| Research Article | ||
Open Vet. J.. 2025; 15(8): 3477-3485 Open Veterinary Journal, (2025), Vol. 15(8): 3477-3485 Research Article Growth progression and hematobiochemical dynamics in broiler chickens treated with antibiotic, probiotic, and acidifierMd. Mominul Islam1, Md. Saiful Islam2, Khaled Mahmud Sujan1 and Mohammad Alam Miah1*1Department of Physiology, Faculty of Veterinary Science, Bangladesh Agricultural University, Mymensingh, Bangladesh 2Department of Biochemistry and Molecular Biology, Faculty of Agriculture, Bangladesh Agricultural University, Mymensingh, Bangladesh *Corresponding Author: Mohammad Alam Miah. Department of Physiology, Faculty of Veterinary Science, Bangladesh Agricultural University, Mymensingh, Bangladesh. Email: mam74 [at] bau.edu.bd Submitted: 26/05/2025 Revised: 01/07/2025 Accepted: 03/07/2025 Published: 31/08/2025 © 2025 Open Veterinary Journal
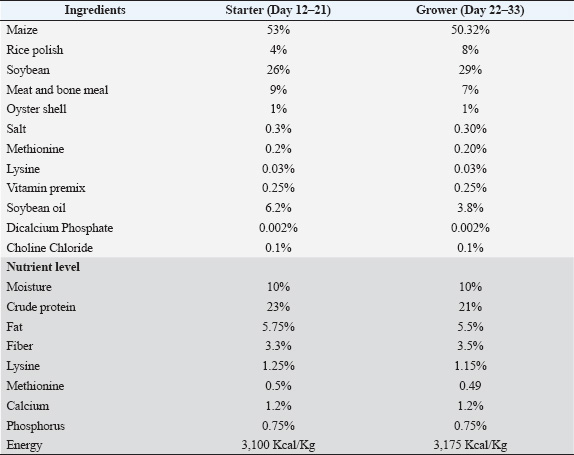
ABSTRACTBackground: Feed additive supplementation for weight gain is a recent trend in poultry farming. Antibiotics, probiotics, and acidifiers are commonly administered to improve weight gain and health status. Understanding their specific effects on growth and hematobiochemical parameters is essential for broiler production optimization. Aim: This research aimed to investigate the effects of antibiotic, probiotic, and acidifier treatments on broiler growth progression and hematobiochemical alterations. Methods: Eighty 12-day-old broiler chicks were randomly divided into four groups: Group A (control), Group B (antibiotic-treated), Group C (probiotic-treated), and Group D (acidifier-treated). The respective feed additives were administered to groups B, C, and D, while group A received a standard diet without additives. Body weights were recorded weekly until day 33, and blood samples were collected for hematological and biochemical analysis on the final day. Results: A significant increase in body weight was observed for all treatment groups compared with the control group across all observation days. The antibiotic-treated group showed a substantial increase in the total erythrocyte count, hemoglobin, and packed cell volume compared with the other groups. The total cholesterol level was significantly reduced in the probiotic- and acidifier-treated groups, and the triglyceride level was remarkably decreased in the acidifier-treated group. Furthermore, high-density lipoprotein levels were significantly increased in the acidifier-treated group and decreased in the antibiotic-treated group. However, low-density lipoprotein was significantly increased in the treated groups. Additionally, the aspartate aminotransferase level was notably elevated in the antibiotic- and acidifier-treated groups, while all treatment groups showed a significant increase in alanine aminotransferase level compared to the control. Conclusion: Antibiotic, probiotic, and acidifier supplementation significantly enhanced weight gain in broilers compared with the control group. Among the treatments, antibiotics notably improved hematological parameters, whereas probiotics and acidifiers positively influenced lipid profiles. Overall, these feed additives had beneficial effects on growth and selected biochemical parameters, with varying impacts on hematological values. Keywords: Antibiotic, Probiotic, Acidifier, Growth performance, Hematobiochemical profile. IntroductionChicken has emerged as the most widely reared poultry species in Bangladesh, largely because of the high demand for its meat and eggs as essential protein sources. The low cost of farming, quick returns on investment, and the country’s favorable climate for raising chickens are key factors contributing to the rise of poultry farming in Bangladesh (Hamid et al., 2017). However, pathogenic microorganisms are a major threat to the growing industry as they cause diseases that hinder growth and productivity, ultimately raising production costs (Hafez and Attia, 2020). Therefore, to combat the detrimental effects of pathogens and ensure better economic returns, farmers often rely on feed additives such as antibiotics, hormones, vitamins, and other growth promoters in poultry feed or drinking water (Granstad et al., 2020). Antibiotics as additives play a crucial role in the effective production of meat and eggs by controlling pathogenic bacteria in the gut and maximizing nutrient utilization, which leads to improved growth rate, reproductive performance, uniformity, and reduced morbidity and mortality rate (Lourenco et al., 2019). Therefore, a wide range of antibiotics are commonly added to the feed or water at subtherapeutic doses (Islam et al., 2016). However, the uncontrolled use of antibiotics as feed additives may contribute to the development of antibiotic-resistant bacteria, potentially jeopardizing the effectiveness of antibiotics in treating bacterial infections in humans (Ghimpețeanu et al., 2022). On the other hand, eliminating antibiotic supplements from diets could adversely affect profitability. Therefore, alternative solutions for disease prevention and growth enhancement are being extensively investigated (Mehdi et al., 2018). A potential substitute for synthetic antibiotics could be the use of probiotics and acidifiers as feed additives (Shi et al., 2019). The administration of probiotics with poultry feed or drinking water has recently gained significant attention (Bai et al., 2013). Probiotics are defined as live microorganisms that are beneficial to the host when ingested in an appropriate dosage (Alagawany et al., 2018). Probiotics are added to animal feed or water to provide a beneficial intestinal microbiota that suppresses the growth of harmful microbes and promotes overall health (El Jeni et al., 2021). The