| Research Article | ||
Open Vet. J.. 2025; 15(9): 4153-4161 Open Veterinary Journal, (2025), Vol. 15(9): 4153-4161 Research Article Validation method for determining the concentration of norfloxacin-tylosin combination in broiler chicken tissue using high-performance liquid chromatographyCahyo Wibisono1, Agustina Dwi Wijayanti2*, Dyah Ayu Widiasih3, Ida Tjahajati4, Ika Nindya Irianti2, Kanya Gabriella Sasri Pamudya5, Ridha Avicena Ila Salsabila1and Luh Made Sudimartini61Veterinary Science Doctoral Program, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia 2Department of Pharmacology, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia 3Department of Veterinary Public Health, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia 4Department of Internal Medicine, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia 5Master of Veterinary Science, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia 6Laboratory of Veterinary Pharmacy, Faculty of Veterinary Medicine, Udayana University, Denpasar, Indonesia *Corresponding Author: Agustina Dwi Wijayanti. Department of Pharmacology, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia. Email: tinabdy [at] ugm.ac.id Submitted: 27/05/2025 Revised: 02/08/2025 Accepted: 16/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
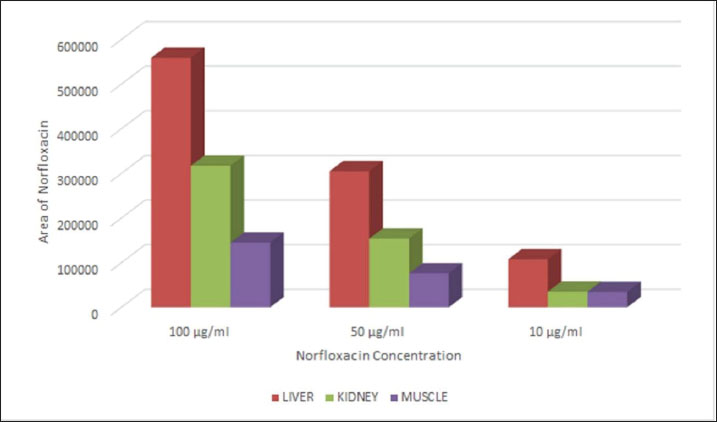
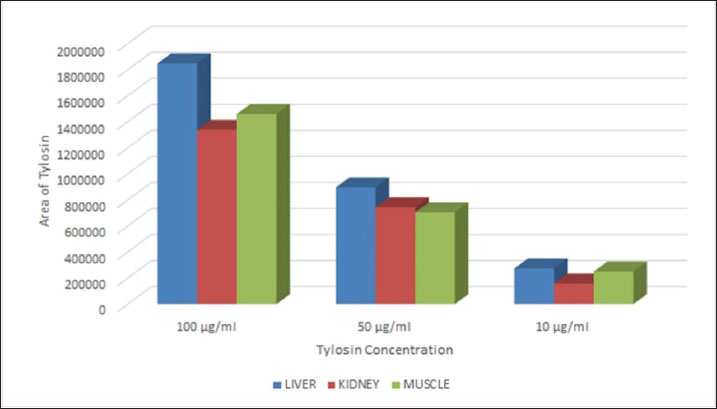
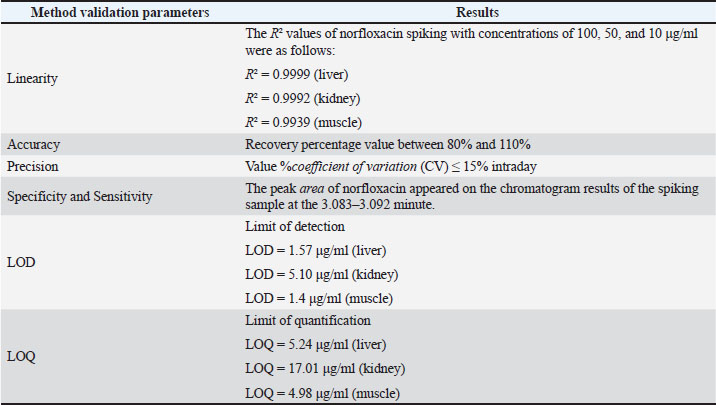
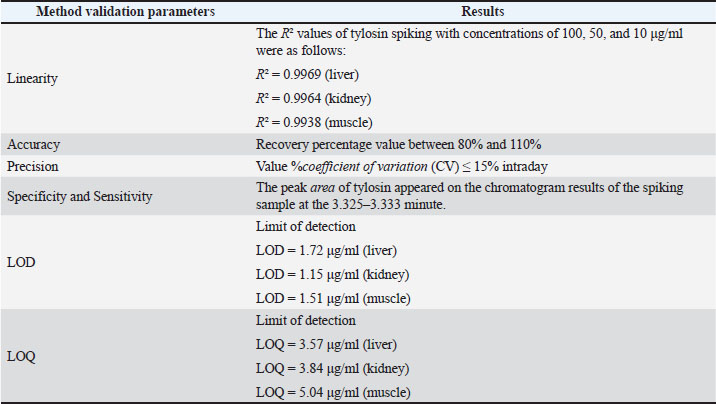
ABSTRACTBackground: The combination of norfloxacin and tylosin is an alternative to prevent antibiotic residue and resistance. Measurement of the levels of the combination of norfloxacin-tylosin in tissue using high-performance liquid chromatography (HPLC) to obtain parameters for further research, such as pharmacokinetics and residue analysis. When broiler chickens are given norfloxacin-tylosin antibiotics, they make antibiotics more effective against bacterial illnesses. These results indicate that the right mix of antibiotics can be used as a single medicine to achieve synergistic effects. Also, reach the SDGs for no hunger, good health, and well-being. Aim: This study aimed to ascertain the correct validation method for the combination antibiotic norfloxacin-tylosin in broiler chicken tissues, specifically the liver, kidney, and muscle. Methods: HPLC consistently detected norfloxacin-tylosin in broiler chicken tissue. Mobile phase methanol/acetonitrile was 50:50 (v/v). The chromatographic equipment utilized a 250 mm Shimpack ODS C18 column with a four μm diameter. Solution detection happened at a 1 ml/minutes flow rate, 30°C oven temperature, and 20 μl injection volume. Light had a wavelength of 279 nm. In Bogor, Indonesia, PT Mensana Aneka Satwa manufactured liquid antibiotics and combined them with broiler chicken tissue from 28-day-old antibiotic-free-fed chicks. Results: The tissue, liver, kidney, and muscle showed linearity values of R2=0.9999, R2=0.9992, and R2=0.9939 for norfloxacin and R2=0.9969, R2=0.9964, and R2=0.9938 for tylosin. Norfloxacin had liver, kidney, and muscle accuracy scores of 100.57%, 101.85%, and 105.41%, respectively, while tylosin had 103.89%, 95.83%, and 105.49%. Norfloxacin showed intraday precision values (%CV) of 0.77%, 4.18%, and 4% in the liver, kidney, and muscle, while tylosin had 0.74%, 1.84%, and 1%. Tylosin retained 3,325–3,358 minutes and norfloxacin 3.083–3.092. The limit of detection values for norfloxacin in the liver, kidney, and muscle were 1.57, 5.10, and 1.4 μg/ml, respectively. Tylosin levels in liver, kidney, and muscle were 1.72, 1.15, and 1.51 μg/ml, while norfloxacin had limit of quantification values of 5.24, 17.01, and 4.98 μg/ml. Conclusion: According to the European Medicines Agency Guideline, the mixed solution of norfloxacin and tylosin demonstrated favourable outcomes regarding