods have been recommended. The procedure described here uses a zinc sulfate solution. This approach relies on solutions with a higher specific gravity than coccidian cysts but lower than the specific gravity of fecal debris, which settles at the bottom. For centrifugal flotation using zinc sulfate, a stool sample weighing 1 g was emulsified in 10 parts of tap water and then passed through wire gauze for sifting. The filtrate was collected in a Wassermann tube and centrifuged at 2,500 rpm. After centrifugation, the sediment is suspended in water, and the supernatant is discarded. This washing process is repeated until the supernatant becomes clear. Next, 3–4 ml of a 33% zinc sulfate solution is added to the sediment, mixed thoroughly, and the tube is topped up with zinc sulfate solution to within half an inch of the rim. Finally, several portions of the supernatant fluid are extracted and examined for parasites using a bacteriological loop. Quantitative methods for fecal examinationThis procedure is used to determine the Oocyst per gram, sporulation percentage, and oocyst dimensions. The McMaster chamber method is a widely applied technique for analyzing the shedding pattern of individual oocysts in infected chickens and quantifying the number of Eimeria oocysts in a fecal sample. The oocyst flotation method, which aligns with the quantitative approach, uses the McMaster slide for simplified counting. Observations were conducted under a light microscope (10× magnification), and the following equation was applied to calculate the oocyst count per gram as per Permin et al. (1997): Number of oocysts per gram=(15 × number of oocysts per chamber)/0.15 Coccidian oocyst sporulationTo diagnose coccidia, it is often necessary to allow oocysts to sporulate, reaching the infectious stage. To facilitate this process, mix the feces containing the coccidia with multiple volumes of solution. Then, a thin layer of the mixture was spread in a Petri dish containing a 5% potassium dichromate solution. Potassium dichromate protects the oocysts from bacterial degradation. Since oxygen is essential for oocyst development, ensure that the fluid layer is only a few millimeters thick. Although most species form sporocysts and sporozoites within a few days, it is advisable to allow at least a week for full development, or even longer for certain species. If immediate examination of the sporulated oocysts is not required, the fecal suspension can be refrigerated and transferred to a bottle. Under these conditions, oocysts can remain viable for several months and up to a year in some species. Identification of Eimeria speciesIdentifying Eimeria sp. involves analyzing a combination of factors, including oocyst size, location within the gut, appearance of lesions, and schizont size, as described by Calnek et al. (1997), Conway and McKenzie (2007), and McDougald and Fitz-Coy (2008). Blood picture and biochemical testsBlood samples were collected from the jugular vein for CBC and serum biochemistry analysis. The blood count included measurements of WBC and RBC. Differential leukocyte counts (DLCs), including LYs, NET, and MO, were assessed using equipment from MS4-Melet Schloesing Laboratories, France. S levels of serum amylase (measured using the α-Amylase Activity Assay Kit E-BC-K007-M) and LDH (analyzed with the Cobas 8000 system from Roche, Germany) were determined through spectrophotometry as described by Haen (1995). Ethical approvalThis study was approved by the Al-Mukhtar Committee for Bio-safety and Bioethics, Omar Al-Mukhtar University; approval number is NBC: 007. A 25 43. ResultsClinical signsAll chickens exhibited several clinical symptoms, including pale combs, ruffled feathers, salivation, diarrhea, drooping wings, and general body weakness (Fig. 1). Mortality was observed across all groups. However, the remaining birds, particularly in the second group, showed signs of recovery and activity.
