| Research Article | ||
Open Vet. J.. 2025; 15(9): 4090-4096 Open Veterinary Journal, (2025), Vol. 15(9): 4090-4096 Research Article Distribution of androgen receptor immunoreactive cells in the adrenal glands of Hystrix javanicaTeguh Budipitojo1*, Arinda Devi Larasati2, Sekar Arum Krisna Putri2, Woro Danur Wendo1 and Yuda Heru Fibrianto31Department of Anatomy, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia 2Sains Veterinary Graduate School, Universitas Gadjah Mada, Yogyakarta, Indonesia 3Department of Physiology, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia *Corresponding Author: Teguh Budipitojo. Department of Anatomy, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Yogyakarta, Indonesia. Email: budipitojo [at] ugm.ac.id Submitted: 16/05/2025 Revised: 01/08/2025 Accepted: 17/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
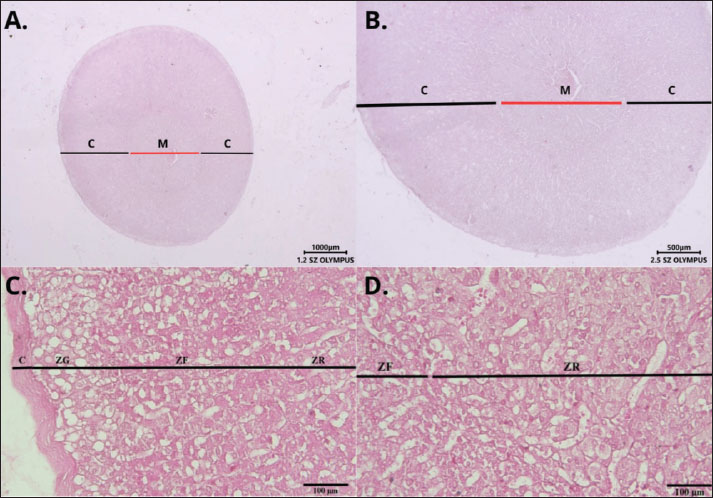
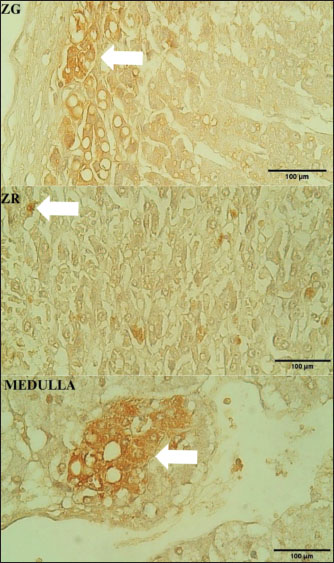
ABSTRACTBackground: The adrenal glands play a vital role in regulating endocrine functions by producing corticosteroids, mineralocorticoids, and androgens. Adrenal androgens contribute to overall systemic androgen levels, primarily through interactions with androgen receptors (ARs) within the gland. Although the distribution of AR has been studied in various mammalian species, no research has yet focused on the Sunda porcupines (Hystrix javanica), a species of considerable ecological importance in Indonesia. Aim: This study aimed to investigate the endocrinal regulation of the adrenal gland of Sunda porcupines (H. javanica) by examining the distribution of AR-immunoreactive (AR-IR) cells. Methods: This study was conducted at the Laboratory of Anatomy, Faculty of Veterinary Medicine, Universitas Gadjah Mada, Indonesia, from January to August 2022. The research involved six adrenal glands sourced from Sunda porcupines (H. javanica), without regard to sex or age. A sample size of six Sunda porcupines is acceptable for the initial descriptive analysis. Normal tissue specimens were collected and subsequently fixed in Bouin’s solution for 24 hours. Tissues were then processed and embedded in paraffin blocks. Histological evaluation was performed in routine staining, and immunohistochemistry (IHC) was performed to detect AR expression. In the IHC procedure, a primary antibody against the AR, produced in rabbits and polyclonal in nature, which specifically binds to the activated form of the receptor, was used. The localization and distribution of AR-IR cells were assessed descriptively across different zones of the adrenal gland cortex, namely, the glomerulosa (ZG), fasciculata (ZF), and reticularis (ZR), including the medulla. Results: AR-IR cells were predominantly localized in the ZG, with a smaller presence observed in the ZF of the adrenal cortex. No AR expression was detected in the ZR. Interestingly, AR-IR cells were also detected in the adrenal medulla. Conclusion: This study indicates species-specific distribution patterns of AR-IR cells, which may clarify the role of ARs in physiological adaptation of Sunda porcupines (H. javanica). Keywords: Adrenal gland, Androgen receptor, Androgen receptor-immunoreactive cells, Adrenal androgen, Sunda porcupines. IntroductionIn most vertebrates, including wild animals, the adrenal glands are typically paired endocrine organs located adjacent to the kidneys (Grossman, 2024; Jennings and Premanandan, 2025). The morphology and function of adrenal glands can vary significantly among different animal classes (Barszcz et al., 2016; Santos et al., 2016). The gland comprises two primary regions: the cortex and the medulla. The outer adrenal cortex, which is responsible for producing steroid hormones, is further divided into three distinct zones: the ZG, which produces mineralocorticoids (e.g., aldosterone) to regulate electrolyte and fluid balance. The ZF, which synthesizes glucocorticoids (e.g., cortisol), plays a crucial role in metabolism and stress response. The ZR is responsible for the secretion of sex steroid hormones, including androgens (Grossman, 2024). The inner adrenal medulla secretes catecholamines (e.g., epinephrine and norepinephrine), which are vital for the acute stress response of the body (Jennings and Premanandan, 2025). Among the hormones produced by the adrenal cortex, adrenal androgens, such as dehydroepiandrosterone (DHEA) and