| Research Article | ||
Open Vet. J.. 2025; 15(9): 4121-4127 Open Veterinary Journal, (2025), Vol. 15(9): 4121-4127 Research Article Potential of microbial-based feed additives on broiler carcass and fat percentagesMohammad Sukmanadi1*, Rochmah Kurnijasanti1, Sunaryo Hadi Warsito2, Aswin Rafif Khairullah3, Imam Mustofa4, Riza Zainuddin Ahmad3, Adeyinka Oye Akintunde5, Bima Putra Pratama6, Siti Rani Ayuti7 and Ertika Fitri Lisnanti81Division of Basic Veterinary Medicine, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 2Division of Animal Husbandry, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 3Research Center for Veterinary Science, National Research and Innovation Agency (BRIN), Bogor, Indonesia 4Division of Veterinary Reproduction, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 5Department of Agriculture and Industrial Technology, Babcock University, Ilishan Remo, Nigeria 6Research Center for Agroindustry, National Research and Innovation Agency (BRIN), South Tangerang, Indonesia 7Faculty of Veterinary Medicine, Universitas Syiah Kuala, Banda Aceh, Indonesia 8Program of Animal Husbandry, Faculty of Agriculture, Universitas Islam Kadiri, Kediri, Indonesia *Corresponding Author: Mohammad Sukmanadi. Division of Basic Veterinary Medicine, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: moh-s [at] fkh.unair.ac.id Submitted: 24/05/2025 Revised: 30/07/2025 Accepted: 07/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
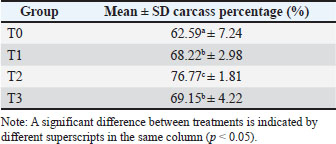
ABSTRACTBackground: Factors before and after slaughter can influence carcass quality. A factor that can affect carcass quality before slaughter is feed that includes additives. One alternative is to administer probiotics. Aim: This study aimed to determine the effects of liquid probiotics Bacillus subtilis and Bacillus coagulans as feed additives on carcass percentage, consumption, feed conversion rate (FCR), and abdominal fat percentage in broilers. Methods: Twenty-four Lohmann broilers (Gallus gallus domesticus) aged 21 days. Birds were randomly divided into four treatment groups. The T0 control group was orally administered 2 ml of distilled water/head/day. T1 was orally administered 2 ml of bacterial isolate/head/day. Group T2 was orally administered with 4 ml of bacterial isolate/head/day. Group T3 was orally administered with 6 ml/head/day of bacterial isolates. Each treatment was conducted for 14 days until the age of 35 days. Broilers were physically weighed to obtain the data sample. Results: All liquid probiotic dosages (2, 4, and 6 ml) had significant differences (p < 0.05) in carcass percentage, intake, FCR, and fat percentage. The carcass percentage resulted from a 4 ml liquid probiotic treatment, which was higher than the 2 and 6 ml doses. Conclusion: Four milliliters of liquid probiotics (B. coagulans and B. subtilis) are recommended for optimal broiler rearing outcomes. Keywords: Abdominal fat, Bacillus spp., Carcass percentage, Feed conversion, Food producers. IntroductionBroiler chickens are still a top priority for meeting the protein needs of humans. It does not require a large area to maintain, is highly nutritious, grows quickly, and efficiently converts food into meat so that it quickly reaches marketable age with a high body weight (Beski et al., 2015). However, it also has a high tendency for fatty traits because it is accompanied by fat-forming genes. Broilers are one of the largest contributors of animal protein from livestock and are a superior commodity. The broiler industry is growing rapidly because chicken meat is the main source of consumer menus. Broiler meat is easy to find in modern and traditional markets. Larger broiler meat production is carried out in modern and traditional chicken slaughterhouses. The broiler farming business aims to produce carcasses with a high weight and low fat content (Cullere et al., 2019). A broiler carcass is the meat and bones of a chicken after being separated from the head to the base of the neck and from the feet to the knees. The feathers have been plucked, and the contents of the stomach cavity have been removed. A good-quality carcass contains little fat (Kokoszyński et al., 2022). Food producers, including livestock entrepreneurs, are required to improve the quality of their products, given the public’s high awareness of food safety. Although carcass quality depends on consumer preferences, specific standards are used as a reference (Barbut and Leishman, 2022). Carcasses that are fit for consumption must comply with Indonesian National Standards, starting from handling methods, carcass cutting methods, size and quality, requirements including origin materials, carcass preparation, post-harvest processing, auxiliary materials, additional materials, final product quality, to packaging. Factors before and after slaughter can influence carcass quality. A factor that can affect carcass quality before slaughter is feed that includes additives (hormones, antibiotics, or minerals). These factors will result in poor carcass quality (Mir et al., 2017). Therefore, it is necessary to choose the right feed ingredients. One alternative is to administer probiotics. Probiotics are living, non-pathogenic microorganisms that are introduced to feed and can improve the balance of bacteria in the digestive tract, which can affect growth rate, boost meat production, improve ration usage efficiency, improve digestibility of feed ingredients, and improve animal health (Kumar et al., 2025). Probiotics are an effort for food security through the production of microbial-based feed to obtain feed products used in animal husbandry, with the addition of microbial ingredients to produce animal-based food ingredients, especially high-quality and measurable animal protein that supports food security through the development of science and technology (Krysiak et al., 2021). This study aimed to determine the effects of probiotics containing Bacillus subtilis and Bacillus coagulans on significant traits in broilers, including carcass percentage, feed consumption, feed conversion rate (FCR), and abdominal fat percentage. The analysis of how these probiotics influence metabolic efficiency, nutrient utilization, and fat deposition provides new insights into their multifaceted role in broiler production system optimization. This approach advances the understanding of probiotic applications, contributing valuable knowledge toward sustainable and health-promoting poultry farming practices. Materials and MethodsLocation and time of researchThis research was conducted for 1 month from November 2022 to January 2023. This research was conducted at the Experimental Animal Cage Laboratory, Faculty of Veterinary Medicine, Airlangga University, Indonesia. Research materials and instrumentsThe liquid probiotic preparation used was a microbial-based prototype containing microorganisms: B. subtilis and B. coagulans (4 × 107 CFU/ml). The doses determined in this study were 2, 4, and 6 ml in each treatment. Research methodsTwenty-four Lohmann Strain broilers (Gallus gallus domesticus) aged 21 days. Birds were randomly divided into four treatment groups. The T0 control group was orally administered 2 ml of distilled water/head/day. T1 was orally administered 2 ml of bacterial isolate/head/day. Group T2 was orally administered with 4 ml of bacterial isolate/head/day. Group T3 was orally administered with 6 ml/head/day of bacterial isolates. Each treatment was conducted for 14 days until the age of 35 days. Carcass percentageThe final body weight of each bird at the end of the study determined the carcass percentage. The selected chickens were slaughtered by cutting the jugular vein, allowing the blood to drain while the legs were positioned above and the head below. Once the blood flow ceased and the bird became immobile, a semi-scalding method was employed by immersing the carcass in hot water for 45 seconds, facilitating the removal of feathers. After feather removal, the contents of the abdominal cavity were removed, and the head and legs were cut. The resulting carcass was weighed to determine its weight. The carcass percentage was calculated using the following formula: Carcass Percentage (%)=(Carcass Weight (g) / Live Body Weight (g) × 100. Feed consumptionThe total feed was administered twice daily, once in the morning and once in the evening, with the remaining feed being weighed accordingly. Feed conversion rateComparison between the amount of feed administered during a specific cycle and the total biomass produced. Live weight was measured based on body weight at the time of weighing after a 12-hour fasting period, with the results expressed in grams per individual at the end of the study (4 weeks). Abdominal fat percentageFat collected from depots surrounding the gizzard and the layer between the abdominal muscles and intestines was weighed to calculate the abdominal fat percentage. These tissues were subsequently weighed (10). The quality of broiler carcasses is assessed based on the amount of abdominal fat; higher fat content influences carcass shrinkage or yield. A good carcass should contain a substantial amount of meat, with a portion suitable for consumption, and should not have excessive fat levels (11). The abdominal fat percentage was calculated using the following formula: Abdominal fat percentage (%)=(weight of abdominal fat (g)/cass weight (g) × 100. Data analysisThe research findings were analyzed using one-way analysis of variance with the SPSS for Windows 26.0 program to determine the true differences between each treatment group. Duncan’s multiple range test is performed with a significance level of 0.05 if a genuine effect is observed. Ethical approvalAll relevant institutional policies on the use and care of animals were followed. The Animal Care and Use Committee of the Faculty of Veterinary Medicine at Airlangga University Surabaya in East Java, Indonesia on 21 October 2019, approved this study (Certificate number: 1.KE.199.12.2019). ResultsCarcass percentageThe carcass percentage was calculated using live and broiler carcass weight data. This information was collected during the most recent study period. The results of statistical investigations on the administration of liquid probiotics, B. subtilis, and B. coagulans showed a significant difference (p < 0.05) in the proportion of broiler chicken carcasses. Table 1 displays the findings of the examination of the carcass percentage of broiler chickens fed with liquid probiotics. Table 1. Mean and SD of carcass percentage.
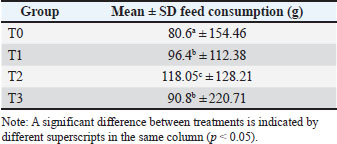
The average broiler carcass percentage in the control group was 62.5%, followed by the T1 group at 68.2%, the T2 group at 76.7%, and the T3 group at 69.1%. Feed consumption The results of the UN measurements of broiler chickens aged 15–35 days with the addition of Soursop leaf flour can be seen in Table 2. Table 2. Mean and SD of feed consumption.
