| Research Article | ||
Open Vet. J.. 2025; 15(9): 4162-4180 Open Veterinary Journal, (2025), Vol. 15(9): 4162-4180 Research Article Shared resistance and virulence of Klebsiella pneumoniae from human and meat sources: A one health perspectiveQasim Zamil Bneed1, Noor Adil Abood2, Orooba Meteab Faja3*, Baneen Najm Alhasanawi3, Zahraa Sameer3and Maryam Ali Alzubaidy31College of Biotechnology, University of Al-Qadisiyah, Al Diwaniyah, Iraq 2College of Pharmacy, Al-Nahrain University, Baghdad, Iraq 3College of Veterinary Medicine, University of Al-Qadisiyah, Al Diwaniyah, Iraq *Corresponding Author: Orooba Meteab Faja. College of Veterinary Medicine, University of Al-Qadisiyah, Al Diwaniyah, Iraq. Email: orooba.faja [at] qu.edu.iq Submitted: 28/05/2025 Revised: 10/08/2025 Accepted: 19/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
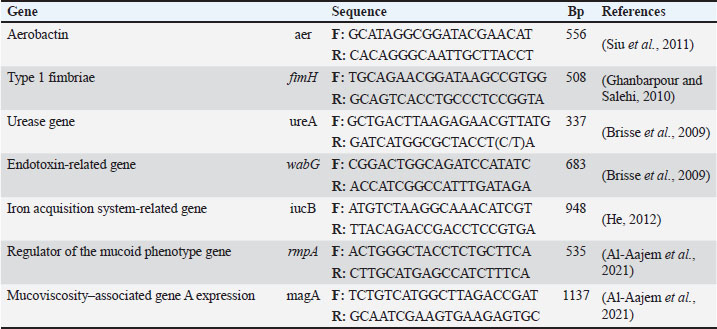
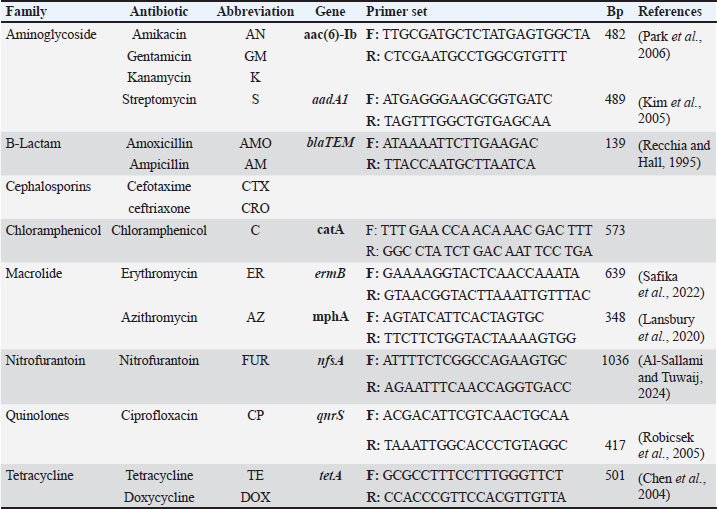
ABSTRACTBackground: Klebsiella pneumoniae is a Gram-negative bacterium that causes several infections, particularly in hospitals and food-borne settings. Aim: This study aimed to investigate the presence of K. pneumoniae in human clinical samples and raw meat from local markets. It also focused on evaluating the antimicrobial resistance patterns, resistance genes, and virulence-related markers. Methods: A total of 198 samples, including 96 from human burns and 102 from raw lamb meat, were analyzed. Klebsiella pneumoniae was isolated and identified using cultural and biochemical tests, followed by polymerase chain reaction (PCR) confirmation of the 16S rRNA gene. Antibiotic susceptibility was assessed using the disk diffusion method, and conventional PCR was used to detect nine resistance genes and six virulence genes. Genetic similarity among isolates was determined using Enterobacterial Repetitive Intergenic Consensus Polymerase Chain Reaction (ERIC-PCR). Results: Of the total samples, 73 were positive for K. pneumoniae, including 37 human isolates and 36 meat isolates. All isolates exhibited high resistance to ampicillin (100%), cefotaxime (94.52%), and erythromycin (89.04%). Common resistance genes included blaTEM (91.78%), sul1 (72.60%), and tetA (57.53%). Virulence genes, such as fimH and use, were also found to be widespread. ERIC-PCR revealed several clusters that contained both human and meat isolates, indicating genetic similarity. Conclusion: These findings confirm the circulation of multidrug-resistant and virulent K. pneumoniae strains across food and human sources. The genetic overlap between sources suggests that food is a potential transmission route. Continuous surveillance and responsible antibiotic use are essential for minimizing public health risks and improving health procedures. Keywords: Beta-lactamase, Capsular, Efflux, Enterobacteriaceae, Nosocomial, Plasmid. IntroductionKlebsiella pneumoniae (K. pneumoniae) is a gram-negative bacterium and the primary pathogen that causes nosocomial infections. Klebsiella pneumoniae can lead to pneumonia, urinary tract infection, bloodstream infection, and meningitis. Klebsiella pneumoniae is also increasingly implicated in community-acquired infections. Community-acquired infections caused by this organism were once thought to be rare. Based on virulence, K. pneumoniae strains can be divided into two morphologically indistinguishable subspecies: classical K. pneumoniae (cKP) and hypervirulent K. pneumoniae (hvKP). cKP strains largely cause nosocomial infections and are therefore endemic in clinical settings. In contrast, hvKP strains can cause community-acquired infections, including liver abscesses, lymphadenitis in neonates, and necrotizing pneumonia, typically in otherwise healthy individuals (Beig et al., 2024). Klebsiella pneumoniae is a bacterial pathogen associated with pneumonia, bloodstream infections, and urinary tract infections. Klebsiella pneumoniae has developed resistance mechanisms to many antibiotics, mainly through horizontal gene transfer, β-lactamases, efflux pumps, and mutations (Karami-Zarandi et al., 2023). The rise of hypervirulent strains carrying plasmid genes such as rmpA/rmpA2 and strong iron uptake systems has made infections more severe and difficult to treat (Kocsis, 2023; Al-Deresawi et al., 2024; Hu et al., 2024). Antibiotic resistance in bacteria and its consequences have become a global public health threat. An alarming increase in antibiotic-resistant bacteria has been reported globally. Klebsiella pneumoniae is one of the organisms with the most serious increased antibiotic resistance, leading to increasing numbers of CPKP-related infections (Li et al., 2023; Rahmat Ullah et al., 2024). Antibiotic resistance is primarily conferred by either chromosomal mutations or plasmid-mediated gene transfer (Li et al., 2024). The recruitment of resistance plasmids is often envisioned as a co-evolution process in which an antibiotic-susceptible bacterial strain acquires an antibiotic-resistant plasmid epidemic strain. One of the greatest concerns is that a CRKP ID50 cultured in the absence of the selective pressure of antibiotics could revert back to a carbapenem-susceptible strain, rendering whole-population antibiotic therapy ineffective (Nang et al., 2024). Klebsiella pneumoniae is equipped with polysaccharide capsules that protect against phagocytic uptake and complement attack, allowing the bacteria to colonize their host. In recent decades, K. pneumoniae infections have become more difficult to treat due to the emergence of antibiotic-resistant strains. Beta-lactams are the most commonly used antibiotics to treat K. pneumoniae infections; however, this pathogen has been equipped with a variety of mechanisms to avoid beta-lactam antibiotics, resulting in treatment failure (Stojowska-Swędrzyńska et al., 2021). Klebsiella pneumoniae resistance to beta-lactam antibiotics primarily results from the production of β-lactamase, allowing for widespread resistance to most β-lactam antibiotics. Klebsiella pneumoniae produces many β-lactamases, including 66 types