primary function of probiotics is to enhance the intestinal environment, promote better nutrient absorption, and strengthen the animal’s immune system. However, the uncontrolled use of probiotics without robust scientific validation raises concerns about their reliable effectiveness and potential adverse effects. Therefore, understanding the mode of action, optimal dosages, and possible interaction of probiotics with poultry feeds is crucial before their generous application in the poultry sector. Similarly, the administration of acidifiers (Organic acids) with poultry feed or water has emerged as another critical nutritional approach in the poultry sector (Abd El-Ghany et al., 2022). Acidifiers help suppress the development of pathogenic intestinal microflora by lowering the pH in poultry feed, the gut, and microbial cytoplasm. This minimizes microbial competition for nutrients and decreases subclinical infections, immune mediator release, and growth-inhibiting microbial metabolite formation (Pearlin et al., 2020). Consequently, protein and energy digestion are improved, facilitating better growth in chickens (Khan et al., 2022). They also prevent epithelial cell damage by reducing microbe colonization on the intestinal wall (Paul et al., 2007). However, similar to probiotics, we should also consider the use of acidifiers in the poultry industry, based on rigorous experimental evidence to ensure their efficacy and safety. Further research is recommended to identify the impacts of antibiotics, probiotics, and acidifiers, their optimal dosage in broiler feed, and their effects on broiler chicken growth and development. The indiscriminate use of feed additives without adequate scientific justification could bring a significant risk to the sustainability of the poultry sector. Considering this, the current study focused on assessing the growth progression and hematobiochemical alterations in broilers supplemented with an antibiotic, probiotic, and acidifier through drinking water. Materials and MethodsEthical approvalThis study, along with all experimental protocols, was approved and conducted in alignment with the animal handling, care, and usage regulations by the Animal Welfare and Experimentation Ethics Committee of Bangladesh Agricultural University, Mymensingh-2202, Bangladesh (AWEEC/BAU/2020-15). The experiment was conducted at the experimental section of the Department of Physiology, Bangladesh Agricultural University, Mymensingh 2202. Experimental frameworkA total of eighty 12-day-old Hubbard Classic strain broiler chicks were randomly separated into four equal groups, with each group containing 20 birds. Group A was considered as the control and provided with non-medicated water; Group B was supplemented with an antibiotic (Renamycin® Vet) at a dose of 1.00 g/l; Group C was supplemented with a probiotic (Avi-Bac) at a dose of 0.50 g/l, and Group D was supplied with an acidifier (Hameco-pH®) at a dose of 1.00 ml/l, every day up to 33 days of age. Feed additives were administered through drinking water, a frequently used method that ensures precise dosing and uniform intake among all birds, minimizes variability due to feed consumption, and allows better control over additive effects while reducing potential interactions with feed components (Vermeulen et al., 2002; Torshizi et al., 2010). The initial body weights of all birds were measured before they were grouped on day 12. Body weight was measured weekly on days 19, 26, and 33. After sacrificing the birds on the last day of the experiment on day 33, blood samples were obtained for hematological alterations [total erythrocyte count (TEC), hemoglobin (Hb), packed cell volume (PCV), and erythrocyte sedimentation rate (ESR)] and serum samples for biochemical dynamics [Total cholesterol, triglycerides, high-density lipoprotein (HDL), low-density lipoprotein (LDL), aspartate aminotransferase (AST), and alanine aminotransferase (ALT)]. Management practicesAll groups were provided with standard broiler starter and grower feeds in compliance with their age (Table 1). Feeds were purchased from a well-known and reliable feed company, Quality Feeds Limited, Dhaka, Bangladesh. Renamycin® Vet, which is manufactured by Renata Limited, Dhaka, Bangladesh, and composed of 227.20 mg of oxytetracycline hydrochloride United States Pharmacopeia (USP) per gram powder, was used as the antibiotic. Oxytetracycline hydrochloride (USP) was chosen for this study due to its widespread use, proven efficacy as a broad-spectrum antibiotic, availability, cost-effectiveness, safety profile, and established role in infection control in broilers (Atiyah and Hamood, 2021; Sheikh et al., 2022). As the probiotic, Avi-Bac of Opsonin Pharma Limited, Dhaka, Bangladesh, was used, which contained Bacillus subtilis, Bifidobacterium longum, Lactobacillus acidophilus, as well as Hemicellulose Extract and Dextrose. Avi-Bac had a minimum concentration of 6 × 1010 colony-forming units per kilogram. Hameco-pH®, by Square Pharmaceuticals Limited, Dhaka, Bangladesh, was used as an acidifier, which contained a combination of Glycerin 0.50%, Propylene glycol 0.50%, Citric acid 2.5%, Sodium formate 8.67%, Formic acid 17.55%, Acetic acid 7.6%, Lactic acid 3.75%, Propionic acid 2.31%, Ammonium Propionate 8.32%, and moisture up to 100%. Medications were prepared daily and provided in the morning. The water consumption and body weight of the birds were considered to calculate the appropriate concentration of medications. The standard broiler-rearing approach was maintained during the experiment. Birds were vaccinated against the Infectious Bursal Disease on days 12 and 22 and the Newcastle Disease Virus on day 20 via the intraocular route. Biosecurity measures were also strictly practiced. Table 1. Broiler feed composition and feed formulation according to age