sensitivity, specificity, linearity, accuracy, and precision. Keywords: Broiler tissue, HPLC, Norfloxacin-tylosin combination, Validation method. IntroductionAntibiotic combinations can effectively treat various infections in broiler chickens, thereby preventing antibiotic residues and resistance. A single antibiotic dose is frequently insufficient; thus, a combination of antibiotics may be administered for the appropriate infection. According to Al-Mustafa and Al-Ghamdi (2000), the incidence of norfloxacin resistance in poultry products in Saudi Arabia in Escherichia coli and Pseudomonas sp. was approximately 45% and 70.6%, respectively. Norfloxacin is a fluoroquinolone-class synthetic antibiotic used to manage bacterial infections in broilers, especially broiler chickens. This broad-spectrum antibiotic targets Gram-negative bacteria. Norfloxacin primarily treats urinary tract infections and effectively addresses gastrointestinal infections (Abu-Bash et al., 2008; Elazab et al., 2020). Norfloxacin inhibits a range of pathogenic bacteria, including E. coli, Staphylococcus aureus, Salmonella spp., and Pasteurella spp. Norfloxacin inhibits bacterial DNA replication and transcription, which requires DNA gyrase and topoisomerase IV. This antibiotic can activate NADPH oxidation in phagocytes and enhance the production of reactive oxygen species. Tylosin is a broad-spectrum macrolide antibiotic that eliminates Gram-positive and Gram-negative bacteria, such as Mycoplasma spp., Streptococcus spp., and Avibacterium spp. (Elazab et al., 2020). Tylosin stops protein synthesis by attaching to the 50S ribosome, similar to erythromycin. Tylosin is effective against Streptococcus and Staphylococcus spp. The combination of norfloxacin and tylosin is a broad-spectrum antibacterial that operates effectively and synergistically in treating bacterial infections within the poultry industry (Aboubakr and Elbadawy, 2017; Fitriana et al., 2020). To date, no research has been conducted on measuring the levels of the norfloxacin-tylosin combination in tissue. No research has been conducted on how to measure the levels of the norfloxacin-tylosin combination. However, only a few studies have conducted methods for measuring the levels of combination antibiotics, such as (Wijayanti et al. (2022), who measured the levels of the enrofloxacin-tylosin combination, and Aboubakr and Elbadawy (2017), who measured the levels of the tylosin-doxycycline combination. Pharmacokinetic simulation explains drug retention in the body before excretion and facilitates the determination of residual levels over time. To conduct pharmacokinetic tests, it is necessary to measure levels in the tissue first, and high-performance liquid chromatography (HPLC) is usually used for level measurements. HPLC is among the several analytical approaches employed in pharmacokinetic and residual research studies. The body’s reaction to medication combinations is typically assessed using a single HPLC validation method (Nwankwo et al., 2025). HPLC validation methods must be established to quantify the concentration of multiple compounds in broiler tissue (Bedor et al., 2007). The validation of analytical methods entails the evaluation of materials, procedures, processes, systems, and tools to confirm that their application reliably produces the anticipated outcomes. The validation of analytical methods involves assessing parameters to establish a standard for testing an ingredient. The parameters used for analysis include sensitivity, accuracy, precision, specificity, linearity, limit of detection (LOD), and quantification (LOQ) (Wijayanti et al., 2022). These parameters are essential for further testing, such as residue analysis in broiler chicken tissue and pharmacokinetic profile studies, especially the combination of norfloxacin and tylosin in broiler chicken tissue. This study used a