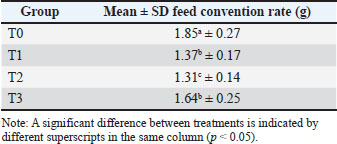
Based on the research, the results of body weight gain at the age of 15–35 days were obtained, namely, T0 (80.6 ± SD 154.46) g/head/day, T1 (96.4 ± SD 112.38) g/head/day, T2 (118.05 ± SD 128.21) g/head/day, and T3 (90.8 ± SD 220.71) g/head/day, with an average overall body weight gain of 1485.5 g/head. Feed conversion rate Feed conversion is a benchmark for determining the feed quality given to livestock to meet the nutritional requirements. Ration conversion is obtained from the ratio of the consumed ration to the increase in body weight over a certain period of time. The higher the feed conversion value, the worse the nutritional value of the feed. The same amount of feed consumption at a greater body weight gain will certainly result in a smaller feed conversion value. Based on Table 3 and the research that has been conducted, the results of feed conversion at the age of 15–35 days are obtained, namely, T0 (1.85 ± SD 0.27), T1 (1.37 ± SD 0.17), T2 (1.31 ± SD 0.14), and T3 (1.64 ± SD 0.25). The overall feed conversion average was 1.61. Table 3. Mean and SD of feed convention rate.
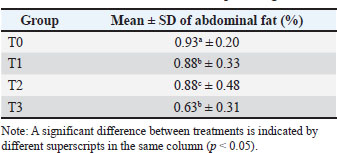
Abdominal fat percentageBased on Table 4 and the results of previous research, the results of the percentage of abdominal fat at the age of 15–35 days are T0 (0.93 ± SD 0.20), T1 (0.88 ± SD 0.33), T2 (0.88 ± SD 0.48), and T3 (0.63 ± SD 0.31) %, with an average overall abdominal fat percentage of 0.83%. Table 4. Mean and SD of abdominal fat percentage.
According to the ANOVA results, oral probiotic therapy significantly impacted the proportion of abdominal fat in broiler chickens (p < 0.05). DiscussionCarcass percentageThe average percentage of broiler carcasses in this study agreed with Murawska’s (2017) findings, which showed that the proportion of broiler carcasses varied between 65% and 75% of body weight. Belly fat weight, carcass weight, final live weight, and fat health significantly influence the percentage of carcasses. The higher carcass weight achieved is the reason for the increased carcass percentage (Jalala et al., 2023). The microbial composition of the chicken intestine was believed to be closely associated with the carcass weight percentage in the treatment groups. The potential for the intestines of broiler chickens to contain more Bacillus bacteria. Increasing the number of B. subtilis and B. coagulans in the intestine will benefit the growth of chickens (Mohamed et al., 2022). Lactobacillus bacteria can break down simple carbohydrates into lactic acid. The pH of the environment drops as lactic acid levels rise, inhibiting the growth of other microorganisms. When Lactobacillus colonizes the surface of the digestive tract, it inhibits the growth of harmful bacteria, such as Escherichia coli, in the small intestine and stops the growth of fungi. Lactobacillus bacteria can preserve the equilibrium of various bacterial communities in the small intestine (Wyszyńska and Godlewska, 2021). Several factors, including strain, live weight, feed quantity and quality, and non-carcass weight, can influence carcass weight (Ikusika et al., 2020). Feed was one of the elements that affected this study because it was not modified to meet the needs of broiler chickens throughout specific growth stages. The nutritional value and availability of the feed to satisfy the needs of broiler chickens during the rearing phase are determined by its quality (Mir et al., 2017). Broiler chickens given a probiotic combination of B. subtilis and B. coagulans 4 ml orally had the highest carcass percentage, as opposed to those given a probiotic combination of B. subtilis and B. coagulans 2 ml and 6 ml orally. The increase in carcass percentage was less pronounced at the maximum dose (6 ml) than at the 4 ml treatment. An excess of probiotic microbes is believed to cause the digestive tract to become too acidic. This may cause the typical flora to become unstable. Additionally, an overly acidic environment can harm the duodenal villi, which can impact the absorption of nutrients (Shehata et al., 2022). The application technique, dosage, type of strain, basal feed, and probiotic concentration all affect the effectiveness of probiotics (Kumar et al., 2025). The effects of probiotics, such as B. subtilis and B. coagulans, on broiler carcass percentage can be molecularly explained through their influence on gut health, nutrient absorption, immune