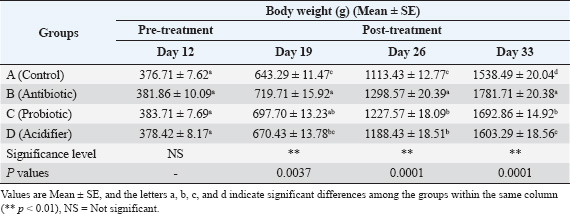
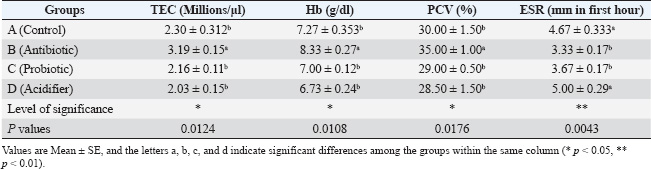
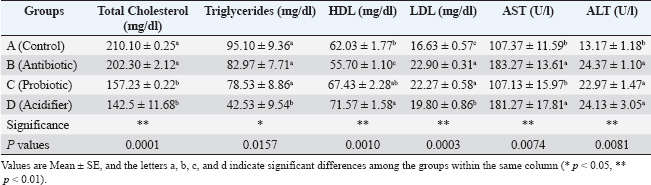
Blood collection and serum preparationApproximately 10 ml of blood was obtained from each bird after slaughter. About 5 ml was transferred to a sterile test tube with an anticoagulant (3.8% Trisodium citrate) at a ratio of 1:10. The rest was kept in another test tube without an anticoagulant and placed in a slanting position at room temperature for clotting. The samples were then refrigerated at 4°C overnight. Centrifugation at 1,000 rpm for 15 minutes separated the serum from the clotted blood. Cell-free serum samples were stored in a screw-capped serum vial and preserved at −20°C for further biochemical analysis (Khalil et al., 2020). Hemato-biochemical analysisHematological characteristics, i.e., TEC, Hb, PCV, and ESR, were assessed within 2 hours of sample collection following standard protocols (Salahuddin et al., 2012). Furthermore, biochemical parameters, i.e., serum total cholesterol, triglycerides, HDL, LDL, AST, and ALT, were measured based on standard methods using a T 80 UV spectrophotometer (PG Instruments, Great Britain) (Haque et al., 2017). Test-specific reagents supplied by High Technology Incorporation, USA, were utilized for all assays. The hemato-biochemical analysis was performed in collaboration with Professor Muhammad Hossain Central Laboratory, Bangladesh Agricultural University, Mymensingh-2202, Bangladesh. Statistical analysisThe results are presented as the mean ± SEM. Data were analyzed using one-way analysis of variance. Tukey’s multiple comparison test was used to determine significant group differences. ResultsEffects of feed additives on body weight progressionTable 2 shows the growth performance of different groups treated with the antibiotic, probiotic, and acidifier. On day 12 of the broiler chicks’ age at the experiment’s onset, the body weights were similar. However, after 1 week of feed additive supplementation on day 19, the antibiotic-treated group (group B) exhibited the highest body weight. In contrast, the body weight was the lowest in the control group (group A). However, the probiotic-treated group (group C) and the acidifier-treated group (group D) were similar. On day 26, all the treatment groups varied remarkably compared to the control group, where group B (antibiotic) showed the maximum body weight, and the body weight of group A (control) was the minimum. By day 33, weight differences remained significantly higher in the treated groups in comparison with the control group, with the antibiotic-treated group still showing the highest body weight, followed by the probiotic- and acidifier-treated groups. Overall, these findings suggest a slower body weight increment in the control group in consecutive observation days, whereas the increment rate was noticeably higher in all the treatment groups, with the antibiotic-treated group being at the top. Effects of feed additives on hematological factorsTable 3 shows the hematological parameters, TEC, Hb, PCV, and ESR of different groups treated with the antibiotic, probiotic, and acidifier. The results indicated that the broiler group fed with the antibiotic (group B) had the maximum TEC, Hb, and PCV values, followed by the control (group A), probiotic (group C), and acidifier (group D) groups. The minimum TEC, Hb, and PCV values were recorded in the acidifier-supplemented group. In contrast, the highest ESR value was found in the acidifier-treated group, followed by the control, probiotic, and antibiotic-treated groups. Although hematological observations were found within the typical range, all the treated groups showed significant differences compared with the control group. The TEC, Hb, and PCV values of the antibiotic-supplemented group were noticeably higher than those of the other groups. However, significant differences within the other groups were not observed. Furthermore, the ESR values of group B (antibiotic) and C (probiotic) were significantly lower than group D (acidifier) and A (control). Effects of feed additives on biochemical profileThe serum biochemical parameters, total cholesterol, triglycerides, HDL, LDL, AST, and ALT are shown in Table 4. The highest total cholesterol and triglyceride levels were found in control group A, and the lowest were in the acidifier-treated group D. The total cholesterol level was significantly reduced in the probiotic-treated group C and the acidifier-treated group D compared with the control group A and the antibiotic-treated group B. On the other hand, triglyceride levels decreased significantly in all groups except the control. Moreover, the highest HDL was found in the acidifier-treated group, whereas the lowest was in the antibiotic-treated group. HDL levels in the birds treated with the acidifier were significantly higher than those in the rest. In contrast, the LDL levels were found to be the highest in the antibiotic-treated group, while broilers of the control group had the lowest LDL value. Although the difference in LDL levels of the antibiotic- and probiotic-treated groups was non-significant, the LDL levels of the control and acidifier-treated groups were significantly lower than those of the antibiotic- and probiotic-treated groups. On the contrary, the highest level of AST was found in the antibiotic-treated group, followed by the acidifier, control, and probiotic-treated groups. Similarly, the highest ALT level was also noticed in the antibiotic-treated group, whereas the lowest value was observed in the control group. Table 2. Effects of antibiotic, probiotic, and acidifier on body weight (g) progression in broilers.