validated analytical method to evaluate the HPLC analytical technique for measuring norfloxacin-tylosin combinations in vitro in chicken tissues. This study used a proven analytical approach to evaluate the HPLC analytical technique for measuring norfloxacin-tylosin concentrations in vitro in broiler chicken tissues, including liver, kidney, and muscle. Therefore, the results of this study can serve as a reference for future research. Materials and MethodsResearch procedure and samplingThis research was conducted at the Pharmacology Laboratory, Department of Pharmacology and Poultry Research Implementation Unit, and Poultry Health Management Training of the Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia. The research started from March 2024 to April 2025. The methods adopted include the treatment of animal and tissue sampling, the fabrication of a standard curve for the composition of norfloxacin (batch number AS4012504002 produced by Pharmacopoeia) - tylosin (batch number AAFCS50024 produced by Sigma Aldrich) in a mobile phase, drug injection in the liver tissue, kidney tissue, and muscle tissue, and a determination of the values of the studied parameters. A combination of products included 210 mg of norfloxacin and 120 mg of tylosin tartrate HCl in 100 ml of liquid formulation, sourced from PT Mensana Aneka Satwa, Bogor, Indonesia. The concentration analysis of norfloxacin-tylosin, derived from a stock solution combination of norfloxacin-tylosin, and in muscle, liver, and kidney specimens was conducted using HPLC. Six broilers, beginning from day-old chicks, were housed in an enclosed facility for experimental animals. Ad libitum drinking water and antibiotic-free BR-1 and BR-2 feed were provided. Halal slaughter and dissection of 1–1.5 kg broilers at 28 days. Broiler chicken tissues, including the liver, kidneys, and muscles, were gathered and stored in a freezer until the extracted tissue samples could be analyzed using HPLC. In the spiking analysis, the drug concentration derived from the blank tissue samples was quantified in the broiler chicken tissue samples. High-performance liquid chromatographyHPLC Shimadzu 6.1 (Kyoto, Japan) used a control system SCL-10 A VP, an SPD 10 AV VP UV-Vis detector, an LC-10 AD VP pump, a DGU-14A degasser, a CTO-10 AC VP oven, and a 250 × 4.6 mm Shim-pack ODS C18 column with a 20 µl injection volume and a 10-minute runtime. The instrument was set to an oven temperature of 30°C, wavelength λ 279 nm, as measured by the detector, and a flow rate of 1 ml/minutes. A 50:50 v/v mixture of methanol (pro analysis for chromatography, JT. Backer) and acetonitrile (pro analysis for chromatography, JT. Backer) is present within the mobile phase. Extraction of broiler chicken tissue samplesNorfloxacin-tylosin levels in the liver, kidney, and muscle were assessed employing modified extraction from Wijayanti et al. (2022) and Wijayanti et al. (2024). The sample was measured to be 1 g, and 1 ml of acetonitrile was added. The sample was then homogenized using a vortex mixer and centrifuged at 6,000 rpm for 10 minutes. The centrifuged sample was subsequently filtered to isolate the supernatant. Subsequently, the solution was subjected to evaporation in a water bath for 10 minutes until it reached a dry state. After drying, 1.5 ml of phosphate buffer at pH 7.4 and 2 ml of n-Hexane were added. Vortexed for 5 minutes and centrifuged at 3,000 g for 10 minutes. The supernatant was collected, and N-hexane was discarded. This process was repeated three times. Subsequently, the supernatant was centrifuged at 2,500 g for 15 minutes. Subsequently, a supernatant was filtered using double filter paper, and 20 µl was injected into the HPLC. Assessment of the methodology’s specificity, accuracy, precision, and sensitivityThe standard curve for broiler chicken tissue samples was created by