that can break down penicillins, cephalosporins, carbapenems, and aztreonam (Lin et al., 2024). Both plasmid-borne enzymes and chromosomal β-lactamases, such as cephalosporinase and penicillinase, are common in this species. In classical strains, resistance to β-lactams is often linked to extended-spectrum β-lactamases, cephalosporinase, or carbapenemases. Recent reports have described hvKP strains with a new mechanism that alters β-lactam–binding proteins, making treatment more difficult. This pathogen can also resist aminoglycosides, fluoroquinolones, and colistin (Al-Deresawi et al., 2022; Qin et al., 2024; García-Cobos et al., 2025). In this study, we investigated K. pneumoniae in human clinical samples and raw meat. It assessed antibiotic resistance, detected resistance and virulence genes, and compared hvKP with cKP. Materials and MethodsStudy area and settingThis study was conducted in Al-Diwaniyah City, Iraq. Human clinical samples were collected from patients admitted to local hospitals in this region. Raw meat samples were purchased from butcher shops and open-air markets in the same city. All laboratory analyses were performed at the College of Veterinary Medicine, University of Al-Qadisiyah. Sample collection and processingPatient consent and ethical approval were provided by the Committee for Research Ethics, College of Veterinary Medicine, University of Al-Qadisiyah, Al-Diwaniyah City, Iraq. A total of 198 samples were collected for this study. These included 96 human burn specimens and 102 raw lamb meat samples obtained from local markets. The human samples consisted of swabs, urine, and wound exudates from hospitalized patients showing signs of infection. All samples were collected using sterile techniques and transferred immediately to cold boxes to the microbiology laboratory for further analysis. Meat samples were purchased from butcher shops and open-air meat vendors. The samples included cuts from beef, lamb, and poultry. Each piece was handled using sterile gloves and placed in sealed sterile bags before transport. Care was taken to avoid cross-contamination during packaging and delivery. All samples were inoculated into nutrient broth and incubated overnight at 37°C. After enrichment, a loopful of the broth was streaked onto MacConkey agar and incubated aerobically for 24 hours at 37°C for 24 hours. Lactose-fermenting colonies with mucoid consistency were selected and subcultured on nutrient agar for purification (Sabeeh et al., 2018). Presumptive K. pneumoniae colonies were identified based on colony morphology, Gram staining, and standard biochemical tests such as indole, citrate utilization, urease activity, and TSI reactions. Only confirmed isolates were subjected to molecular analysis. All bacterial isolates were stored in tryptic soy broth supplemented with 20% glycerol at 80°C until further testing. Storage conditions were carefully maintained to preserve the samples’ genetic and phenotypic integrity. Molecular confirmation by polymerase chain reaction (PCR)Genomic DNA was extracted from the confirmed isolates using a commercial DNA extraction kit according to the manufacturer’s instructions. The DNA concentration and purity were assessed using a NanoDrop spectrophotometer. The extracted DNA samples were stored at 20°C for downstream PCR. A conventional polymerase chain reaction assay targeting the 16S rRNA gene was used to confirm the identity of K. pneumoniae. The specific primers used for amplification were designed based on conserved regions of the 16S rRNA gene. The PCR reactions were performed in a thermal cycler with an initial denaturation step at 94°C for 5 minutes, followed by 35 cycles of denaturation at 94°C, annealing at 55°C, and extension at 72°C, with a final extension of 10 minutes. The PCR products were analyzed by agarose gel electrophoresis using a 1.5% ethidium bromide-stained gel. The expected amplicon size was approximately 1,500 bp. The presence of a sharp band at this size confirmed that the isolate was K. pneumoniae. A positive control (previously confirmed K. pneumoniae DNA) and a negative control (nuclease-free water) were included in each run. Only isolates that produced the expected band were considered valid and included in further molecular studies. All PCR runs were repeated at least twice to ensure the accuracy of the results. The gel images were documented using a gel documentation system for records and reference. Antibiotic susceptibility testingAntimicrobial resistance profiles were determined using the Kirby-Bauer disk diffusion method. Mueller-Hinton agar plates were prepared, and sterile saline was used to adjust bacterial suspensions to a 0.5 McFarland standard. Each suspension was evenly spread across the agar plate surface using sterile swabs. A total of 12 antibiotic discs were used, including ampicillin (10 µg), amoxicillin (20 µg), cefotaxime (30 µg), ciprofloxacin (5 µg), gentamicin (10 µg), imipenem (10 µg), tetracycline (30 µg), streptomycin (10 µg), erythromycin (15 µg), sulfamethoxazole (25 µg), chloramphenicol (30 µg), and ceftriaxone (30 µg). All discs were obtained from a commercial supplier. Plates were incubated at 37°C for 18–24 hours. The diameter of each inhibition zone was measured in millimeters and compared to Clinical and Laboratory Standards Institute guidelines to interpret results as sensitive, intermediate, or resistant. Isolates resistant to three or more classes of antibiotics were defined as MDR. The number and combination of resistances were recorded for each isolate. We ensured quality control using Escherichia coli ATCC 25922 as a reference strain. To confirm reproducibility, all tests were performed in duplicate. Detection of resistance and virulence genesPCR was used to detect nine resistance and six virulence genes in the isolates. The resistance genes included blaTEM, blaSHV, tetA, sul1, aadA1, catA1, qnrS, nfsA, and ermB. The virulence genes screened were fimH, use, wabG, fu, rmpA, and entB. Each gene was amplified using specific primers