responses, and microbial balance. Bacillus spores germinate in the gastrointestinal tract, colonizing the gut and producing enzymes that modify the microbial ecosystem (Khalid et al., 2022). These probiotics suppress pathogenic bacteria (e.g., Salmonella and E. coli) through competitive exclusion, antimicrobial substance production (bacteriocins and lipopeptides), and niche occupation. This results in a more favorable microbiota balance, promoting a healthier gut environment (Naeem and Bourassa, 2025). Bacillus spp. produce enzymes such as amylases, proteases, lipases, and cellulases (Latorre et al., 2016). These enzymes break down feed components into simpler molecules at the molecular level, improving digestibility and nutrient absorption efficiency in the small intestine (Ravindran and Abdollahi, 2021). Molecularly, increased enzyme activity enhances the hydrolysis of complex carbohydrates and proteins, leading to better amino acid and lipid uptake (Ma et al., 2024). In vitro studies indicated that probiotics stimulate the expression of tight junction proteins (e.g., occludin and claudins), thereby strengthening the integrity of the intestinal barrier at the molecular level. This prevents pathogen translocation and reduces inflammation, thereby optimizing nutrient absorption and overall health (di Vito et al., 2022). Bacillus probiotics interact with the gut-associated lymphoid tissue (GALT), influencing cytokine expression (e.g., increased IL-10, decreased pro-inflammatory cytokines like TNF-α). They may activate Toll-like receptors on epithelial and immune cells, modulating immune responses at the molecular level (Kulkarni et al., 2022). These effects reduce inflammatory stress, allowing more nutrients to be diverted toward growth and carcass development. Li et al. (2024) reported that Bacillus spp. may regulate genes involved in lipid metabolism, such as those coding for fatty acid synthase or lipoprotein lipase, favoring leaner carcasses. At the molecular level, this can result in decreased adipogenesis and improved muscle-to-fat ratios. Overall, the molecular effects of B. subtilis and B. coagulans contribute to improved feed efficiency, better nutrient utilization, and enhanced growth performance, resulting in an increased broiler carcass percentage. Feed consumptionHigher probiotic doses resulted in higher feed consumption. High feed consumption stimulates faster growth, resulting in higher body weight gain (Idan et al., 2020). The consumption of large amounts of rations sometimes supports the rapid growth of broilers. Broiler chickens’ weight gain describes the ability of broiler chickens to convert the feed they consume into meat (Rocha et al., 2022). The molecular effects of probiotics, such as B. subtilis and B. coagulans, on broiler feed consumption are primarily mediated through their influence on gut health, nutrient-sensing mechanisms, and appetite-regulating pathways. Bacillus spp. colonize the gut and produce beneficial metabolites, including short-chain fatty acids (SCFAs), such as acetate, propionate, and butyrate. These SCFAs serve as signaling molecules that can stimulate enteroendocrine cells to secrete appetite-regulating hormones, thereby influencing feed intake at the molecular level (Liu et al., 2021). By producing enzymes (amylases, proteases, and lipases), Bacillus spp. increase the breakdown of feed into absorbable molecules. Improved digestion leads to more efficient nutrient absorption, which can enhance the activity of nutrient sensors in the gut and brain, thereby affecting the regulation of feed intake (Predescu et al., 2024). Probiotics strengthen the intestinal barrier by upregulating tight junction proteins (e.g., occludin and claudins), thereby reducing gut inflammation (Predescu et al., 2024). A healthier gut with better barrier function supports optimal nutrient sensing and signaling, potentially reducing feed-related discomfort and influencing consumption patterns (Emami et al., 2020). Probiotics influence the expression of gut hormones such as ghrelin, peptide YY (PYY), cholecystokinin (CCK), and glucagon-like peptide-1 (GLP-1) (Melo-Duran et al., 2018). For example, increased SCFAs can stimulate PYY and GLP-1 release, which communicate with the hypothalamus to suppress appetite or, in some cases, enhance feed intake depending on the context. These hormones act via G-protein-coupled receptors (GPCRs) on target cells, modulating neural circuits