androstenedione, serve as precursors for more potent sex steroids, including testosterone and estrogen (Dumontet and Martinez, 2021). The biological effects of these adrenal-derived androgens are mediated through the androgen receptor (AR). AR expression is present in various zones of the adrenal cortex. The findings demonstrate that AR expression directly contributes to the local regulation of adrenal function (Gannon et al., 2019). Androgen signaling via AR plays a crucial role in the development of male-specific traits during embryonic growth. It also supports spermatogenesis, regulates sexual behavior, and maintains fertility throughout adulthood. Interestingly, this signaling pathway is also essential for the development and functioning of female reproductive organs, influencing processes such as ovarian folliculogenesis, embryo implantation, and uterine and mammary gland maturation (Chang et al., 2013). AR activation in the adrenal gland begins with the binding of androgens, primarily testosterone and dihydrotestosterone, to the AR (Dart et al., 2013). This receptor, a ligand-dependent nuclear transcription factor, is expressed in all adrenal cortex zones in both male and female mammals. AR activation in the adrenal gland is essential for maintaining hormonal balance and overall adrenal health (Gannon et al., 2019). Given the important roles of AR in adrenal physiology, investigating how the adrenal glands are organized across different species, particularly in the context of their functional adaptations, is essential. Several studies have explored the morphology of adrenal glands across a range of species, including both domestic and wild animals. Extensive research has focused on domestic species such as cattle (Jelinek and Konecny, 2011) and the one-humped camel (Camelus dromedarius) (Abdalla and Ali, 1988), as well as wild ruminants such as the European bison (Barszcz et al., 2016), and other wild animals such as the mongoose (Herpestes auropunctatus) (Tomich, 1965). Despite these efforts, studies focusing specifically on the adrenal gland morphology in the Sunda porcupines (Hystrix javanica) are still lacking. Although direct investigations on the adrenal glands of porcupines remain limited, related studies on rodents, such as guinea pigs (Cavia porcellus), have provided valuable insights. The adrenal glands in these animals display structural adaptations in response to stress, with exposure to stressors inducing notable morphological changes in the adrenal cortex (Santos et al., 2016). These findings emphasize the crucial role of the adrenal gland in environmental adaptation. Based on the general organization of mammalian adrenal glands, the structure in porcupines is expected to follow a similar basic pattern, with specific modifications reflecting their unique ecological pressures. Studies on closely related species demonstrate that porcupines possess adrenal characteristics adapted to their environmental demands. In this context, the ecological significance of the Sunda porcupine (H. javanica), a rodent species endemic to Indonesia, should be considered. Sunda porcupines (H. javanica) play a crucial role in maintaining the ecosystem’s balance and sustainability. They contribute to seed dispersal, which is vital for forest regeneration and plant diversity preservation. In addition, their digging and foraging activities position them as actantial ecosystem engineers. This complex ecological contribution makes the Sunda porcupine a keystone species that deserves attention in conservation efforts aimed at preserving biodiversity (Louw et al., 2019; Farida et al., 2022). Characterizing AR expression in H. javanica adrenal glands provides fundamental knowledge of endocrine adaptations in wild mammals facing natural and anthropogenic pressures. Since the Sunda porcupine (H. javanica) is classified as a species of Least Concern, it is important to expand our understanding of its physiology. To the best of our knowledge, the present study represents the first report describing the distribution of AR-immunoreactive (AR-IR) cells within the adrenal gland of Sunda porcupines (H. javanica), providing foundational information for future research into the species’ endocrine adaptations. Materials and MethodsThis study was conducted from January to August 2022 at the Laboratory of Anatomy within the Faculty of Veterinary Medicine