previously published. The PCR protocol was optimized for each target gene. All reactions were performed in 25 µl volumes, with 1 µl of template DNA, 10 pmol of each primer, and a commercial PCR master mix. The thermal cycling conditions varied slightly between genes based on the primer melting temperatures. Each experiment included both positive and negative controls. Amplified products were visualized on 1.5% agarose gels and compared with a DNA ladder to confirm the expected band sizes. The prevalence of each gene among the isolates was recorded. The co-occurrence of resistance and virulence genes within the isolates was also documented. Data are summarized in comparative tables showing frequencies and distributions between human and meat samples. These results were later used in the correlation analysis (Tables 1 and 2). Molecular typing using Random Amplified Polymorphic DNA (RAPD)- and Enterobacterial Repetitive Intergenic Consensus Polymerase Chain Reaction (ERIC-PCR)To explore the genetic diversity and relatedness among K. pneumoniae isolates, RAPD-PCR and ERIC-PCR methods were employed. These typing techniques were used in parallel to increase the fingerprinting resolution and ensure reliability. RAPD-PCR was conducted using a single arbitrary primer, which was selected based on its ability to produce clear and reproducible band patterns. The reaction was performed in a 25 µl volume containing template DNA, the RAPD primer, Taq polymerase, MgCl₂, and buffer. Cycling conditions were standardized across all samples and included a low annealing temperature to promote random amplification. ERIC1 and ERIC2 primers (Macrogen, Korea) were used to perform ERIC-PCR. The reaction components and PCR program were optimized as previously described. The amplified products were separated on 2% agarose gels and visualized under UV light after staining with ethidium bromide. Table 1. Occurrence of virulence genes in K. pneumoniae.
Table 2. Occurrence of antibiotic-resistant genes among K. pneumoniae isolates.
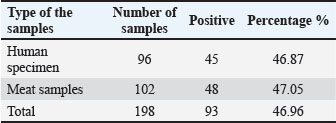
Digital images of the RAPD and ERIC gels were analyzed using gel documentation software. Banding patterns were compared using the Dice similarity coefficient. Dendrograms were created using the UPGMA clustering algorithm to visualize genetic relationships. Strains showing ≥80% similarity were considered part of the same cluster. Mixed clusters that included both meat and human isolates were marked for further analysis, as they might suggest zoonotic transmission or common environmental exposure. Statistical analysisStatistical analysis was performed using simple descriptive methods. Chi-square test was used to compare results, and p-values of 0.05 were considered significant. Ethical approvalThis study was approved by the Ethics Committee of the College of Veterinary Medicine, University of Al-Qadisiyah (approval no. 128/22-1-2024). Informed consent was obtained from all patients or their guardians prior to sample collection. ResultsIsolation and identification of K. pneumoniaeOf the 198 total samples examined, K. pneumoniae was isolated from 93 cases. This included 45 (46.87%) isolates from human clinical samples and 48 (47.05%) from raw meat samples. The overall detection rate was approximately 93 (46.96%). Large, moist, mucoid, and pink lactose-fermenting colonies were observed on MacConkey agar. Microscopic examination revealed short, plump, Gram-negative rods. Biochemical testing confirmed the isolates’ identity. All were indole-negative, urease-positive, and citrate-positive and produced gas in TSI slants. These characteristics support the preliminary identification of K. pneumoniae. Most of the positive samples from human sources were urine, wound swabs, and blood cultures. In the meat samples, isolates were recovered mainly from chicken and beef cuts, with fewer from lamb cuts. The distribution was nearly even between human and food sources, indicating a shared prevalence. The purified isolates were stored in glycerol broth at −80°C for further molecular analysis. All isolates maintained viability upon subculturing, exhibiting stable growth and colony morphology. The recovery rate from clinical and food samples suggests widespread environmental and host association with this pathogen. These findings are essential for further molecular confirmation and profiling (Table 3). Molecular confirmation by polymerase chain reaction and sequencingAll 73 presumptive isolates were tested for 16S rRNA gene amplification by PCR. Each isolate produced a clear band at approximately 1,500 bp, confirming their identity as K. pneumoniae. No amplification was observed in negative controls, whereas positive controls consistently produced the expected band (Figs. 1 and 2). The gel images showed single, sharp bands without smearing, indicating high-quality amplification. The DNA purity, assessed by NanoDrop readings, ranged between 1.75 and 2.05 for the A260/280 ratio. This confirms the suitability of the DNA for downstream applications. Repeat PCR runs for randomly selected isolates produced identical results, demonstrating good test conditions repeatability and accuracy. The DNA from both the meat and human isolates showed similar banding intensities and sizes. No contamination or false positives were observed in any of the PCR samples. The absence of non-specific bands confirmed the assay’s primer specificity and overall robustness. This step allowed for the confident classification of all isolates as K. pneumoniae. Following confirmation, each isolate was assigned a unique code and moved forward for AMR testing, gene detection, and molecular typing. This helped ensure traceability throughout the study (Table 4). Table 3. Distribution of positive samples from different types of samples
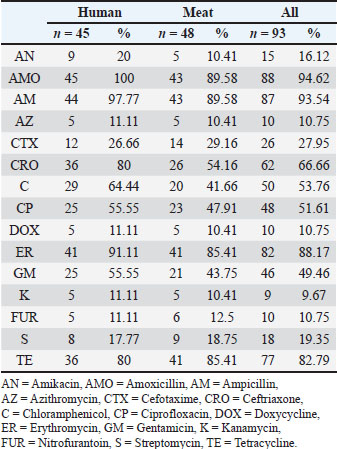
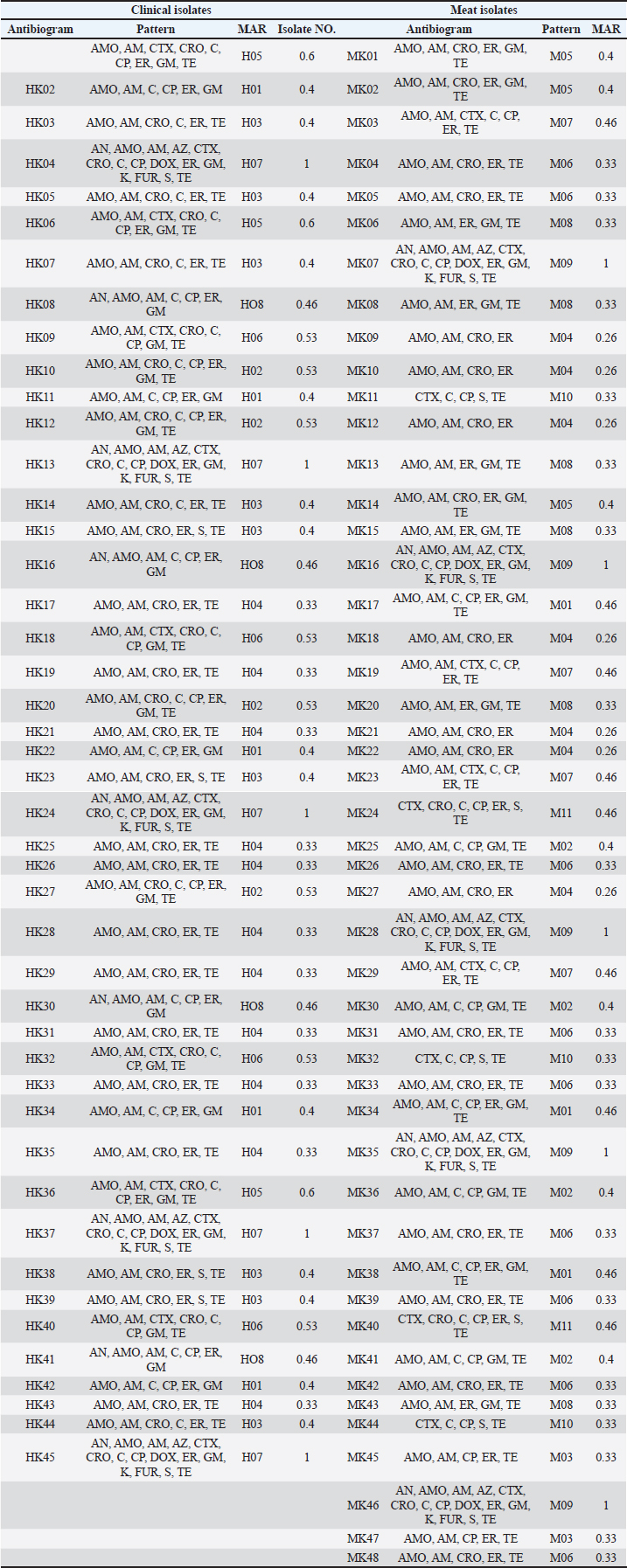
