| Research Article | ||
Open Vet. J.. 2025; 15(8): 3486-3504 Open Veterinary Journal, (2025), Vol. 15(8): 3486-3504 Original Research The protective effects of the combination of Simmondsia chinensis extract and vitamin E on nephrotoxicity and hepatotoxicity in rats exposed to cisplatin: An anti-inflammatory and antioxidant studyAli Mohammed Khafaji and Sura Safi Khafaji*Physiology, Biochemistry and Pharmacology Department, College of Veterinary Medicine, Al-Qasim Green University, Babylon, Iraq *Corresponding Author: Sura Safi Khafaji. Department of Physiology, Pharmacology and Chemistry, College of Veterinary Medicine, Al-Qasim Green University, Al-Qasim City, Iraq. Email: sura.khafaji [at] vet.uoqasim.edu.iq Submitted: 20/05/2025 Revised: 21/07/2025 Accepted: 30/07/2025 Published: 31/08/2025 © 2025 Open Veterinary Journal
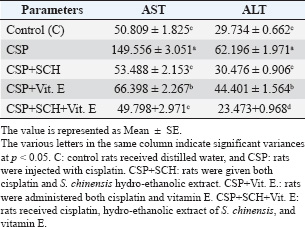
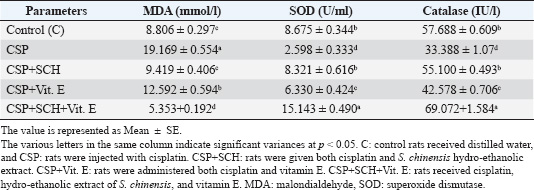
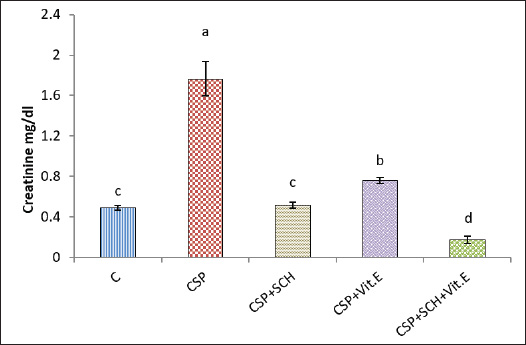
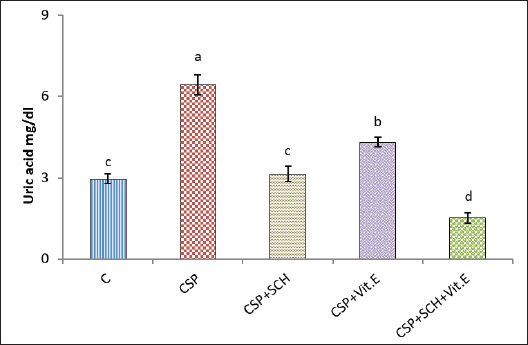
ABSTRACTBackground: Cisplatin (CSP), a chemotherapy drug widely used for treating solid tumors, can cause cellular toxicity in various percentages. Natural antioxidants, such as phenols, help reduce free radicals and inflammation. Aim: This study aimed to investigate the synergistic protective effects of Simmondsia chinensis extract, vitamin E, or their combination, particularly in cases of CSP-induced renal and hepatotoxicity. Methods: Fifty adult male rats were randomly grouped into five groups (n=10): control group (C): each rat received (2 ml/day/orally) distilled water; CSP group: each rat was injected intraperitoneally (IP) with a single dose of (2 mg/kg) CSP for eight consecutive weeks; and CSP+SCH group: all rats in this group were first injected with cisplatin via IP injection, similar to the CSP group, at the same time administered S. chinensis hydro-ethanolic extract at a dose of (0.6 g/kg/day/orally). In the CSP + Vitamin E group, each rat was treated the same as in the CSP group and then received vitamin E at a dose of 100 mg/kg/day/orally. In the (CSP+SCH+Vit. E) group, rats were IP-injected with cisplatin and then administered both S. chinensis hydro-ethanolic extract and vitamin E, using the same doses as in the third and fourth groups; all treatment administrations were administered over eight consecutive weeks. Results: The hepatic enzyme levels of alanine transaminase and aspartate transaminase, as well as malondialdehyde, creatinine, uric acid, cystatin C, and tumor necrosis factor-alpha (TNF-α) were significantly increased in the CSP group compared with all experimental groups. These parameters significantly declined (p < 0.05) in the CSP + SCH + Vit. E group compared with other experimental groups. The injection of cisplatin caused a decrease in the serum concentrations of superoxide dismutase, catalase, and interleukin-10 compared to all treatment groups. All these parameters were significantly elevated (p < 0.05) in the CSP + SCH + Vit. E group and CSP + SCH groups compared with the CSP group. The histological alterations of the renal and hepatic tissues were improved and enhanced in patients with CSP + SCH + Vit. E group compared with all experimental groups. Conclusion: Simmondsia chinensis alone or in combination with vitamin E can mitigate cisplatin-induced hepatic and renal toxicity in rats through its synergistic antioxidant and anti-inflammatory properties. Keywords: Cisplatin, Simmondsia chinensis extract, Vitamin E, Nephrotoxicity, Hepatotoxicity. IntroductionThe increasing prevalence of cancer in Iraq necessitates the development of therapeutic programs aimed at reducing the toxins produced by these medicines and alleviating the stress they place on the body’s vital systems. In 1965, researchers discovered that platinum complexes exhibited a distinctive and intriguing effect, which later served as the cornerstone for one of the most commonly used classes of anticancer medications (Rosenberg et al., 1965). Cisplatin is a platinum-based chemotherapeutic agent that was approved by the FDA in 1978. It remains one of the most widely used anticancer drugs, showing strong efficacy against cancers of the kidney, liver, testis, ovary, head, neck, and lung (Prestayko et al., 1979). Cisplatin, scientifically known as cis-dichlorodiammineplatinum (Cis-DDM) (II), is a coordination complex consisting of a central platinum atom bound to two chloride ions and two ammonia molecules in a cis configuration (Tchounwou et al., 2021). It has been shown to be effective in treating various solid tumors (Romani, 2022). Cisplatin is a cytotoxic drug that can cause cellular toxicity in various percentages, including nephrotoxicity (72%), ototoxicity (75%–100%), hepatotoxicity (36%), cardiotoxicity (6%), and toxicity to other organs (Abd Rashid et al., 2021). Cisplatin exerts its anticancer effects through several mechanisms. It destroys cancer cells by damaging DNA, inhibiting DNA synthesis and mitosis, and inducing apoptosis (Dasari and Tchounwou, 2014; Brown et al., 2019). The adverse side effects of cisplatin depend on the dosage and duration of cisplatin administration, resulting in increased toxicity, oxidative stress, and free radicals (Hamaya et al., 2023), subsequent mitochondrial damage, and activation of cell death pathways (Fawzy et al., 2022). Cisplatin nephrotoxicity is one of the most significant side effects associated with cisplatin treatment. Cisplatin accumulation in renal tubular cells causes damage to these cells (Hakiminia et al., 2019). Simmondsia chinensis, commonly known as Jojoba, is a desert shrub native to Mexico and the United States. It is now cultivated in arid regions worldwide for its economically valuable seed waxy ester (Gentry, 1958). Simmondsia chinensis extracts have anti-inflammatory, antimicrobial, antioxidant, and wound-healing properties; therefore, they are used as a supportive topical agent in impaired wound healing conditions to accelerate wound healing delayed by dexamethasone in a preclinical rat model. Its beneficial effects have been attributed to its anti-inflammatory and pro-healing properties (Siddique et al., 2023; Begum et al., 2024). Furthermore, jojoba topical application significantly increased collagen III mRNA and protein levels and hyaluronic acid synthesis and formed a protective skin barrier that aids in healing and infection prevention (Tietel et al., 2024). The beneficial effects of S. chinensis are largely attributed to its polyphenols, phytosterols, and tocopherols (Feki et al., 2022), which act as antioxidants to protect cells against reactive oxygen species (ROS) and free radical-induced tissue damage. Such oxidative stress is linked to diseases such as cancer, diabetes, and rheumatoid arthritis, induces inflammation, and suppresses immunity. As confirmed by recent phytochemical studies, Jojoba (S. chinensis) extract is rich in bioactive compounds, including polysaccharides, lignans, triterpenoids, flavonoids, phenolic acids, and phytosterols (Zhu et al., 2021; El Gendy et al., 2023). Phytochemicals are gaining attention as natural antioxidants used in food, health, and cosmetics, offering safer and more eco-friendly alternatives to synthetic compounds. Flavonoids are characterized by a remarkable diversity in biochemical and antioxidant properties, making them powerful agents in fighting various ailments, including cardiovascular disease, cancer, and neurodegenerative disorders (Baptista et al., 2024). Vitamin E (alpha-tocopherol) is important in its physiological functions, which include battling oxidation, regulating immunity, acting as an anti-inflammatory agent, managing chronic diseases such as diabetes and arthritis, and protecting nerves (Ribeiro et al., 2021). Besides its role in the food, cosmetics, pharmaceutical, and dermatology sectors, its application as a therapeutic supplement could also provide potential benefits in the fields of dermatology, oncology, and infertility (Fatima et al., 2025), which is a class of fat-soluble chemicals with antioxidant properties that exist naturally in eight chemical forms (alpha-, beta-, gamma-, and delta-tocopherol and alpha-, beta-, gamma-, and delta-tocotrienol) (Alghamdi et al., 2020). Vitamin E is a lipophilic antioxidant that protects cell membranes from oxidative damage and has been used in dermatology for over 50 years (Cascella et al., 2017). Vitamin E, a fat-soluble antioxidant, is vital for defending polyunsaturated fatty acids (PUFAs) within cell membranes against oxidative stress damage. In particular, alpha-tocopherol, a specific type of vitamin E, maintains cell membrane stability by inhibiting reactions involving lipid peroxides. This protective mechanism also extends to cell components such as mitochondria, proteins, and DNA. Moreover, vitamin E can help lower the expression of pro-inflammatory cytokines (Traber, 2021; Zhang et al., 2024). Vitamin E also plays a major role in reducing the apoptosis that occurs due to oxidative stress in some organs, such as the kidney, nerve, and liver cells (Jain et al., 2022). Given the rising prevalence of cancer in Iraq and the associated toxicity of chemotherapeutic agents such as cisplatin, the current study aims to explore the protective effects of S. chinensis (jojoba) extract and vitamin E, alone or in combination, against cisplatin-induced nephrotoxicity and hepatotoxicity in rats, with further emphasis on their potential antioxidant and anti-inflammatory properties. Materials and MethodsSteps of preparation of the Jojoba S. chinensis extractThe Jojoba (S. chinensis L.) plant was classified in the University of Karbala/College of Education for Pure Sciences/Department of Life Sciences, and its classification was issued 4/6/1252 on 26/2/2025. The S. chinensis seeds were purchased from the medical herbarium. S. chinensis seeds were prepared as previously described (Abdel-Wahhab et al., 2016; Abdulraheem et al., 2019; Tietel et al., 2024). Weigh 100 g of S. chinensis seeds and grind them with an electric blender. Add 1,000 ml of 70% ethanol to the crushed seeds and cover the beaker completely with aluminum foil to avoid light exposure. The beaker with the mixture was placed on a hot plate at 220 rpm and temperature (30 °C–50 °C) for 12 hours. The mixture was then filtered with gauze to get rid