inserting the medicine into the blank sample. The extracted broiler chicken tissues, including liver, kidney, and muscle samples, were added with norfloxacin-tylosin stock solutions at 100, 50, and 10 µg/ml concentrations. Subsequently, 20 µl of each produced sample was extracted and injected into the HPLC system. The determination of the standard curve values in broiler chicken liver, kidney, and muscle samples was conducted in the same manner as for norfloxacin and tylosin, using characteristics such as linearity, precision, specificity, accuracy, sensitivity, LOD, and limit of quantification. The accuracy and precision metrics of the analytical methodology were created for broiler chicken tissues, such as kidney, muscle, and liver, for the norfloxacin-tylosin standard curve. The accuracy value, expressed as a percentage of recovery, is determined by taking the concentration reading, dividing it by the measured concentration, and multiplying the result by 100%. The coefficient of variation (CV%), which is determined by comparing standard deviations to the mean measured concentration and multiplying by 100%, indicates the accuracy value. The profile area of the peak and retention time of the standard norfloxacin-tylosin solution at 10, 50, and 100 μg/ml concentrations were analyzed to evaluate specificity. The blank chromatogram was compared with the spiking solution at a 100 μg/ml concentration in liver, kidney, and muscle tissues. Because of LOQ and LOD readings, HPLC sensitivity may be ascertained. The LOQ is the minimum concentration of analytes in a sample that satisfies specific criteria. The LOD is the minimum quantity of detectable metabolites in a sample. Nevertheless, it may not always be quantifiable, resulting in a substantial reaction compared to the void. Ethical approvalThe Ethics Commission of the Faculty of Veterinary Medicine of Universitas Gadjah Mada, Yogyakarta, Indonesia, approved the experimental animal therapy with number 26/EC-FKH/int./2024. ResultsMethodology for measuring norfloxacin-tylosin in tissues using a preliminary method Tables 1 and 2 outline the accuracy, sensitivity, linearity, precision, specificity, LOD, and LOQ outcomes of broiler chicken tissue. Figures 1 and 2 illustrate the calibration curve linearity graph and validation outcomes for quantifying norfloxacin-tylosin levels in broiler tissues. The linearity of a calibration curve, as evidenced by a deviation value (r² ≥ 0.99), illustrates the linear relationship between the drug concentration and the peak area.
Fig. 1. Calibration graph for norfloxacin in the liver, kidney, and muscle at concentrations of 100, 50, and 10 μg/ml.
Fig. 2. Calibration graph for tylosin in the liver, kidney, and muscle at concentrations of 100, 50, and 10 μg/ml. Table 1. Validated method for quantifying norfloxacin concentration in broiler tissues.
Table 2.Validated method for quantifying tylosin concentration in broiler tissues.
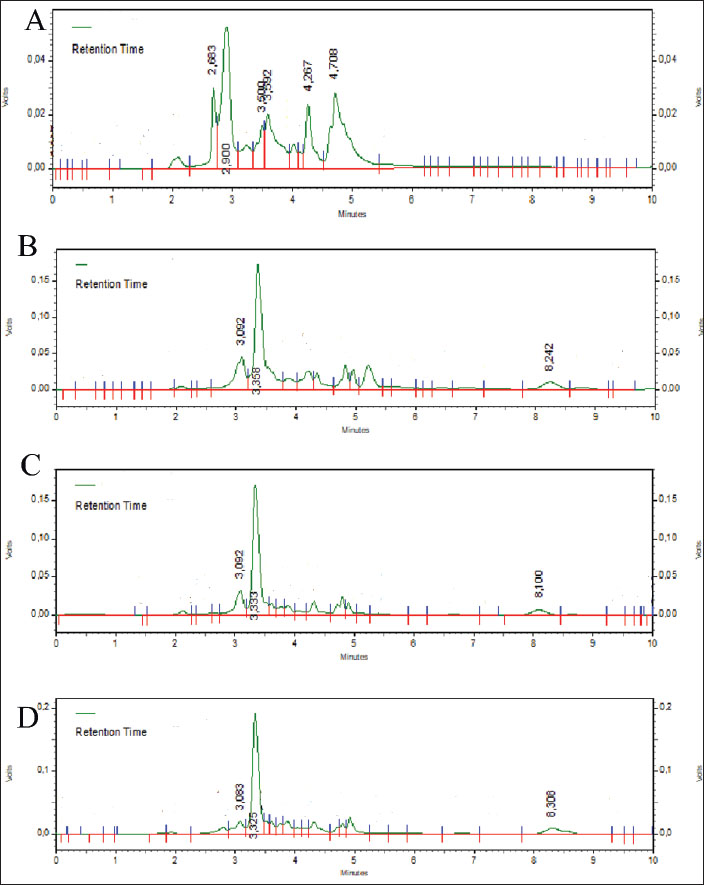
The intraday coefficient of variation (CV%) for norfloxacin in the liver, kidneys, and muscles was 100, 50, and 10 μg/ml, respectively, 0.77%, 4.18%, and 4%. The tylosin precision levels in the liver, kidneys, and muscles were 0.74%, 1.84%, and 1%, respectively. Precision is deemed acceptable if it is less than 15%. For the liver, kidney, and muscle, norfloxacin had accuracy values of 100.57%, 101.85%, and 105.41%, respectively, while tylosin had accuracy values of 103.89%, 95.83%, and 105.49%, respectively. Ahmad et al. (2010) asserted that HPLC is an appropriate technique for quantifying drug concentrations in broiler chicken tissue, provided the recovery rate falls between 80% and 110%. The distinct peaks of norfloxacin at 3,083–3,092 minutes and tylosin at 3,325–3,358 minutes demonstrate norfloxacin-tylosin specificity detection in broiler chicken tissues (Fig. 3).