Antibiotic susceptibility profilesAll 73 isolates were ampicillin-resistant (73/73, 100%). High resistance was also found for cefotaxime (69/73, 94.5%) and erythromycin (65/73, 89.0%). Tetracycline resistance was observed in 56 of 73 isolates (76.7%). Sulfamethoxazole resistance was observed in 47 of 73 isolates (64.4%) and gentamicin resistance in 44 of 73 isolates (60.3%). Ciprofloxacin resistance was recorded in 39 of 73 isolates (53.4%), whereas streptomycin resistance was noted in 37 of 73 isolates (50.7%). Chloramphenicol was effective in 52 of 73 isolates (71.2%) and imipenem in 47 of 73 isolates (64.4%). These results indicate that most isolates were multidrug-resistant. More than 85% of the isolates were resistant to three or more classes of antibiotics, qualifying them as MDR. Several isolates were resistant to up to eight different antibiotics. This pattern was consistent across both the human and meat samples. The zone diameter readings varied across the antibiotics. For example, in all cases, the mean inhibition zone for ampicillin was 8 mm. In contrast, imipenem showed wider zones in many isolates, particularly those from meat. Some meat isolates were unexpectedly fluoroquinolones resistant. Escherichia coli ATCC 25922 showed proper sensitivity to all antibiotics, confirming the reliability of the test. These findings underline the serious resistance levels in both clinical and foodborne K. pneumoniae strains (Tables 5 and 6).
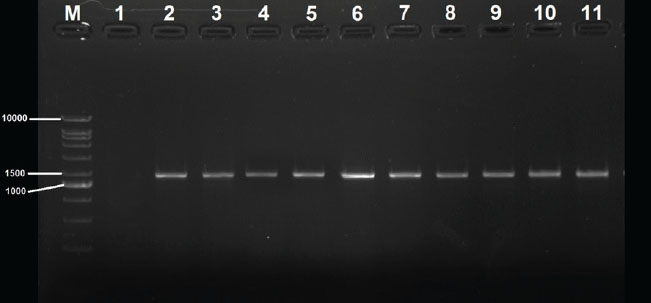
Fig. 1. Amplification of 16S rRNA of 10 K. pneumoniae isolates was fractionated on 1% agarose gel electrophoresis stained with ethidium bromide, Lane M: 1kb ladder marker; Lane1: negative control; Lane2: HK04; Lane3: HK13; Lane4: HK24; Lane5: HK37; Lane6: HK45; Lane7: MK07; Lane8: MK16; Lane9: MK28; Lane9: MK35; Lane10: MK46.
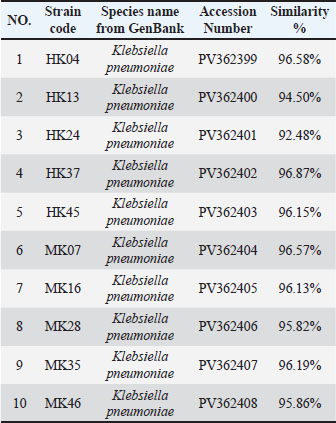
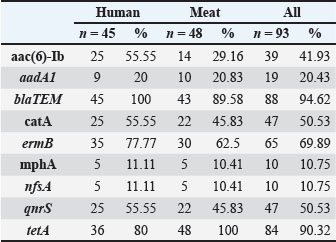
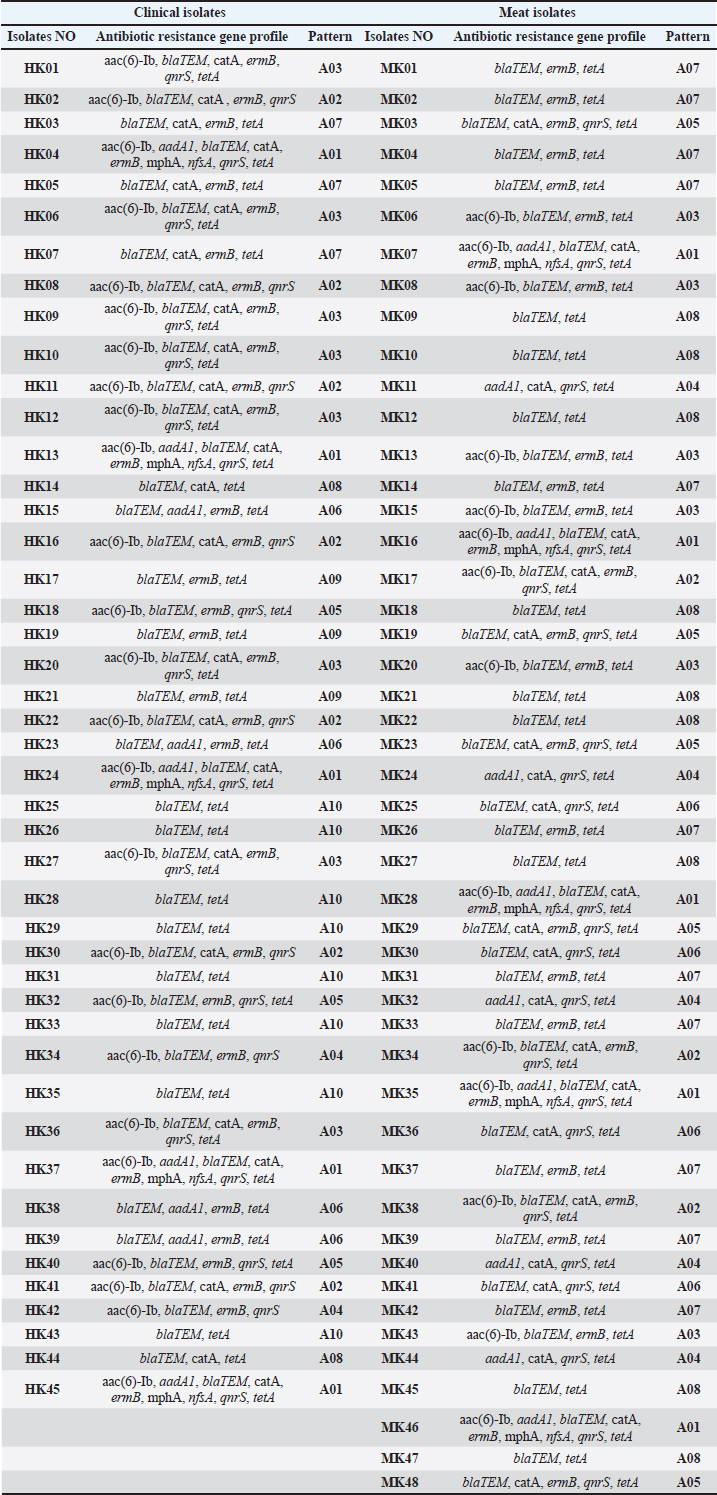
Fig. 2. Phylogenetic tree showing the evolutionary relationships of the 16S rRNA gene of eight strains of K. pneumoniae. Molecular detection of resistance genesThe blaTEM gene was the most frequently detected in 67 of 73 isolates (91.8%). The sul1 gene was found in 53 of 73 isolates (72.6%), while tetA was identified in 42 of 73 isolates (57.5%). aadA1 was present in 36 of 73 isolates (49.3%), and ermB was present in 34 of 73 isolates (46.6%). catA1 was present in 32 of 73 isolates (43.8%), qnrS in 26 of 73 isolates (35.6%), and nfsA in 19 of 73 isolates (26.0%). Several isolates carried more than one resistance gene. This highlights the presence of combined resistance traits within single strains. Several isolates carried four or more resistance genes. These multidrug gene profiles were common among clinical samples, although some meat isolates showed a similar pattern. blaTEM, sul1, and tetA were the most frequent combinations. Resistance genes were not limited to a single sample type. Many of the same gene profiles were observed in both human and meat sources, suggesting horizontal gene transfer and common environmental exposures. Gel electrophoresis of PCR amplicons showed distinct bands for each gene, with sizes matching the expected base pair lengths. Controls confirmed the accuracy of each amplification, and all positive results were verified by repeat testing (Fig. 3 and Tables 7, 8). Table 4. Comparison of K. pneumoniae isolate sequences with GenBank sequences based on the percentage of similarity using BLAST software.
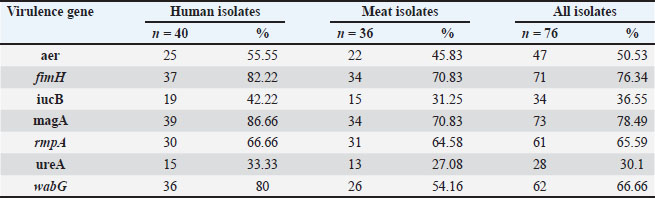
Detection of virulence genesVirulence factors were widespread, and fimH was present in 57 of 73 isolates (78.1%). Uge was detected in 52 of 73 isolates (71.2%), and wabG was detected in 47 of 73 isolates (64.4%). The entB gene was identified in 42 (57.5%) of 73 isolates. rmpA was found in 30 of 73 isolates (41.1%) and kfu in 29 of 73 isolates (39.7%). Many isolates carried multiple virulence genes, with some harboring up to five. This shows that both the clinical and meat isolates had high pathogenic potential. The coexistence of virulence genes was common. Some isolates harbored up to five different virulence genes. These strains were distributed among both human and meat samples, suggesting the widespread occurrence of highly virulent strains. Clinical isolates had a slightly higher average number of virulence genes than meat isolates, but the difference was not statistically significant. Meat isolates from poultry samples showed the highest presence of virulence genes among the food groups. Amplicons for each gene appeared as well-defined single bands. All gene sizes matched the published reference values. The results were reproducible, and each set included appropriate controls to validate amplification (Tables 9, 10 and Fig. 4). Table 5. Antibiotic resistance profiles of K. pneumoniae isolates.
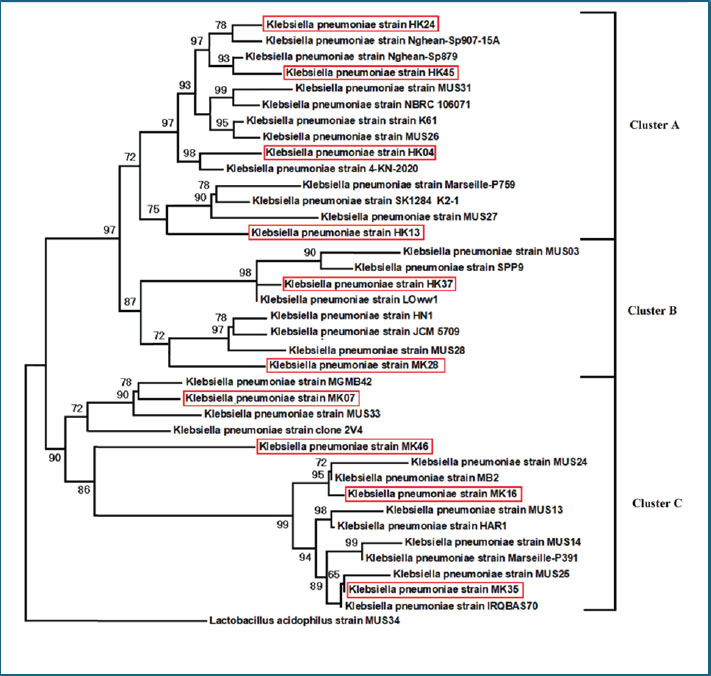
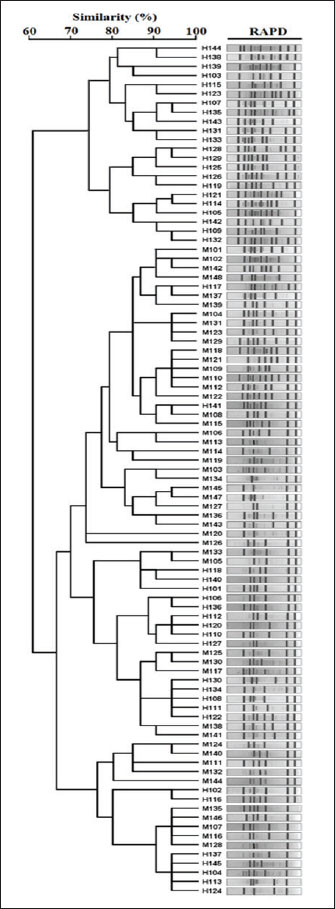
Molecular fingerprinting using RAPD-PCR and ERIC-PCRRAPD-PCR generated variable patterns across all isolates. The number of bands per isolate ranged from 3 to 10, with band sizes ranging from 200 to 2,000 bp. Banding profiles differed noticeably among isolates from different sources. ERIC-PCR produced more consistent and reproducible results. Most isolates showed 5–7 bands, with clearer separation between clusters. ERIC-PCR was more effective in grouping closely related strains based on similarity. Dendrogram analysis of combined RAPD and ERIC data revealed 11 major clusters. Some clusters included both human and meat isolates, indicating possible genetic similarity and shared ancestry. This was most evident in clusters A, C, and F. Several meat isolates were found in the same clades as hospital isolates, suggesting either contamination or a common origin. Some isolates remained distinct, showing no close genetic links to others in the dataset. The dual-typing approach using RAPD and ERIC-PCR improved strain discrimination resolution and confidence. Genetically related and highly resistant K. pneumoniae strains are circulating in both food and health care environments (Fig. 5). Table 6. Antibiotic resistance pattern and MAR of K. pneumoniae isolates.
