| Research Article | ||
Open Vet. J.. 2025; 15(9): 4097-4105 Open Veterinary Journal, (2025), Vol. 15(9): 4097-4105 Research Article Characterization of antimicrobial-resistant Escherichia coli from poultry birds in Ebonyi North, NigeriaAgatha Ifunanya Ugbo1, Theophilus Ikechukwu Nnenwa2, Emmanuel Nnabuike Ugbo2,3, Mustofa Helmi Effendi3*, Boniface Oke2, Bernard Nnabuife Agumah2, Hartanto Mulyo Raharjo4, Wiwiek Tyasningsih4, Budiastuti Budiastuti5 and Saifur Rehman61Department of Microbiology and Parasitology, Faculty of Allied Health Sciences, David Umahi Federal University of Health Sciences, Uburu, Nigeria 2Department of Applied Microbiology, Faculty of Science, Ebonyi State University, Abakaliki, Nigeria 3Division of Veterinary Public Health, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 4Division of Veterinary Microbiology, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia 5Study Program of Pharmacy Science, Faculty of Health Science, Universitas Muhammadiyah Surabaya, Indonesia 6Department of Veterinary Microbiology, The University of Veterinary and Animal Sciences, Swat, Pakistan *Corresponding Author: Mustofa Helmi Effendi. Division of Veterinary Public Health, Faculty of Veterinary Medicine, Universitas Airlangga, Surabaya, Indonesia. Email: mustofa-h-e [at] fkh.unair.ac.id Submitted: 17/05/2025 Revised: 15/08/2025 Accepted: 24/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
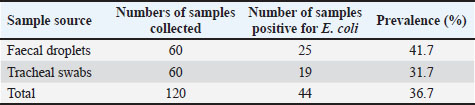
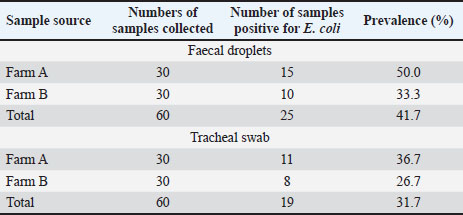
ABSTRACTBackground: The widespread use of antibiotics for disease prevention and control has inadvertently upsurged the development of antibiotic resistance worldwide. Aim: This study aimed to determine the occurrences and spread of antimicrobial-resistant Escherichia coli in poultry birds in Ebonyi North. Methods: A total of 120 samples (faecal droplet and tracheal swab) were collected from two different poultry farms in the rural area of Ebonyi North. Standard microbiological methods were used to isolate and identify the isolates, and confirmation was achieved by matrix-assisted laser desorption ionization–time of flight mass spectrometry. Disc diffusion tests were used to determine antimicrobial susceptibility profiles. Statistical analysis was performed using SPSS version 20.0 software. Results: This study revealed an overall prevalence of E. coli of 36.7% in poultry birds. The highest prevalence of E. coli (41.7%) was observed from fecal droplets, and least in trachea swabs (31.7%). The isolates were highly susceptible to gentamicin and ciprofloxacin, with a range of 80.0%–100%. High level of resistance was observed among the isolates to ampicillin and tetracycline, with a range of 45.5%–75.0%. Thus, erythromycin and streptomycin showed average inhibitory activity on the E. coli with a range of 60.0%–81.8%. Multidrug resistance (MDR) was reported in 35.3% of the E. coli isolates. The Multiple Antibiotic Resistance Index was reported to range from 0.5 to 0.8, with an average index of 0.66. There was no statistically significant difference between the sample source and E. coli prevalence (p < 0.05) in the two farms. Conclusion: The widespread use of antibiotics in poultry farming as growth promotion and disease prevention is a key factor in the rise of MDR-producing E. coli in poultry birds. To combat this growing problem, there is an urgent need for better surveillance, stricter regulations, and improved farm practices, including biosecurity measures and alternative disease control strategies. Keywords: Antimicrobial resistance, E. coli, MALDI-TOF MS, Poultry birds, Public health. IntroductionEscherichia coli are Gram-negative, rod-shaped bacteria that are typically present in the digestive systems of humans and animals (Pradika et al., 2019; Islam et al., 2020). While many strains are harmless, certain pathogenic types of E. coli are responsible for diseases in both species. These pathogenic strains can cause conditions such as colibacillosis, a major contributor to financial losses in the poultry sector (Raji et al., 2018; Ansharieta et al., 2021; Putri et al., 2023; Kendek et al., 2024). The growing presence of antimicrobial-resistant (AMR) E. coli on poultry farms poses serious risks to animal health and public safety (Zalewska et al., 2021; Ahadini et al., 2025). These resistant bacteria, found throughout the poultry supply chain, can be transmitted to humans via contaminated meat or through direct exposure (Viegas et al., 2021; Agumah et al., 2025). In Sub-Saharan Africa, poultry represents an essential source of dietary protein and accounts for roughly 24.0% of all meat from livestock (FAOSTAT, 2021). Poultry farming is generally affordable and accessible, providing meat and eggs to a broad population (Islam et al., 2020). To boost growth, enhance productivity, and prevent illness, farmers often administer antibiotics, both in therapeutic doses and as growth promoters (Abdel-Tawab et al., 2022; Ugbo et al., 2023a,b). This widespread antibiotic usage has significant implications for food safety, dietary habits, and the broader food system (Roth et al., 2019). As a result, poultry is now recognized as a major contributor to the spread of antibiotic resistance (Zalewska et