of the residue and further filtered using Whatman paper (0.5 mm). The filtrate was dried in an incubator at 40 °C. After drying extraction, the net extract was 25.6 g per 100 g of jojoba powder. The final extract was kept frozen at (−20 °C) until use. Experimental animalsThe experiment was conducted in the College of Veterinary Medicine/Al-Qasim Green University and the animal house of the College of ScienceUniversity of Babil. The experiment was extended from September 25, 2024, to January 1, 2025. A total of 50 mature male Wistar rats (aged 75 days old and weighing 254-260 g) were used in the current experiment. In wire-plastic cages that measure 40 × 60 cm and were well ventilated, the animals were kept at 23°C–25°C, 73%–76% relative humidity, and a 12:12 hours dark/light cycle. The animals had free access to water and laboratory food at all times. After 10 days of acclimatization, the animals were weighed before treatment (0 day) and after each period of the experiment. Experimental designFifty adult male rats were randomly grouped into five groups (n=10): First group: Each rat received (2 ml/day/orally) distilled water for eight weeks and served as a control group (C); second group (CSP): each rat was injected with (2 mg/kg) cisplatin (CSP) (Accord®, United Kingdom) as a single dose/week intraperitoneally (IP) for eight weeks; third group (CSP + SCH): each rat was treated as the second group and received (0.6 g/kg/day/orally) S. chinensis hydro-ethanolic extract (SCH) seeds purchased from the medical herbarium for eight consecutive weeks; fourth group (CSP + Vitamin E): each rat treated as the second group then received vitamin E (100 mg/kg/day/orally) (NOW®, USA) for eight consecutive weeks; and fifth group (CSP + SCH + Vitamin E): each rat treated as the second group then received both S. chinensis hydro-ethanolic extract and vitamin E at the same as the doses in third and fourth groups for eighth consecutive weeks. In addition to cisplatin, jojoba extract and vitamin E were used according to previous studies (Amate et al., 1996; Feki et al., 2022; Peña-Corona et al., 2023). At the end of the experimental period, each rat was anesthetized with ketamine (40 mg/kg) / Xylazine (10 mg/kg) body weight (Gaertner et al., 2008). Blood samples were collected, and the liver and kidneys were dissected and weighed. Kidney samples were harvested and preserved in 10% formalin for histopathological examination. Blood sample collectionFollowing anesthesia, 3 ml of blood was taken directly from the heart of each rat. Blood samples were placed in non-heparinized tubes and left at room temperature until clotting before being centrifuged at 5,000 rpm for 15 minutes to separate serum. The sera were collected in Eppendorf containers and stored at (−20°C) (Parasuraman et al., 2010) until use for assessment of the biomarkers ALT and AST, marker oxidants malondialdehyde (MDA), antioxidant markers superoxide dismutase (SOD), catalase (CAT), uric acid, creatinine, cystatin C, tumor necrosis factor alpha (TNF-α), and interleukin 10 (IL-10). Determination of liver enzyme activitiesSerum alanine aminotransferase (ALT) activity was measured using the Elabscience® kit and the ARCHITECT c4000 device. A colorimetric method is used to accurately determine ALT activity through the following reactions according to the methodology described by Young (1990). Serum aspartate aminotransferase activity (AST) was measured using an Elabscience® kit from China and the ARCHITECT C4000 device. The principle involves a colorimetric assessment to determine AST activity through specific reactions according to the methodology described by Young (1990). Determination of oxidant and antioxidant markersThe activities of antioxidant markers, such as superoxide dismutase (SOD), CAT, and MDA, were determined according to the method described by Mohandas et al. (1984), Aebi (1984), and Kei (1978). Determination of creatinine, uric acid, and cystatin C markersThe serum creatinine level was measured using a Biolabo Creatinine Kit (ACHITECT C4000, France). The creatinine level was determined as described previously (Toora and Rajagopal, 2002). Uric acid concentration in serum was determined using a Biolabo-Urea Kit (ACHITECT C4000, France). The measurement principle is presented as described by (Pachla et al., 1987). Cystatin C was measured according to the method described by Onopiuk et al. (2015). Determination of cytokine levelsThe levels of interleukin (IL-10) and TNF-α were assessed using enzyme-linked immunosorbent assay kits (Elabscience®, USA) following the manufacturer’s instructions (Fortis et al., 1996; Barbara et al., 1996). Histological studyThe kidney and liver were fixed in 10% buffered formaldehyde for 48 h and then preserved in 70% ethanol until processed histologically according to the method adopted by Kiernan (2015), as follows: dehydration, clearing, embedding in paraffin, and staining with hematoxylin and eosin (H&E) after sectioning into 5-µm slices, as follows: dehydration, clearing, infiltration paraffin, embedding, cutting, staining, and examination at (100x) magnification, then all histological sections were photographed. Statistical analysisStatistical analysis of the outcomes of the current experiment was conducted using version 16 of the Statistical Package for Social Science (SPSS). The mean (M) ± SE represents the data. Statistically, the differences at p < 0.05 are significant. To evaluate significant differences between the groups, post hoc Tukey’s test and one-way ANOVA I were used (Schefler, 1980). Ethical approvalThe ethical and scientific committees of Veterinary Medicine at Al-Qasim Green University approved the current experiment, ensuring proper care and use of laboratory animals (ESCVM, No. 2134.1292024). ResultsEffects of the combination of S. chinensis and vitamin E on liver enzymes in cisplatin-exposed ratsThe effects of S. chinensis extract, vitamin E, and cisplatin on liver enzymes are presented in Table 1. The intraperitoneal injection of cisplatin for only 8 weeks caused a significant elevation (p < 0.05) in aspartate transaminase (AST) and alanine aminotransferase (ALT) levels compared with the control and other treatment groups. In contrast, the concentration of each AST and ALT significantly declined (p < 0.05) in the CSP+SCH+Vit. E group in comparison with CSP, CSP + Vit. E, and CSP + SCH groups, with non-significant variances (p > 0.05) compared with the control. Interestingly, the administration of both S. chinensis extract and cisplatin (CSP + SCH) and both vitamin E and cisplatin (CSP + Vit. E) caused a significant decrement (p < 0.05) in ALT and AST concentrations when compared with rats that received cisplatin only, with non-significant differences (p > 0.05) in AST values between CSP + SCH and control groups, as well as in ALT values among CSP + SCH, control, and CSP + SCH + Vit. E groups. Impact of the combination of S. chinensis and vitamin E on oxidant and antioxidant markers in cisplatin-exposed ratsTable 2 explains the impact of S. chinensis hydro-ethanolic extract, vitamin E, and cisplatin on oxidant and antioxidant parameters. The statistical analysis of MDA results showed a significant increase (p < 0.05) in CSP-treated rats compared with other treatment rats. Rats that received both vitamin E and S. chinensis extract recorded a significant decline (p < 0.05) in serum MDA concentration compared with other treatment groups. Importantly, the level of MDA registered a significant decrement (p < 0.05) in CSP-SCH-treated rats when compared with CSP and Vit. E, whereas a non-significant variance (p > 0.05) was recorded between SCH and control rats. The statistical analysis of SOD and CAT results recorded a significant reduction (p < 0.05) in CSP-treated rats compared with other treatment rats. Rats that received both S. chinensis extract and Vit. E registered a significant elevation (p < 0.05) in serum SOD and CAT concentrations compared with other treatment groups (Table 2). Importantly, compared with the control group, CSP-Vit. E-treated rats registered a significant decrement (p < 0.05) in the serum SOD and CAT concentrations (Table 2). The levels of SOD and CAT recorded a non-significant variance (p > 0.05) in CSP-SCH-treated rats compared with control rats (Table 2). Effects of S. chinensis, vitamin E, and cisplatin on creatinine, uric acid, and cystatin C levels in ratsThe results of the creatinine (mg/dl) and uric acid concentrations are explained in Figures 1 and 2, respectively; rats that were injected with cisplatin showed a significant (p < 0.05) increment in serum concentrations of creatinine and uric acid (1.767 ± 0.167 and 6.425 ± 0.364), respectively, in comparison with other treatment groups. Concentrations of creatinine and uric acid in rats that received a combination of SCH + Vit. E recorded a significant decrement (p < 0.05), with values of 0.174 ± 0.080 and 1.516 ± 0.187, respectively, when compared with other treatment groups. The serum concentrations of UA and creatinine were significantly decreased (p < 0.05) in SCH-treated rats (3.132 ± 0.281 and 0.516 ± 0.03), respectively, compared with the Vit. E groups and CSP, with non-significant (p > 0.05) differences with the control group. The creatinine and uric acid levels declined significantly (p < 0.05) in CSP+Vit. E-treated rats, with values of 0.489 ± 0.024 and 4.313 ± 0.179, respectively, compared with the CSP group, while they increased significantly (p < 0.05) in comparison with other treatment groups. Table 1. Effect of S. chinensis hydro-ethanolic extract and vitamin E on liver enzymes in response to cisplatin-induced hepato-nephrotoxicity in male rats.
Table 2. Effect of S. chinensis hydro-ethanolic extract and vitamin E on liver enzymes in response to cisplatin-induced hepato-nephrotoxicity in male rats.
Fig. 1. Serum creatinine concentration (mg/dl) in rats. The various letters in the same column indicate significant variances at p < 0.05. C: control rats received distilled water, and CSP: rats were injected with cisplatin. CSP+SCH: rats were given both cisplatin and S. chinensis hydro-ethanolic extract. CSP+Vit. E: rats were administered both cisplatin and vitamin E. CSP+SCH+Vit. E: rats received cisplatin, hydro-ethanolic extract of S. chinensis, and vitamin E.