Table 3. Effects of antibiotic, probiotic, and acidifier on hematology in broilers.
Table 4. Effects of antibiotic, probiotic, and acidifier on biochemical studies in broilers.
DiscussionIn this study, we investigated the effects of the antibiotic, probiotic, and acidifier on growth progression, hematological parameters, and biochemical profiles in broiler chickens. In the findings of our study, all the treatment groups of broiler chickens (antibiotic, probiotic, and acidifier) exhibited increased body weight progression than the non-treated group (control). This finding aligns with previous studies despite differences in dosage, duration, and route of administration (Leone and Ferrante, 2023; Hossain et al., 2024). On the contrary, negligible effects of dietary probiotics supplements on body weight, weight gain, and feed intake in broilers were also noticed (Sarangi et al., 2016). However, probiotic supplementation at an adequate level significantly outperformed antibiotic supplementation in terms of body weight gain in broiler chickens (Nath et al., 2023). Moreover, body weight gain and feed conversion ratio were reported to be improved in a dose-dependent manner (Manvatkar et al., 2022). Probiotic treatment may have increased body weight gain by increasing the height and width of the duodenum and jejunum villi (Elsayed et al., 2024). Furthermore, better growth performance in the treatment groups of broilers in comparison with the control group might be associated with better feed utilization, digestion, absorption, and metabolism facilitated by the supplemented antibiotic, probiotic, and acidifier (He et al., 2019). Antibiotics may exert systemic effects that influence cardiac hypertrophy or increase metabolic demands (Dibner and Richards 2005). The higher weight gain in the antibiotic group could indicate enhanced liver metabolism, driven by better nutrient absorption and greater detoxification activity (Apajalahti and Vienola, 2016). These findings highlight the trade-off between optimizing growth and addressing livestock health concerns. Although the probiotic and acidifier offer safer alternatives, further optimization may be needed to match the growth performance of the antibiotic. The hematology findings of our study revealed that the TEC, Hb, and PCV values were significantly higher in the antibiotic-treated group than in the non-antibiotic group. The reason might be the effect of the antibiotic in the gut to increase nutrient absorption and utilization and decrease inflammation and infections, which helps reduce stress and enhance essential nutrient availability, especially iron and protein, promoting robust support for red blood cell production and other blood parameters. Proper antibiotic treatment with an appropriate dose can positively influence certain blood parameters such as Hb, PCV, and TEC values (Hasan et al., 2021). However, no significant differences were recorded in those hematological parameters after the discriminate and indiscriminate use of antibiotics in broilers (Islam et al., 2019). Interestingly, antibiotic treatment was also observed to reduce the blood parameters (Elkomy et al., 2018). It is noteworthy that the type, dosage, and duration of antibiotics and the health condition of birds can influence the hematological profile. In this study, we did not observe any significant changes in TEC, Hb, and PCV in the probiotic and acidifier-treated groups. Since the ultimate function of probiotics and acidifiers is to improve gut health and nutrient digestion, they may not always be directly linked with erythropoiesis. Strengthening our results, similar findings were also outlined in other studies (Shareef and Al-Dabbagh, 2009; Khalil et al., 2020). However, an increase in Hb concentration and PCV was observed in the treated groups, although the impact on TEC was not significant (Ferdous et al., 2019). As a general indicator of inflammation and possible subclinical stress or infection in broilers, we examined the ESR value that reflects the rate at which red blood cells settle, which can be influenced by plasma protein composition and inflammatory processes (Pelagalli et al., 2023). In our study, ESR values were significantly reduced in the antibiotic- and probiotic-treated groups compared with the control, indicating the potential anti-inflammatory effects of these additives. This reduction likely reflects the immunomodulatory action of these additives, which not only suppress pathogenic bacteria but also enhance immune function, thereby limiting inflammatory responses (Rehman et al., 2020; Jankowski et al., 2022; Sattar et al., 2023). In contrast, the acidifier-supplemented group showed a slight but insignificant increase in ESR compared with the control, possibly due to mucosal adaptation caused by acid supplementation, which can modulate the gut environment and immune responses of broilers, affecting inflammatory markers like ESR (Abd El-Ghany, 2024). Regarding the biochemical profiles, the outcomes of our research depicted that the total cholesterol levels were significantly reduced in the groups treated with the probiotic and acidifier, as compared to the control group. Similar findings were also observed in the case of triglyceride levels, with the