al., 2021). The frequent, low-dose use of antibiotics in intensive poultry farming is a key factor behind this growing issue (Roth et al., 2019). Such unregulated practices, aimed at enhancing growth and feed conversion efficiency, represent a public health threat (Gupta et al., 2021), exacerbated by the increasing demands for animal-based proteins resulting from population growth and economic development (Hedman et al., 2020). Products derived from poultry and other food-producing animals serve as major transmission pathways for zoonotic and foodborne pathogens, including E. coli (Hafez and Attia, 2020). The widespread use and misuse of antimicrobials in animal production aggravate this risk by driving the emergence of AMR strains, thereby heightening the public health threat of foodborne infections (Ajibola et al., 2025). The African continent experiences a high incidence of foodborne diseases, with about 91 million cases and nearly 137,000 deaths every year (Andrew Selaledi et al., 2020). Antibiotic-resistant strains of E. coli, Salmonella, Staphylococcus aureus, Campylobacter, and Proteus mirabilis have been widely detected across human, animal, and environmental sources, underscoring their role in the interconnected One Health cycle of antimicrobial resistance (Le et al., 2016; Bushen et al., 2021; Ngogang et al., 2021; Ugbo et al., 2023a,b; Yanestria et al., 2023). The misuse of antibiotics in both human and veterinary medicine is a driving force behind resistance in disease-causing microbes as well as in normal microbial communities of exposed organisms (Yunita et al., 2020; Rafif Khairullah et al., 2022; Hochmuth et al., 2023). Furthermore, the release of antibiotic residues into the environment continuously subjects bacteria to selective pressure, facilitating the development of resistance (Putra et al., 2019; Widodo et al., 2022). This trend complicates the treatment and control of infections in both humans and animals (Khairullah et al., 2020; Khan et al., 2024). In the rural areas of Abakaliki in Ebonyi North, poultry farmers often rely on antibiotics for disease management without professional veterinary guidance and generally operate under substandard hygiene conditions. Given these circumstances, it is essential to evaluate the consequences of antibiotic misuse in livestock farming and the potential role poultry may play in the transmission of zoonotic bacteria. Identifying AMR E. coli in poultry birds provides early warning of zoonotic risks, helping to protect consumers from foodborne infections and also generates data will guides responsible antibiotic use in veterinary practice and poultry farming. Therefore, this research focuses on assessing the occurrence and spread of AMR E. coli in poultry within the Ebonyi North region. Materials and MethodsA cross-sectional study was carried out in private farms located in the rural area of Abakaliki in Ebonyi North zone. A sample size of 120 was estimated based on previous prevalence reports using Cochran’s formula {n=[Z2(pq)]/e2} based on the previously reported prevalence (Mtonga et al., 2021). Samples of fresh chicken faecal droplets and tracheal swabs were randomly collected from the poultry farms and analyzed. Sample collectionExactly two poultry farms of broiler birds were selected and studied between February to April 2025. A total of 120 samples were collected, with 30 samples obtained from each source (fecal and tracheal swabs) across both poultry farms. The samples were aseptically obtained using sterile swab stick. It was stored in 5 ml buffered peptone water (BPW) for pre-enrichment purposes and transported in an icebox to the Department of Applied Microbiology Laboratory of Ebonyi State University, Abakaliki, within a few hours of collection for microbiological analysis. Isolation and identification of E. coliThe E. coli isolates were isolated and identified using standard microbiological techniques (Bushen et al., 2021). The pre-enriched samples in BPW were inoculated into a freshly prepared nutrient broth and incubated for 24 hours at 37°C. The nutrient broth containing the suspected organisms was plated onto MacConkey, Eosin Methylene Blue agar (Sigma Aldrich, Germany) and incubated for 24 hours at 37°C. The probable E. coli colonies were further sub-cultured onto nutrient ager to obtain a pure culture. The fresh pure colonies of the presumptive E. coli were further identified using Gram staining and major biochemical tests, which included indole, oxidase, methyl red, catalase, Voges-Proskauer test, and sugar utilization test via triple sugar iron agar. Additionally, the biochemically identified isolates were all confirmed by matrix-assisted laser desorption ionization–time of flight mass spectrometry (Bruker MALDI Biotyper database, Germany) (Khater et al., 2021). Antimicrobial susceptibility testingThe susceptibility patterns of the bacterial isolates were phenotypically determined by the Kirby and Bauer disc diffusion method as recommended by Clinical Laboratory Standards Institute guidelines (CLSI, 2018). The fresh colonies of bacterial isolates were resuspended in nutrient broth, and the bacterial growth was adjusted to match a 0.5 McFarland turbidity standard. A sterile swab