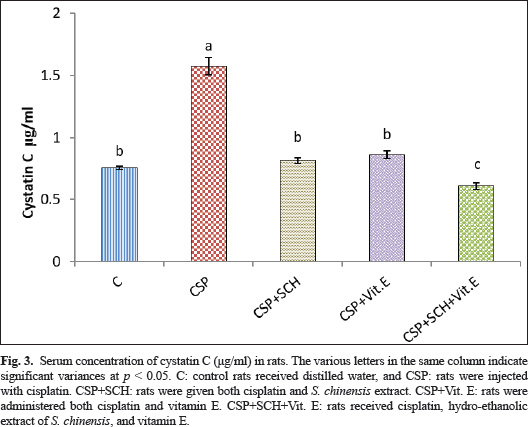
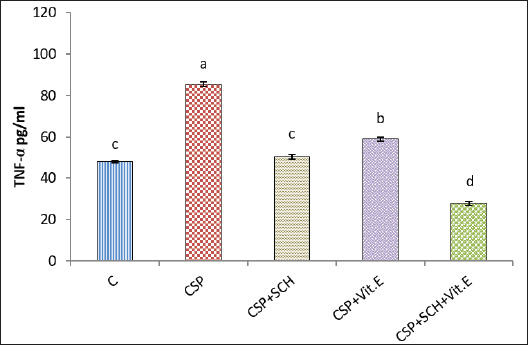
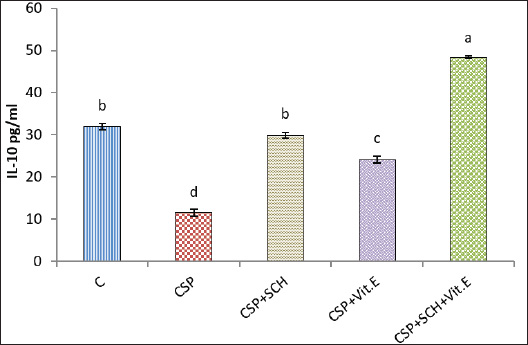
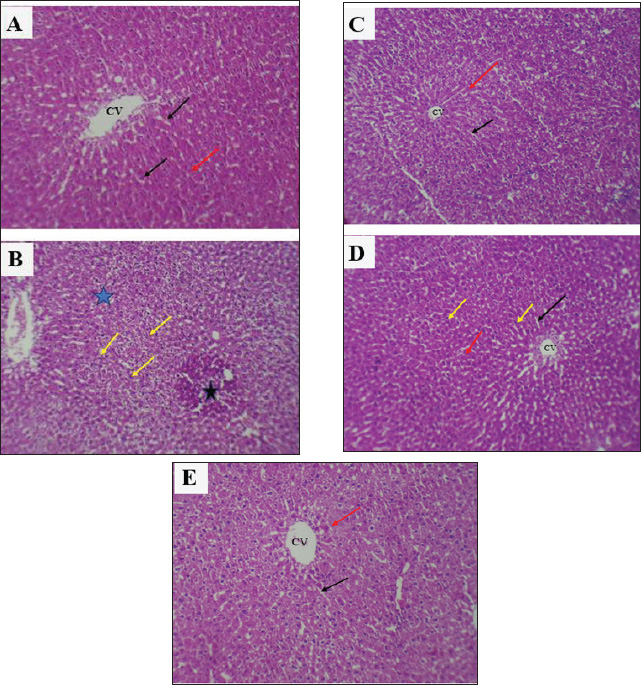
Fig. 2. Serum concentration of UA (mg/dl) in rats. The various letters in the same column indicate significant variances at p < 0.05. C: control rats received distilled water, and CSP: rats were injected with cisplatin. CSP+SCH: rats were given both cisplatin and S. chinensis extract. CSP+Vit. E: rats were administered both cisplatin and vitamin E. CSP+SCH+Vit. E: rats received cisplatin, hydro-ethanolic extract of S. chinensis, and vitamin E. The results of cystatin C are explained in Figure 3. The IP-injection of cisplatin caused a significant (p < 0.05) elevation in cystatin C concentration in the CSP group, with a value (1.574 ± 0.069), compared with other treatment groups. Interestingly, the administration of both S. chinensis extract and vitamin E in the CSP-SCH-Vit. E group caused a significant (p < 0.05) decline in the serum level of cystatin C with a value (0.608 ± 0.029) when compared with the control and other treated groups. The statistical analysis of serum cystatin C concentration revealed a return to control levels, with no significant (p > 0.05) differences among SCH plus cisplatin, Vit. E plus cisplatin, and control rats, with values of 0.814 ± 0.022, 0.859 ± 0.030 and 0.754 ± 0.012, respectively. Effects of vitamin E and S. chinensis hydro-ethanolic extract on cytokine parameters in rats exposed to cisplatinThe level of TNF-α was significantly (p < 0.05) increased in CSP rats, with a value of 85.466 ± 1.123, compared with that in all experimental groups. Interestingly, TNF-α was significantly (p < 0.05) decreased in the CSP + CSH + Vit. E group, with a value of 27.828 ± 1.085, compared to all experimental rats. The administration of S. chinensis hydro-ethanolic extract to CSP-treated rats caused a significant decrease (p < 0.05) in that mediator, TNF-α (50.314 ± 1.094), compared with that of CSP and Vit. E groups, whereas the CSP-Vit. E-treated rats recorded a significant increment (p < 0.05), with a value of 58.965 ± 0.943, compared with the CSP plus SCH group, control group, and CSP-SCH-Vit. E group, and a significant reduction (p < 0.05) compared with CSP rats (Fig. 4). The level of IL-10 lowered significantly (p <0.05) in CSP- and CSP-Vit. E-treated rats compared with the CSP+SCH+Vit. E, control, and SCH+CSP groups (Fig. 5). It was significantly elevated (p < 0.05) in rats that received a combination of S. chinensis hydro-ethanolic extract and vitamin E after exposure to cisplatin compared to all experimental groups (Fig. 5). Compared with CSP and CSP+Vit. E, the IL-10 level was increased (p < 0.05) in the CSP+ SCH rats (Fig. 5). The IL-10 values of CSP+SCH+Vit. E, CSP+SCH, CSP+Vit. E, CSP, and control rats were 48.415 ± 0.363, 29.897 ± 0.598, 24.153 ± 0.880, 11.544 ± 0.869, and 31.951 ± 0.722, respectively. Histopathological changesThe evaluation of histological slides from rats exposed to cisplatin only (CSP group; Fig. 6B) and stained with hematoxylin and eosin (H&E) revealed a hydropic degeneration around the central vein and portal area, congestion, sinusoidal dilation, and signs of inflammation in hepatic tissues characterized by the aggregation of Kupffer cells and inflammatory cell foci. Some hepatocytes exhibited pyknotic nuclei, and their disorganized architecture of the hepatic cords was more prominent than that of the control group (Fig. 6A), which showed preserved hepatic features without any signs of injury, congestion, or inflammation. Observations of the hepatic sections after Vit. E administration or S. chinensis extract after injection of cisplatin showed a reduction in the histological changes, and hepatic tissues displayed mild congestion with fewer infiltration of inflammatory cells; hepatic cords and sinusoids seemed to be a normal appearance (Fig. 6C and D). Interestingly, the hepatic sections of SCH-Vit. E-CSP rats (Fig. 6E) showed normal sinusoids, normal portal tracts with normal hepatic cords, which arranged radially around central veins, similar to the appearance in control rats. The histological sections of rats treated with cisplatin (Fig. 7B) showed nephrotoxic lesions, which appeared as severe degenerative changes and congestion, expansion of Bowman’s space, dilation in the glomerular capillaries, and apoptosis in the tubular lining epithelium when compared with the control group (Fig. 7A), which showed a normal appearance in renal corpuscles and tubules. However, this disturbance was partially reversed with the administration of either S. chinensis hydro-ethanolic extract or Vit. E, where mild congestion and the Bowmans’ space persisted, a slight bit bigger when compared to the control group (Fig. 7C, D). Importantly, the administration of S. chinensis hydro-ethanolic extract with Vit. E to rats exposed to cisplatin seemed to restore to normal architecture as control groups, with normal Bowman’s space and glomerular capillaries (Fig. 7E). DiscussionCisplatin is commonly utilized as an antitumor medication for treating several tumors, including lung, ovarian, testicular, head, neck, and colorectal cancers. However, it can cause several cellular alterations and ROS generation that affect metabolisms inside cells (Abdel-Daim et al., 2020). The present results showed that the rats exposed to cisplatin exhibited significant alterations in hepatic function markers, as reported by a significant elevation in the levels of AST and ALT in the CSP group. Although cisplatin is effective against tumors, it can cause cellular toxicity, and its hepatotoxicity remains a significant dose-dependent side effect, observable even after a single low-dose administration (Dkhil et al., 2013). These biochemical markers serve as critical indicators of hepatocellular injury, as alterations in hepatocyte cell permeability lead to the release of these enzymes into the bloodstream (Louisa et al., 2023). The current experiment was consistent with the findings obtained by Hosack et al. (2023), who correlated the structural damage of hepatic tissues with an elevated level of hepatic enzymes located in the cytosol and mitochondria and leakage to the blood circulation following cellular injury, indicating the onset of hepatotoxicity.
Fig. 3. Serum concentration of cystatin C (μg/ml) in rats. The various letters in the same column indicate significant variances at p < 0.05. C: control rats received distilled water, and CSP: rats were injected with cisplatin. CSP+SCH: rats were given both cisplatin and S. chinensis extract. CSP+Vit. E: rats were administered both cisplatin and vitamin E. CSP+SCH+Vit. E: rats received cisplatin, hydro-ethanolic extract of S. chinensis, and vitamin E.
Fig. 4. Serum concentration of tumor necrosis factor-alpha (TNF-α) (pg/ml) in rats. The various letters in the same column indicate significant variances at p < 0.05. C: control rats received distilled water, and CSP: rats were injected with cisplatin. CSP+SCH: rats were given both cisplatin and S. chinensis extract. CSP+Vit. E: rats were administered both cisplatin and vitamin E. CSP+SCH+Vit. E: rats received cisplatin, hydro-ethanolic extract of S. chinensis, and vitamin E.
Fig. 5. Serum interleukin-10 (IL-10) concentration (pg/ml) in rats. The various letters in the same column indicate significant variances at p < 0.05. C: control rats received distilled water, and CSP: rats were injected with cisplatin. CSP+SCH: rats were given both cisplatin and S. chinensis extract. CSP+Vit. E: rats were administered both cisplatin and vitamin E. CSP+SCH+Vit. E: rats received cisplatin, hydro-ethanolic extract of S. chinensis, and vitamin E. Various experimental studies have shown that following exposure to cisplatin, these parameters were significantly elevated, and this rise was accompanied by histological alterations in the liver’s structure due to the exposure of the hepatocyte cellular membrane to excessive amounts of free radicals, causing cell membrane damage as well as AST and ALT leak from hepatocytes (Gazwi et al., 2024; Satyam et al., 2024). The present experiment reported that S. chinensis extract could reduce AST and ALT enzyme levels due to its ability to diminish and treat liver damage caused by cisplatin and preserve hepatocyte integrity. This finding was supported by El-Saeed and El-Khawaga (2024), who reported enhancement and protection of hepatic tissues from damage caused by lead acetate, resulting in improvement of hepatic enzymes, including ALP, ALT, and AST. Interestingly, the administration of vitamin E to rats in the CSP+Vit. E group could enhance the hepatic functions and reduce the activities of both AST and ALT enzymes via its antioxidant features. These findings align with those of Palipoch et al. (2014), who concluded that pretreatment with tocopherol could safeguard against cisplatin-induced hepatotoxicity as AST and ALT. Additionally, Vit. E is considered a good hepatoprotective supplement due to its ability to maintain the hepatocellular membrane integrity from oxidative damage caused during treatment with cisplatin (Alghamdi et al., 2020). The combined therapy of S. chinensis extract and Vit. E reported a more noticeable reduction in AST and ALT levels. This suggests a potential synergism between the S. chinensis extract and Vit. E in the amelioration of cisplatin-induced hepatotoxicity. In comparing S. chinensis extract with Vit. E, both compounds reported significant hepatoprotective effects, but S. chinensis extract appeared to be more effective in reducing AST and ALT levels. These results are noteworthy as AST and ALT are vital enzymes that reflect mitochondrial damage, and this reduction indicates the ability of natural antioxidant constituents of S. chinensis extract such as polyphenols (e.g., flavonoids) and kaempferol to protect against mitochondrial injury via a potential antioxidant free radical scavenging capacity (Borgne-Sanchez and Fromenty, 2018; Feki et al., 2022; Khafaji, 2023a).