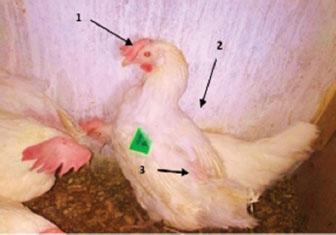
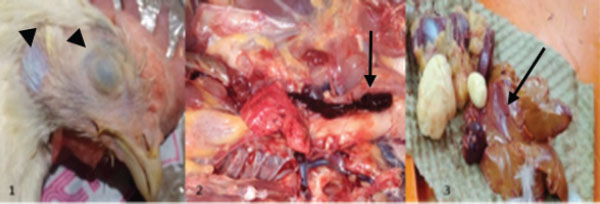
Fig. 1. Pale combs (arrow 1), ruffled feathers (arrow 2), and drooping wings (arrow 3). Gross lesionsDuring the experiment, sudden death was observed in four chickens. The pathological anatomy of the chickens revealed symptoms such as suffocation, oxygen deprivation, and a bluish discoloration of the skin in cases of acute? Death. Fresh blood accumulation was noted in the abdominal cavity, while the cecum contained blood and displayed damage to the mucous membrane. The liver appeared less firm, pale, and fragile, and exhibited pathological damage along with the presence of blood, which led to the production of green feces. Additionally, fluid buildup in the lungs may have contributed to respiratory difficulties (Figs. 2 and 3).
Fig. 2. Pathological anatomy of a chicken.
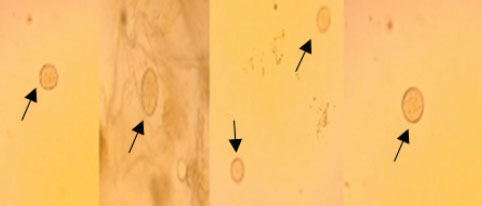
Fig. 3. The cecum is filled with blood, and the mucous is damaged. Direct examination of the fecal samplesMicroscopic diagnosis, which focuses on evaluating the oocyst size and shape (Fig. 4).
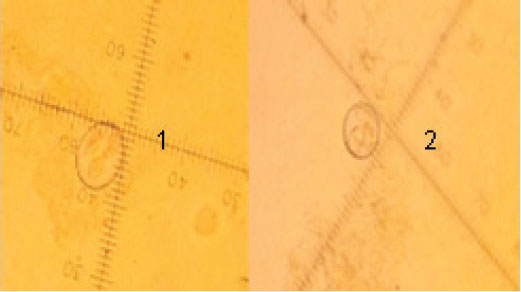
Fig. 4. Oocysts of Emeria sp. (black arrows) from fecal samples of the birds (400×). Identification of Eimeria speciesThe Eimeria oocysts were identified as Eimeria tenella and Eimeria mitis, which were distinguished based on their size and shape morphology. E. tenella appeared ovoid with a smooth surface texture, measuring 22.5 × 15 μl. Meanwhile, E. mitis exhibited a subspherical shape, with a smooth surface texture, but was smaller in size, measuring 17.5 × 17.5 μl (Fig. 5).
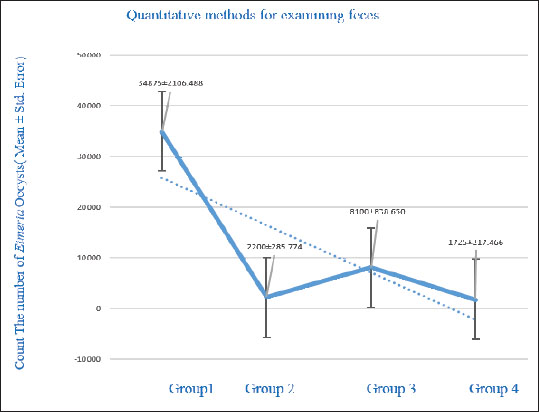
Fig. 5. Mature oocysts of Eimeria sp. E. tenella (1) and E. mitis (2) (100×). Counting the oocysts of Eimeria in the fecal samplesThe current findings revealed a significant reduction (p < 0.05) in the number of Eimeria oocysts excreted in feces among the groups infected with the parasite and treated with potassium permanganate and S. costus compared with the positive control group. The highest number of oocysts excreted in the positive control group was 34,875 oocysts per gram. Group 3, which received potassium permanganate and 0.5 ml of S. costus, with 8,100 oocysts per gram. Group 2, treated only with potassium permanganate, recorded 2,200 oocysts per gram. The lowest level was observed in group 4, which was treated with potassium permanganate and 1 ml of S. costus, yielding 1,725 oocysts per gram. Figure 6 illustrates the effect of potassium permanganate and S. costus on the number of Eimeria oocysts in various groups at the end of the study. There was a significant reduction in the number of Eimeria oocysts (p < 0.05) (mean ± SE).