acidifier-treated group demonstrating the lowest value. These findings are consistent with those of some previous studies, which denoted that probiotics and acidifiers significantly decreased serum total cholesterol and triglyceride levels (Hossain et al., 2024; Sardar et al., 2024). The mechanism of how probiotics might reduce cholesterol and triglyceride levels is that they acclimate cholesterol originating from various sources in the intestinal tract and abolish the expression of Niemann-Pick C1-Like 1, a protein related to cholesterol absorption and homeostasis (Yazhini et al., 2018). In contrast with our results, no considerable impacts on total cholesterol were reported upon providing the organic acid supplementation with the poultry feed (Adil et al., 2010). However, a notable decline in cholesterol and triglyceride contents was observed in chickens supplemented with probiotics (Mansoub, 2011). However, in the present study, HDL cholesterol, which is well-known for cardiovascular health benefits, was higher in the probiotic and acidifier-supplemented groups, whereas LDL cholesterol, which is considered harmful, was significantly increased in the antibiotic-treated group and decreased in the acidifier-treated group. Similar findings were reported that acidifiers significantly reduced LDL value in the serum of broilers (Dong et al., 2024; Sedghi et al., 2024). This could be associated with improved feed nutrient use due to reduced microbial nutrient loss and accelerated anabolic activity. Acidifiers have been found to enhance immune response and lower gut pH to promote stabilization of gut microflora (Engberg et al., 2000). The increased LDL levels in antibiotics might be due to the interference of antibiotics in lipid homeostasis (Rotimi et al., 2015). In the case of the liver function test in our study, ALT and AST values were more or less increased in the treatment groups than in the control group. The marked increase in AST and ALT activity may indicate hepatocellular stress or elevated hepatic metabolic activity resulting from the treatment groups. Consistent results were found in other studies suggesting that the supplemented antibiotics increased serum AST and ALT levels in broilers, possibly due to hepatic enzyme induction (Makled et al., 2023; Abd El-Ghany, 2024). However, these elevations do not necessarily indicate liver damage because mild increases in transaminases can occur due to increased hepatic workload or metabolic adjustment rather than hepatocellular injury. In contrast, it was reported that the AST value decreased by the acidifier treatment in synergy with dietary toxin binders (Heidari et al., 2018), while no significant impact of acidifiers on the concentration of ALT and AST was also found (Khalil et al., 2020). Furthermore, probiotic treatments were also reported to decline the AST and ALT values (Hussein et al., 2020); however, some studies have not found any significant difference (Qiu et al., 2022). These findings highlight the trade-off between optimizing growth and addressing poultry health concerns. Although antibiotic supplementation yielded the highest weight gain in our study, its long-term use raises safety concerns and possible liver toxicity, warranting careful consideration. In contrast, probiotics and acidifiers can be used as safer alternatives as they significantly enhance growth performance by improving gut health, nutrient absorption, and lipid profiles without causing significant hepatocellular damage. Previous studies have also confirmed that probiotics and acidifiers are generally safe and well-tolerated in broilers, without contributing to antimicrobial resistance or liver stress, supporting their potential as sustainable alternatives to antibiotics (Alagawany et al., 2018; Abd El-Ghany et al., 2022). Additionally, many organic acids and their salts used as acidifiers are approved by the Food and Drug Administration as Generally Recognized as Safe (Sorathiya et al., 2025), further ensuring their use for responsible and sustainable broiler production. ConclusionThis research highlights the significant benefits of incorporating antibiotics, probiotics, and acidifiers into the diets of broilers. These additives enhanced overall growth performance, improved feed efficiency, and positively influenced hematological markers, such as TEC, Hb, and PCV. Additionally, they lower cholesterol and triglyceride levels compared with the control group. However, the changes observed in LDL, AST, ALT, and HDL levels demonstrate the need for a deeper investigation into their specific impacts. The study indicates that these improvements may result from the additives’ ability to modulate feed intake, digestion, and hemopoietic functions alongside their potential influence on gut microbiota. Although the findings offer promising insights into poultry nutrition, deeper research is obligatory to reveal the underlying mechanisms, dose-response relationships, and potential long-term effects. AcknowledgmentsThe authors sincerely thank the Department of Physiology, Faculty of Veterinary Science, Bangladesh Agricultural University, Mymensingh-2202, Bangladesh, and Professor Muhammad Hossain Central Laboratory, Bangladesh Agricultural University, Mymensingh-2202, Bangladesh, for