involved in appetite regulation (Zhang et al., 2019). Gut hormone signals are transmitted via the vagus nerve to the hypothalamus, where they influence neuropeptides such as NPY and pro-opiomelanocortin (Tachibana and Tsutsui, 2016). Bacillus probiotics may indirectly modulate the expression of these probiotics and neuropeptides at the molecular level, thereby affecting the motivation of birds to consume feed (Naeem and Bourassa, 2025). At the molecular level, B. subtilis and B. coagulans affect broiler feed consumption by modifying gut microbiota and metabolites, enhancing enzyme activity, strengthening gut barrier integrity, and modulating enteroendocrine hormones and neural pathways involved in appetite regulation. These combined effects can lead to changes in feed intake, thereby optimizing growth performance. Feed conversion rateProbiotics B. subtilis and B. coagulans are more effective than the control in increasing the feed conversion value of broiler chickens. A low feed conversion value indicates good feed use efficiency because the chicken uses its ration for growth more efficiently when its feed conversion value is low. Feed conversion indicates whether high-quality feed was chosen or assembled (Rocha et al., 2022). The effect of probiotics, such as B. subtilis and B. coagulans, on the FCR of broilers involves a series of interconnected molecular mechanisms that enhance nutrient use and metabolic efficiency. First, Bacillus spp. produce extracellular enzymes—amylases, proteases, lipases, and cellulases—that catalyze the hydrolysis of complex feed components into simpler, absorbable molecules, thereby increasing digestion and nutrient absorption in the small intestine (Latorre et al., 2016). Probiotics also promote the expression of tight junction proteins (e.g., occludin, claudins), strengthening intestinal barrier integrity, reducing nutrient loss, and preventing pathogen infiltration. Furthermore, Bacillus spp. modulates the gut microbiota, favoring beneficial bacteria that produce SCFAs such as acetate, propionate, and butyrate. These metabolites serve as energy sources and signaling molecules that influence gene expression related to carbohydrate, lipid, and protein metabolism in intestinal and muscle tissues (Ali et al., 2022). Additionally, probiotics interact with the GALT (Brisbin et al., 2010), upregulating anti-inflammatory cytokines like IL-10 and TGF-β, which reduces intestinal inflammation, thus conserving energy and nutrients for growth (Duangnumsawang et al., 2022). Collectively, these mechanisms optimize nutrient digestion, absorption, immune function, and energy metabolism, resulting in more efficient feed conversion—fewer feed resources are needed per unit of body mass gain. Overall, Bacillus probiotics enhance the molecular pathways underlying digestion, immune modulation, and metabolic regulation, resulting in improved FCR in broilers. Abdominal fat percentageThe low percentage of abdominal fat produced indicates that the condition of the resulting fat tends to improve. Abdominal fat is a recognized byproduct that can lower carcass quality (Fouad and El-Senousey, 2014). Therefore, the carcass quality increases with a decrease in the percentage of abdominal fat. The quantity of abdominal fat in broiler chickens contain determines the quality of their carcasses (Kokoszyński et al., 2025). The influence of probiotics, such as B. subtilis and B. coagulans, on the abdominal fat percentage of broilers involves complex molecular mechanisms that regulate lipid metabolism, energy utilization, and inflammatory responses (Kumar et al., 2025). Initially, probiotics modulate gene expression by decreasing the activity of lipogenic enzymes, such as fatty acid synthase and acetyl-CoA carboxylase, in adipose tissue and liver, ultimately reducing lipogenesis (Fouad and El-Senousey, 2014). This process is mediated by microbial metabolites, such as SCFAs, which influence transcription factors, such as SREBP-1c, promoting decreased fat synthesis. Concurrently, probiotics enhance the expression of lipolytic enzymes such as HSL and ATGL, shifting the balance toward fat breakdown (Yang et al., 2025). Furthermore, probiotics stimulate gut hormones, such as PYY and CCK, which signal satiety through GPCRs. This hormonal response reduces feed intake, thereby limiting fat deposition (Wessels, 2022). On the inflammatory front, Bacillus spp. decrease pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β