Fig. 6. Photomicrographs of hepatic sections. A: Rats of the control group showed normal histologic architecture of the central vein and hepatic cords. B: Rats receiving cisplatin (CSP) showed clear congestion (black star) with inflammatory cells and Kupffer cell infiltration (yellow arrow) with disorganization in the hepatic cord and apoptosis (blue star). C: Rats receiving S. chinensis hydro-ethanolic extract (CSP + SCH) showed a normal histologic appearance of hepatic tissue as a control group. D: Rats administered vitamin E showed mild congestion with light inflammatory cell infiltration, and the hepatic cords and sinusoids appeared normal. E: Rats receiving a combination of S. chinensis hydro-ethanolic extract and vitamin E (CSP + SCH + Vitamin E) showed normal sinusoids, normal portal tracts, and normal hepatic cords, which were arranged radially around the central veins, similar to the appearance in control rats. CV: central vein, black arrow: sinusoids, red arrow: hepatic cords. (H&E) (100X). The current finding revealed that the I.P. injection of cisplatin caused a rise in serum MDA in CSP-rats, which might be due to the ability of cisplatin to induce free radical generation via several pathways, including induction of protein denaturation, DNA damage, lipid peroxidation, inflammation, and programed cell death (Ahmed and Ghobara, 2016). CSP-induced generation of NOS and ROS caused exacerbation of oxidative stress by reducing the generation of enzymatic antioxidants, including CAT and SOD, and non-enzymatic antioxidants, such as glutathione, which led to toxicity (Abdel-Daim et al., 2020). Consistent with previous studies (Abo-Elmaaty et al., 2020; Habib et al., 2021), these studies reported that CSP administration in rats and mice, respectively, could cause an elevation in MDA levels in the kidneys and liver, accompanied by a reduction in SOD, CAT, and GSH levels. The present results were supported by the findings of Cesur et al. (2025), who concluded that the administration of cisplatin as a single dose per six weeks could elevate MDA levels and reduce glutathione levels.
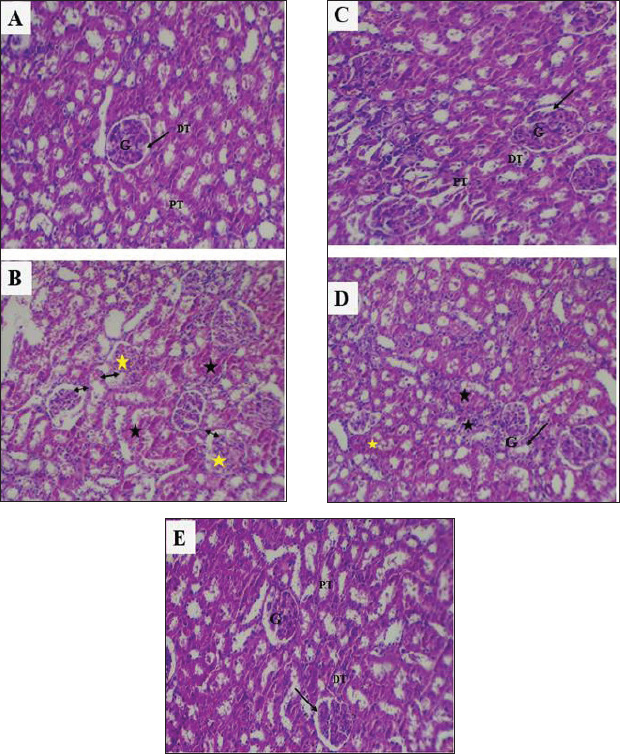
Fig. 7. Photomicrographs of kidney sections. A: Control rats showing normal histologic appearance of kidney glomeruli and renal tubules. B: rats injected with CSP showed severe degenerative changes and congestion (black star) with dilation of Bowman’s space in the glomerular capillaries (black double-headed arrow), with apoptosis in glomerular capillaries and filtration inflammatory cells (yellow star). C: Rats that received S. chinensis hydro-ethanolic extract (CSP + SCH) showed a histologic structure of renal tissue similar to the histological appearance of the control group. D: Rats administered vitamin E showed a slight congestion, and the Bowman’s space was slightly enlarged. E: Rats administered a combination of S. chinensis hydro-ethanolic extract and vitamin E showed normal histological architecture of renal tissue with normal glomeruli and renal tubules. G: glomeruli, black arrow: Bowman’s space DT: distal convoluted tubule. PT: proximal convoluted tubule. (H&E). (100X) The present findings of a decrease in MDA levels in vitamin E-treated rats were consistent with those of Hakiminia et al. (2019), who reported that the administration of vitamin E could reduce oxidative stress biomarkers, such as decreasing the levels of MDA and NO and enhance the activities of renal antioxidant enzymes, including renal CAT and SOD. Importantly, Vit. E could reduce and/or prevent lipid peroxidation by eliminating free radical generation due to its chromanol hydroxyl group (Niki, 2014). The antioxidant capacity of vitamin E, which is known to inhibit lipid peroxidation, is positively reflected in the increase in SOD and CAT activities, which is in agreement with the previous findings of Alghamdi and colleagues (2020), who concluded that vitamin E boosts antioxidant defenses and mitigates the toxic effects of cisplatin in the liver and kidneys by ameliorating the activities of antioxidant, CAT, and glutathione peroxidase and reducing lipid peroxidation. In fact, tocopherols reduce oxidative damage primarily due to their ability to neutralize and remove lipid peroxyl radicals more rapidly than these radicals can react with proteins or fatty acid side chains. This is positively reflected in the levels of SOD and CAT, which scavenge the ROS involving superoxide anion (O2) and hydrogen peroxide (H2O2), respectively (Hedayati-Moghadam et al., 2023; Zishan et al., 2025). Administration of the S. chinensis extract or Vit. E during rats’ intoxication with cisplatin, lipid peroxidation could be reduced and the hepatocellular and renal cellular antioxidant capacity could be improved (Zhang et al., 2021), due to the biological components of S. chinensis that possessed potential roles as antioxidant agents, such as flavonoids and phenolic acid, which can provide hepatoprotection and renal protection against oxidative toxicity caused by free radicals generated during injection with cisplatin (Badr et al., 2017). Lipoxygenase inhibitors in S. chinensis extract can suppress leukotriene synthesis and thereby reduce ROS generation and oxidative stress (Abdel-Mageed et al., 2014; Siahaan, 2020). Importantly, the findings obtained by Okasha et al. (2025) that improved the potential antioxidant capacity of S. chinensis via the elevation of SOD and CAT activities more than the administration of vitamin D are consistent with the present findings. Interestingly, S. chinensis extract could enhance the generation of antioxidant enzymes by promoting upregulation of Nrf2 gene expression, a protein that regulates antioxidant enzymes (Salama et al., 2025). Therefore, it can protect the hepatocellular and renal cellular membranes by several pathways, including a direct mechanism via scavenging ROS and NOS, reducing lipid peroxidation and protecting cellular membrane integrity, and an indirect mechanism via induction of antioxidant synthesis in hepatocellular cells (Kandil et al., 2022; Zhou et al., 2022). Additionally, S. chinensis seed extract is rich in natural antioxidants, including hydroxytoluene derivatives, omega-3 fatty acids, and phytic acid. Phytic acid prevents the Fenton reaction by binding to iron ions, which are crucial for the activity of the toxic compound MPP+ (Abou-Zeid et al., 2021; Abdel-Wahhab et al., 2016). By inhibiting this reaction, phytic acid reduces the production of harmful free radicals, including superoxide and hydroxyl radicals, in the mitochondria (Khattab et al., 2010; Abdel-Wahhab et al., 2016). In addition, the presence of γ, δ, and α tocopherols in S. chinensis seed, along with the double bonds in unsaturated fatty acids such as oleic, linoleic, linolenic, docosenoic, and eicosanoic acids, makes the extract a potentially powerful antioxidant that can help to remove free radicals and reduce oxidative stress (Atteya et al., 2021; Awad et al., 2022; Ma et al., 2023). Clinically, CSP is one of the main contributors to AKI in patients with cancer (Yang et al., 2018). The results of the current study are in line with the results of recent studies that have shown that cisplatin causes direct damage to renal nephrons, resulting in a decrease in the glomerular filtration rate, resulting in an accumulation of creatinine in the blood (McSweeney et al., 2021; Imam et al., 2023; Mahmoud and Shams, 2024). In addition, the indirect pathway of damage was the activation of inflammatory pathways such as NF-κB and TNF-α by cisplatin, resulting in worsening cellular damage and renal fibrosis and necrosis. In addition, high creatinine levels were often present within a few days after taking cisplatin and increased with cumulative doses or risk factors (Fang et al., 2021). Vit. E can reduce oxidative stress in kidney cells by enhancing the body’s ability to eliminate free radicals and boosting antioxidant enzymes, such as GPx, CAT, and SOD (Fang et al., 2021; Ni et al., 2023; Bogacka et al., 2024). These enzymes protect renal tubular cells and capsules from oxidative damage. Vit. E also suppresses pro-inflammatory cytokines such as TNF-α, increasing cellular resistance to inflammation. This leads to improved glomerular filtration and reduced waste accumulation, resulting in lower blood creatinine levels (Koay et al., 2021; Jin et al., 2024; Meng et al., 2025). The concentration of uric acid was increased in cisplatin-treated animals due to increased cell degradation and oxidative stress, leading to a higher rate of purine production and conversion to uric acid, as well as the inhibitory effect of cisplatin on the kidneys’ ability to secrete this acid (Jana et al., 2023). Furthermore, cisplatin can disrupt organic anion transporters (OAT1/OAT3) and urate transporters responsible for UA reabsorption and secretion in the proximal tubules of the kidney (Hu et al., 2017; Oliveira et al., 2024). The administration of jojoba seed extract had a valuable impact on decreasing UA levels through multiple mechanisms. Among these mechanisms was the biological role of the bioactive components of S. chinensis seed extract, including phenols and tocopherols, which can inhibit the activity of xanthine oxidase, an enzyme responsible for the conversion of xanthine into uric acid (Selamoglu et al., 2017), thereby reducing the production of uric acid in the cell and enhancing cell membrane stability and protecting mitochondria from oxidative stress (Baptista et al., 2024). Jojoba seed extract suppresses the activity of inflammatory pathways that can increase the production of nitrogenous waste products, including UA. Additionally, a previous study suggested that ellagic acid, a component of S. chinensis extract, could reduce renal inflammation and oxidative stress, resulting in improved kidney function and preserving the integrity of kidney tissue mitochondria against ROS (Belhadj et al., 2018). According to a previous study (Baptista et al., 2024), the phenolic compounds in S. chinensis seed extract have the potential to play a role in the treatment of chronic kidney disease (CKD) by reducing uric acid levels, a risk factor for the development of CKD. Polyphenols, the primary bioactive components of S. chinensis seed extract, can donate hydrogen atoms to neutralize free