Fig. 3. Standard norfloxacin-tylosin chromatogram. (A) Chromatogram of blank liver (without drugs). (B) Norfloxacin-tylosin spiking chromatogram concentration of 100 μg/ml in the liver (norfloxacin retention time of 3,092 minutes and tylosin retention time of 3,358 minutes); (C) norfloxacin-tylosin spiking chromatogram concentration of 100 μg/ml in the kidney (norfloxacin retention time of 3,092 minutes and tylosin retention time of 3,333 minutes); (D) norfloxacin-tylosin spiking chromatogram concentration of 100 μg/ml in the muscle (norfloxacin retention time of 3,083 minutes and tylosin retention time of 3,325 minutes). The LOD of norfloxacin for broiler chicken tissues in the liver, kidneys, and muscle is 1.57, 5.10, and 1.4 μg/ml, respectively, and the LOD of tylosin for all tissues in the liver, kidneys, and muscle is 1.72, 1.15, and 1.51 μg/ml, respectively. The LOQ of norfloxacin for all tissues in the liver, kidneys, and muscle was 5.24, 17.01, and 4.98 μg/ml, respectively. The LOQ tylosin for all liver, kidneys, and muscle tissues is 3.87, 3.84, and 5.04 μg/ml, respectively, demonstrating adequate sensitivity and specificity to detect and quantify norfloxacin-tylosin in broiler tissue. DiscussionThe findings indicated that HPLC configuration detection at a wavelength of 279 nm is applicable for quantifying norfloxacin and tylosin levels. Chierentin (2013) employed an optimal wavelength of 277 nm for detecting norfloxacin, with a constant temperature for the column maintained at 28°C–30°C and a flow rate of 1 ml/minutes. Wijayanti et al. (2022) used a wavelength of 280 nm at a temperature of 30°C and a flow rate of 1 ml/minutes to detect tylosin. Norfloxacin-tylosin’s analytical peak is readily discernible from the impurity peak of an unidentified compound in the feed matrix. The linearity of the calibration curve was evaluated using three concentrations: 100, 50, and 10 μg/ml. The linearity of norfloxacin in liver, kidney, and muscle tissues was determined as R²=0.9999, R²=0.9992, and R²=0.9939, respectively. In contrast, the linearity of tylosin in broiler tissues, such as liver, kidney, and muscle tissues, was established as R²=0.9969, R²=0.9964, and R²=0.9938, respectively. Ahmad et al. (2010) asserted that a satisfactory linearity criterion is that the coefficient value fulfills the correlation (r) > 0.9999, ensuring the robustness of the outcomes of this investigation. Accuracy is a measurement parameter indicated by the degree of proximity of the analysis results to the analyte level and is expressed as the percentage of analyte recovery added (Singh et al., 2013). The accuracy test was performed using a minimum of three dilution levels or three serial dilutions with each concentration level (Clark et al., 2009). The accuracy of the norfloxacin-tylosine combination sample was determined in three standards with known concentrations of 100, 50, and 10 μg/ml. Norfloxacin and tylosin levels in muscle, kidney, and liver tissue had high accuracy values in the validation findings. According to the findings of (Wijayanti et al., 2022) the measurement of tylosin levels using HPLC with an octadesil-silica column configuration with a diameter of 5 μm and a length of 150 mm with a motion phase of a mixture of monobasic sodium phosphate 0.05 M (pH 2.5) and acetonitrile (65:35 v/v) showed an accuracy value of 90%–110%. However, it is based on the findings of Osman et al. (2022), the detected of norfloxacin using HPLC with a mobile phase consisting of a 1% triethanolamine mixture and 80% acetonitrile as a suitable mobile phase. The flow rate is up to 1.2 ml/minutes, the temperature is 25°C, and the wavelength is 280 nm, which shows an accuracy of 101.12%. According to Sousa Maia et al. (2008), the accuracy of the sample extraction method must be at least 75%, and the systematic value should not exceed 25%. An accuracy value between 90% and 110% indicates good results for measuring norfloxacin-tylosin levels in liver, kidney, and muscle tissue. The results of the %recovery calculation at the three norfloxacin standards in this study’s liver, kidneys, and muscles were between 100.57% and 105.41%. These results show that the method for detecting norfloxacin levels is reliable. In contrast, the chlosyne results were obtained at the three tylosin standards in this study’s liver, kidneys, and muscles, which were between 95.83% and 105.49%. To find the accuracy value (%CV), divide the standard deviation of the test results by their average and then multiply that number by 100%. (Mendes et al., 2015). A precision value (%CV) is determined by measuring the analyte concentration with a minimum of six repetitions