by downregulating the NF-κB pathway, leading to improved insulin sensitivity and less fat accumulation (Shehata et al., 2022). Additionally, probiotics upregulate antioxidant genes such as Superoxide dismutaseand Glutathione peroxidase, mitigating oxidative stress associated with adiposity (Oke et al., 2024). Lastly, bacterial fermentation-derived SCFAs, particularly butyrate, activate nuclear receptors like PPAR-α and PPAR-γ. PPAR-α enhances fatty acid oxidation in liver and muscle tissues, reducing fat storage, while PPAR-γ regulates adipogenesis (Wen et al., 2024). Collectively, these pathways reprogram energy partitioning from fat storage toward lean tissue growth in broilers. In summary, Bacillus probiotics influence gene expression, hormone secretion, inflammatory pathways, and microbial metabolites to reduce abdominal fat percentage, promoting healthier and more efficient broiler production. ConclusionLiquid probiotics B. coagulans and B. subtilis at a dose of 4 ml to obtain the best effects and results in broiler rearing. AcknowledgmentsThe authors would like to thank the Directorate of Research and Community Service, Deputy of Strengthening Research and Development Ministry of Research and Technology/BRIN of the Republic of Indonesia, for their assistance. Conflict of interestThe authors declare no conflict of interest. FundingThe research was funded by Venetian ungulae faults, Universitas piranga tabun 2022 (letter of appointment agreement number: 340/UN3.1.6/PT/2022). Author’s contributionsMS, SHW, ARK, and IM: conceived the idea, designed the mainframe of this manuscript, and acquired, analyzed, and interpreted the data. BPP, RZA, and SRA: drafted the manuscript. RK, EFL, and AOA: critically read and revised the manuscript for intellectual content. All authors have read and approved the final version of the manuscript. Data availabilityAll data are available in the revised manuscript. ReferencesAli, Q., Ma, S., La, S., Guo, Z., Liu, B., Gao, Z., Farooq, U., Wang, Z., Zhu, X., Cui, Y., Li, D. and Shi, Y. 2022. Microbial short-chain fatty acids: a bridge between dietary fibers and poultry gut health—a review. Anim. Biosci. 35(10), 1461–1478. Barbut, S. and Leishman, E.M. 2022. Quality and processability of modern poultry meat. Animals 12(20), 2766. Beski, S.S.M., Swick, R.A. and Iji, P.A. 2015. Specialized protein products in broiler chicken nutrition: a review. Anim. Nutr. 1(2), 47–53. Brisbin, J.T., Gong, J., Parvizi, P. and Sharif, S. 2010. Effects of lactobacilli on cytokine expression by chicken spleen and cecal tonsil cells. Clin. Vaccine Immunol. 17(9), 1337–1343. Cullere, M., Schiavone, A., Dabbou, S., Gasco, L. and Zotte, A.D. 2019. Meat quality and sensory traits of finisher broiler chickens fed with black soldier fly (Hermetia Illucens L.) Larvae fat as alternative fat source. Animals 9(4), 140. di Vito, R., Conte, C. and Traina, G. 2022. A multi-strain probiotic formulation improves intestinal barrier function by the modulation of tight and adherent junction proteins. Cells 11(16), 2617. Duangnumsawang, Y., Zentek, J., Vahjen, W., Tarradas, J. and Boroojeni, F.G. 2022. Alterations in bacterial metabolites, cytokines, and mucosal integrity in the caecum of broilers caused by feed additives and host-related factors. Front. Physiol. 13(1), 935870. Emami, N.K., Calik, A., White, M.B., Kimminau, E.A. and Dalloul, R.A. 2020. Effect of probiotics and multi-component feed additives on microbiota, gut barrier and immune responses in broiler chickens during subclinical necrotic enteritis. Front. Vet. Sci. 7(1), 572142. Fouad, A.M. and El-Senousey, H.K. 2014. Nutritional factors affecting abdominal fat deposition in poultry: a review. Asian-Australas J. Anim. Sci. 27(7), 1057–1068. Idan, F., Nortey, T.N.N., Paulk, C.B., Beyer, R.S. and Stark, C.R. 2020. Evaluating the effect of feeding starters crumbles on the overall performance of broilers raised for 42 days. J. Appl. Poult. Res. 29(3), 692–699. Ikusika, O., Falowo, A., Mpendulo, C., Zindove, T. and Okoh, A. 2020. Effect of strain, sex and slaughter weight on growth performance, carcass yield and quality of broiler meat. Open Agric. 5(1), 607–616. Jalala, M.A.R., Zakariaa, H.A.H., Hayajneha, F.M. and Mehyarb, G.M. 2023. Performance, Carcass characteristics, and meat quality of broiler chickens Fed β-mannanase and two levels of energy. Trop. Anim. Sci. J. 46(2), 190–200. Khalid, A., Khalid, F., Mahreen, N., Hussain, S.M., Shahzad, M.M., Khan, S. and Wang, Z. 2022. Effect of spore-forming probiotics on the poultry production: a review. Food Sci. Anim. Resour. 