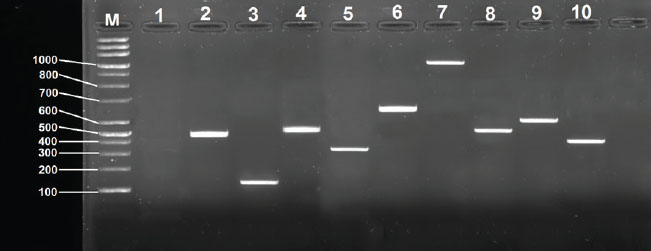
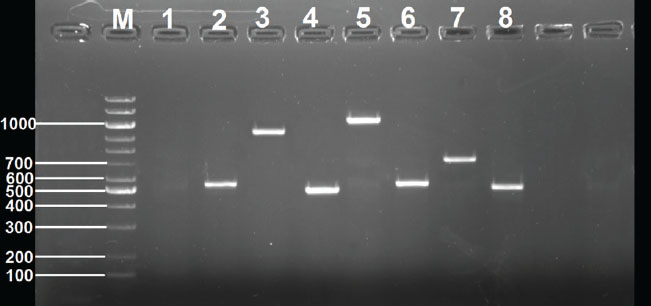
Fig. 3. Detection of antibiotic resistance genes in K. pneumoniae isolate MK16 by PCR, electrophoresed on 1.5 % (w/v) agarose gel. Lane M: 100-bp DNA ladder. Lane 1: Negative control. Lane 2: aac(6)-Ib gene. Lane 3: the blaTEM gene. Lane 4: aadA1 gene Lane 5: mphA gene. Lane 6: ermB Lane 7: nfsA gene. Lane 8: tetA gene Lane 9: catA gene. Lane 10: qnrS gene Table 7. Antimicrobial resistance genes in Klebsiella pneumoniae.
DiscussionThe results confirmed a high occurrence of K. pneumoniae in both human and meat samples (Morante et al., 2021). The nearly equal distribution of isolates between clinical and food sources highlights this bacterium’s wide environmental adaptability. These findings are consistent with those of prior work in India, where K. pneumoniae was isolated from a variety of sources with similar prevalence and resistance trends (Karim et al., 2018). The high recovery rate in meat samples is consistent with reports from Iran (Eghbalpoor et al., 2019) and China (Yang et al., 2021) showing that retail meat can act as a vehicle for K. pneumoniae transmission to humans, especially when food hygiene is poor. Identification of this bacterium in food products calls for more rigorous monitoring of animal-sourced foods in markets and slaughterhouses. All isolates in this study were confirmed by 16S rRNA PCR, and the confirmation rate supported the reliability of classical and biochemical methods as a first-line identification tool. However, molecular testing remains essential for definitive classification and should be incorporated into surveillance programs (Bobbadi et al., 2021). Antibiotic susceptibility data revealed concerning levels of MDR. The universal resistance to ampicillin and high resistance to cephalosporins and macrolides agree with global trends showing increasing treatment challenges in both hospitals and communities (Yang et al., 2021). Resistance was high across both human and meat isolates. This reinforces the possibility of antibiotic misuse in livestock production, contributing to resistant strains that enter the food chain and affect human health, as noted in previous meat-related K. pneumoniae studies (Robicsek et al., 2005; Siu et al., 2011). The detection of blaTEM, sul1, and tetA genes in most isolates confirms the molecular basis of the observed resistance. These genes are widely distributed in gram-negative pathogens and are often located on mobile genetic elements, supporting the idea of gene transfer between clinical and environmental strains (Morante et al., 2021). The presence of qnrS and ermB genes in a considerable number of isolates indicates resistance to quinolones and macrolides. In recent years, these genes have been frequently reported in hospital-acquired and foodborne K. pneumoniae isolates (Pham et al., 2024). Their spread is linked to plasmid transfer and antibiotic pressure. Identification of multiple resistance genes in single isolates is of particular concern. This indicates the co-selection and persistence of resistance traits under antibiotic exposure. Similar findings have been described in bloodstream and urinary isolates in other regions (Yang et al., 2021). The genetic basis of resistance in our isolates aligns with genomic studies that have found large plasmids harboring resistance and virulence gene clusters. These mobile elements increase bacterial adaptability and complicate treatment (Li et al., 2022). Table 8. Antimicrobial resistance gene pattern of K. pneumoniae.
Table 9. Virulence gene in K. pneumoniae isolates.
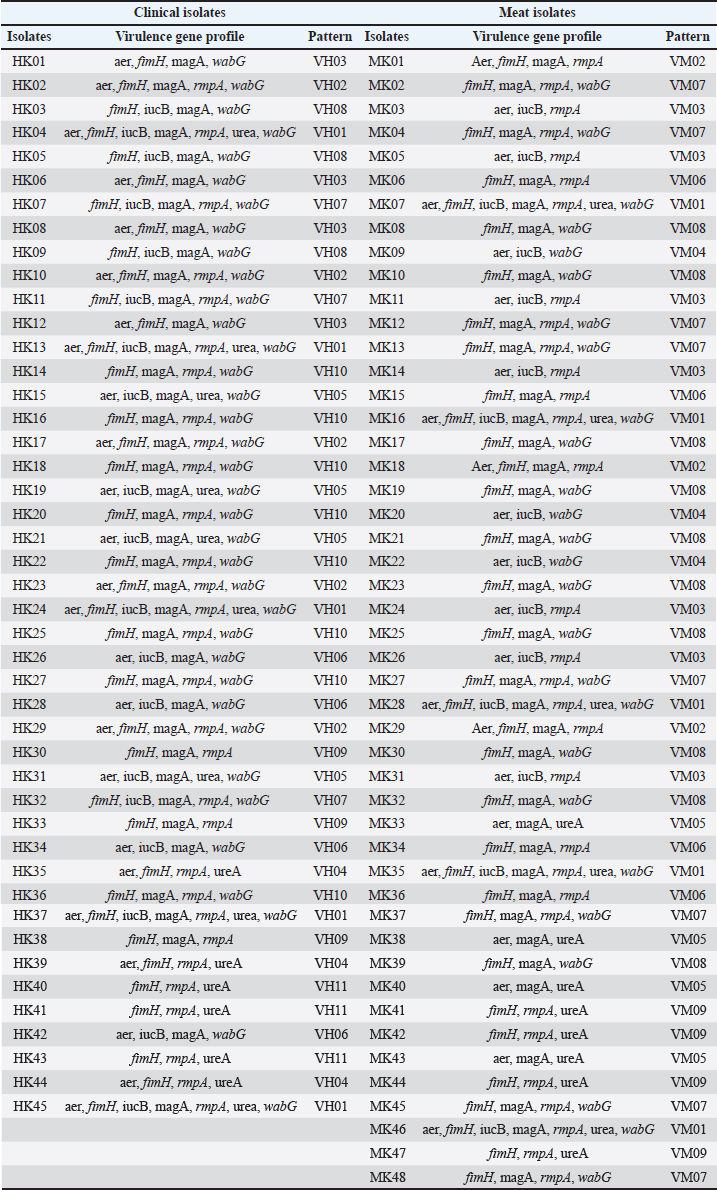
Table 10. Virulence gene pattern in K. pneumoniae isolates from clinical sources and meat from the market.
Fig. 4. Detection of virulence genes in K. pneumoniae isolate MK07 by PCR, electrophoresed on 1.5 % (w/v) agarose gel. Lane M: 100-bp DNA ladder. Lane 1: Negative control. Lane 2: aer gene. Lane 3: iucB gene. Lane 4: fimH gene. Lane 5: magA gene. Lane 6: rmpA gene. Lane 7: wabG gene. Lane 8: ureA gene. The presence of fimH and uge virulence genes in most isolates suggests strong adhesion and colonization potential. These traits are critical for initiating urinary tract and bloodstream infections, as supported by other studies from Southeast Asia and Europe (Håkonsholm et al., 2022). The wabG gene, which is involved in lipopolysaccharide synthesis, was also common among our isolates. Its presence enhances the bacterium’s ability to evade host immunity and is associated with severe forms of infection (Håkonsholm et al., 2022; Voellmy et al., 2022). The detection of rmpA and kfu genes in meat and human isolates reflects the increasing spread of hypervirulent K. pneumoniae (hvKP). These genes are often found in strains capable of causing liver abscesses and invasive disease even in healthy individuals (Lansbury et al., 2020; Li et al., 2022; Kocsis, 2023). The entB gene was present in more than half of the isolates. It plays a major role in iron acquisition via siderophore systems, which are essential for host tissue survival. Previous studies have shown that iron uptake systems are key virulence features of both classical and hvKP (Voellmy et al., 2022). The coexistence of multiple virulence genes in food isolates is alarming. Foodborne strains are not only resistant but also potentially hypervirulent. This agrees with findings from food protection studies where virulent and resistant strains were recovered from ready-to-eat products (Siu et al., 2011).