at 100% or nine repetitions, specifically three concentrations with three repetitions (Ghanem and Abu-Lafi, 2013). Zheng et al. (2018) defined a precision value (%CV) as “less than good” if it exceeded 15%. The precision value (%CV) measurement results are presented as follows: The norfloxacin level detection method has an average precision value (%CV) of 0.77%, 4.18%, and 4% in liver, kidney, and muscle tissues, respectively. The precision value (%CV) of tylosin in the same tissues was 0.74%, 1.84%, and 1%. According to Wijayanti et al. (2022), this study’s precision value measurements (%CV) were classified as acceptable, with a value of less than 15%. Specificity is the ability of an analytical method to accurately measure the compound being measured without interference from other compounds. The specificity of the analytical method used in this study was determined by examining the peak area profiles in the blank chromatogram and spiking standard solutions of norfloxacin and tylosin in the liver, kidney, and chest muscle (Ghanem and Abu-Lafi, 2015). Figure 1 displays the results of the blank chromatogram and the mobile phase diluted with norfloxacin-tylosin. According to the chromatogram findings, Figure 1A does not exhibit a peak region with a retention duration between the much third minute. Figure 1B–D illustrate the appearance of the norfloxacin and tylosin peak regions at 3,0823–3,092 minutes and 3,333–3,358 minutes, respectively. The peak area is generated at each concentration of norfloxacin-tylosin. A higher concentration of injected norfloxacin-tylosin results in an increased peak area. According to Pietro et al. (2011), the chromatogram findings exhibit high specificity, as they properly differentiate norfloxacin-tylosin molecules at a given retention period without the interference of extraneous compounds in the peak region. This study determined the sensitivity of the test method by identifying the LOQ and LOD values. The LOQ represents the minimum concentration of substances in an extract that satisfies the accuracy criteria. In contrast, the LOD denotes the minimum concentration that can be detected, albeit not necessarily measured, and yields a significant response relative to a blank sample (Pandey et al., 2020). Osman et al. (2022) reported that the LOD and LOQ values for norfloxacin were 0.0008 and 0.0024 μg/ml, respectively. In contrast, Singh et al. (2013) reported LOD and LOQ values of 0.35 and 1.16 μg/ml, respectively. The LOD and LOQ values in this study were comparable to those reported in previous literature, indicating that the method employed is suitable for detecting norfloxacin. Pietro et al. (2011) measured tylosin using HPLC, yielding LOD and LOQ values of 0.87 and 2.2 ng/ml, respectively. According to the study by Ghanem and Abu-Lafi (2015) determined the LOD and LOQ for norfloxacin to be 5.16 and 18.7 μg/ml, respectively. The LOD and LOQ of this study were consistent with those of previous studies, indicating that the method effectively detects norfloxacin and tylosin. The European Commission specifies that the maximum residue limits for norfloxacin and tylosin in broiler chicken tissue are between 100 and 300 μg/kg. Current research necessitates enhancements in measuring the LOQ and LOD due to existing limitations in assessing the residue values of these two drug compounds. ConclusionThe optimum parameters for in vitro analysis of the norfloxacin-tylosin combination in liver, kidney, and muscle tissues using HPLC require a Shimpack ODS C18 column with dimensions of 4 × 250 mm. The mobile phase consisted of a 50:50 v/v blend of acetonitrile and methanol, maintained at a temperature of 30°C, a wavelength of λ 279 nm, and a flow rate of 1 ml/minutes. The validation results demonstrate that our bioanalytical method satisfies the criteria of linearity, accuracy, precision, specificity, and sensitivity as outlined in the European Medicines Agency Guideline. Consequently, it is pertinent for the analysis of the norfloxacin-tylosin combination in the liver, kidney, and muscle tissues of broiler chickens. This study may facilitate further studies, including residue level analysis and pharmacokinetic investigations with a lower concentration of the norfloxacin-tylosin combination. AcknowledgmentsThe authors thank the Indonesian Ministry of Finance, Indonesia Endowment Fund for Education/Lembaga Pengolola Dana Pendidikan (LPDP) Scholarship, for support. In addition, the authors would like to thank the Department of Pharmacology, Laboratory of Pharmacology, Faculty of Veterinary Medicine at Universitas Gadjah Mada for providing the facilities needed for this research. FundingThe Ministry of Finance and the LPDP Scholarship fund this research. Conflict of interestThe authors have no conflicts of interest to declare. Authors’ contributionsCW, ADW, DAW, and IT Planned the study, conducted CW and INI research in the field, and conducted test samples in the laboratory. The finished draft was written, reviewed, read, and approved by all the authors. Data availabilityAll data are presented in this study. ReferencesAboubakr, M. and Elbadawy, M. 2017. Pharmacokinetics, tissue residues and efficacy of D-Tylo50/25® (tylosin-doxycycline combination) in broiler chickens. Int. J. Basic Clin. Pharmacol. 