stick was aseptically removed and soaked in the standardized isolates; the excess was tip off, and freshly prepared Muller-Hinton agar (Oxoid, UK) was spread using a swab stick containing the inoculum. The inoculated plates were allowed to stand for 10–15 minutes. The following antibiotics: gentamycin (500 µg), streptomycin (10 µg), ciprofloxacin (5 µg), tetracycline (30 µg), erythromycin (15 µg), and ampicillin (10 µg) (Oxoid, UK) were placed on the inoculated plates using sterile forceps. The plates were incubated at 37°C for 24 hours after which the zones of inhibition around each disc were measured to the nearest mm with a metre rule, recorded, and interpreted as resistant and non-resistant (sensitive and intermediate) according to the Clinical Laboratory Standard Institute (CLSI, 2018) guidelines. Determination of bacterial multidrug resistance (MDR) indexThe bacterial isolates were interpreted as multi-drug resistant (MDR) when the strain was resistant to at least three various classes of antibiotics (Magiorakos et al., 2012; Ugbo et al., 2023a,b). The MDR index for a single bacterial isolate was calculated as the number of antibiotics to which an isolate is resistant (a) divided by the total number of antimicrobial agents to which the isolate was assayed (b) according to the previous method (Aworh et al., 2019). Statistical analysisData on E. coli prevalence across farms and sample sources were analyzed using the chi-square (χ²) test of independence to evaluate potential associations between categorical variables. For each comparison, observed frequencies of positive and negative samples were arranged into contingency tables, and expected frequencies were calculated based on marginal totals. Separate analyses were performed to compare (i) farms within each sample source, (ii) overall prevalence between Farm A and Farm B, and (iii) overall prevalence between faecal and tracheal samples. The chi-square statistic, degrees of freedom (df), and corresponding p-values were reported. Statistical significance was set at p < 0.05. Ethical approvalNot needed for this study. The poultry farm owners were approached before the collection of samples, and oral consent was obtained from them. ResultsThe bacterial isolates obtained from both faecal droppings and tracheal swabs were identified as E. coli based on their morphological and biochemical properties. These isolates exhibited Gram-negative, rod-shaped characteristics and produced pink colonies on MacConkey agar and a green metallic sheen on EMB agar, typical indicators of E. coli. Of the 120 samples processed, only 44 (36.7%) were confirmed as E. coli (Table 1). Of the 44 isolates, 25 (41.7%) belonged to fecal drops and 19 (31.7%) to tracheal swabs (Table 2). The breakdown by farm revealed that out of the 25 faecal dropping samples positive for E. coli, 15 (50.0%) were from Farm A and 10 (33.3%) from Farm B. Of 19 E. coli obtained from tracheal swabs, 11 (36.7%) were from Farm A and 8 (26.7%) from Farm B (Table 2). Table 1. Overall occurrence of E. coli isolates from poultry farms
Table 2.Distribution of E. coli isolates from faecal droplets and tracheal swab of chickens.
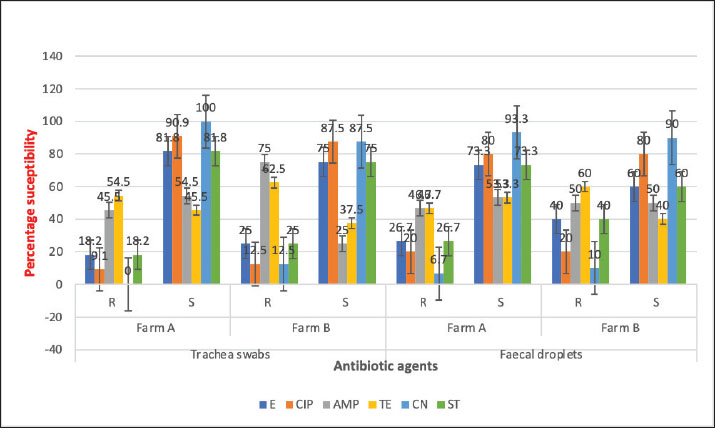
Chi-square analysis revealed no significant association between E. coli positivity and sample source or farm. When comparing farms within each sample type, no significant difference was observed (χ²=1.40, df=3, p=0.70). Similarly, the overall prevalence of E. coli between Farm A (43.3%) and Farm B (30.0%) did not differ significantly (χ²=2.30, df=1, p=0.13). Comparison of sample sources also showed no significant variation, with E. coli detected in 41.7% of faecal samples and 31.7% of tracheal samples (χ²=1.29, df=1, p=0.25). Thus, the distribution of E. coli across farms and sample sources appeared comparable. Antibiotic susceptibility testing of the E. coli isolates revealed varying resistance patterns. Isolates from faecal samples demonstrated high sensitivity to gentamicin and ciprofloxacin, with susceptibility rates between 87.5% and 100%. In contrast, notable resistance was observed against ampicillin (45.5%–50.0%) and tetracycline (46.7%–62.5%) among the isolates. Erythromycin and streptomycin displayed moderate inhibitory effects, with susceptibility rates ranging from 75.0% to 81.8%. For isolates from tracheal swabs, susceptibility to gentamicin and ciprofloxacin ranged from 80.0% to 90.0%, while erythromycin showed effectiveness in 60.0% to 73.3% of cases. However, these isolates also showed resistance to ampicillin and tetracycline, with resistance levels between 46.7% and 60.0% (Fig. 1).
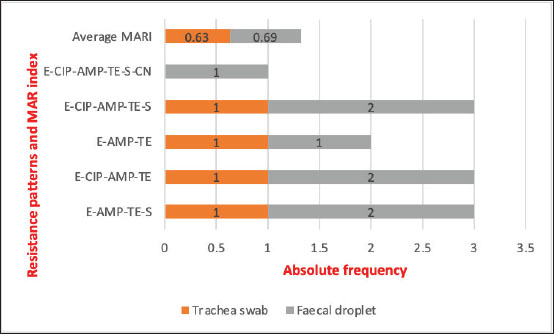
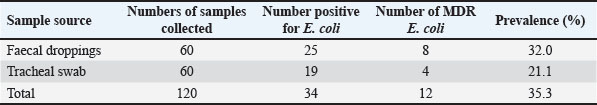
Fig. 1. Antimicrobial resistance and susceptibility profiles of E. coli isolates from of chickens. Key: AMP=Ampicillin, CIP=Ciprofloxacin, CN=Gentamicin, E=Erythromycin, R=Resistant, S=Susceptible, ST=Streptomycin, TE=Tetracycline. Out of the 34 E. coli isolates tested for MDR, 12 (35.3%) were found to be MDR strains, 8 (32.0%) from faecal droppings, and 4 (21.1%) from tracheal swabs (Table 3). The multiple antibiotic resistance (MAR) index for these isolates ranged from 0.5 to 0.8, with an average MAR index of 0.6 (Fig. 2).