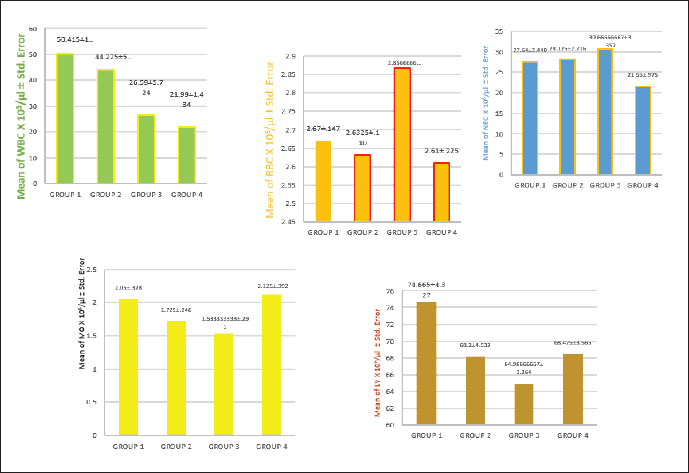
Fig. 6. Effect of treatments on coccidia. Blood picture and biochemical testsThe blood test results outlined in Figure 7 reveal significant differences (p < 0.05) in the WBC count across the groups. The positive control group showed the highest WBC level at 50.42 × 103/μl, while group 4 recorded the lowest value at 21.99 × 103/μl. Group 2 presented a value of 44.23 × 103/μl, and group 3 displayed a level of 26.59 × 103/μl. For total RBC counts, significant differences (p < 0.05) were observed among the groups, indicating the effects of the treatments on these values. Group 3 had the highest RBC value at 2.87 × 106/μl, followed by the positive control group with a value of 2.67 × 106/μl. The lowest value was recorded in group 4 at 2.61 × 106/μl, while group 2 registered a level of 2.63 × 106/μl, as shown in Figure 7. In this study, DLC for chickens infected with E. tenella and E. mitis revealed an increase in LY numbers in the positive control group, reaching 74.66 × 103/μl, which was higher than that in the other groups. The lowest LY count was observed in group 3 at 64.97 × 103/μl, whereas groups 2 and 4 recorded values of 68.20 × 103/μl and 68.48 × 103/μl, respectively. For MO, group 4 recorded a value of 2.13 × 103/μl, slightly higher than that of the positive control group at 2.05 × 103/μl. Group 2 showed a monocyte count of 1.73 × 103/μl, while group 3 had the lowest level at 1.53 × 103/μl.
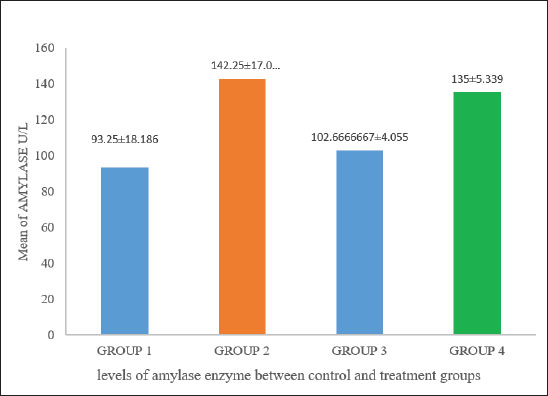
Fig. 7. Comparison of the number of cells in the blood count. Figure 7 indicates significant differences (p < 0.05) in neutrophil counts among the groups. Group 3 had the highest value, registering 30.67 × 103/μl, whereas group 4 recorded the lowest value at 21.55 × 103/μl. Meanwhile, the positive control and group 2 presented values of 27.64 × 103/μl and 28.13 × 103/μl, respectively. Biochemical tests illustrate a comparison of amylase activity across different groups, with values recorded at 93.25, 142.25, 102.66, and 135 U/l. A significant difference in amylase activity was observed between the positive control and treatment groups (p < 0.05) (Fig. 8).