6(2), 383. Abu-Bash, E.A., Gharaibeh, S.M., Abudabos, A.M., F. Shunnaq, A. and Al-Ma, A.M. 2008. Pharmacokinetics and bioequivalence of two norfloxacin oral dosage forms (vapcotril - 10%® and mycomas 10%®) in healthy broiler chickens. Int. J. Poultry Sci. 7(3), 289–293. Ahmad, M., Murtaza, G., Khiljee, S. and Madni, M.A. 2010. Validation and application of a new optimized RP-HPLC-fluorescent detection method for norfloxacin. Int. J. Pharmacol. Pharm. Sci. 4(5), 195–198. Al-Mustafa, Z.H. and Al-Ghamdi, M.S. 2000. Use of norfloxacin in poultry production in the eastern province of Saudi Arabia and its possible impact on public health. Int. J. Environ. Health Res. 10(4), 291–299. Bedor, D.C.G., Gonçalves, T.M., Bastos, L.L., Sousa, C.E.M.D., Abreu, L.R.P.D., Oliveira, E.D.J. and Santana, D.P.D. 2007. Development and validation of a new method for the quantification of norfloxacin by HPLC-UV and its application to a comparative pharmacokinetic study in human volunteers. Braz. J. Pharm. Sci. 43(2), 231–238. Chierentin, L. 2013. Development and validation of a simple, rapid and stability-indicating high performance liquid chromatography method for quantification of norfloxacin in a pharmaceutical product. J. Chromatogr. Sep. Tech. 04, 171. Clark, C.R., Dowling, P.M. and Boison, J.O. 2009. Development and validation of a method for determination of tilmicosin residues in equine plasma and tissues using HPLC. J. Liquid Chromatogr. Rel. Technol. 32, 2839–2856. Elazab, S.T., Elshater, N.S., Hashem, Y.H., Park, S.C. and Hsu, W.H. 2020. Pharmacokinetics, tissue residues, and ex vivo pharmacodynamics of tylosin against Mycoplasma anatis in ducks. J. Vet. Pharmacol. Ther. 43(1), 57–66. Fitriana, I., Chotimah, A.C., Wijayanti, A.D., Kristianingrum, Y.P. and Pratama, A.M. 2020. Antibiotics combination effects of tylosin and enrofloxacin on liver and renal functions of broiler. J. Kedokt. Hewan. 1(1), 24–27. Ghanem, M. and Abu-Lafi, S. 2015. Development and validation of RP-HPLC method for the simultaneous determination of trimethoprim, sulfadimidine sodium and tylosin tartrate in injectable solution formulation. J. Appl. Pharm. Sci. 5(1), 94–98. Ghanem, M.M. and Abu-Lafi, S.A. 2013. Development and validation of a stability-indicating HPLC method for the simultaneous determination of sulfadiazine sodium and trimethoprim in injectable solution formulation. Sci. Pharm. 81(1), 167–182. Mendes, C., Buttchevitz, A., Kruger, J.H., Bernardi, L.S., Oliveira, P.R. and Silva, M.A.S. 2015. Quantitative analysis of norfloxacin in β-cyclodextrin inclusion complexes—development and validation of a stability-indicating HPLC method. Anal. Sci. 31(10), 1083–1089. Nwankwo, I., Onwumere-Idolor, S., Ezenduka, E., Nwanta, J. and Anaga, A. 2025. Tylosin residue in chicken: detection with ELISA, four plate test, HPLC, effect of heat treatment and implications for human health. Iran. J. Vet. Sci. Tech. 17(1), 55–63. Osman, S., Elgendy, K., Saad, M. and Turkey, A. 2022. Rapid hplc determination of norfloxacin, levofloxacin, and moxifloxacin alone or in a mixture. Egypt. J. Chem. 0(0), 0–0. Pandey, S., Dhanani, J., Lipman, J., Roberts, J.A., Wallis, S.C. and Parker, S.L. 2020. Development and validation of LC-MS/MS methods to measure tobramycin and lincomycin in plasma, microdialysis fluid and urine: application to a pilot pharmacokinetic research study. Clin. Chem. Labo. Med. 58(2), 274–284. Pietro, W., Cybulski, W., Kos, K. and Mitura, A. 2011. Analytical procedure for the determination of tylosin A in feedingstuff by liquid chromatography-ultraviolet detection. Bull. Vet. Inst. Pulawy 55, 725–729. Singh, R.N., Sahoo, S., Mishra, U., Garnaik, B., Sahoo, S.K. and Hati, D. 2013. Stability indicating RP-HPLC method development and validation of norfloxacin. Am. J. Adv. Drug Deliv. 1, 743–758. Sousa Maia, M.B., Martins, I.L., Nascimento, D.F.D., Cunha, A.N., De Lima, F.E.G., Bezerra, F.A.F., Moraes, M.O. and Moraes, M.E.A. 2008. Validation of a reversed-phase high-performance liquid chromatography method with fluorescence detection for the bioequivalence study of norfloxacin in plasma samples. Therap. Drug Monit. 