Fig. 2. Patterns of MARI of E. coli isolated from tracheal swabs and faecal dropping of chickens. Table 3. Frequency of multidrug resistance E. coli isolates from chickens.
DiscussionThis study investigated the occurrence and dissemination of AMR E. coli in poultry birds. The findings confirmed the presence of E. coli in poultry farms, with an overall prevalence of 36.7% among sampled chickens. The highest prevalence (41.7%) was recorded in faecal droplet samples, while the lowest (31.7%) was observed in tracheal swabs. These results align with previous studies, such as the 33.0% prevalence reported in Nepal (Saud et al., 2019) and 31.1% in Taif, Saudi Arabia (Altalhi et al., 2010). Similarly, a study in Romania found E. coli in 30.0% of poultry chicken samples (Brătfelan et al., 2023), while a lower prevalence of 14.3% was reported in Nigeria (Aniokette et al., 2016). Another study recorded prevalence rates of 56.0% in chicken faecal samples and 32.0% in poultry products (Mensah et al., 2022). Higher prevalence rates have been reported elsewhere, including 46.9% in Ghana (Boamah et al., 2016), 64.3% (Rasmussen et al., 2015), and up to 80.0% (Adzitey et al., 2020). Differences in prevalence across studies may be attributed to variations in sampling procedures and microbiological techniques. E. coli in poultry is of particular public health concern not only because it indicates faecal contamination but also due to its association with foodborne illnesses (Agyare et al., 2018; Adzitey et al., 2020). The 36.7% prevalence found in this study is lower than the 63.6% reported by Amadi et al. (2018), the 82.0% reported by Bolan et al. (2020), and 100.0% prevalence found by Ejeh et al. (2017). This finding highlights a notable level of E. coli contamination, suggesting poor sanitary conditions in poultry farms in rural Ebonyi North, and underscores the potential public health risks from contaminated poultry products. The higher prevalence in faecal droplet samples compared to tracheal swabs supports earlier findings by Raji et al. (2018). The antibiotic susceptibility profile of the E. coli isolates revealed variable resistance patterns. Isolates from both faecal and tracheal samples exhibited high susceptibility to gentamicin and ciprofloxacin, with susceptibility rates ranging from 80.0% to 100.0%. In contrast, significant resistance was observed against ampicillin and tetracycline, ranging from 45.5% to 75.0%. Erythromycin and streptomycin showed notable antimicrobial activity, with susceptibility rates between 60.0% and 81.8%. These findings are consistent with earlier studies reporting high resistance to tetracycline and ampicillin (84.9%–89.5%) and strong susceptibility to gentamicin and ciprofloxacin (53.5%–91.9%) (Abdel-Tawab et al., 2022). Similarly, other studies have reported high resistance to tetracycline (62.5%–82.1%) and ampicillin (62.5%–78.6%), while noting low resistance to gentamicin (8.0%) and ciprofloxacin (12.0%) in poultry-derived E. coli isolates (Agyare et al., 2018; Adzitey et al., 2020). The high resistance to ampicillin and tetracycline observed here is likely due to their frequent use for infection control in poultry farms. Likewise, resistance to erythromycin and streptomycin may result from their inappropriate application in disease management. Multidrug resistance was detected in 35.3% of E. coli isolates from faecal and tracheal samples. This aligns with earlier studies, although higher MDR rates were reported by Mensah et al. (2022) at 57.1%, and others noted even higher rates of 62.6% (Johnson et al., 2012) and 68.3% (Adzitey et al., 2020). Such variation in MDR prevalence may reflect differences in antibiotic usage at the farm level, national antimicrobial policies, or the selection of antibiotics used in susceptibility testing. The excessive use of antibiotics for growth promotion, treatment, and prophylaxis in poultry is a likely contributor to this resistance trend. The multiple antibiotic resistance index (MARI) of the E. coli isolates in this study was 0.6, a notably high value. This is comparable to other reports showing MARI values of 0.5 (Raji et al., 2018), 0.6 (Viegas et al., 2021), and 0.7 (Bolan et al., 2020). A MARI of 0.66 is concerning, as MDR pathogens are increasingly recognized as significant foodborne threats that complicate treatment in both animals and humans. The potential for zoonotic transmission through contaminated poultry products or direct contact raises serious public health concerns, especially in regions with unregulated antibiotic use (Wibisono et al., 2020; Yanestria et al., 2022). Therefore, enhancing the detection and monitoring of MDR E. coli in poultry farms is essential for agricultural safety and as