Fig. 5. Dendrogram of typeable K. pneumoniae isolates produced from RAPD analysis using the average linkage unweighted group pair method with arithmetic averages (UPGMA). ERIC-PCR and RAPD-PCR genotyping revealed several genetic clusters containing both human and meat isolates. These results strongly suggest that the two environments share sources or transmission. Similar clustering patterns have been reported in typing studies from India and Africa (Robicsek et al., 2005; Bobbadi et al., 2021). The observed overlap in fingerprint profiles confirms the idea of genetic relatedness and supports the One Health perspective. Due to poor biosecurity and antimicrobial stewardship, the same strains may circulate in animals, humans, and food products (Morante et al., 2021; Håkonsholm et al., 2022). The high genetic diversity of the isolates reflects the ongoing evolution and adaptation of K. pneumoniae. This diversity is driven by antibiotic exposure, mobile elements, and selective pressure in clinical and agricultural environments (Yang et al., 2021; Li et al., 2022). Some isolates exhibited unique banding patterns and did not cluster with others. These outliers might represent new or imported strains or strains that have undergone significant genetic shifts. Whole-genome sequencing would provide more clarity on this (Håkonsholm et al., 2022). Bacteria can acquire new genes and survive in multiple hosts, making them formidable pathogens (Al-Aajem et al., 2021). These exposures often result in the selection of partially resistant populations (Habeeb, 2024). The evolution of resistance in response to disinfectants such as isopropanol and chlorhexidine is another worrying trend in K. pneumoniae strains (Morante et al., 2021). Surveillance data now highlight the importance of simultaneously detecting resistance and virulence genes. Some carbapenem-resistant strains carry high virulence genes, making them more difficult to treat and more likely to cause outbreaks (Lansbury et al., 2020; Li et al., 2022). The emergence of resistant K. pneumoniae in livestock, food products, water, and hospital settings presents a significant challenge from a public health perspective. The presence of resistance genes in meat isolates from our study is consistent with the risks posed by such practices (Håkonsholm et al., 2022; Li et al., 2022; Lu et al., 2022; Luna-Pineda et al., 2024; Xu et al., 2024). Improved sanitation, proper food handling, and consumer education are also vital. Bacterial contamination of meat can be reduced by improving hygiene practices during slaughter, transport, and retail, which are all critical steps in the transmission pathway (Habeeb, 2018; Yaseen et al., 2020; Ghazi et al., 2024). ConclusionThis study revealed a high prevalence of K. pneumoniae in both human clinical samples and raw meat, emphasizing the widespread presence of this pathogen in the environment and food chain. Resistance genes such as blaTEM, sul1, and tetA were frequently detected, confirming the molecular basis of this resistance. In addition, many isolates harbored multiple resistance genes, which increased the risk of treatment failure. The presence of key virulence genes, including fimH, uge, wabG, and rmpA, in human and meat isolates indicates the potential of these strains to cause serious infections. The detection of these genes in foodborne strains is particularly concerning because it suggests that contaminated food could serve as a vehicle for hypervirulent pathogens. Molecular typing using RAPD-PCR and ERIC-PCR revealed that several isolates from different sources were genetically similar. This genetic overlap points to the possibility of transmission through the food chain or environmental routes and highlights the urgent need for one health-based surveillance. This study shows that the same types of K. pneumoniae were found in both people and raw meat. This means that food could be a way for bacteria to spread. This is a warning for public health. These results suggest a risk of cross-transmission between humans and food. Better food safety and careful use of antibiotics are required. One limitation of this study is that it did not use whole-genome sequencing. This could provide deeper information about how the bacteria are related. AcknowledgmentThe authors thank the College of Veterinary Medicine, University of Al-Qadisiyah, for their support in this study. FundingThe authors self-funded the study. No external funding source is available. Authors’ contributionsAll authors participated in the study. Conflict of interestThe authors declare no conflicts of interest. Data availabilityData are available when requested by the corresponding author. ReferencesAbd-Alhassen, J.K., Janabi, A.H.D. and Aboktifa, M.A. 2021. Antioxidant and antimicrobial evaluation of lycopene isolated from watermelon. Biochem. Cell. Arch. 21, 2905–2910. Ahmadi, M., Ranjbar, R., Behzadi, P. and Mohammadian, T. 2022. Virulence factors, antibiotic resistance patterns, and molecular types of clinical isolates of Klebsiella pneumoniae. Expert Rev. Anti-Infect. Therapy 20(3), 463–472; doi:10.1080/14787210.2022.1990040 Al-Aajem, B.M.R., Jasim, H.M. and Saleem, A.J. 2021. Detection of virulent genes Khe, iuc, rmp, magA in Klebsiella pneumoniae isolated from urinary tract infection. Int. J. Drug Deliv. Technol. 11(4), 1470–1473; doi: 10.25258/ijddt.11.4.60 Al-Deresawi, T.S. 2024. Isolation and characterization of fermenting bacterial isolates from vinegar industry waste in local markets of Wasit Province. Karbala Int. J. Modern Sci. 10(3), 442–448. Al-Deresawi, T.S., Mohammed, M.K. and Khudhair, S.H. 2022. The use of bacterial cellulose produced from the local isolate Komagataeibacter xylinus TELE as an antibacterial for wounds. Int. J. Drug Del. Technol. 12(4), 1825–1830; doi:10.25258/ijddt.12.4.56 Al-Fahady, M.Q. and Hameed, H.M. 2023. Bioceutical role of nano and organic selenium on certain reproductive value of laying hen during force molting. Iraqi J. Vet. Sci. 37(2), 325–331; doi:10.33899/ijvs.2022.134401.2364 Al-Sallami, Z.S.M. and Tuwaij, N.S.S. 2024. Molecular study of antibiotic resistance gene among nitrofurantoin-resistant gram-negative bacteria isolates from pregnant women. BIO Web Conf. 139, 06023. doi:10.1051/bioconf/202413906023 Ballén, V., Gabasa, Y., Ratia, C., Ortega, R., Tejero, M. and Soto, S. 2021. Antibiotic resistance and virulence profiles of Klebsiella pneumoniae strains isolated from different clinical sources. Front. Cell. Infect. Microbiol. 11, 738223; doi:10.3389/fcimb.2021.738223 Beig, M., Aghamohammad, S., Majidzadeh, N., Asforooshani, M.K., Rezaie, N., Abed, S., Khiavi, E.H.G. and Sholeh, M. 2024. Antibiotic resistance rates in hypervirulent Klebsiella pneumoniae strains: a systematic review and meta-analysis. J. Global Antimicrob. Resist. 38, 376–388; doi:10.1016/j.jgar.2024.06.018 Bobbadi, S., Chinnam, B.K., Reddy, P.N. and Kandhan, S. 2021. Analysis of antibiotic resistance and virulence patterns in Klebsiella pneumonia isolated from human urinary tract infections in India. Lett. Appl. Microbiol. 73(5), 590–598; doi:10.1111/lam.13544 Brisse, S., Fevre, C., Passet, V., Issenhuth-Jeanjean, S., Tournebize, R., Diancourt, L. and Grimont, P. 2009. Virulent clones of Klebsiella pneumoniae: identification and evolutionary scenario based on genomic and phenotypic characterization. PLoS. One 4(3), e4982; doi:10.1371/journal.pone.0004982 Chen, J.W., Lu, J.Y., Zhang, R. and Cai, J.C. 2021. Antibiotic resistance and virulence characteristics analysis of a carbapenem-resistant hypervirulent Klebsiella pneumoniae. Zhonghua Yi Xue Za Zhi 101(31), 2478–2484; doi:10.3760/cma.j.cn112137-20201119-03143 Chen, L., Deng, M., Wang, J., Wu, T., Zhou, S., Yang, R., Zhang, D. and Zou, M. 2024. Antibiotic resistance and epidemiological characteristics of polymyxin-resistant Klebsiella pneumoniae. J. Cent. South Univ. Med. Sci. 49(5), 737–747; doi: 10.11817/j.issn.1672-7347.2024.230567 Chen, S., Zhao, S., White, D.G., Schroeder, C.M., Lu, R., Yang, H., Mcdermott, P.F., Ayers, S. and Meng, J. 2004. Characterization of multiple-antimicrobial-resistant Salmonella serovars isolated from retail meats. Appl. Environ. Microbiol. 