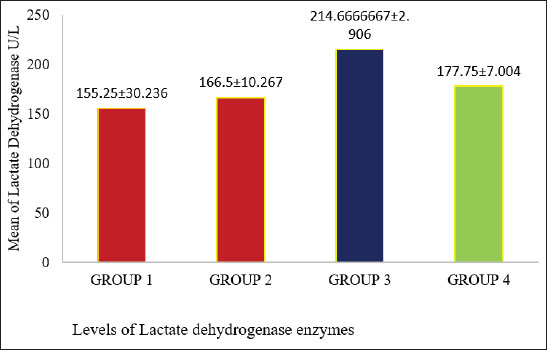
Fig. 8. Different amylase enzyme levels between the positive control and treatment groups (p < 0.05). Figure 9 demonstrates a comparison of LDH activity levels across different groups. The LDH activities were significantly different between the positive control and treatment groups (p < 0.05). Enzyme activity was highest in group 3, whereas the positive control group had the lowest activity.
Fig. 9. Displays different values of LDHEs and compares the levels between the study groups (p < 0.05). DiscussionThe cecum epithelium, capillary blood vessels, necrosis, and bleeding can be observed in the affected specimens. The most severe macroscopic lesions were noted in the positive control group, where the cecum appeared filled with blood and contained a cheesy pulp (McDougald, 2003). This group did not receive treatment with potassium permanganate or S. costus, which are used to mitigate the harmful effects of the E. tenella parasite. The presence of many oocysts in this group compared to others highlights the infection severity. Variations in the number of excreted oocysts serve as a key indicator for evaluating the intensity of infection and the effectiveness of coccidiosis treatments. The gradual decline in the number of excreted oocysts may be attributed to the introduction of potassium permanganate into drinking water in the second group and its combination with S. costus extract (1 ml) in the treated group. LYs, MO, and NET play crucial roles as defensive WBCs, with their numbers fluctuating in response to infections. These cells are believed to can engulf or counteract infectious agents or foreign substances. An increase in LYs was noted in the blood of chickens within the positive control group, while the third group exhibited a lower percentage of these cells. In contrast, an elevated monocyte count was identified in the fourth group and an increase in NET in the third group (Burton and Guion, 1986). These findings align with the observations of Kogut et al. (2005) and Meskerem et al. (2013), who documented an elevated differential leukocyte count in coccidia-infected broiler chickens. The heightened LY count may result from an activated immune response in the infected birds, likely triggered by increased lymphopoiesis as an initial defense mechanism against infection. The total WBC count showed a marked increase in the positive control group compared with the other groups, likely due to extensive exposure to high numbers of parasite oocysts. This rise in leukocyte count may reflect the immune system’s reaction to coccidial infection in chickens and the resulting severe tissue damage. Such an increase indicates a significant escalation in disease severity (p < 0.05). The elevated WBC levels in the positive control group were attributed to the immune response triggered by the presence of cysts, which act as antigens. This antigenic presence stimulated both humoral and cellular immune responses, leading to higher WBC counts as part of the body’s defense mechanism, as noted by Islam et al. (2004). Conversely, the groups treated with potassium permanganate and *Saussurea costus* exhibited relatively lower WBC counts compared to the positive control group. This reduction can be attributed to the effects of the treatments. These interventions appeared to mitigate poultry coccidiosis by curbing oocyst proliferation and inhibiting the growth of Eimeria species in the gastrointestinal tissue