42(6), 968–980. Kokoszyński, D., Włodarczyk, K., Żochowska-Kujawska, J., Kotowicz, M., Wegner, M., Stęczny, K. and Cygan-Szczegielniak, D. 2025. Effect of intramuscular fat level on carcass composition, physicochemical characteristics, texture, and microstructure of breast muscle of broiler chickens. Poult. Sci. 104(2), 104772. Kokoszyński, D., Żochowska-Kujawska, J., Kotowicz, M., Sobczak, M., Piwczyński, D., Stęczny, K., Majrowska, M. and Saleh, M. 2022. Carcass characteristics and selected meat quality traits from commercial broiler chickens of different origin. Anim. Sci. J. 93(1), e13709. Krysiak, K., Konkol, D. and Korczyński, M. 2021. Overview of the use of probiotics in poultry production. Animals 11(6), 1620. Kulkarni, R.R., Gaghan, C., Gorrell, K., Sharif, S. and Taha-Abdelaziz, K. 2022. Probiotics as alternatives to antibiotics for the prevention and control of necrotic enteritis in chickens. Pathogens (Basel) 11(6), 692. Kumar H., Bhardwaj I., Nepovimova E., Dhanjal D.S., Shaikh S.S, Knop R., Atuahene D., Shaikh, A.M. and Béla, K. 2025. Revolutionising broiler nutrition: the role of probiotics, fermented products, and paraprobiotics in functional feeds. J. Agric. Food Res. 21(1), 101859 Latorre, J.D., Hernandez-Velasco, X., Wolfenden, R.E., Vicente, J.L., Wolfenden, A.D., Menconi, A., Bielke, L.R., Hargis, B.M. and Tellez, G. 2016. Evaluation and selection of Bacillus species based on enzyme production, antimicrobial activity, and biofilm synthesis as direct-fed microbial candidates for poultry. Front. Vet. Sci. 3(1), 95. Li, L., Yang, L., Zhang, L., He, F., Xia, Z. and Xiang, B. 2024. Multi-omic analysis reveals that Bacillus licheniformis enhances pekin ducks growth performance via lipid metabolism regulation. Front. Pharmacol. 15(1), 1412231. Liu, L., Li, Q., Yang, Y. and Guo, A. 2021. Biological function of short-chain fatty acids and its regulation on intestinal health of poultry. Front. Vetv Sci. 8(1), 736739. Ma, C.L., Yin, Z.C., Zhang, X.Y., Zhang, C.X., Zhang, W.Y., Li, Y.X. and Yang, X.J. 2024. Early addition of enzyme-treated soybean in the diet improves amino acid absorption and protein digestibility by promoting digestive enzyme activity in broilers. Animal 18(12), 101364. Melo-Duran, D., Gonzalez-Ortiz, G., Sola-Oriol, D., Martinez-Mora, M., Perez, J.F. and Bedford, M.R. 2018. Relationship between peptide YY, cholecystokinin and fermentation products in fasted, re-fed and ad libitum fed broiler chickens. Anim. Feed Sci. Technol. 247(1), 141–148. Mir, N.A., Rafiq, A., Kumar, F., Singh, V. and Shukla, V. 2017. Determinants of broiler chicken meat quality and factors affecting them: a review. J. Food Sci. Technol. 54(10), 2997–3009. Mohamed, T.M., Sun, W., Bumbie, G.Z., Elokil, A.A., Mohammed, K.A.F., Zebin, R., Hu, P., Wu, L. and Tang, Z. 2022. Feeding Bacillus subtilis ATCC19659 to broiler chickens enhances growth performance and immune function by modulating intestinal morphology and cecum microbiota. Front. Microbiol. 12(1), 798350. Murawska, D. 2017. The effect of age on growth performance and carcass quality parameters in different poultry species. In book: Poultry Science. Ed., Manafi, M. pp: 33–50. Naeem, M. and Bourassa, D. 2025. Probiotics in poultry: unlocking productivity through microbiome modulation and gut health. Microorganisms 13(2), 257. Oke, O.E., Akosile, O.A., Oni, A.I., Opowoye, I.O., Ishola, C.A., Adebiyi, J.O., Odeyemi, A.J., Adjei-Mensah, B., Uyanga, V.A. and Abioja, M.O. 2024. Oxidative stress in poultry production. Poult. Sci. 103(9), 104003. Predescu, N.C., Stefan, G., Rosu, M.P. and Papuc, C. 2024. Fermented feed in broiler diets reduces the antinutritional factors, improves productive performances and modulates gut microbiome—a review. Agriculture 14(10), 1752. Ravindran, V. and Abdollahi, M.R. 2021. Nutrition and digestive physiology of the broiler chick: State of the art and outlook. Animals 11(10), 2795. Rocha, A.G., Dilkin, P., Neto, R.M., Schaefer, C. and Mallmann, C.A. 2022. Growth performance of broiler chickens fed on feeds with varying mixing homogeneity. Vet. Anim. Sci. 17(1), 100263. Shehata, A.A., Yalçın, S., Latorre, J. D., Basiouni, S., Attia, Y.A., Abd El-Wahab, A., Visscher, C., El-Seedi, H.R., Huber, C., Hafez, H.M., Eisenreich, W. and Tellez-Isaias, G. 2022. Probiotics, prebiotics, and phytogenic substances for optimizing gut health in poultry. Microorganisms 10(2), 395. Tachibana, T. and Tsutsui, K. 2016. Neuropeptide control of feeding behavior in birds and its difference with mammals. Front. Neurosci. 