granting access to their facilities and resources, which made this work possible. Conflicts of interestThe authors declare no conflicts of interest. FundingThis research was conducted without any external financial funding. Author’s contributionsMd. Mominul Islam conducted the experiment, collected and analyzed the data, and drafted the manuscript. Md. Saiful Islam and Khaled Mahmud Sujan contributed to the statistical analysis and data interpretation. Mohammad Alam Miah supervised the entire study, critically reviewed the manuscript for intellectual content, and approved the final version for publication. All authors have read and approved the final version of the manuscript. Data availabilityThe datasets generated and analyzed during the current study are available upon reasonable request from the corresponding author. ReferencesAbd El-Ghany, W.A. 2024. Applications of organic acids in poultry production: an updated and comprehensive review. Agriculture 14(10), 1756. Abd El-Ghany, W.A., Abdel-Latif, M.A., Hosny, F., Alatfeehy, N.M., Noreldin, A.E., Quesnell, R.R., Chapman, R., Sakai, L. and Elbestawy, A.R. 2022. Comparative efficacy of postbiotic, probiotic, and antibiotic against necrotic enteritis in broiler chickens. Poult. Sci. 101, 101988. Adil, S., Banday, T., Bhat, G.A., Mir, M.S. and Rehman, M. 2010. Effect of dietary supplementation of organic acids on performance, intestinal histomorphology, and serum biochemistry of broiler chicken. Vet. Med. Int. 2010, 479485. Alagawany, M., Abd El-Hack, M.E., Farag, M.R., Sachan, S., Karthik, K. and Dhama, K. 2018. The use of probiotics as eco-friendly alternatives for antibiotics in poultry nutrition. Environ. Sci. Pollut. Res. 25, 10611–10618. Apajalahti, J. and Vienola, K. 2016. Interaction between chicken intestinal microbiota and protein digestion. Anim. Feed Sci. Technol. 221, 323–330. Atiyah W.R. and Hamood M.F. 2021. Enhancing the productive performance of broiler chickens by adding Spirulina platensis compared with probiotic, prebiotics, and oxytetracycline. Iraqi J. Vet. Med. 45(1), 31–36. Bai, S.P., Wu, A.M., Ding, X.M., Lei, Y., Bai, J., Zhang, K.Y. and Chio, J.S. 2013. Effects of probiotic-supplemented diets on growth performance and intestinal immune characteristics of broiler chickens. Poult. Sci. 92, 663–670. Dibner, J.J. and Richards, J.D. 2005. Antibiotic growth promoters in agriculture: history and mode of action. Poult. Sci. 84(4), 634–643. Dong, Y., Gao, X., Qiao, C., Han, M., Miao, Z., Liu, C., Yan, L. and Li, J. 2024. Effects of mixed organic acids and essential oils in drinking water on growth performance, intestinal digestive capacity, and immune status in broiler chickens. Animals 14(15), 2160. El Jeni, R., Dittoe, D.K., Olson, E.G., Lourenco, J., Corcionivoschi, N., Ricke, S.C. and Callaway, T.R. 2021. Probiotics and potential applications for alternative poultry production systems. Poult. Sci. 100, 101156. Elsayed, M.A.M., Abdelrahman, M.A.M., Darwish, M.H.A., Mohammed, A.A.A., Negm, E.A. and Abdelsamea, N.A. 2024. Effect of dietary probiotic supplementation on blood parameters, behavior and health performance of broilers. J. Adv. Vet. Res. 14(7), 1269–1275. Elkomy, A., Belih, S., Aboubakr, M. and Morad, M. 2018. Effect of amoxicillin on some hematological parameters in broiler chickens. Res. J. Poult. Sci. 11(2-4), 9–11. Engberg, R.M., Hedemann, M.S., Leser, T.D. and Jensen, B.B. 2000. Effect of zinc bacitracin and salinomycin on intestinal microflora and performance of broilers. Poult. Sci. 79(9), 1301–1309. Ferdous, M.F., Arefin, M.S., Rahman, M.M., Ripon, M.M.R., Rashid, M.H., Sultana, M.R., Hossain, M.T., Ahammad, M.U. and Rafiq, K. 2019. Beneficial effects of probiotic and phytobiotic as growth promoter alternative to antibiotic for safe broiler production. J. Adv. Vet. Anim. Res. 6(3), 409–415. Ghimpețeanu, O.M., Pogurschi, E.N., Popa, D.C., Dragomir, N., Drăgotoiu, T., Mihai, O.D. and Petcu, C.D. 2022. Antibiotic use in livestock and residues in food—a public health threat: a review. Foods 11, 1430. Granstad, S., Kristoffersen, A.B., Benestad, S.L., Sjurseth, S.K., David, B., Sorensen, L., Fjermedal, A., Edvardsen, D.H., Sanson, G., Lovland, A., Kaldhusdal, M. 2020. Effect of feed additives as alternatives to in-feed antimicrobials on production performance and intestinal Clostridium perfringens counts in broiler chickens. Animals 10, 240. Hafez, H.M. and Attia, Y.A. 2020. Challenges to the poultry industry: current perspectives and strategic future after the COVID-19 outbreak. Front. Vet. Sci. 7, 516. Hamid, M.A., Rahman, M.A., Ahmed, S. and Hossain, K.M. 2017. Status of poultry industry in Bangladesh and the role of private sector for its development. Asian J. Poult. Sci. 11, 1–13. Haque, M.I., Ahmad, N. and Miah, M.A. 2017. Comparative analysis of body weight and serum biochemistry in broilers supplemented with some selected probiotics and antibiotic growth promoters. J. Adv. Vet. Anim. Res. 4, 288–294. Hasan, M.N., Islam, M.S., Hasan, M.R. and Islam, K.R. 2021. Effects of colistin sulfate on hematological parameters in broiler. Asian J. Med. Biol. Res. 7(2), 113–117. He, T., Long, S., Mahfuz, S., Wu, D., Wang, X., Wei, X. and Piao, X. 2019. Effects of probiotics as antibiotics