of chickens. In addition, they improved intestinal health by promoting increased epithelial turnover and reducing intestinal permeability. The findings from the current experiment revealed an increase in the total number of RBCs in the groups treated with potassium permanganate and S. costus (0.5 ml) compared to the positive control groups. This can be attributed to the beneficial effects of the treatments, which aid in cleaning the digestive tract and repairing the damaged cecum lining by stimulating cellular growth and division. These processes enhance the body’s ability to regulate growth, digestion, and absorption, allowing for improved utilization of nutrients essential for vital functions. This positively impacts the overall RBC count by reducing cecal bleeding caused by parasitic infection (Boa-Amponsem et al., 2000). In contrast, the reduction in the RBC count observed in the positive control group was primarily linked to cecal bleeding, which resulted in blood loss and a subsequent decline in RBC levels. In addition, this condition disrupts normal digestion and nutrient absorption, leading to a deficiency in essential nutrients required for growth, tissue repair, and renewal (Bogado et al., 2010). In the chicken experiment, the treatment group exhibited a highly significant increase in serum amylase levels compared with the positive control group infested with coccidian parasites, particularly in groups 2 and 4. This outcome aligns with findings by Major and Ruff (1978), who observed a reduction in pancreatic weight and a general decrease in amylolytic activity within the pancreas in broiler chickens infected with Eimeria acervulina, Eimeria maxima, and Eimeria necatrix. Among these, only E. acervulina and E. maxima reduced amylolytic activity in the affected areas’ surface mucosa. A decline in Enzyme activity Instead activity was directly correlated with the increasing severity of infection, as reflected by lesion scores linked to E. acervuline infections. Biochemical analyses revealed a significant increase in serum LDH activities in the treated groups, particularly in group 3. Plasma LDH and amylase activity were elevated, likely due to the toxic effects of interventions. Previous studies have documented a notable increase in serum LDH activity in chickens infected with fowl typhoid treated with neem leaf extract. As described by Raheja and Jakhar (2005), this elevated LDH activity is commonly associated with processes involving cellular necrosis, such as hepatocellular necrosis, myocardial damage, renal necrosis, pancreatic necrosis, and hemolysis Abboud and Storey (2013). AcknowledgmentsNone. Conflict of interestThe authors declare that there is no conflict of interest. FundingNone. Authors’ contributionsNagia A.S. Abdalsalam: Corresponding author, choosing a research idea, caring for chickens, distributing groups and dosing chickens, collecting samples, conducting necessary analyses, dissection, writing and statistical analysis, and financial support. Somia A. Alsanousi: Confirmation of results, writing. Hanan A. Alkilani: Concerning chickens, distributing groups and dosing chickens, collecting samples, conducting necessary analysis, and dissection, financial support. Ibrahim Saleh Milad: Confirmation of results, writing. Data availabilityAll data were provided in the manuscript. ReferencesAbadi, A., Arya, M. and Shahid, N. 2012. Prevalence and aetiology of poultry coccidiosis and associated risk factors in White Leghorn Grower chickens at Kombolcha poultry farm, Ethiopia. J. Words. Poult. Res. 2(3), 54–59. Abboud, J. and Storey, K.B. 2013. Novel control of lactate dehydrogenase from the freeze tolerant wood frog: role of posttranslational modifications. Peer J. 1, e12. Allen, J.E. and Maizels, R.M. 2011. Diversity and dialogue in immunity to helminths. Nat. Rev. Immunol. 