radicals and prevent cellular oxidative damage, which is reflected in the suppression of uric acid generation (Abou-Zeid et al., 2021; Tsuchimoto et al., 2022). The administration of S. chinensis extract and Vit. E, alone or in combination, in rats exposed to cisplatin resulted in the suppression of uric acid and creatinine production due to the potential synergistic protective effects of these compounds and their bioactive gradients against cisplatin-induced renal toxicity (Baptista et al., 2024; Ramadan et al., 2024). The rise in cystatin C levels in the CSP group, an indicator of kidney function injury, was consistent with the results of Benoit et al. (2020). In addition, recent research showed that cisplatin administration caused damage to kidney tubular cells, resulting in increased levels of cystatin C in the blood (Abo-Elmaaty et al., 2020), which was in agreement with the study conducted by Conrad et al. (2023), who observed that serum cystatin C levels rise significantly in cisplatin-treated rats before the increase in creatinine, indicating early kidney dysfunction, highlighting cystatin C as a sensitive indicator of glomerular filtration rate (GFR) and a reliable marker for the early detection of acute kidney failure associated with chemotherapy, especially cisplatin (Mody et al., 2024). Importantly, S. chinensis seed extract could reduce the production of cystatin C by preventing the generation of ROS within renal cells, minimizing damage to glomerular membranes (Kara, 2017; Alrubaie and Khafaji, 2025), and maintaining the glomerular filtration rate, thereby preventing cellular inflammation, inhibiting the activation of the nuclear transcription factors NF-kB and TNF-α, and protecting the integrity of renal cells from oxidative damage (El Gendy et al., 2023). The current administration of alpha-tocopherol may prevent oxidative damage to cellular proteins and membranes in the glomeruli and renal tubules by interrupting ROS cascade reactions at the cellular level (Khafaji et al., 2019; Galli et al., 2022). On the other hand, Vit. E prevented the expression of inflammatory cytokines, including TNF-α and IL-6, which are responsible for elevated cystatin C in toxic exposures that may cause nephritis, by inhibiting the activation of nuclear transcription factor NF-κB (Tripathi and Alshahrani, 2021). Recent research has indicated that Vit. E enhances the generation of antioxidant enzymes, such as SOD and CAT, in kidney cells, thereby improving their self-defense capabilities and preventing persistent oxidative stress (Zhang et al., 2024). Interestingly, the present administration of Vit. E. with cisplatin-treated rats was linked with a reduction in the level of cystatin C due to its ability to protect renal cells from oxidative stress and cellular inflammation, maintaining the integrity of glomerular filtration. These results are consistent with previous findings (Dada et al., 2023; Park et al., 2025). Cisplatin is metabolically converted to a strong potential toxic agent that causes oxidative stress that influences the cellular respiration pathway, causing mitochondrial DNA damage and nuclear DNA injury, resulting in a cascade of inflammation responses and activation of apoptotic pathways, as reported by Amirshahrokhi and Khalili (2015), who noted suppressed IL-10 and an elevation in pro-inflammatory cytokines, TNF-α and IL-1β, as indicative of a link between oxidation and inflammation in cisplatin to induced nephrotoxicity in rats (Madhusudhana Rao et al., 2011; Gatea et al., 2022). The current results are in line with the findings obtained by Gazwi et al. (2024), who reported that the administration of cisplatin for 21 days increased the levels of MDA and nitric oxide (NO) and pro-inflammatory cytokines, TNF-α and IL-6, with a reduction in IL-10, indicating oxidative stress and subsequent inflammation. Importantly, the present results indicated that cisplatin injection could elevate the serum concentration of TNF-α due to the ability of cisplatin to induce the extrinsic pathway via upregulation of TNF-α with its receptors, as well as FAS ligand/receptor system activation, leading to activation of caspase 8 and 3, subsequently triggering cell death “apoptosis” (Yu et al., 2018; Patel et al., 2025). TNF-α serves as a key mediator in the extrinsic pathway and also plays a role in the inflammatory processes associated with cisplatin nephrotoxicity (Elmorsy et al., 2024). This involves the activation of pro-inflammatory cytokines and the recruitment of leukocytes, which contribute to oxidative stress (Khafaji et al., 2024; Li et al., 2024). TNF-α acts as an inducer of ROS formation, which triggers the activation of nuclear factor κB (NFκB), which subsequently initiates the transcription of inflammatory cytokines, including TNF-α (Patel et al., 2025). The findings of this study are consistent with the conclusions of Khafaji and Khafaji (2023) and Zheng et al. (2024). Intriguingly, the administration of vitamin E in the current experiment, particularly as α-tocopherol, can reduce inflammatory pathways by suppressing the NF-kB pathway, as suggested by the researchers (Wu et al., 2024), who documented that the production of pro-inflammatory cytokines, such as TNF-α and IL-6, was decreased, accompanied by increased IL-10 production, which is crucial in regulating the immune response and inflammation (Wallert et al., 2021). Additionally, the present results are consistent with the findings of Abdel-Wahhab et al. (2016), who demonstrated the protective role of ethanolic jojoba seed extract on the livers of rats fed a fumonisin-contaminated diet. Their results showed that incorporating this polyphenol-rich extract into the diet of rats resulted in a reduction in inflammatory cytokines, including TNF-α, IL-1α, and NO, along with an increase in serum IL-10. The current findings are in line with those of Feki et al. (2022), who reported the protective and anti-inflammatory role of S. chinensis seed extract in paracetamol-treated rats via down-regulation of TNF-α, and Bax, a pro-apoptotic protein, accompanied by up-regulation of IL-10 and Bcl-2, an anti-apoptotic protein (Abou-Zeid et al., 2021; Khafaji, 2023b). The CSP injection in the current experimental rats caused histopathological changes in hepatocytes, aligning with the findings of previous studies (Folayan et al., 2022; Cesur et al., 2025). Importantly, these alterations might be due to the excessive accumulation of cisplatin in hepatocytes, causing hepatotoxic oxidative stress and apoptosis, resulting in hepatocyte damage, hyperemia, and sinusoidal and vascular dilation (Ekincı-̇Akdemır et al., 2020; Rady et al., 2023). The current findings are in agreement with the study by Yılmaz (2025), who demonstrated the ability of cisplatin to cause expansion in the sinusoids, hydropic degeneration that spreads throughout the hepatic tissues, and excessive infiltration of inflammatory cells (Kaymak et al., 2022; El-Hak et al., 2022). The histological results of S. chinensis extract-treated groups (CSP + SCH) indicated the effectiveness of S. chinensis extract to recover and protect the portal tracts, sinusoids, and hepatic cords from any damage that might be caused by the action of cisplatin. This confirms the ability of S. chinensis extract to sustain the integrity of hepatocytes and protect against free radicals, oxidative stress, and hepatotoxicity induced by cisplatin. This is supported by the findings of Abdel-Wahhab et al. (2015), who proved the hepatoprotective roles of jojoba (S. chinensis) ethanolic seed extract in an in vivo study and concluded that both low and high doses (0.5 and 1 mg/kg, respectively) could reduce oxidative stress in a mycotoxin-induced liver damage model. The current findings are in line with those of a previous study (Salama et al., 2025), which concluded that the anti-inflammatory effect of jojoba seeds with hepatoprotective effects could improve the hepatocellular appearance without necrosis or hemorrhage. This suggests that the presence of unsaturated fatty acids and δ, α, and γ tocopherols (Atteya et al., 2021; Awad et al., 2022) renders jojoba seeds one of the most powerful antioxidants, enabling them to eliminate free radicals and thereby reduce oxidative damage in tissues (Ma et al., 2023). Investigations of the hepatic slides of Vit. E-cisplatin showed some healthy appearance, which confirmed the protective effects of Vit. E-cisplatin on inflammation reduction, as well as enhancing the pathological alteration in hepatic tissues, which agrees with the findings of Mohamed et al. (2022). Vitamin E acts as a potential suppressant of lipid peroxidation and protects against free radical damage to fatty acids that exist as unsaturated phospholipid membranes of hepatocytes (Hedayati-Moghadam et al., 2023). A previous study reported that Vit. E could protect hepatocytes from oxidative stress induced by cisplatin (Omar et al., 2023). Importantly, the current histopathological alterations of hepatic tissues in the cisplatin-treated S. chinensis-Vit. E-treated rats showed more improvement and enhancement, which might be attributed to the synergistic effect of S. chinensis hydro-ethanolic extract with vitamin E, which collaboratively acts as anti-inflammatory, antiapoptotic, and antioxidant factors that diminish the oxidative stress and avoid exhaustion of the endogenous antioxidants such as CAT, SOD, GSH, and vitamins C and E, which support the integrity of hepatocytes membrane via diminishing lipid peroxidation induced by ROS in hepatic tissues (Hedayati-Moghadam et al., 2023; Salama et al., 2025). The intraperitoneal injection of cisplatin to rats (CSP group) caused severe damage in renal tissues, which is consistent with the findings of a previous study (Darwish et al., 2017) that reported necrosis in proximal and distal tubules after cisplatin injection via its ability to induce more potential of caspase-3, caspase-8, and caspase-9 gene expression in tubular epithelial cells in the cortex and medulla, leading to cell membrane degeneration (Mostafa et al., 2018; Li et al., 2023; Cesur et al., 2025). Several studies have suggested that the balance of oxidants/antioxidants is the basic cause of renal tissue injury during cisplatin chemotherapy. It has also been reported