substitutes on growth performance, serum biochemical parameters, intestinal morphology, and barrier function of broilers. Animals 9(11), 985. Heidari, M.R., Sadeghi, A.A. and Rezaeipour, V. 2018. Effects of acidifier supplementation and toxin binder on performance, carcass, blood metabolites, intestinal morphology, and microbial population in broiler chickens. Iran J. Appl. Anim. Sci. 8(3), 469–476. Hossain, M.T., Sardar, D., Afsana, S., Datta, M. and Habib, M.A. 2024. Comparative analysis between multi-strain probiotics and antibiotic as starter feed supplement of poultry on growth performance, serum metabolites and meat quality. Vet. Anim. Sci. 24, 100346. Hussein, E.O.S., Ahmed, S.H., Abudabos, A.M., Aljumaah, M.R., Alkhlulaifi, M.M., Nassan, M.A., Suliman, G.M., Naiel, M.A.E. and Swelum, A.A. 2020. Effect of antibiotic, phytobiotic and probiotic supplementation on growth, blood indices and intestine health in broiler chicks challenged with Clostridium perfringens. Animals 10(3), 507. Islam, M.S., Siddiqui, M.N., Sayed, M.A., Tahjib-Ul-Arif, M., Islam, M.A., Hossain, M.A. 2016. Dietary effects of buckwheat (Fagopyrum esculentum) and black cumin (Nigella sativa) seed on growth performance, serum lipid profile and intestinal microflora of broiler chicks. S. Afr. J. Anim. Sci. 46, 103–111. Islam, M.S., Islam, M.Z. and Islam, M.S. 2019. Discriminate and indiscriminate use of amoxicillin and its effects on hematological parameters of broiler. Asian J. Med. Biol. Res. 5(2), 153–157. Jankowski, J., Tykałowski, B., Stępniowska, A., Konieczka, P., Koncicki, A., Matusevičius, P. and Ognik, K. 2022. Immune parameters in chickens treated with antibiotics and probiotics during early life. Animals 12(9), 1133. Khalil, K.K.I., Islam, M.A., Sujan, K.M., Mustari, A., Ahmad, N., Miah, M.A. 2020. Dietary acidifier and lysozyme improve growth performances and hemato-biochemical profile in broiler chicken. J. Adv. Biotechnol. Exp. Ther. 3, 241–247. Khan, R.U., Naz, S., Raziq, F., Qudratullah, Q., Khan, N.A., Laudadio, V., Tufarelli, V. and Ragni, M. 2022. Prospects of organic acids as safe alternative to antibiotics in broiler chickens diet. Environ. Sci. Pollut. Res. Int. 29, 32594–32604. Leone, F. and Ferrante, V. 2023. Effects of prebiotics and precision biotics on performance, animal welfare and environmental impact: a review. Sci. Total Environ. 901, 165951. Lourenco, J.M., Seidel, D.S. and Callaway, T.R. 2019. Antibiotics and gut function: historical and current perspectives. In Improving gut health in poultry. Eds., Ricke S.C. Cambridge, UK: Francis Dodds Science Publishing, pp: 172–189. Makled, M.N., Eldeeb, M.A., Abouelezz, K., Amen, O.K. and Habib, M.A. 2023. Impacts of probiotics with or without organic acids as dietary supplements on growth performance, carcass quality, digestibility, intestinal development, and gut microbiota of broiler chicks. Trop. Subtrop. Agroecosys. 26(3), 1–12. Mansoub, N.H. 2011. Comparison of effects of using yogurt and probiotic on performance and serum composition of broiler chickens. Ann. Biol. Res. 2(3), 121. Manvatkar, P.N., Kulkarni, R.C., Awandkar, S.P., Chavhan, S.G., Durge, S.M., Avhad, S.R., Channa, G.R. and Kulkarni, M.B. 2022. Performance of broiler chicken on dietary supplementation of protected organic acids blend. Br. Poult. Sci. 63(5), 633–640. Mehdi, Y., Letourneau-Montminy, M.P., Gaucher, M.L., Chorfi, Y., Suresh, G., Rouissi, T., Brar, S.K., Cote, C., Avalos Ramirez, A. and Godbout, S. 2018. Use of antibiotics in broiler production: global impacts and alternatives. Anim. Nutr. 4, 170–178. Nath, S.K., Hossain, M.T., Ferdous, M., Siddika, M.A., Hossain, A., Maruf, A.A., Chowdhory, A.T. and Nath, T.C. 2023. Effects of antibiotic, acidifier, and probiotic supplementation on mortality rates, lipoprotein profile, and carcass traits of broiler chickens. Vet. Anim. Sci. 22, 100325. Paul, S.K., Halder, G., Mondal, M.K. and Samanta, G. 2007. Effect of organic acid salt on the performance and gut health of broiler chicken. Jpn. Poult. Sci. 44, 389–395. Pearlin, B.V., Muthuvel, S., Govidasamy, P., Villavan, M., Alagawany, M., Farag, M.R., Dhama, K. and Gopi, M. 2020. Role of acidifiers in livestock nutrition and health: a review. J. Anim. Physiol. Anim. Nutr. 104, 558–569. Pelagalli, M., Tomassetti, F., Nicolai, E., Giovannelli, A., Codella, S., Iozzo, M., Massoud, R., Secchi, R., Venditti, A., Pieri, M. and Bernardini, S. 2023. The role of erythrocyte sedimentation rate (ESR) in myeloproliferative and lymphoproliferative diseases: comparison between DIESSE CUBE 30 TOUCH and Alifax Test 1. Diseases 11(4), 169. Qiu, K., Wang, X., Zhang, H., Wang, J., Qi, G. and Wu, S. 2022. Dietary supplementation of a new probiotic compound improves the growth performance and health of broilers by altering the composition of cecal microflora. Biology 11(5), 633. Rehman, A., Arif, M., Sajjad, N., Al-Ghadi, M.Q., Alagawany, M., Abd El-Hack, M.E., Alhimaidi, A.R., Elnesr, S.S., Almutairi, B.O., Amran, R.A., Hussein, E.O.S. and Swelum, A.A. 2020. Dietary effect of probiotics and prebiotics on broiler performance, carcass, and immunity. Poult. Sci. 99(12), 6946–6953. Rotimi, S.O., Ojo, D.A., Talabi, O.A., Ugbaja, R.N., Balogun, E.A. and Ademuyiwa, O. 2015. Amoxillin- and pefloxacin-induced