part of the broader global strategy to combat antimicrobial resistance. Limitations of the studyThis study focused on the characterization of AMR E. coli from poultry birds, some limitations must be documented for future study direction. The study findings are limited to Ebonyi North and may not generalize to other regions in Nigeria, and only poultry birds were included; the absence of environmental and human samples limits One Health inferences. A cross-sectional study may not capture seasonal variation in carriage or resistance patterns, and not all the antibiotic classes were tested. Culture-based methods and phenotypic methods were used, and this may create bias. However, further studies should incorporate molecular techniques such as polymerase chain reaction and whole-genome sequencing. ConclusionThis research revealed a notably high occurrence of E. coli in poultry farms situated in rural parts of Ebonyi North. A considerable number of the E. coli isolates demonstrated resistance to more than three different antibiotic classes, accompanied by high MAR index values. The detection of MDR E. coli in poultry birds points to the widespread misuse and over-reliance on antibiotics, raising serious concerns for both veterinary and human health in the context of antimicrobial resistance. Addressing this issue requires strengthened monitoring efforts, improved diagnostic capabilities, increased involvement of veterinary experts, and the enforcement of stricter regulations regarding antibiotic application in poultry farming practices to help prevent the further spread of MDR pathogens and protect public health. AcknowledgmentsThe authors appreciate the Universitas Airlangga and Ebonyi State University. FundingThe authors would like to thank Ebonyi State University, Abakaliki Nigeria, and Lembaga Penelitian dan Pengabdian Masyarakat Universitas Airlangga, Indonesia, for their support. This study was partly supported by the International Research Consortium, Lembaga Penelitian dan Pengabdian Masyarakat, Universitas Airlangga, Surabaya, Indonesia Year 2025 with grant number: 478/B/UN3.FKH/PL.14.01/2025. Conflict of interestThe authors declare no conflict of interest. Authors’ contributionsENU and AIU: Conceived, designed, and coordinated the study. BNA, BO, and TIN: Designed data collection tools, supervised the field sample and data collection, and laboratory work, as well as data entry. WT, HMR, BB, and MHE: Validation, supervision, and formal analysis. ENU and MHE: Contributed reagents, materials, and analysis tools. ENU, AIU, SR, and MHE: Carried out the statistical analysis, interpretation, and participated in writing and editing the manuscript. All authors have read, reviewed, and approved the final manuscript. Data availabilityAll data were provided in the manuscript. ReferencesAbdel-Tawab, A.A., Ammar, A.M., Nasef, S.A. and Reda, R.M. 2022. Prevalence E. coli in diseased chickens with its antibiogram pattern. Benha Vet. Med. Jour. 28(2), 224–230. Adzitey, F., Assoah-Peprah, P., Teye, G.A., Somboro, A.M., Kumalo, H.M. and Amoako, D.G. 2020. Prevalence and antimicrobial resistance of Escherichia coli isolated from various meat types in the Tamale Metropolis of Ghana. Int. Jour. Food Sci. 2020, 887–896. Agumah, N.B., Effendi, M.H., Tyasningsih, T., Witaningrum, A.M., Ugbo, E.N., Agumah, O.B., Ahmad, R.Z., Nwagwu, C.S., Ugbo, A.I., Khairullah, A.R., Moses, I.B., Yanestria, S.M., Riwu, K.H.P. and Wasito, W. 2025. Distribution of cephalosporin-resistant Campylobacter species isolated from meat sold in Abakaliki, Nigeria. Open Vet. J. 15(4), 1615–1623. Agyare, C., Boamah, V.E., Zumbi, C.N. and Osei, F.B. 2018. Antibiotic use in poultry production and its effects on bacterial resistance. In Antimicrob. Resist. Global Threat. Intech Open. Kumar, Y . Kumasi, Ghana: IntechOpen, 16, pp: 79–88. Ahadini, S.N., Tyasningsih, W., Effendi, M.H., Khairullah, A.R., Kusala, M.K.J., Fauziah, I., Latifah, L., Moses, I.B., Yanestria, S.M., Fauzia, K.A., Kurniasih, D.A.A. and Wibowo, S. 2025. Molecular detection of blaTEM-encoding genes in multidrug-resistant Escherichia coli from cloacal swabs of ducks in Indonesia farms. Open Vet. J. 15(1), 92–97. Ajibola, A.T., De Lagarde, M., Ojo, O.E., Balogun, S.A., Vanier, G., Fairbrother, J.M. and Shittu, O.B. 2025. Antimicrobial resistance and virulence gene profiles of Escherichia coli isolated from poultry farms using One Health perspective in Abeokuta, Nigeria. BMC. Microbiol. 