70(1), 1–7; doi:10.1128/AEM.70.1.1-7.2004 Clinical and Laboratory Standards Institute. 2023. Performance standards for antimicrobial susceptibility testing (33rd ed., CLSI supplement M100). Wayne, PA: Clinical and Laboratory Standards Institute. Eghbalpoor, F., Habibi, M., Azizi, O., Asadi Karam, M.R. and Bouzari, S. 2019. Antibiotic resistance, virulence and genetic diversity of Klebsiella pneumoniae in community- and hospital-acquired urinary tract infections in Iran. Acta Microbiol. Immunol. Hungarica 66(3), 349–366; doi:10.1556/030.66.2019.006 García-Cobos, S., Oteo-Iglesias, J. and Pérez-Vázquez, M. 2025. Hypervirulent Klebsiella pneumoniae: epidemiology outside Asian countries, antibiotic resistance association, methods of detection and clinical management. Enfermedades Infecc. Microbiol. Clín. 43(2), 102–109; doi: 10.1016/j.eimce.2024.12.008 Ghanbarpour, R. and Salehi, M. 2010. Determination of Adhesin encoding genes in Escherichia coli Isolates from omphalitis of chicks. Am. J. Anim. Vet. Sci. 5(2), 91–96; doi: 10.3844/ajavsp.2010.91.96 Ghazi, A.M., Ali Al-bayati, M.A. and Janabi, A.H. 2024. Metabolomics-detected alterations generated by phytosomal propolis and phytosomal lycopene in male rats with induced benign prostatic hyperplasia. Iraqi J. Vet. Sci. 38(Suppl I–IV), 7–15; doi:10.33899/ijvs.2024.147764.3531 Giri, S., Shekar, M., Shetty, A.V., G, P.T. and Shetty, A.K. 2021. Antibiotic resistance and random amplified polymorphic DNA typing of Klebsiella pneumoniae isolated from clinical and water samples. Water Environ. Res. 93(11), 2740–2753; doi:10.1002/wer.1630 Golsha, R., Montazeri, M., Razaghi, N. and Zade, M.E. 2021. Frequency of beta-lactamase antibiotic resistance genes in Escherichia coli and Klebsiella pneumoniae. Ethiopian J. Health Sci. 31(3), 663–672; doi:10.4314/ejhs.v31i3.24 Habeeb, A.A.. 2018. Detection of drug resistance gene expression in Candida albicans isolated from oral thrush of children via real-time PCR technique. J. Pharm. Sci. Res. 10(3), 594–596. Habeeb, A.A.. 2024. Status of common communicable diseases in children tested using ELISA methods in Wasit Province, Iraq: a retrospective study. J. Commun. Dis. 56(1), 16–20; doi:10.24321/0019.5138.202404 Håkonsholm, F., Hetland, M.A.K., Svanevik, C.S., Lunestad, B.T., Löhr, I.H. and Marathe, N.P. 2022. Insights into the genetic diversity, antibiotic resistance and pathogenic potential of Klebsiella pneumoniae from the Norwegian marine environment using whole-genome analysis. Int. J. Hygiene Environ. Health 242, 113967; doi:10.1016/j.ijheh.2022.113967 Hartantyo, S.H.P., Chau, M.L., Koh, T.H., Yap, M., Yi, T., Cao, D.Y.H., Gutiérrez, R.A. and Ng, L.C. 2020. Foodborne Klebsiella pneumoniae: virulence potential, antibiotic resistance, and risks to food safety. J. Food Prot. 83(7), 1096–1103; doi:10.4315/JFP-19-520 Hassan, W.S., Abdulrazzaq, K.M., Al-Obaidi, Q.T. and Al-Azow, K.A. 2024. Molecular detection of Anaplasma platys in dogs in Nineveh province, Iraq. Iraqi J. Vet. Sci. 38(3), 677–682; doi:10.33899/ijvs.2024.148465.3592 He, J.Y. 2012. Study on serotype and distribution on characterization of virulence genes of Klebsiella pneumonia, Doctoral dissertation, Chongqing Medical University, Chongqing. Hu, F., Pan, Y., Li, H., Han, R., Liu, X., Ma, R., Wu, Y., Lun, H., Qin, X., Li, J., Wang, A., Zhou, M., Liu, B., Zhou, Z. and He, P. 2024. Carbapenem-resistant Klebsiella pneumoniae capsular types, antibiotic resistance and virulence factors in China: a longitudinal, multi-centre study. Nat. Microbiol. 9(3), 814–829; doi:10.1038/s41564-024-01612-1 Karami-Zarandi, M., Rahdar, H.A., Esmaeili, H. and Ranjbar, R. 2023. Klebsiella Pneumoniae: an update on antibiotic resistance mechanisms. Future Microbiol. 18, 65–81; doi:10.2217/fmb-2022-0097 Karim, S., Mansour, K., Janabi, A. and Al-Nakeeb, N. 2019. First phylogenetic characterization of Pseudocowpox virus from cattle in Al-Qadisiyah province/ Iraq. Iraqi J. Vet. Sci. 33(1), 123–126; doi:10.33899/ijvs.2019.125525.1047 Kim, S.H., Wei, C.I., Tzou, Y.M. and An, H. 2005. Multidrug-resistant Klebsiella pneumoniae isolated from farm environments and retail products in Oklahoma. J. Food Prot. 68(10), 2022–2029; doi:10.4315/0362-028X-68.10.2022 Kocsis, B. 2023. Hypervirulent Klebsiella pneumoniae: an update on epidemiology, detection and antibiotic resistance. Acta Microbiol. Immunol. Hung. 70(4), 278–287; doi:10.1556/030.2023.02186 Lansbury, L., Lim, B., Baskaran, V. and Lim, W.S. 2020. Co-infections in people with COVID-19: a systematic review and meta-analysis. J. Infection 81(2), 266–275; doi:10.1016/j.jinf.2020.05.046 Li, J., Li, Y., Tang, M., Xia, F., Min, C., Hu, Y., Wang, H., Zhang, J. and Zou, M. 2022. Distribution, characterization, and antibiotic resistance of hypervirulent Klebsiella pneumoniae isolates in a Chinese population with asymptomatic bacteriuria. BMC. Microbiol. 22(1), 29; doi:10.1186/s12866-021-02413-w Li, L., Gao, X., Li, M., Liu, Y., Ma, J., Wang, X., Yu, Z., Cheng, W., Zhang, W., Sun, H., Song, X. and Wang, Z. 2024. Relationship between biofilm formation and antibiotic resistance of Klebsiella pneumoniae and updates on antibiofilm therapeutic strategies. Front. Cell. Infect. Microbiol. 14, 1324895; doi:10.3389/fcimb.2024.1324895 Li, L., Ma, J., Cheng, P., Li, M., Yu, Z., Song, X., Yu, Z., Sun, H., Zhang, W. and Wang, Z. 2023. Roles of two-component regulatory systems in Klebsiella pneumoniae: regulation of virulence, antibiotic resistance, and stress responses. Microbiol. Res. 272, 127374; doi:10.1016/j.micres.2023.127374 Li, L., Xu, X., Cheng, P., Yu, Z., Li, M., Yu, Z., Cheng, W., Zhang, W., Sun, H. and Song, X. 2025. Klebsiella pneumoniae derived outer membrane vesicles mediated bacterial virulence, antibiotic resistance, host immune responses and clinical applications. Virulence 16(1), 2449722; doi:10.1080/21505594.2025.2449722 Lin, J.Y., Zhu, Z.C., Zhu, J., Chen, L. and Du, H. 2024. Antibiotic heteroresistance in Klebsiella pneumoniae: definition, detection methods, mechanisms, and combination therapy. Microbiological Res. 283, 127701; doi:10.1016/j.micres.2024.127701 Lin, Z., Yu, J., Liu, S. and Zhu, M. 2022. Prevalence and antibiotic resistance of Klebsiella pneumoniae in a tertiary hospital in Hangzhou, China, 2006–2020. J. Int. Med. Res. 50(2), 3000605221079761; doi:10.1177/03000605221079761 López-Sampedro, I., Hernández-Chico, I., Gómez-Vicente, E., Expósito-Ruiz, M., Navarro-Marí, J.M. and Gutiérrez-Fernández, J. 2023. Evolution of antibiotic resistance in Escherichia coli and Klebsiella pneumoniae from urine cultures. Arch. Español. Urol. 76(3), 203–214; doi:10.56434/j.arch.esp.urol.20237603.24 Lu, J., Chen, J., Liu, C., Zeng, Y., Sun, Q., Li, J., Shen, Z., Chen, S. and Zhang, R. 2022. Identification of antibiotic resistance and virulence‐encoding factors in Klebsiella pneumoniae by Raman spectroscopy and deep learning. Microb. Biotechnol. 