that cisplatin administration could generate nitric oxide (NOx), MDA, and myeloperoxidase (MPO) and further upregulate the gene expression of endothelial nitric oxide synthase and inducible nitric oxide synthase (iNOS) in renal corpuscles and tubular cells, resulting in excessive oxidative stress and cell damage (McSweeney et al., 2021; Makled et al., 2025; Rasheed et al., 2025). The administration of S. chinensis hydro-ethanolic extract could improve the histopathological damage by mitigating the cellular inflammation response by inhibiting ROS generation, oxidative toxicity, and apoptosis. The findings of Abou-Zeid et al. (2021) supported the current results. A previous study concluded that treatment with S. chinensis seed could restore the normal renal structure and remove the toxicity in the kidney, suggesting that the S. chinensis seed contains several antioxidant compounds, including anthocyanins and tannins, presenting compelling evidence to elucidate the observed beneficial effects in combating oxidative stresses (Belhadj et al., 2020). The protective role of vitamin E in rats co-treated with cisplatin was supported by evidence from Darwish et al. (2017), who concluded that the supplementation of 75 mg/kg of Vit. E, along with a single dose of cisplatin for 4 weeks, could reduce renal inflammation and apoptosis by suppressing ROS generation and lipid peroxidation that caused renal nephron and tubular damage and apoptosis due to the potential antioxidant role of Vit. E against free radical (Raouf, 2020) and Vit. Vit. E could improve nephron integrity and reduce the generation of MPO, NOx, and iNOS (Nasiri et al., 2020; Obaid et al., 2022). Intriguingly, the renal histology observations of co-administration of SCH and Vit. E in cisplatin-treated rats were improved due to a synergistic protective role of both Vit. E and S. chinensis extract, which had antiapoptotic and anti-inflammatory properties, caused the regeneration of renal tissues, as well as the recovery and restoration of the structural components of Bowman’s capsule and glomerulus, which reflected positively on the enhancement of the current studied biomarkers of renal functions (Abdalla et al., 2024; Meng et al., 2025). LimitationsAlthough this study offers valuable insights into the observed protective effects, certain limitations merit consideration. The exclusive use of male rats and a single-dose cisplatin model may limit broader applicability and clinical relevance. In addition, the absence of molecular pathway analysis constrains mechanistic interpretation. Furthermore, further studies on the molecular mechanisms are required to identify the protective role of S. chinensis. ConclusionThe current study concluded that the administration of S. chinensis alone or with vitamin E can mitigate cisplatin-induced renal and hepatic toxicity in rats via its potent antioxidant and anti-inflammatory properties. The current research is the first to demonstrate the synergistic protective effects of the combination of S. chinensis with Vit. E, which can exert a preventive role in renal and hepatic toxicity. Further studies are essential to clarify the therapeutic potential of S. chinensis nanoparticles in reproductive and cancer-related disorders. In addition, investigating their role in modulating inflammatory and apoptotic pathways will enhance our understanding of their mechanisms. Moreover, cytotoxicity assays such as MTT and LDH are recommended to validate their cellular safety and efficacy. List of AbbreviationALT: alanine aminotransferase, AST: serum aspartate aminotransferase, BWG: Body weight change, CAT: Catalase, CSP: Cisplatin, GPx: Glutathione peroxidase, IL-10: Interleukin-10, IP: intraperitoneally, MDA: Malondialdehyde, NF-κB: Nuclear factor-kappa B, NO: Nitric oxide, NOS: Nitric oxide synthase, RNS: Reactive nitrogen species, ROS: Reactive oxygen species, S. chinensis: Simmondsia chinensis, SCH: Simmondsia chinensis, SOD: superoxide dismutase, TNF-α: Tumor necrosis factor alpha, Vit. E: Vitamin E. AcknowledgmentsThe authors appreciate the efforts of the dean of Veterinary Medicine at Al-Qasim Green University in supporting this experiment. Conflict of interestThe authors have no conflicts of interest to declare. FundingNo specific grant was provided for this research. Data availabilityAll data supporting the findings of this study can be found in the manuscript. ReferencesAbd Rashid, N., Abd Halim, S.A.S., Teoh, S.L., Budin, S.B., Hussan, F., Ridzuan, N.R.A. and Jalil, N.A.A. 2021. The role of natural antioxidants in cisplatin-induced hepatotoxicity. Biomed. Pharmacother. 144, 112328. Abdalla, S., Aroua, M.K. and Gew, L.T. 2024. A comprehensive review of plant-based cosmetic oils (virgin coconut oil, olive oil, argan oil, and jojoba oil): chemical and biological properties and their cosmeceutical applications. ACS Omega 9(44), 44019–44032. Abdel-Daim, M.M., Abdel-Rahman, H.G., Dessouki, A.A., El-Far, A.H., Khodeer, D.M., Bin-Jumah, M., Alhader, M.S., Alkahtani, S. and Aleya, L. 2020. Impact of garlic (Allium sativum) oil on cisplatin-induced hepatorenal biochemical and histopathological alterations in rats. Sci. Total Environ. 710, 136338; doi:10.1016/j.scitotenv.2019.136338 Abdel-Mageed, W.M., Bayoumi, S.A.L.H., Salama, A.A.R., Salem-Bekhit, M.M., Abd-Alrahman, S.H. and Sayed, H.M. 2014. Antioxidant lipoxygenase inhibitors from the leaf extracts of Simmondsia chinensis. Asian Pacific J. Trop. Med. 7, S521–S526. Abdel-Wahhab, M., Joubert, O., El-Nekeety, A., Sharaf, H., Abu-Salem, F. and Rihn, B. 2015. Dietary incorporation of jojoba extract eliminates oxidative damage in livers of rats fed fumonisin-contaminated diet. Hepatoma Res. 0, 0–86. Abdulraheem, I.F., Al-Rubaye, R.T., Abed, K.M. and Abdulmajeed, B.A. 2019. Extraction of jojoba oil using various concentrations of two different solvents. Iraqi J. Agric. Sci. 50(5). Abo-Elmaaty, A.M.A., Behairy, A., El-Naseery, N.I. and Abdel-Daim, M.M. 2020. The protective efficacy of vitamin E and cod liver oil against cisplatin-induced acute kidney injury in rats. Environ. Sci. Pollut. Res. 27(35), 44412–44426. Abou-Zeid, S.M., Tahoun, E.A. and Abubakr, H.O. 2021. Ameliorative effects of jojoba oil on fipronil-induced hepatorenal- and neuro-toxicity: the antioxidant status and apoptotic markers expression in rats. Environ. Sci. Pollut. Res. Int. 28, 25959–25971. Aebi, H., 1984. [13] Catalase in vitro. In Methods in enzymology. Ed., Packer, L. San Diego, CA: Academic Press, pp: 121–126; doi: 10.1016/s0076-6879(84)05016-3 Alghamdi, F., Al-Seeni, M.N. and Ghoneim, M.A. 2020. Potential synergistic antioxidant effect of thymoquinone and vitamin E on cisplatin-induced acute nephropathy in rats. Clin. Nutr. Exp. 32, 29–37. Alrubaie, M.F.K. and Khafaji, S.S. 2025. Impacts of Pinus pinaster extract and L. carnitine on gene expression and reproductive efficacy against Mancozeb-induced testicular toxicity in male rats. Open Vet. J. 15(3), 1166. Amate, J.Y., Ishida, A., Tsujino, K., Tatsumi, M., Nakatsuji, S., Kuwamura, M., Kotani, T. and Sakuma, S. 1996. Immunohistochemical study of rat renal interstitial fibrosis induced by repeated injection of cisplatin, with special reference to the kinetics of macrophages and myofibroblasts. Toxicol. Pathol. 24(2), 199–206. Amirshahrokhi, K. and Khalili, A.R. 2015. Thalidomide ameliorates cisplatin-induced nephrotoxicity by inhibiting renal inflammation in an experimental model. Inflammation 38, 476–484. Atteya, A.K.G., Sami, R., Al-Mushhin, A.A.M., Ismail, K.A. and Genaidy, E.A.E. 2021. Response of seeds, oil yield and fatty acids percentage of Jojoba Shrub strain EAI to mycorrhizal fungi and Moringa leaves extract. Horticulturae 7, 395. Awad, N.A., Eliraq, M., El-Bassel, E.H., Ismail, A.S.M., Abd El-aziz, Y.S.G., Gawish, M.S., Zewail, R.M.Y., Sami, R., Khojah, E., Hilary, U., Al-Moalem, M.H. and Sayed-Ahmed, K. 2022. Evaluation of the effect of elite Jojoba lines on the chemical properties of their seed oil. Molecules 27, 3904. Badr, A.N., Shehata, M.G. and Abdel-Razek, A.G. 2017. Antioxidant activities and potential impacts to reduce aflatoxins utilizing jojoba and jatropha oils and extracts. Int. J. Pharmacol. 2017, 13(8), 1103–1114; doi:10.3923/ijp.2017.1103.1114 Baptista, F., Paié-Ribeiro, J., Almeida, M. and Barros, A.N. 2024. Exploring the role of phenolic compounds in chronic kidney disease: a systematic review. Molecules 29(11), 2576. Barbara, J.A., Van Ostade, X. and Lopez, A.F. 1996. Tumour necrosis factor-alpha (TNF-α): the good, the bad and potentially very effective. Immunol. cell Biol. 74(5), 434–443. Begum, F., Kotian, P.J., Hiremath, S., Ramdasi, A., Sharma, A., Beegum, F., Gurram, P.C., Nampoothiri, M., Krishnadas, N. and Shenoy, R.R. 2024. Jojoba oil hastens dexamethasone induced delayed wound healing: a preclinical study. Pharm. Sci. 30(3), 332–338. Belhadj, S., Dal, S., Khaskhoussi, F., Maillard-Pedracini, E., Hentati, O. and Sigrist, S. 2020. Anorexic and metabolic effect of jojoba: potential treatment against metabolic syndrome and hepatic complications. Nutr. Metab. 17, 1–10. Brown, A., Kumar, S. and Tchounwou, P.B. 2019. Cisplatin-based chemotherapy of human cancers. J. Cancer Sci. Therapy 11(4), 97. Bunel, V., Tournay, Y., Baudoux, T., De Prez, E., Marchand, M., Mekinda, Z., Maréchal, R., Roumeguère, T., Antoine, M.H. and Nortier, J.L. 2017. Early detection of acute cisplatin nephrotoxicity: interest of urinary monitoring of proximal tubular biomarkers. Clin. Kidney J. 10(5), 639–647. Cascella, M., Palma, G., Barbieri, A., Bimonte, S., Amruthraj, N.J., Muzio, M.R., Del Vecchio, V., Rea, D., Falco, M., Luciano, A., Arra, C. and Cuomo, A. 2017. Role of Nigella sativa and its constituent thymoquinone on chemotherapy-induced nephrotoxicity: evidences from experimental animal studies. Nutrients 9(6), 625. Cesur, S., Yalinbas-Kaya, B., Tureyen, A., Zemheri-Navruz, F., Demirel, H.H. and Ince, S. 2025. Obtusifolin improves cisplatin-induced hepatonephrotoxicity via the Nrf2/HO-1 signaling pathway. Naunyn-Schmiedeb. Arch. Pharmacol. 1–16. Conrad, S., Gant Kanegusuku, A. and Conklin, S.E. 2023. Taking a step back from testing: preanalytical considerations in molecular infectious disease diagnostics. Clin. Biochem. 