11, 375–388. Anderson, G. and Jorgensen, W.K. 2004. Live vaccines for three species of Eimeria RIRDC publication No. 03/143 RIRDC project No. DAQ-259. Ausralian: Research and Development Corporation Boa-Amponsem, K., Price, S.E., Picard, M., Geraert, P.A. and Siegel, P.B. 2000. Vitamin E and immune Responses of broiler pure line chickens. Poult. Sci. 79, 466–476. Bogado, A.L.G., Garcia, J.L., Nunesda-Silra, P.F., Balarin, M.R.S. and Junior, J.G. 2010. Post challenge hematological evaluation with virulent B strain of Eimeria tenella in broilers immunized With attenuated or sporozoite proteins from homologous strain. Rev. Bras. Parasitol. Vet. Jabot. 19(1), 1–6. Burton, R. and Guion, C.W. 1986. The differential leucocyte blood count: its precision and individuality in the chicken. Poult. Sci. 47, 1945–1949. Calnek, B.W., Barnes, H.J., Beard, C.W., McDougald, L.R. and Saif, Y.M. 1997. Disease of poultry, Conway, D.P. and McKenzie, M.E. 2007. Poultry coccidiosis: diagnostic and testing procedures, Cynthia, M.K. and Scott, L. 2000. The Merck veterinary manual. Coccidiosis. El-Shall, N.A., Abd El-Hack, M.E., Albaqami, N.M., Khafaga, A.F., Taha, A.E., Swelum, A.A., El-Saadony, M.T., Salem, H.M., El-Tahan, A.M., Abuqamar, S.F., El-Tarabily, K.A. and Elbestawy, A.R. 2022. Phytochemical control of poultry coccidiosis: a review. Poult. Sci. 101, 101542. Haen, P.J. 1995. Principles of hematology. Harris Young, pp: 400–421. Herborn, J.B. (1973) Phytochemical methods. A guide to modern techniques of plant analysis. Avian Dis. 27, 1043–1050. Islam, M.S., Lucky, N.S., Islam, M.R., Ahad, A., Das, B.R., Rahman, M.M. and Siddiqui, M.S.I. 2004. Haematological parameters of fayonmi, assil and local chickens reared in Sylhet region in Bangladesh. Int. J. Poult. Sci 3, 144–147. Kogut, M.H., Gore, T.C. and Long, P.L. 2005. E. tenella, E. necatrix and E. adenoeides: peripheral blood leukocyte response of chickens and turkeys to strains adapted to the turkey embryo. Exp. Parasitol. 58, 63–71. Levine, N.D. 1985. Apicomplexa. In An illustrated guide to the protozoa. Eds., Lee, J.J., Hutner, S.H. and Boves, E.C. Lawrence, KS: Society of protozoologists, pp: 349–52. Major, J.R. and Ruff, M.D. 1978. Eimeria Spp.: influence of Coccidia on digestion (amylolytic activity) in broiler chickens. Exp. Parasitology 45(2), 234–240. McDonald, V. 1999. Gut intraepithelial lymphocytes and immunity to Coccidia. Parasitol. Today 15, 483–487. McDougald, L.R. 2003. Coccidiosis. In Disease of poultry, 11th ed. Ed., Saif, Y.M. Ames, IA: Iowa State Press, pp: 973–990. McDougald, L.R. and Fitz-Coy, S. 2008. Coccidiosis. 12th ed. In diseases of poultry. Ed., Saif, Y.M. Ames, IA: Black well Publishing. Meskerem, A., Chaiwat, B., Nirat, G. and Montakan, V. 2013. Haematological, biochemical and histopathological changes caused by coccidiosis in chickens. Kasetsart J. (Nat. Sci). 47, 238–246. Permin, A., Hansen, J.W. and Ikjaer, L.C. 1997. Epidemiology, diagnosis and positive control of poultry parasites. Rome, Italy: FAO. Raheja, S. and Jakhar, K.K. 2005. Studies on the effects of neem (Azadirachta indica) leaf extract on pathology of experimental fowl typhoid in broiler chickens. Indian J. Vet. Pathol. 