25(1), 239–254. Altalhi, A.D., Gherbawy, Y.A. and Hassan, S.A. 2010. Antibiotic resistance in Escherichia coli isolated from retail raw chicken meat in Taif, Saudi Arabia. Foodborne Path. 7, 281–285. Amadi, V.A., Watson, N., Onyegbule, O.A., Belmar, V., Avendano, E., Tiwari, K., Sharma, R. and Hariharan, H. 2018. Antimicrobial resistance profiles of Escherichia coli recovered from feces of healthy free-range chickens in Grenada, West Indies. Int. J. Microbiol. Appl. Sci. 4(6), 168–175. Andrew Selaledi, L., Mohammed Hassan, Z., Manyelo, T.G. and Mabelebele, M. 2020. The current status of the alternative use to antibiotics in poultry production: an African perspective. Antibiotics 9, 594–602. Aniokette, U., Iroha, C., Ajah, M. and Nwakaeze, A. 2016. Occurrence of multi-drug-resistant Gram-negative bacteria from poultry and poultry products sold in Abakaliki. Jour. Agric. Sci. Food Tech. 2, 119–124. Ansharieta, R., Effendi, M.H. and Plumeriastuti, H. 2021. Genetic identification of Shiga toxin encoding gene from cases of multidrug resistance (MDR) Escherichia coli isolated from raw milk. Trop. Anim. Sci. J. 44(1), 10–15. Aworh, M.K., Kwaga, J., Okolocha, E., Mba, N. and Thakur, S. 2019. Prevalence and risk factors for multi-drug resistant Escherichia coli among poultry workers in the Federal Capital Territory, Abuja, Nigeria. PLos One 14(11), e0225379. Boamah, V.E., Agyare, C., Odoi, H. and Dalsgaard, A. 2016. Practices and factors influencing the use of antibiotics in selected poultry farms in Ghana. J Antimicro. 2(2), 120–127. Bolan, N.S., Szogi, A.A., Chuasavathi, T., Seshadri, B., Rothrock, M.J. and Panneerselvam, P. 2020. Uses and management of poultry litter. World’s. Poultry Sci. Jour. 66, 673–698. Brătfelan, D.O., Tabaran, A., Colobatiu, L., Mihaiu, R. and Mihaiu, M. 2023. Prevalence and antimicrobial resistance of Escherichia coli isolates from chicken meat in Romania. Animals 13, 3488–3496. Bushen, A., Tekalign, E. and Abayneh, M. 2021. Drug- and multidrug-resistance pattern of Enterobacteriaceae isolated from droppings of healthy chickens on a poultry farm in Southwest Ethiopia. Infect. Drug Resist. 14, 2051–2058. Clinical and Laboratory Standards Institute. 2018. M100 performance standards for antimicrobial susceptibility testing. 28th ed. Wayne City, PA: Clinical and Laboratory Standards Institute. Available from: www.clsi.org Ejeh, F.E., Lawan, F.A., Abdulsalam, H., Mamman, P.H. and Kwanashie, C.N. 2017. Multiple antimicrobial resistance of Escherichia coli and Salmonella species isolated from broilers and local chickens retailed along the roadside in Zaria, Nigeria. Sokoto Jour. Vet. Sci. 15(3), 45–53. FAOSTAT. 2021. Food and Agriculture Organization of the United Nations. Available from: http://nigeria.countrystat.org/search-and-visualize-data/en/ (Accessed 8 December 2021). Gupta, C.L., Blum, S.E., Kattusamy, K., Daniel, T., Druyan, S., Shapira, R., Krifucks, O., Zhu, Y.G., Zhou, X.Y., Su, J.Q. and Cytryn, E. 2021. Longitudinal study on the effects of growth-promoting and therapeutic antibiotics on the dynamics of chicken cloacal and litter microbiomes and resistomes. Microbiome 9, 178–185. Hafez, H.M. and Attia, Y.A. 2020. Challenges to the Poultry Industry: current Perspectives and Strategic Future After the COVID-19 Outbreak. Frontier. Vet. Sci. 7, 516–523. Hedman, H.D., Vasco, K.A. and Zhang, L. 2020. A review of antimicrobial resistance in poultry farming within low-resource settings. Animals 10, 264–270. Hochmuth, G., Hochmuth, R. and Mylavarapu, R. 2023. Using composted poultry manure (Litter) in mulched vegetable production. Gainesville, FL: University of Florida, pp. 1–8. Islam, M.S., Sabuj, A.A.M., Haque, Z.F., Pondit, A., Hossain, M.G. and Saha, S. 2020. Seroprevalence and risk factors of avian reovirus in backyard chickens in different areas of Mymensingh district in Bangladesh. J. Adv. Vet. Anim. Res. 7(3), 546–553. Johnson, T.J., Logue, C.M., Johnson, J.R., Kuskowski, M.A., Sherwood, J.S., Barnes, H.J., Debroy, C., Wannemuehler, Y.M., Obata-Yasuoka, M., Spanjaard, L. and Nolan, L.K. 2012. Associations between multidrug resistance, plasmid content, and virulence potential among extraintestinal pathogenic and commensal Escherichia coli from humans and poultry. Foodborne Path. Dis. 9, 37–46. Kendek, I.A., Putri, M.F.R., Wibisono, F.J., Effendi, M.H., Tyasningsih, W., Ugbo, E.N. and Agumah, N.B. 2024. Molecular detection of hlyF gene on multidrug resistance of avian pathogenic Escherichia coli isolated from ducks on wet markets of Surabaya, Indonesia. Biodiversitas 25(3), 1246–1253. Khairullah, A.R., Ramandinianto, S.C. and Effendi, M.H. 2020. A review of livestock-associated methicillin-resistant Staphylococcus aureus (LA-MRSA) on bovine mastitis. Syst. Rev. Pharm. 11(7), 172–183. Khan, M.S., Muhammad, N.A., Haque, E., Barua, A., Chowdhury, T., Mullick, R. and Mahmud, A.M. 2024. Isolation and identification of non-plasmid multidrug resistant E. coli from poultry wastes in Chittagong region, Bangladesh. Jour. Bacteriol. Parasitol. 