at Universitas Gadjah Mada. The subjects of the study consisted of six adrenal glands extracted from Sunda porcupines (H. javanica), regardless of sex or age. These specimens were sourced from porcupine hunters in the Ngawi region of East Java, Indonesia. The sample size in this study was determined based on ethical considerations regarding the use of animals in research and the limited availability of Javan porcupine (H. javanica) specimens in their natural habitat. Furthermore, the sample size was deemed adequate to describe the observed anatomical and histological structures because this study was descriptive and did not involve inferential statistical analysis. The number of animal samples followed the requirements in qualitative exploratory studies without strict statistical rules as in experimental or quantitative studies. A sample size of six Sunda porcupine is acceptable for the initial descriptive analysis (Dunk, 2007; Fischer et al., 2008). The glands were collected and fixed in Bouin’s solution for 24 hours. After fixation, the samples were trimmed, placed in tissue cassettes, and labeled. The tissues were then processed for Hematoxylin-Eosin (H&E) staining (Alturkistani et al., 2015). A polyclonal rabbit primary antibody anti-AR (bs-12471R, Bioss Antibodies, USA) at a 1:100 dilution was used for immunohistochemistry (IHC) evaluation. Additional reagents included the Starr Trek Universal HRP® kit, aquadest, PBS solution, and Canada balsam. For immunohistochemical staining, tissue slides were deparaffinized using xylene and rehydrated using alcohol. To block endogenous peroxidase activity, the slides were immersed in 3% H2O2 in methanol for 20–30 minutes and then incubated with Biocare’s Background Sniper® for 20–30 minutes to prevent non-specific antibody reactions. Slides were incubated with the AR primary antibodies overnight at 4°C. Trekkie Universal Link® secondary antibodies were added to bind the primary antibodies and incubated for 20–30 minutes. After that, slides were incubated with Trek Avidin-HRP® for 20–30 minutes, and 3-3’-Diaminobenzidine chromogen was applied to visualize immunoreactive cells. Harris hematoxylin solution was used for the staining. The slides were then washed with PBS and rinsed with distilled water, dehydrated through a graded series of alcohol, cleared with xylene, and mounted using Canada balsam. We observed the distribution of AR-IR cells using a descriptive method. Six slides were examined under a light microscope (BX51, Olympus, Tokyo, Japan) and a stereo binocular microscope (SZ51, Olympus, Tokyo, Japan) at various magnifications to identify the distribution of AR-IR cells based on their histological and morphological characteristics. This observation aims to detect the localization of the distribution of AR-IR cells in the adrenal glands of Sunda porcupines (H. javanica). The observation results were documented by taking microscopic images using the AmScope HD310 Microscope Camera attached to the microscope. Ethical approvalSample collection was conducted under the ethical clearance number 326/KEC-LPPT/IX/2022. ResultsThe adrenal glands of H. javanica are small, typically triangular-shaped organs at the kidney cranial poles. Each gland is encapsulated by a fibrous capsule and consists of two distinct parts: the adrenal cortex and medulla (Fig. 1A and B). The adrenal cortex in H. javanica is divided into three main zones: ZG, located in the outermost layer of the adrenal cortex, just beneath the fibrous capsule; ZF, positioned in the middle of the adrenal cortex beneath ZG; and ZR, situated adjacent to the adrenal medulla, which is the innermost and largest layer of the adrenal cortex. The adrenal medulla is composed of large cells with polygonal or polyhedral shapes, arranged in groups and separated by blood capillaries. The cells in the medulla are eosinophilic, appear reddish on eosin staining, and contain clear cytoplasmic granules (Fig. 1C and D). Immunohistochemical analysis revealed cells that are immunoreactive to AR-IR in the adrenal glands of H. javanica. The distribution of AR-IR cells is most localized in the ZG. Immunoreactivity was also detected in the ZF, although the number was less than that in the ZG. In addition, strong AR-IR reactivity was observed in the adrenal medulla, indicating that cells in the medulla also significantly express ARs (Fig. 2).