cholesterogenesis and phospholipidosis in rat tissues. Lipids Health Dis. 14, 13. Salahuddin, M., Miah, M.A. and Ahmad, N. 2012. Effects of protein and vitamin A&D on growth performance and haemato biochemical profile in broiler. Bangl. J. Vet. Med. 10, 9–14. Sarangi, N.R., Babu, L.K., Kumar, A., Pradhan, C.R., Pati, P.K. and Mishra, J.P. 2016. Effect of dietary supplementation of prebiotic, probiotic, and synbiotic on growth performance and carcass characteristics of broiler chickens. Vet. World 9, 313–319. Sardar, D., Afsana, S., Habib, A. and Hossain, T. 2024. Dietary effects of multi-strain probiotics as an alternative to antibiotics on growth performance, carcass characteristics, blood profiling and meat quality of broilers. Vet. Integr. Sci. 23(2), 1–17. Sattar, A., Nime, J., Azmal, S.A., Rahaman, A., Matin, S.M.A., Haque, A., Ullah, H., Hossen, L. and Ahmad, N. 2023. Effects of probiotic and organic acids with yeast extract on body weight gain and hemato-biochemical parameters in broilers. Int. J. Anim. Sci. Technol. 7(1), 5–10. Sedghi, M., Azghadi, M.A., Mohammadi, I., Ghasemi, R., Sarrami, Z. and Abbasi, M. 2024. The effects of acidifier inclusion in the diet on growth performance, gastrointestinal health, ileal microbial population, and gene expression in broilers. Braz. J. Poult. Sci. 26(2), 1–20. Shareef, M. and Al-Dabbagh, A.S.A. 2009. Effect of probiotic (Saccharomyces cerevisiae) on performance of broiler chicks. Iraqi J. Vet. Sci. 23, 23–29. Sheikh, I.S., Shah, S.H., Kakar, N., Tariq, M.M., Kakar, M.E., Mustafa, M.Z. 2022. Use of growth promoter in feed: tylosin phosphate and oxytetracycline Di-Hydrate show synergistic effect on the haematological parameters and biochemical components of broiler chicken blood. Pak. J. Zool. 54(6), 2903–2908. Shi, Z., Rothrock, M.J.Jr. and Ricke, S.C. 2019. Applications of microbiome analyses in alternative poultry broiler production systems. Front. Vet. Sci. 6, 157. Sorathiya, K.B., Melo, A., Hogg, M.C. and Pintado, M. 2025. Organic acids in food preservation: exploring synergies, molecular insights, and sustainable applications. Sustainability 17(8), 3434. Torshizi, M.A.K., Moghaddam A.R., Rahimi, S. and Mojgani, N. 2010. Assessing the effect of administering probiotics in water or as a feed supplement on broiler performance and immune response. Bri. Poult. Sci. 51(2), 178–184. Vermeulen, B., De Backer, P. and Remon, J.P. 2002. Drug administration to poultry. Adv. Drug Deliv. Rev. 54(6), 795–803. Yazhini, P., Visha, P., Selvaraj, P. and Chandran, V. 2018. Dietary encapsulated probiotic effect on broiler serum biochemical parameters. Vet. World 11(9), 1344–1348. | ||
| How to Cite this Article |
| Pubmed Style Islam MM, Islam MS, Sujan KM, Miah MA. Growth progression and hematobiochemical dynamics in broiler chickens treated with antibiotic, probiotic, and acidifier. Open Vet. J.. 2025; 15(8): 3477-3485. doi:10.5455/OVJ.2025.v15.i8.11 Web Style Islam MM, Islam MS, Sujan KM, Miah MA. Growth progression and hematobiochemical dynamics in broiler chickens treated with antibiotic, probiotic, and acidifier. https://www.openveterinaryjournal.com/?mno=260787 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i8.11 AMA (American Medical Association) Style Islam MM, Islam MS, Sujan KM, Miah MA. Growth progression and hematobiochemical dynamics in broiler chickens treated with antibiotic, probiotic, and acidifier. Open Vet. J.. 2025; 15(8): 3477-3485. doi:10.5455/OVJ.2025.v15.i8.11 Vancouver/ICMJE Style Islam MM, Islam MS, Sujan KM, Miah MA. Growth progression and hematobiochemical dynamics in broiler chickens treated with antibiotic, probiotic, and acidifier. Open Vet. J.. (2025), [cited January 25, 2026]; 15(8): 3477-3485. doi:10.5455/OVJ.2025.v15.i8.11 Harvard Style Islam, M. M., Islam, . M. S., Sujan, . K. M. & Miah, . M. A. (2025) Growth progression and hematobiochemical dynamics in broiler chickens treated with antibiotic, probiotic, and acidifier. Open Vet. J., 15 (8), 3477-3485. doi:10.5455/OVJ.2025.v15.i8.11 Turabian Style Islam, Md. Mominul, Md. Saiful Islam, Khaled Mahmud Sujan, and Mohammad Alam Miah. 2025. Growth progression and hematobiochemical dynamics in broiler chickens treated with antibiotic, probiotic, and acidifier. Open Veterinary Journal, 15 (8), 3477-3485. doi:10.5455/OVJ.2025.v15.i8.11 Chicago Style Islam, Md. Mominul, Md. Saiful Islam, Khaled Mahmud Sujan, and Mohammad Alam Miah. "Growth progression and hematobiochemical dynamics in broiler chickens treated with antibiotic, probiotic, and acidifier." Open Veterinary Journal 15 (2025), 3477-3485. doi:10.5455/OVJ.2025.v15.i8.11 MLA (The Modern Language Association) Style Islam, Md. Mominul, Md. Saiful Islam, Khaled Mahmud Sujan, and Mohammad Alam Miah. "Growth progression and hematobiochemical dynamics in broiler chickens treated with antibiotic, probiotic, and acidifier." Open Veterinary Journal 15.8 (2025), 3477-3485. Print. doi:10.5455/OVJ.2025.v15.i8.11 APA (American Psychological Association) Style Islam, M. M., Islam, . M. S., Sujan, . K. M. & Miah, . M. A. (2025) Growth progression and hematobiochemical dynamics in broiler chickens treated with antibiotic, probiotic, and acidifier. Open Veterinary Journal, 15 (8), 3477-3485. doi:10.5455/OVJ.2025.v15.i8.11 |