5(1), 76–85. Khater, D.F., Lela, R.A., El-Diasty, M., Moustafa, S.A. and Wareth, G. 2021. Detection of harmful foodborne pathogens in food samples at the points of sale by MALDT-TOF MS in Egypt. BMC Res. Notes 14(112), 1–6. Le, T.H., Ng, C., Chen, H., Yi, X.Z., Koh, T.H., Barkham, T.M.S., Zhou, Z. and Gin, K.Y.H. 2016. Occurrences and characterization of antibiotic-resistant bacteria and genetic determinants of hospital wastewater in a tropical country. Antimicrob. Agents Chem. 60(12), 7449–7456. Magiorakos, A.P., Srinivasan, A., Carey, R.B., Carmeli, Y., Falagas, M.E., Giske, C.G., Harbarth, S., Hindler, J.F., Kahlmeter, G., Olsson-Liljequist, B., Paterson, D.L., Rice, L.B., Stelling, J., Struelens, M.J., Vatopoulos, A., Weber, J.T. and Monnet, D.L. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Eur. Soc. Clin. Microbiol. Infect. Dis. 18, 268–281. Mensah, G.I., Adjei, V.Y., Vicar, E.K., Atsu, P.S., Blavo, D.L., Johnson, S.A.M. and Addo, K.K. 2022. Safety of retailed poultry: analysis of antibiotic resistance in Escherichia coli from raw chicken and poultry fecal matter from selected farms and retail outlets in Accra, Ghana. Microbiol. Insights 15, 1–5. Mtonga, S., Nyirenda, S.S. and Mulemba, S.S. 2021. Epidemiology and antimicrobial resistance of pathogenic E. coli in chickens from selected poultry farms in Zambia. Jour. Zoonotic Dis. 4, 18–28. Ngogang, M.P., Ernest, T., Kariuki, J., Mouliom Mouiche, M.M., Ngogang, J., Wade, A. and Van Der Sande, M.A.B. 2021. Microbial contamination of chicken litter manure and antimicrobial resistance threat in an urban area setting in Cameroon. Antibiotics 10, 20. Pradika, A.Y., Chusniati, S., Purnama, M.T.E., Effendi, M.H., Yudhana, A. and Wibawati, P.A. 2019. Total test of Escherichia coli on fresh cow milk at dairy farmer cooperative (KPSP) Karyo Ngremboko Purwoharjo Banyuwangi. J. Med. Vet. 2(1), 1–6. Putra, A.R.S., Effendi, M.H., Koesdarto, S. and Tyasningsih, W. 2019. Molecular identification of extended spectrum beta-lactamase (ESBL) producing Escherichia coli isolated from dairy cows in East Java Province, Indonesia. Indian Vet. J. 96(10), 26–30. Putri, M.F.R., Kendek, I.A., Wibisono, F.J., Effendi, M.H., Rahardjo, D., Tyasningsih, W. and Ugbo, E.N. 2023. Molecular detection of iron gene on multidrug resistant avian fecal Escherichia coli isolated from broiler on traditional markets, Surabaya, Indonesia. Biodiversitas 24(12), 6454–6460. Rafif Khairullah, A., Rehman, S., Agus Sudjarwo, S., Helmi Effendi, M., Chasyer Ramandinianto, S., Aega Gololodo, M., Widodo, A., Hendriana Priscilia Riwu, K. and Ayu Kurniawati, D. 2022. Detection of mecA gene and methicillin-resistant Staphylococcus aureus (MRSA) isolated from milk and risk factors from farms in Probolinggo, Indonesia. F1000Research 11(1), 722. Raji, M., Adekeye, J., Kwaga, J., Bale, J. and Henton, M. 2018. Serovars and biochemical characterization of Escherichia coli isolated from colibacillosis cases and dead-in-shell embryos in poultry in Zaria-Nigeria. Vet. Archiv. 77(6), 495–505. Rasmussen, M.M., Opintan, J.A., Frimodt-Møller, N. and Styrishave, B. 2015. Beta-lactamase producing Escherichia coli isolates in imported and locally produced chicken meat from Ghana. PLos One 10, e0139706. Roth, N., Käsbohrer, A., Mayrhofer, S., Zitz, U., Hofacre, C. and Domig, K.J. 2019. The application of antibiotics in broiler production and the resulting antibiotic resistance in Escherichia coli: a global overview. Poultry Sci. 98, 1791–1804. Saud, B., Paudel, G., Khichaju, S., Bajracharya, D., Dhungana, G., Awasthi, M.S. and Shrestha, V. 2019. Multidrug-resistant bacteria from raw meat of buffalo and chicken, Nepal. Vet. Med. Int. 2019, 7960268. Ugbo, E.N., Effendi, M.H., Witaningrum, A.M., Tyasningsih, W., Agumah, B.N., Ugbo, A.I., Nnabugwu, C.C. and Okata-Nwali, D.O. 2023a. Antimicrobial resistance pattern of Salmonella spp. isolated from poultry farms in Abakaliki, Nigeria. Biodiversitas 24(9), 5207–5214. Ugbo, E.N., Jacob, J.I., Effendi, M.H., Witaningrum, A.M., Agumah, B.N., Ugbo, A.I. and Moses, B.I. 2023b. Poultry slaughterhouse wastewater as reservoirs for spreading extended-spectrum beta-lactamase-producing Escherichia coli in Abakaliki, Nigeria. Biodiversitas 24(9), 4960–4966. Viegas, C., Carolino, E., Malta-Vacas, J., Sabino, R., Viegas, S. and Veríssimo, C. 2021. Fungal contamination of poultry litter: a public health problem. J. Toxicol. Environ. Health Part A 75, 1341–1350. Wibisono, F.J., Sumiarto, B., Untari, T., Effendi, M.H., Permatasari, D.A. and Witaningrum, A.M. 2020. The presence of extended-spectrum beta-lactamases (ESBL) producing Escherichia coli on layer chicken farms in Blitar Area, Indonesia. Biodiversitas 21(6), 