115, 22–32. Dada, S., Fabiyi-Edebor, T., Akintoye, O., Ezekpo, O., Dada, O., Bamikefa, T. and Sanya, J. 2023. Α-tocopherol ameliorates redox equilibrium disorders and reduces inflammatory response caused by diclofenac-induced nephrotoxicity in male Wistar Rats. Cureus 15(12), 15. Dasari, S. and Bernard Tchounwou, P. 2014. Cisplatin in cancer therapy: molecular mechanisms of action. Eur. J. Pharmacol. 740, 364–378. Dkhil, M.A., Abdel-Baki, A.S., Al-Quraishy, S. and Abdel-Moneim, A.E. 2013. Hepatic oxidative stress in Mongolian gerbils experimentally infected with Babesia divergens. Ticks Tick-borne Dis. 4(4), 346–351. Duan, M., Leng, S. and Mao, P. 2024. Cisplatin in the era of PARP inhibitors and immunotherapy. Pharmacol. Therapeut. 258, 108642. El Gendy, S.N., Elmotayam, A.K., Samir, R., Ezzat, M.I. and El Sayed, A.M. 2023. A review of the desert gold jojoba (Simmondsia chinensis) whole plant, oil, and meal: phytochemical composition, medicinal uses, and detoxification. J. Am. Oil Chem. Soc. 100(8), 591–614. Elmorsy, E.A., Saber, S., Hamad, R.S., Abdel-Reheim, M.A., El-Kott, A.F., AlShehri, M.A., Morsy, K., Salama, S.A. and Youssef, M.E., 2024. Advances in understanding cisplatin-induced toxicity: molecular mechanisms and protective strategies. Eur. J. Pharm. Sci. 203, 106939. El Sherif, F., Aldayel, M., Ismail, M.B., Alrajeh, H.S., Younis, N.S. and Khattab, S. 2023. Bio-stimulant for improving Simmondsia chinensis secondary metabolite production, as well as antimicrobial activity and wound healing abilities. Plants 12(18), 3311. El-Saeed, R.A. and El-Khawaga, O.Y. 2024. Hepatoprotective effect of jojoba oil against lead-induced toxicity in rats. Egypt. Pharm. J. 23(2), 299–308. Fang, C.Y., Lou, D.Y., Zhou, L.Q., Wang, J.C., Yang, B., He, Q.J., Wang, J.J. and Weng, Q.J. 2021. Natural products: potential treatments for cisplatin-induced nephrotoxicity. Acta Pharmacol. Sin. 42(12), 1951–1969. Fatima, G., Dzupina, A., Mahdi, A.A., Fedacko, J., Magomedova, A. and Yousif, N.G. 2025. Role of vitamin-E as a vital nutrient in health and diseases. Indian J. Clin. Biochem., 1–14. Fawzy, M., Khodeer, D., El-Sayed, N., Saeed, N. and Ahmed, Y. 2022. Molecular mechanisms of cisplatin induced nephrotoxicity. Records Pharm. Biomed. Sci. 6(3), 128–135. Feki, F., Mahmoudi, A., Denev, P., Feki, I., Ognyanov, M., Georgiev, Y., Choura, S., Chamkha, M., Trendafilova, A. and Sayadi, S. 2022. A jojoba (Simmondsia chinensis) seed cake extracts express hepatoprotective activity against paracetamol-induced toxicity in rats. Biomed. Pharmacother. 153, 113371. Gaertner, D.J., Hallman, T.M., Hankenson, F. C., and Batchelder, M. A. 2008. Anesthesia and analgesia for laboratory rodents. In Anesthesia and analgesia in laboratory animals. Eds., Fish, R., Brown, M.J., Danneman, P.J., and Karas, A.Z. San Diego, USA; American College of Laboratory, Animal Medicine Series, Academic press: Elsevier, pp: 239–297. Fortis, C., Foppoli, M., Gianotti, L., Galli, L., Citterio, G., Consogno, G., Gentilini, O. and Braga, M. 1996. Increased interleukin-10 serum levels in patients with solid tumours. Cancer Lett. 104(1), 1–5. Galli, F., Bonomini, M., Bartolini, D., Zatini, L., Reboldi, G., Marcantonini, G., Gentile, G., Sirolli, V. and Di Pietro, N. 2022. Vitamin E (alpha-tocopherol) metabolism and nutrition in chronic kidney disease. Antioxidants 11(5), 989. Gatea, S.M., Altaie, S.M., Khafaji, S.S., Aljanabi, T.K. and Nafel, N.M. 2022. Effect of in Ovo injection of vitamins E and D3 on hatchability and embryos development of poultry. REDVET 23(3), 550–560. Gazwi, H.S.S., Zaki, A.H., Abd Allah, N.A.R., Gomaa, A.T., Milošević, M., Al-Rejaie, S.S., Mohany, M. and Yassien, E.E. 2024. Mitigation of cisplatin-induced hepatotoxicity by Salvia officinalis: attenuation of oxidative damage and inflammation in rats. Free Radic. Biol. Med. 222, 62–71; doi:10.1016/j.freeradbiomed.2024.06.005 Gazwi, H.S.S., Zaki, A.H., Abd Allah, N.A.R., Gomaa, A.T., Milošević, M., Al-Rejaie, S.S., Mohany, M. and Yassien, E.E. 2024. Mitigation of cisplatin-induced hepatotoxicity by Salvia officinalis: attenuation of oxidative damage and inflammation in rats. Free Rad. Biol. Med. 222, 62–71. Gentry, H.S. 1958. The natural history of Jojoba (Simmondsia chinensis) and its cultural aspects. Econ. Botany 12(3), 261–295. Habib, S.A., Suddek, G.M., Abdel Rahim, M. and Abdelrahman, R.S. 2021. The protective effect of protocatechuic acid on hepatotoxicity induced by cisplatin in mice. Life Sci. 277, 119485; doi:10.1016/j.lfs.2021.119485 Hakiminia, B., Goudarzi, A. and Moghaddas, A. 2019. Has vitamin E any shreds of evidence in cisplatin-induced toxicity. J. Biochem. Mol. Toxicol. 33(8), 22349. Hakiminia, B., Goudarzi, A. and Moghaddas, A. 2019. Has vitamin E any shreds of evidence in cisplatin-induced toxicity. J. Biochem. Mol. Toxicol. 33(8), 22349; doi:10.1002/jbt.22349 Hamaya, S., Oura, K., Morishita, A. and Masaki, T. 2023. Cisplatin in liver cancer therapy. Int. J. Mol. Sci. 24(13), 10858. Hedayati-Moghadam, M., Baghcheghi, Y., Beheshti, F., Shabgah, A.G., Salmani, H. and Hosseini, M. 2023. Vitamin E prevented hepatic and renal tissue damage in hypothyroid rats. Adv. Biomed. Res. 12(1), 75. Hosack, T., Damry, D. and Biswas, S. 2023. Drug-induced liver injury: a comprehensive review. Therap. Adv. Gastroenterol. 16, 17562848231163410; doi:10.1177/17562848231163410 Hu, S., Leblanc, A.F., Gibson, A.A., Hong, K.W., Kim, J.Y., Janke, L.J., Li, L., Vasilyeva, A., Finkelstein, D.B., Sprowl, J.A., Sweet, D.H., Schlatter, E., Ciarimboli, G., Schellens, J., Baker, S.D., Pabla, N. and Sparreboom, A. 2017. Identification of OAT1/OAT3 as contributors to cisplatin toxicity. Clin. Transl. Sci. 10(5), 412–420. Imam, F., Kothiyal, P., Alshehri, S., Afzal, M., Iqbal, M., Khan, M.R., Alanazi, A.A.H. and Anwer, M.K. 2023. Hirsutidin prevents cisplatin-evoked renal toxicity by reducing oxidative stress/inflammation and restoring the endogenous enzymatic and non-enzymatic level. Biomedicines 11(3), 804. Jain, P., Singh, I., Surana, S.J., Shirkhedkar, A.A. 2022.Tocopherols and tocotrienols: the essential vitamin E. In Bioactive foodcomponents activity in mechanistic approach. Eds., Baú Betim Cazarin, C., Lemos Bicas, J., Pastore, G.M. and Marostica, M.R., Jr. Amsterdam, The Netherlands: Academic Press. pp: 139–154. Jana, S., Mitra, P., Dutta, A., Khatun, A., Kumar Das, T., Pradhan, S., Kumar Nandi, D. and Roy, S. 2023. Early diagnostic biomarkers for acute kidney injury using cisplatin-induced nephrotoxicity in rat model. Curr. Res. Toxicol. 5, 100135. Jiang, Q. 2014. Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Rad. Biol. Med. 72, 76–90. Kandil, N., Kandil, L., Mohamed, R., Selima, M., El Nemr, M., Barakat, A.R. and Alwany, Y. 2022. The role of miRNA-182 and FOXO3 expression in breast cancer. Asian Pac. J. Cancer Prev. 23(10), 3361–3370. Kara, Y. 2017. Phenolic contents and antioxidant activity of Jojoba (Simmondsia chinensis (Link). Schindler. Int. J. Second. Metab. 4(2), 142–142. Kaymak, E., Ünsal, M., Akın, A.T., Öztürk, E., Ceylan, T., Kuloğlu, N., Karabulut, D. and Yakan, B. 2022. Protective effect of melatonin on cisplatin-induced liver injury in rats. Çukurova Med. J. 47(1), 250–258. Kei, S. 1978. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta. 90(1), 37–43. Khafaji, S.S. 2023a. Antioxidant, anti-inflammatory, and anti-reprotoxic effects of kaempferol and vitamin E on lead acetate-induced testicular toxicity in male rats. Open Vet. J. 13(12), 1683–1695. Khafaji, S.S. 2023b. Comparing the effects of Cyperus esculentus hydroethanolic extract and Euterpe oleracea on reproductive efficacy against cadmium-induced testicular toxicity in male rats. J. Adv. Vet. Anim. Res. 10(4), 685–695. Khafaji, S.S. and Khafaji, R.S. 2023. Effects of coriander (Coriandrum sativum L.) leaf and seeds on biochemical and hematological feature. IOP Conf. Ser. Earth Environ. Sci. 1241(1), 12–132. Khafaji, S.S., Hasan, A.G., Ali, M.A., Mo, A.J. and Farhood, A.F. 2024. Study the effects of Foeniculum vulgare on serological and biochemical traits in broiler chicks. Minar Int. Conf. 11(11), 179–196. Khafaji, S.S.O., Aljanabi, T.K. and Suhailaltaie, S.M. 2018. Evaluation the impact of different levels of propolis on some reproductive features in Iraqi local roosters. Adv. Anim. Vet. Sci. 7(2), 82–87. Khattab, R., Goldberg, E., Lin, L. and Thiyam, U. 2010. Quantitative analysis and free-radical-scavenging activity of chlorophyll, phytic acid, and condensed tannins in canola. Food Chem. 122, 1266–1272. Kiernan, J. 2015. Histological and histochemical methods. Theory and Practice, 5th edition, Gdansk, Poland: Medical University of Gdansk, pp: 58–59; doi: 10.5603/FHC.a2016.0007 Lin, S.Y., Chang, C.L., Liou, K.T., Kao, Y.K., Wang, Y.H., Chang, C.C., Kuo, T.B.J., Huang, H.T., Yang, C.C.H., Liaw, C.C. and Shen, Y.C. 2024. The protective role of Achyranthes aspera extract against cisplatin-induced nephrotoxicity by alleviating oxidative stress, inflammation, and PANoptosis. J. Ethnopharmacol. 319, 117097. Louisa, M., Putera, A.M., Sidqi, A.A., Mahaputra, D.K., Firmansyah, E.R., Ammar, M.F., Talya, N., Aryadevi, N.N.B., Sandhiutami, N.M.D., Sukmawati, D. and Soetikno, V. 2022. Attenuation of cisplatin-induced hepatotoxicity by nanocurcumin through modulation of antioxidative and anti-inflammatory pathways. J. Appl. Pharm. Sci. 13(3), 60–70. Madhusudhana Rao, A., Anand, U. and Anand, C.V. 2011. Myeloperoxidase in chronic kidney disease. Indian J. Clin. Biochem. 26, 28–31. Makled, M.N., Makled, N.N., Abdel-Rahman, A.M. and Sharawy, M.H. 2025. Inhibition of p75NTR/p53 axis by ambrisentan suppresses apoptosis and oxidative stress-mediated renal damage in a cisplatin AKI model. Chemico-Biol. Interact. 408, 111408. Mcsweeney, K.R., Gadanec, L.K., Qaradakhi, T., Ali, B.A., Zulli, A. and Apostolopoulos, V. 2021. Mechanisms of cisplatin-induced acute kidney injury: pathological mechanisms, pharmacological interventions, and genetic mitigations. Cancers 13(7), 1572. Meng, L., Liu, S. and Cui, W. 2025. Renal protective effects of vitamin E for drug-induced kidney injury: a meta-analysis. Front. Pharmacol. 16, 1461792. Mody, H., Nair, S., Rump, A., Vaidya, T.R., Garrett, T.J., Lesko, L. and Ait-Oudhia, S. 2024. Identification of novel and early biomarkers for cisplatin-induced nephrotoxicity and the nephroprotective role of cimetidine using a pharmacometabolomic-based approach coupled with in vitro toxicodynamic modeling and simulation. J. Pharm. Sci. 113(1), 268–277. Mohandas, J., Marshall, J.J., Duggin, G.G., Horvath, J.S. and Tiller, D.J. 1984. Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney: possible implications in analgesic nephropathy. Biochem. Pharmacol. 33(11), 1801–1807. Niki, E. 2014. Role of vitamin E as a lipid-soluble peroxyl radical scavenger: in vitro and in vivo evidence. Free Radic. Biol. Med. 66, 3–12; doi:10.1016/j.freeradbiomed.2013.03.022 Obaid, A.A., Al-Sammak, M.A. and Fadhil, M.S. 2022. The effect of vitamin E on the histological structure of kidney in rats treated with cyclophosphamide. Iraqi J. Vet. Sci. 36(2), 513–517. Oliveira, C.A., Mercês, E.A.B., Portela, F.S., Malheiro, L.F.L., Silva, H.B.L., De Benedictis, L.M., De Benedictis, J.M., Silva, C.C.D.E., Santos, A.C.L., Rosa, D.P., Velozo, H.S., De Jesus Soares, T. and De Brito Amaral, L.S. 2024. An integrated view of cisplatin-induced nephrotoxicity, hepatotoxicity, and cardiotoxicity: characteristics, common molecular mechanisms, and current clinical management. Clin. Exp. Nephrol. 28, 711–727. Palipoch, S., Punsawad, C., Koomhin, P. and Suwannalert, P. 2014. Hepatoprotective effect of curcumin and alpha-tocopherol against cisplatin-induced oxidative stress. BMC Complem. Altern. Med. 14, 1–8. Parasuraman, S., Raveendran, R. and Kesavan, R. 2010. Blood sample collection in small laboratory animals. J. Pharmacol. pharmacother. 1(2), 87. Park, I., Kim, S., Um, Y.W., Kim, H.E., Lee, J.H., Kim, S., Kim, P. and Jo, Y.H. 2025. Intravital imaging of peritubular microcirculation impairment in cisplatin-induced acute kidney injury. JCI. Insight 10, e178689. Patel, S., Sathyanathan, V. and Salaman, S.D. 2024. Molecular mechanisms underlying cisplatin-induced nephrotoxicity and the potential ameliorative effects of essential oils: a comprehensive review. Tissue Cell 88, 102377. Peña-Corona, S.I., Vargas-Estrada, D., Chávez-Corona, J.I., Mendoza-Rodríguez, C.A., Caballero-Chacón, S., Pedraza-Chaverri, J., Gracia-Mora, M.I., Galván-Vela, D.P., García-Rodríguez, H., Sánchez-Bartez, F., Vergara-Onofre, M. and Leyva-Gómez, G. 2023. Vitamin E (α-tocopherol) does not ameliorate the toxic effect of bisphenol S on the metabolic analytes and pancreas histoarchitecture of diabetic rats. Toxics 11(7), 626. Prestayko, A.W., D’Aoust, J.C., Issell, B.F. and Crooke, S.T. 1979. Cisplatin (cis-diamminedichloroplatinum II). Cancer Treat. Rev. 6(1), 17–39. Ramadan, O.I., S Ali, L., M Abd-allah, F., E A Ereba, R., S Humeda, H., A Damanhory, A., E Moustafa, A., M Younes, A., M Y Awad, M. and A A Omar, N. 2024. Co-administration of either curcumin or resveratrol with cisplatin treatment decreases hepatotoxicity in rats via anti-inflammatory and oxidative stress-apoptotic pathways. PeerJ 12, e17687. Ribeiro, A.M., Estevinho, B.N. and Rocha, F. 2021. The progress and application of vitamin E encapsulation – a review. Food Hydrocoll. 121, 106998. Romani, A.M.P. 2022. Cisplatin in cancer treatment. Biochem. Pharmacol. 206, 115323. Rosenberg, B., Van Camp, L. and Krigas, T. 1965. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature 205(4972), 698–699. Salama, R.H., Abd-Elghaffar, S.K., Elgalaly, R.M. and Maghraby, N. 2025. Potential protective and therapeutic effects of jojoba oil against chlorpyrifos-induced hepatotoxicity in rats. Assiut Vet. Med. J. 71(185), 289–301. Satyam, S.M., Bairy, L.K., Rehman, A., Farook, M., Khan, S., Nair, A.A., Binu, N.N., Yehya, M. and Khan, M.M. 2024. Dapagliflozin: a promising strategy to combat cisplatin-induced hepatotoxicity in Wistar rats. Biology 13(9), 672; doi:10.3390/biology13090672 Schefler, W.C. (1980). Statistics for the biological sciences. Addison-Wesley Pub. Co. Siahaan, A. 2020. Antioxidant activity of Jojoba (Simmondsia chinensis) seed residue extract. Biosaintifika. J. Biol. Biol. Educ. 12(3), 350–355. Siddique, S., Sultana, S., Akhtar, N., Sethi, A. and Chishti, A.W. 2023. An overview on medicinally important plant: jojoba (Simmondsia chinensis link) schneider. Int. J. Natl. Med. Health Sci. 3(1), 7–12. Tang, C., Livingston, M.J., Safirstein, R. and Dong, Z. 2023. Cisplatin nephrotoxicity: new insights and therapeutic implications. Nat. Rev. Nephrol. 19(1), 53–72. Tchounwou, P.B., Dasari, S., Noubissi, F.K., Ray, P. and Kumar, S. 2021. Advances in our understanding of the molecular mechanisms of action of cisplatin in cancer therapy. J. Exp. Pharmacol. 13, 303–328. Tietel, Z., Melamed, S., Galilov, I., Ben-Gal, A., Dag, A. and Yermiyahu, U. 2024. Elevated nitrogen fertilization differentially affects jojoba wax phytochemicals, fatty acids and fatty alcohols. Front. Plant Sci. 15, 1425733. Tietel, Z., Melamed, S., Ogen-Shtern, N., Eretz-Kdosha, N., Silberstein, E., Ayzenberg, T., Dag, A. and Cohen, G. 2024. Topical application of jojoba (Simmondsia chinensis L.) wax enhances the synthesis of pro-collagen III and hyaluronic acid and reduces inflammation in the ex-vivo human skin organ culture model. Front. Pharmacol. 15, 1333085. Traber, M.G. 2021. Vitamin E: necessary nutrient for neural development and cognitive function. Proc. Nutr. Soc. 80(3), 319–326. Tripathi, P. and Palshahrani, S. 2021. Mitigation of IL-1β, IL-6, TNF-α, and markers of apoptosis by ursolic acid against cisplatin-induced oxidative stress and nephrotoxicity in rats. Hum. Exp. Toxicol. 40(12_suppl), S397–S405. Tsuchimoto, S., Sakai, H. and Fukui, K. 2022. Oxidative stability and antioxidant activity of crude jojoba oil. BPB Rep. 5(6), 121–124. Wallert, M., Börmel, L. and Lorkowski, S. 2021. Inflammatory diseases and vitamin E—what do we know and where do we go?. Mol. Nutr. Food Res. 65(1), 2000097. Yu, B., Gao, W., Zhou, H., Miao, X., Chang, Y., Wang, L., Xu, M. and Ni, G. 2018. Propofol induces apoptosis of breast cancer cells by downregulation of miR-24 signal pathway. Cancer Biomarkers 21(3), 513–519. Yu, X., Meng, X., Xu, M., Zhang, X., Zhang, Y., Ding, G., Huang, S., Zhang, A. and Jia, Z. 2018. Celastrol ameliorates cisplatin nephrotoxicity by inhibiting NF-κB and improving mitochondrial function. EBioMedicine 36, 266–280. Zhang, M., Zhang, J., Ma, Y., Jin, Y., Li, Y. and Wu, X. 2024. Nephropathy induced by cisplatin results from mitochondrial disruption, impaired energy metabolism, altered expression of renal transporters, and accumulation of urinary toxins. J. Trace Elements Med. Biol. 86, 127553. Zheng, Z.L., Ma, J.W., Luo, Y., Liang, G.J., Lei, S.J., Yan, K.J., Meng, H.B. and Liu, X.J. 2024. Mechanism of dexmedetomidine protection against cisplatin induced acute kidney injury in rats. Renal Failure 46(1), 2337287. Zhou, J., Nie, R.-C., Yin, Y.-X., Cai, X.-X., Xie, D. and Cai, M. 2022. Protective effect of natural antioxidants on reducing Cisplatin-Induced nephrotoxicity. Dis. Mark. 1, 1612348. Zhu, H., Wang, X., Wang, X., Pan, G., Zhu, Y. and Feng, Y. 2021. The toxicity and safety of Chinese medicine from the bench to the bedside. J. Herbal Med. 28, 100450. Zishan, M., Sharma, A. and Alam, Z. 2025. Nephroprotective screening of delonix regia flowers and leaves against cisplatin-induced nephrotoxicity in female Wistar rats. World J. Pharm. Res. 14(7), 714–734. | ||
| How to Cite this Article |
| Pubmed Style Khafaji AM, Khafaji SS. The protective effects of the combination of Simmondsia chinensis extract and vitamin E on nephrotoxicity and hepatotoxicity in rats exposed to cisplatin: An anti-inflammatory and antioxidant study. Open Vet. J.. 2025; 15(8): 3486-3504. doi:10.5455/OVJ.2025.v15.i8.12 Web Style Khafaji AM, Khafaji SS. The protective effects of the combination of Simmondsia chinensis extract and vitamin E on nephrotoxicity and hepatotoxicity in rats exposed to cisplatin: An anti-inflammatory and antioxidant study. https://www.openveterinaryjournal.com/?mno=259438 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i8.12 AMA (American Medical Association) Style Khafaji AM, Khafaji SS. The protective effects of the combination of Simmondsia chinensis extract and vitamin E on nephrotoxicity and hepatotoxicity in rats exposed to cisplatin: An anti-inflammatory and antioxidant study. Open Vet. J.. 2025; 15(8): 3486-3504. doi:10.5455/OVJ.2025.v15.i8.12 Vancouver/ICMJE Style Khafaji AM, Khafaji SS. The protective effects of the combination of Simmondsia chinensis extract and vitamin E on nephrotoxicity and hepatotoxicity in rats exposed to cisplatin: An anti-inflammatory and antioxidant study. Open Vet. J.. (2025), [cited January 25, 2026]; 15(8): 3486-3504. doi:10.5455/OVJ.2025.v15.i8.12 Harvard Style Khafaji, A. M. & Khafaji, . S. S. (2025) The protective effects of the combination of Simmondsia chinensis extract and vitamin E on nephrotoxicity and hepatotoxicity in rats exposed to cisplatin: An anti-inflammatory and antioxidant study. Open Vet. J., 15 (8), 3486-3504. doi:10.5455/OVJ.2025.v15.i8.12 Turabian Style Khafaji, Ali Mohammed, and Sura Safi Khafaji. 2025. The protective effects of the combination of Simmondsia chinensis extract and vitamin E on nephrotoxicity and hepatotoxicity in rats exposed to cisplatin: An anti-inflammatory and antioxidant study. Open Veterinary Journal, 15 (8), 3486-3504. doi:10.5455/OVJ.2025.v15.i8.12 Chicago Style Khafaji, Ali Mohammed, and Sura Safi Khafaji. "The protective effects of the combination of Simmondsia chinensis extract and vitamin E on nephrotoxicity and hepatotoxicity in rats exposed to cisplatin: An anti-inflammatory and antioxidant study." Open Veterinary Journal 15 (2025), 3486-3504. doi:10.5455/OVJ.2025.v15.i8.12 MLA (The Modern Language Association) Style Khafaji, Ali Mohammed, and Sura Safi Khafaji. "The protective effects of the combination of Simmondsia chinensis extract and vitamin E on nephrotoxicity and hepatotoxicity in rats exposed to cisplatin: An anti-inflammatory and antioxidant study." Open Veterinary Journal 15.8 (2025), 3486-3504. Print. doi:10.5455/OVJ.2025.v15.i8.12 APA (American Psychological Association) Style Khafaji, A. M. & Khafaji, . S. S. (2025) The protective effects of the combination of Simmondsia chinensis extract and vitamin E on nephrotoxicity and hepatotoxicity in rats exposed to cisplatin: An anti-inflammatory and antioxidant study. Open Veterinary Journal, 15 (8), 3486-3504. doi:10.5455/OVJ.2025.v15.i8.12 |