30(3), 341–346. Wijayanti, A.D., Ardiansyah, R.D., Pratama, A.M., Haryanto, A. and Fitriana, I. 2022. Validation method for determining enrofloxacin and tylosin levels in broiler liver, kidney, and muscle using high-performance liquid chromatography. Vet. World 15, 268–274. Wijayanti, A.D., Muzaki, A.Y., Wibisono, C. and Widiasih, D.A. 2024. Therapeutic effects of lincomycin and level of drug degradation in broiler tissues after treatment. Vet. World 1026, 1026–1034; doi:10.14202/vetworld.2024.1026-1034 Zheng, W., Park, J.A., Abd El-Aty, A.M., Kim, S.K., Cho, S.H., Choi, J.M., Warda, M., Wang, J., Shim, J.H. and Shin, H.C. 2018. Development and validation of a simple solid-phase extraction method coupled with liquid chromatography–triple quadrupole tandem mass spectrometry for simultaneous determination of lincomycin, tylosin A and tylosin B in royal jelly. Biomed. Chromatogr. 32(4). | ||
| How to Cite this Article |
| Pubmed Style Wibisono C, Wijayanti AD, Widiasih DA, Tjahajati I, Irianti IN, Pamudya KGS, Salsabila RAI, Sudimartini LM. Validation method for determining the concentration of norfloxacin-tylosin combination in broiler chicken tissue using high-performance liquid chromatography. Open Vet. J.. 2025; 15(9): 4153-4161. doi:10.5455/OVJ.2025.v15.i9.21 Web Style Wibisono C, Wijayanti AD, Widiasih DA, Tjahajati I, Irianti IN, Pamudya KGS, Salsabila RAI, Sudimartini LM. Validation method for determining the concentration of norfloxacin-tylosin combination in broiler chicken tissue using high-performance liquid chromatography. https://www.openveterinaryjournal.com/?mno=260928 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i9.21 AMA (American Medical Association) Style Wibisono C, Wijayanti AD, Widiasih DA, Tjahajati I, Irianti IN, Pamudya KGS, Salsabila RAI, Sudimartini LM. Validation method for determining the concentration of norfloxacin-tylosin combination in broiler chicken tissue using high-performance liquid chromatography. Open Vet. J.. 2025; 15(9): 4153-4161. doi:10.5455/OVJ.2025.v15.i9.21 Vancouver/ICMJE Style Wibisono C, Wijayanti AD, Widiasih DA, Tjahajati I, Irianti IN, Pamudya KGS, Salsabila RAI, Sudimartini LM. Validation method for determining the concentration of norfloxacin-tylosin combination in broiler chicken tissue using high-performance liquid chromatography. Open Vet. J.. (2025), [cited January 25, 2026]; 15(9): 4153-4161. doi:10.5455/OVJ.2025.v15.i9.21 Harvard Style Wibisono, C., Wijayanti, . A. D., Widiasih, . D. A., Tjahajati, . I., Irianti, . I. N., Pamudya, . K. G. S., Salsabila, . R. A. I. & Sudimartini, . L. M. (2025) Validation method for determining the concentration of norfloxacin-tylosin combination in broiler chicken tissue using high-performance liquid chromatography. Open Vet. J., 15 (9), 4153-4161. doi:10.5455/OVJ.2025.v15.i9.21 Turabian Style Wibisono, Cahyo, Agustina Dwi Wijayanti, Dyah Ayu Widiasih, Ida Tjahajati, Ika Nindya Irianti, Kanya Gabriella Sasri Pamudya, Ridha Avicena Ila Salsabila, and Luh Made Sudimartini. 2025. Validation method for determining the concentration of norfloxacin-tylosin combination in broiler chicken tissue using high-performance liquid chromatography. Open Veterinary Journal, 15 (9), 4153-4161. doi:10.5455/OVJ.2025.v15.i9.21 Chicago Style Wibisono, Cahyo, Agustina Dwi Wijayanti, Dyah Ayu Widiasih, Ida Tjahajati, Ika Nindya Irianti, Kanya Gabriella Sasri Pamudya, Ridha Avicena Ila Salsabila, and Luh Made Sudimartini. "Validation method for determining the concentration of norfloxacin-tylosin combination in broiler chicken tissue using high-performance liquid chromatography." Open Veterinary Journal 15 (2025), 4153-4161. doi:10.5455/OVJ.2025.v15.i9.21 MLA (The Modern Language Association) Style Wibisono, Cahyo, Agustina Dwi Wijayanti, Dyah Ayu Widiasih, Ida Tjahajati, Ika Nindya Irianti, Kanya Gabriella Sasri Pamudya, Ridha Avicena Ila Salsabila, and Luh Made Sudimartini. "Validation method for determining the concentration of norfloxacin-tylosin combination in broiler chicken tissue using high-performance liquid chromatography." Open Veterinary Journal 15.9 (2025), 4153-4161. Print. doi:10.5455/OVJ.2025.v15.i9.21 APA (American Psychological Association) Style Wibisono, C., Wijayanti, . A. D., Widiasih, . D. A., Tjahajati, . I., Irianti, . I. N., Pamudya, . K. G. S., Salsabila, . R. A. I. & Sudimartini, . L. M. (2025) Validation method for determining the concentration of norfloxacin-tylosin combination in broiler chicken tissue using high-performance liquid chromatography. Open Veterinary Journal, 15 (9), 4153-4161. doi:10.5455/OVJ.2025.v15.i9.21 |