15(4), 1270–1280; doi:10.1111/1751-7915.13960 Luna-Pineda, V.M., Rodríguez-Martínez, G., Salazar-García, M. and Romo-Castillo, M. 2024. Plant-origin components: new players to combat antibiotic resistance in Klebsiella pneumoniae. Int. J. Mol. Sci. 25(4), 2134; doi:10.3390/ijms25042134 Mijović, G. 2020. Antibiotic consumption in hospitals and resistance rate of Klebsiella pneumoniae and Escherichia coli in Montenegro. Acta Clin. Croatica 59(3), 469–479; doi:10.20471/acc.2020.59.03.11 Morante, J., Quispe, A.M., Ymaña, B., Moya-Salazar, J., Luque, N., Soza, G., Ramos Chirinos, M. and Pons, M.J. 2021. Tolerance to disinfectants (chlorhexidine and isopropanol) and its association with antibiotic resistance in clinically-related Klebsiella pneumonia isolates. Pathog. Global Health 115(1), 53–60; doi:10.1080/20477724.2020.1845479 Nang, S.C., Lu, J., Yu, H.H., Wickremasinghe, H., Azad, M.A.K., Han, M., Zhao, J., Rao, G., Bergen, P.J., Velkov, T., Sherry, N., Mccarthy, D.T., Aslam, S., Schooley, R.T., Howden, B.P., Barr, J.J., Zhu, Y. and Li, J. 2024. Phage resistance in Klebsiella pneumoniae and bidirectional effects impacting antibiotic susceptibility. Clin. Microbiol. Infection 30(6), 787–794; doi:10.1016/j.cmi.2024.03.015 Park, C.H., Robicsek, A., Jacoby, G.A., Sahm, D. and Hooper, D.C. 2006. Prevalence in the United States of aac(6 ′ ) - Ib - cr encoding a ciprofloxacin-modifying enzyme. Antimicrob. Agents Chemother. 50(11), 3953–3955; doi:10.1128/AAC.00915-06 Pham, H.N., Than, T.D.N., Nguyen, H.A., Vu, D.H., Phung, T.H. and Nguyen, T.K. 2024. Antibiotic resistance, biofilm formation, and persistent phenotype of Klebsiella pneumoniae in a Vietnamese Tertiary Hospital: a Focus on Amikacin. Microbial Drug Resist. 30(5), 203–209; doi:10.1089/mdr.2023.0267 Qin, K., Shi, X., Yang, K., Xu, Q., Wang, F., Chen, S., Xu, T., Liu, J., Wen, W., Chen, R., Liu, Z., Cui, L. and Zhou, K. 2024. Phage-antibiotic synergy suppresses resistance emergence of Klebsiella pneumoniae by altering the evolutionary fitness. mBio 15(10), 139324; doi:10.1128/mbio.01393-24 Qui, N.H. 2023. Baker’s yeast (Saccharomyces cerevisiae) and its application on poultry’s production and health: a review. Iraqi J. Vet. Sci. 37(1), 213–221; doi:10.33899/ijvs.2022.132912.2146 Rahmat Ullah, S., Jamal, M., Rahman, A. and Andleeb, S. 2024. Comprehensive insights into Klebsiella pneumoniae: unravelling clinical impact, epidemiological trends and antibiotic-resistance challenges. J. Antimicrob. Chemotherapy 79(7), 1484–1492; doi:10.1093/jac/dkae184 Recchia, G.D. and Hall, R.M. 1995. Gene cassettes: a new class of mobile element. Microbiology 141(12), 3015–3027; doi:10.1099/13500872-141-12-3015 Robicsek, A., Sahm, D.F., Strahilevitz, J., Jacoby, G.A. and Hooper, D.C. 2005. Broader distribution of plasmid-mediated quinolone resistance in the United States. Antimicrob. Agents Chemother. 49(7), 3001–3003; doi:10.1128/AAC.49.7.3001-3003.2005 Sabeeh, S.A., Esraa, T.M., Alelaah Ali, F.A., Al-Baghdadi, R.J.T. and Janabi, A.H.D. 2018. Metronidazole as a feed additive targeting archaea to potentially reduce frothy bloat in Iraqi local cows. Biochem. Cellular Arch. 18(2), 2023–2025. Safika, S., Nilasari, Z. and Pasaribu, F.H. 2022. Detection of antibiotic resistance coding gene in Klebsiella pneumoniae bacteria isolated from broiler chickens in West Java, Indonesia. J. Appl. Pharm. Sci. 12(7), 190–198; doi:10.7324/JAPS.2022.120722 Siu, L.K., Fung, C.P., Chang, F.Y., Lee, N., Yeh, K.M., Koh, T.H. and Ip, M. 2011. Molecular typing and virulence analysis of serotype K1 Klebsiella pneumoniae strains isolated from liver abscess patients and stool samples from noninfectious subjects in Hong Kong, Singapore, and Taiwan. J. Clin. Microbiol. 49(11), 3761–3765; doi:10.1128/JCM.00686-11 Stojowska-Swędrzyńska, K., Łupkowska, A., Kuczyńska-Wiśnik, D. and Laskowska, E. 2021. Antibiotic heteroresistance in Klebsiella pneumoniae. Int. J. Mol. Sci. 23(1), 449; doi:10.3390/ijms23010449 Tan, D., Zhang, Y., Cheng, M., Le, S., Gu, J., Bao, J., Qin, J., Guo, X. and Zhu, T. 2019. Characterization of Klebsiella pneumoniae ST11 Isolates and Their Interactions with Lytic Phages. Viruses 11(11), 1080; doi:10.3390/v11111080 Tao, H., Liu, L., Chen, X. and Peng, L. 2023. Coexistence of Klebsiella pneumoniae and Acinetobacter baumannii contributes to antibiotic resistance. Int. J. Antimicrob. Agents 62(5), 106993; doi:10.1016/j.ijantimicag.2023.106993 Xu, Y., Liu, D., Han, P., Wang, H., Wang, S., Gao, J., Chen, F., Zhou, X., Deng, K., Luo, J., Zhou, M., Kuang, D., Yang, F., Jiang, Z., Xu, S., Rao, G., Wang, Y. and Qu, J. 2024. Rapid inference of antibiotic resistance and susceptibility for Klebsiella pneumoniae by clinical shotgun metagenomic sequencing. Int. J. Antimicrob. Agents 64(2), 107252; doi:10.1016/j.ijantimicag.2024.107252 Yang, Y., Yang, Y., Chen, G., Lin, M., Chen, Y., He, R., Galvão, K.N., El-Gawad El-Sayed Ahmed, M.A., Roberts, A.P., Wu, Y., Zhong, L.L., Liang, X., Qin, M., Ding, X., Deng, W., Huang, S., Li, H. Y., Dai, M., Chen, D.Q., Zhang, L. and Tian, G.B. 2021. Molecular characterization of carbapenem-resistant and virulent plasmids in Klebsiella pneumoniae from patients with bloodstream infections in China. Emerg. Microb. Infect. 10(1), 700–709; doi: 10.1080/22221751.2021.1906163 Yaseen, M.M., Karawan, A.C., Alfatlawi, M.A.A. and Janabi, A.H.D. 2020. The role of gut bacterial cytochrome-P450 of mosquito larvae in degradation of temephos insecticide. Ann. Trop. Med. Public Health 23(1), S412 ; doi:10.36295/ASRO.2020.23126 | ||
| How to Cite this Article |
| Pubmed Style Bneed QZ, Abood NA, Faja OM, Alhasanawi BN, Sameer Z, Alzubaidy MA. Shared resistance and virulence of Klebsiella pneumoniae from human and meat sources: A one health perspective. Open Vet. J.. 2025; 15(9): 4162-4180. doi:10.5455/OVJ.2025.v15.i9.22 Web Style Bneed QZ, Abood NA, Faja OM, Alhasanawi BN, Sameer Z, Alzubaidy MA. Shared resistance and virulence of Klebsiella pneumoniae from human and meat sources: A one health perspective. https://www.openveterinaryjournal.com/?mno=261255 [Access: January 26, 2026]. doi:10.5455/OVJ.2025.v15.i9.22 AMA (American Medical Association) Style Bneed QZ, Abood NA, Faja OM, Alhasanawi BN, Sameer Z, Alzubaidy MA. Shared resistance and virulence of Klebsiella pneumoniae from human and meat sources: A one health perspective. Open Vet. J.. 2025; 15(9): 4162-4180. doi:10.5455/OVJ.2025.v15.i9.22 Vancouver/ICMJE Style Bneed QZ, Abood NA, Faja OM, Alhasanawi BN, Sameer Z, Alzubaidy MA. Shared resistance and virulence of Klebsiella pneumoniae from human and meat sources: A one health perspective. Open Vet. J.. (2025), [cited January 26, 2026]; 15(9): 4162-4180. doi:10.5455/OVJ.2025.v15.i9.22 Harvard Style Bneed, Q. Z., Abood, . N. A., Faja, . O. M., Alhasanawi, . B. N., Sameer, . Z. & Alzubaidy, . M. A. (2025) Shared resistance and virulence of Klebsiella pneumoniae from human and meat sources: A one health perspective. Open Vet. J., 15 (9), 4162-4180. doi:10.5455/OVJ.2025.v15.i9.22 Turabian Style Bneed, Qasim Zamil, Noor Adil Abood, Orooba Meteab Faja, Baneen Najm Alhasanawi, Zahraa Sameer, and Maryam Ali Alzubaidy. 2025. Shared resistance and virulence of Klebsiella pneumoniae from human and meat sources: A one health perspective. Open Veterinary Journal, 15 (9), 4162-4180. doi:10.5455/OVJ.2025.v15.i9.22 Chicago Style Bneed, Qasim Zamil, Noor Adil Abood, Orooba Meteab Faja, Baneen Najm Alhasanawi, Zahraa Sameer, and Maryam Ali Alzubaidy. "Shared resistance and virulence of Klebsiella pneumoniae from human and meat sources: A one health perspective." Open Veterinary Journal 15 (2025), 4162-4180. doi:10.5455/OVJ.2025.v15.i9.22 MLA (The Modern Language Association) Style Bneed, Qasim Zamil, Noor Adil Abood, Orooba Meteab Faja, Baneen Najm Alhasanawi, Zahraa Sameer, and Maryam Ali Alzubaidy. "Shared resistance and virulence of Klebsiella pneumoniae from human and meat sources: A one health perspective." Open Veterinary Journal 15.9 (2025), 4162-4180. Print. doi:10.5455/OVJ.2025.v15.i9.22 APA (American Psychological Association) Style Bneed, Q. Z., Abood, . N. A., Faja, . O. M., Alhasanawi, . B. N., Sameer, . Z. & Alzubaidy, . M. A. (2025) Shared resistance and virulence of Klebsiella pneumoniae from human and meat sources: A one health perspective. Open Veterinary Journal, 15 (9), 4162-4180. doi:10.5455/OVJ.2025.v15.i9.22 |