29(2), 151. Sivarajan, V.V. and Balachandran, I. 1994. Ayurvedic drugs and their plant sources. New Delhi, India: Oxford & IBH Publishing Co. Pvt. Ltd., p: 439. Vermeulen, A.N., Schaap, D.C. and Schetters, T.P.M. 2001. Positive control of coccidiosis in chickens by vaccination. Vet. Parasitol. 100, 13–20. Williams, R.B. and Catchpol, J. 2000. A new protocol for challenge test to assess the efficacy of live anticoccidial vaccine. Vaccine 18, 1178–1185. Zhang, J.J., Wang, L.X., Ruan, W.K. and An, J. 2013. Investigation into the prevalence of coccidiosis and maduramycin drug resistance in chickens in China. Vet. Parasitol. 191, 29–34. | ||
| How to Cite this Article |
| Pubmed Style Abdalsalam NA, Alkallani HA, Milad IS, Alsanousi SA. Therapeutic effect of potassium permanganate and ethanolic extract of Saussurea costus roots on Leghorn chicken coccidiosis. Open Vet. J.. 2025; 15(9): 4181-4189. doi:10.5455/OVJ.2025.v15.i9.23 Web Style Abdalsalam NA, Alkallani HA, Milad IS, Alsanousi SA. Therapeutic effect of potassium permanganate and ethanolic extract of Saussurea costus roots on Leghorn chicken coccidiosis. https://www.openveterinaryjournal.com/?mno=261261 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i9.23 AMA (American Medical Association) Style Abdalsalam NA, Alkallani HA, Milad IS, Alsanousi SA. Therapeutic effect of potassium permanganate and ethanolic extract of Saussurea costus roots on Leghorn chicken coccidiosis. Open Vet. J.. 2025; 15(9): 4181-4189. doi:10.5455/OVJ.2025.v15.i9.23 Vancouver/ICMJE Style Abdalsalam NA, Alkallani HA, Milad IS, Alsanousi SA. Therapeutic effect of potassium permanganate and ethanolic extract of Saussurea costus roots on Leghorn chicken coccidiosis. Open Vet. J.. (2025), [cited January 25, 2026]; 15(9): 4181-4189. doi:10.5455/OVJ.2025.v15.i9.23 Harvard Style Abdalsalam, N. A., Alkallani, . H. A., Milad, . I. S. & Alsanousi, . S. A. (2025) Therapeutic effect of potassium permanganate and ethanolic extract of Saussurea costus roots on Leghorn chicken coccidiosis. Open Vet. J., 15 (9), 4181-4189. doi:10.5455/OVJ.2025.v15.i9.23 Turabian Style Abdalsalam, Nagia A.s., Hanan A. Alkallani, Ibrahim Saleh Milad, and Somia A. Alsanousi. 2025. Therapeutic effect of potassium permanganate and ethanolic extract of Saussurea costus roots on Leghorn chicken coccidiosis. Open Veterinary Journal, 15 (9), 4181-4189. doi:10.5455/OVJ.2025.v15.i9.23 Chicago Style Abdalsalam, Nagia A.s., Hanan A. Alkallani, Ibrahim Saleh Milad, and Somia A. Alsanousi. "Therapeutic effect of potassium permanganate and ethanolic extract of Saussurea costus roots on Leghorn chicken coccidiosis." Open Veterinary Journal 15 (2025), 4181-4189. doi:10.5455/OVJ.2025.v15.i9.23 MLA (The Modern Language Association) Style Abdalsalam, Nagia A.s., Hanan A. Alkallani, Ibrahim Saleh Milad, and Somia A. Alsanousi. "Therapeutic effect of potassium permanganate and ethanolic extract of Saussurea costus roots on Leghorn chicken coccidiosis." Open Veterinary Journal 15.9 (2025), 4181-4189. Print. doi:10.5455/OVJ.2025.v15.i9.23 APA (American Psychological Association) Style Abdalsalam, N. A., Alkallani, . H. A., Milad, . I. S. & Alsanousi, . S. A. (2025) Therapeutic effect of potassium permanganate and ethanolic extract of Saussurea costus roots on Leghorn chicken coccidiosis. Open Veterinary Journal, 15 (9), 4181-4189. doi:10.5455/OVJ.2025.v15.i9.23 |