10(1), 485. Wen, C., Wang, Q., Gu, S., Jin, J. and Yang, N. 2024. Emerging perspectives in the gut-muscle axis: the gut microbiota and its metabolites as important modulators of meat quality. Microb. Biotechnol. 17(1), e14361. Wessels, A.G. 2022. Influence of the gut microbiome on feed intake of farm animals. Microorganisms 10(7), 1305. Wyszyńska, A.K. and Godlewska, R. 2021. Lactic acid bacteria—a promising tool for controlling chicken campylobacter infection. Front. Microbiol. 12(1), 703441. Yang, X., Bist, R. B., Subedi, S., Guo, Y. and Chai, L. 2025. The application of probiotics and prebiotics in poultry production and impacts on environment: a review. Encyclopedia 5(1), 35. Zhang, J.M., Sun, Y.S., Zhao, L.Q., Chen, T.T., Fan, M.N., Jiao, H.C., Zhao, J.P., Wang, X.J., Li, F.C., Li, H.F. and Lin, H. 2019. SCFAs-induced GLP-1 secretion links the regulation of gut microbiome on hepatic lipogenesis in chickens. Front. Microbiol. 10(1), 2176. | ||
| How to Cite this Article |
| Pubmed Style Sukmanadi M, Kurnijasanti R, Warsito SH, Khairullah AR, Mustofa I, Ahmad RZ, Akintunde AO, Pratama BP, Ayuti SR, Lisnanti EF. Potential of microbial-based feed additives on broiler carcass and fat percentages. Open Vet. J.. 2025; 15(9): 4121-4127. doi:10.5455/OVJ.2025.v15.i9.17 Web Style Sukmanadi M, Kurnijasanti R, Warsito SH, Khairullah AR, Mustofa I, Ahmad RZ, Akintunde AO, Pratama BP, Ayuti SR, Lisnanti EF. Potential of microbial-based feed additives on broiler carcass and fat percentages. https://www.openveterinaryjournal.com/?mno=260329 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i9.17 AMA (American Medical Association) Style Sukmanadi M, Kurnijasanti R, Warsito SH, Khairullah AR, Mustofa I, Ahmad RZ, Akintunde AO, Pratama BP, Ayuti SR, Lisnanti EF. Potential of microbial-based feed additives on broiler carcass and fat percentages. Open Vet. J.. 2025; 15(9): 4121-4127. doi:10.5455/OVJ.2025.v15.i9.17 Vancouver/ICMJE Style Sukmanadi M, Kurnijasanti R, Warsito SH, Khairullah AR, Mustofa I, Ahmad RZ, Akintunde AO, Pratama BP, Ayuti SR, Lisnanti EF. Potential of microbial-based feed additives on broiler carcass and fat percentages. Open Vet. J.. (2025), [cited January 25, 2026]; 15(9): 4121-4127. doi:10.5455/OVJ.2025.v15.i9.17 Harvard Style Sukmanadi, M., Kurnijasanti, . R., Warsito, . S. H., Khairullah, . A. R., Mustofa, . I., Ahmad, . R. Z., Akintunde, . A. O., Pratama, . B. P., Ayuti, . S. R. & Lisnanti, . E. F. (2025) Potential of microbial-based feed additives on broiler carcass and fat percentages. Open Vet. J., 15 (9), 4121-4127. doi:10.5455/OVJ.2025.v15.i9.17 Turabian Style Sukmanadi, Mohammad, Rochmah Kurnijasanti, Sunaryo Hadi Warsito, Aswin Rafif Khairullah, Imam Mustofa, Riza Zainuddin Ahmad, Adeyinka Oye Akintunde, Bima Putra Pratama, Siti Rani Ayuti, and Ertika Fitri Lisnanti. 2025. Potential of microbial-based feed additives on broiler carcass and fat percentages. Open Veterinary Journal, 15 (9), 4121-4127. doi:10.5455/OVJ.2025.v15.i9.17 Chicago Style Sukmanadi, Mohammad, Rochmah Kurnijasanti, Sunaryo Hadi Warsito, Aswin Rafif Khairullah, Imam Mustofa, Riza Zainuddin Ahmad, Adeyinka Oye Akintunde, Bima Putra Pratama, Siti Rani Ayuti, and Ertika Fitri Lisnanti. "Potential of microbial-based feed additives on broiler carcass and fat percentages." Open Veterinary Journal 15 (2025), 4121-4127. doi:10.5455/OVJ.2025.v15.i9.17 MLA (The Modern Language Association) Style Sukmanadi, Mohammad, Rochmah Kurnijasanti, Sunaryo Hadi Warsito, Aswin Rafif Khairullah, Imam Mustofa, Riza Zainuddin Ahmad, Adeyinka Oye Akintunde, Bima Putra Pratama, Siti Rani Ayuti, and Ertika Fitri Lisnanti. "Potential of microbial-based feed additives on broiler carcass and fat percentages." Open Veterinary Journal 15.9 (2025), 4121-4127. Print. doi:10.5455/OVJ.2025.v15.i9.17 APA (American Psychological Association) Style Sukmanadi, M., Kurnijasanti, . R., Warsito, . S. H., Khairullah, . A. R., Mustofa, . I., Ahmad, . R. Z., Akintunde, . A. O., Pratama, . B. P., Ayuti, . S. R. & Lisnanti, . E. F. (2025) Potential of microbial-based feed additives on broiler carcass and fat percentages. Open Veterinary Journal, 15 (9), 4121-4127. doi:10.5455/OVJ.2025.v15.i9.17 |