2667–2671. Widodo, A., Lamid, M., Effendi, M.H., Khairullah, A.R., Riwu, K.H.P., Yustinasari, L.R., Kurniawan, S.C., Ansori, A.N.M., Silaen, O.S.M. and Dameanti, F.N.A.E.P. 2022. Antibiotic sensitivity profile of multidrug-resistant (MDR) Escherichia coli isolated from dairy cow’s milk in Probolinggo, Indonesia. Biodiversitas 23(10), 4971–4976. Yanestria, S.M., Dameanti, F.N.A.E.P., Musayannah, B.G., Pratama, J.W.A., Witaningrum, A.M., Effendi, M.H. and Ugbo, E.N. 2022. Antibiotic resistance pattern of Extended-Spectrum β-Lactamase (ESBL) producing Escherichia coli isolated from a broiler farm environment in Pasuruan district, Indonesia. Biodiversitas 23(9), 4460–4465. Yanestria, S.M., Effendi, M.H., Tyasningsih, W., Mariyono, M. and Ugbo, E.N. 2023. First report of phenotypic and genotypic (blaOXA-61) betalactam resistance in Campylobacter jejuni from broilers in Indonesia. Vet. World 16(11), 2210–2216. Yunita, M.N., Effendi, M.H., Rahmaniar, R.P., Arifah, S. and Yanestria, S.M. 2020. Identification of spa gene for strain typing of methicillin resistant Staphylococcus aureus (MRSA) isolated from nasal swab of dogs. Biochem. Cellular Arch. 20(1), 2999–3004. Zalewska, M., Błazejewska, A., Czapko, A. and Popowska, M. 2021. Antibiotics and antibiotic resistance genes in animal manure: consequences of its application in agriculture. Front. Microbiol. 12, 610–656. | ||
| How to Cite this Article |
| Pubmed Style Ugbo AI, Nnenwa TI, Ugbo EN, Effendi MH, Oke B, Agumah BN, Raharjo HM, Tyasningsih W, Budiastuti B, Rehman S. Characterization of antimicrobial-resistant Escherichia coli from poultry birds in Ebonyi North, Nigeria. Open Vet. J.. 2025; 15(9): 4097-4105. doi:10.5455/OVJ.2025.v15.i9.14 Web Style Ugbo AI, Nnenwa TI, Ugbo EN, Effendi MH, Oke B, Agumah BN, Raharjo HM, Tyasningsih W, Budiastuti B, Rehman S. Characterization of antimicrobial-resistant Escherichia coli from poultry birds in Ebonyi North, Nigeria. https://www.openveterinaryjournal.com/?mno=258928 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i9.14 AMA (American Medical Association) Style Ugbo AI, Nnenwa TI, Ugbo EN, Effendi MH, Oke B, Agumah BN, Raharjo HM, Tyasningsih W, Budiastuti B, Rehman S. Characterization of antimicrobial-resistant Escherichia coli from poultry birds in Ebonyi North, Nigeria. Open Vet. J.. 2025; 15(9): 4097-4105. doi:10.5455/OVJ.2025.v15.i9.14 Vancouver/ICMJE Style Ugbo AI, Nnenwa TI, Ugbo EN, Effendi MH, Oke B, Agumah BN, Raharjo HM, Tyasningsih W, Budiastuti B, Rehman S. Characterization of antimicrobial-resistant Escherichia coli from poultry birds in Ebonyi North, Nigeria. Open Vet. J.. (2025), [cited January 25, 2026]; 15(9): 4097-4105. doi:10.5455/OVJ.2025.v15.i9.14 Harvard Style Ugbo, A. I., Nnenwa, . T. I., Ugbo, . E. N., Effendi, . M. H., Oke, . B., Agumah, . B. N., Raharjo, . H. M., Tyasningsih, . W., Budiastuti, . B. & Rehman, . S. (2025) Characterization of antimicrobial-resistant Escherichia coli from poultry birds in Ebonyi North, Nigeria. Open Vet. J., 15 (9), 4097-4105. doi:10.5455/OVJ.2025.v15.i9.14 Turabian Style Ugbo, Agatha Ifunanya, Theophilus Ikechukwu Nnenwa, Emmanuel Nnabuike Ugbo, Mustofa Helmi Effendi, Boniface Oke, Bernard Nnabuife Agumah, Hartanto Mulyo Raharjo, Wiwiek Tyasningsih, Budiastuti Budiastuti, and Saifur Rehman. 2025. Characterization of antimicrobial-resistant Escherichia coli from poultry birds in Ebonyi North, Nigeria. Open Veterinary Journal, 15 (9), 4097-4105. doi:10.5455/OVJ.2025.v15.i9.14 Chicago Style Ugbo, Agatha Ifunanya, Theophilus Ikechukwu Nnenwa, Emmanuel Nnabuike Ugbo, Mustofa Helmi Effendi, Boniface Oke, Bernard Nnabuife Agumah, Hartanto Mulyo Raharjo, Wiwiek Tyasningsih, Budiastuti Budiastuti, and Saifur Rehman. "Characterization of antimicrobial-resistant Escherichia coli from poultry birds in Ebonyi North, Nigeria." Open Veterinary Journal 15 (2025), 4097-4105. doi:10.5455/OVJ.2025.v15.i9.14 MLA (The Modern Language Association) Style Ugbo, Agatha Ifunanya, Theophilus Ikechukwu Nnenwa, Emmanuel Nnabuike Ugbo, Mustofa Helmi Effendi, Boniface Oke, Bernard Nnabuife Agumah, Hartanto Mulyo Raharjo, Wiwiek Tyasningsih, Budiastuti Budiastuti, and Saifur Rehman. "Characterization of antimicrobial-resistant Escherichia coli from poultry birds in Ebonyi North, Nigeria." Open Veterinary Journal 15.9 (2025), 4097-4105. Print. doi:10.5455/OVJ.2025.v15.i9.14 APA (American Psychological Association) Style Ugbo, A. I., Nnenwa, . T. I., Ugbo, . E. N., Effendi, . M. H., Oke, . B., Agumah, . B. N., Raharjo, . H. M., Tyasningsih, . W., Budiastuti, . B. & Rehman, . S. (2025) Characterization of antimicrobial-resistant Escherichia coli from poultry birds in Ebonyi North, Nigeria. Open Veterinary Journal, 15 (9), 4097-4105. doi:10.5455/OVJ.2025.v15.i9.14 |