Fig. 1. Histological structure of the adrenal gland of Hystrix javanica stained with hematoxylin and eosin (H&E), observed under light microscopy at 12× (A); 25× (B), and 400× (C and D). Transverse section of the adrenal gland divided into the cortex (C) and medulla (M). The cortex comprises the ZG, ZF, and ZR, which border the medulla. The gland is enclosed by a dense connective tissue capsule (C). Scale bar: 100, 500, and 1,000 µm.
Fig. 2. Distribution of AR immunoreactive cells in Hystrix javanica adrenal glands. White arrows indicate immunoreactive cells in the ZG, ZF, and medulla at 400× magnification. Scale bar: 100 µm. DiscussionThis study has several limitations, notably the small sample size (n=6), which was primarily constrained by ethical considerations and the limited availability of Sunda porcupine (H. javanica) specimens, owing to their conservation status. The statistical power of the findings may be restricted, and the results should be interpreted with caution. Future research involving larger sample sizes or comparative analyses across different populations or related species is recommended to validate and expand upon these findings. Nonetheless, this study offers a valuable preliminary account of the distribution of ECs in the Sunda porcupine. The inclusion of the key components of the endocrine system in the adrenal glands represents a significant aspect of this investigation and provides a foundation for more comprehensive future analyses. The adrenal gland, also referred to as the suprarenal gland, is a vital endocrine organ located superior to each kidney. In mammals, including Sunda porcupines (H. javanica), the adrenal glands are encapsulated by thin fibrovascular connective tissue and are structurally divided into two main regions: the cortex and the medulla. The adrenal cortex consists of three distinct zones with well-defined functional roles: the ZG, ZF, and ZR. This zoning is not only structural but also has specialized and dynamic endocrine functions, including the involvement of systemic and local hormone signals and the actantial influence of AR. The ZG is the outermost layer of the adrenal cortex, which is histologically characterized by the arrangement of small cells in a circular formation, similar to the glomeruli (Vinson, 2016). The role of AR in this zone can affect the regulation of mineralocorticoid secretions, maintaining the structural and functional integrity of ZG (Beéry et al., 2003; Benmouloud et al., 2014). Studies in animal models, such as sand rats, have shown that AR activation can suppress ZG cell hypertrophy and reduce lipid accumulation. This effect is thought to occur through the interaction of AR with angiotensin II signaling pathways (Benmouloud et al., 2014). Thus, AR not only acts as a local regulator but also as a stable custodian of mineralocorticoid production, such as aldosterone, which is crucial in maintaining blood pressure and electrolyte balance. This influence indicates that androgens have a broader physiological role through AR, not only in reproductive function but also in the regulation of the cardiovascular system and the homeostasis of body fluids. The ZF is the largest layer of the adrenal cortex and is composed of large cells containing many lipids arranged in a radial column that extends toward the medulla. This zone is responsible for the synthesis of glucocorticoids, such as cortisol (in humans) and corticosterone (in mice), and may play a role in adrenal development (Vinson, 2016; Dutt et al., 2025; Gannon et al., 2019; Dutt et al., 2025;). In terms of androgen influence, AR in ZF plays a role in modulating glucocorticoid synthesis and regulating apoptosis. AR activation can suppress hyperactivity due to excessive ACTH stimulation, thereby preventing the excess glucocorticoid production that can result in metabolic and immune disorders. Studies in sand rats have shown that castration induces increased apoptosis of ZF cells, but testosterone administration can restore this condition to its normal state (Benmouloud et al., 2014). This confirms that androgens play a protective role in the normal continuity and function of ZF. Thus, AR helps maintain a balance between cell proliferation and death in ZF, which is important in the body’s response to stress and the maintenance of the circadian rhythm of glucocorticoid hormones. The innermost layer, the ZR, is the deepest layer of the adrenal cortex and is characterized by an arrangement of small cells that form a mesh-like tissue (reticulum). This zone produces adrenal androgens, such as DHEA and androstenedione. Histology revealed cells with active steroidogenic organelles but lower lipid content than ZF. These cells secrete adrenal androgens, including DHEA and androstenedione, which are converted to testosterone in the peripheral tissues. Although the adrenal gland contributes approximately 1% to small circulating testosterone levels in men, it accounts for 30%–50% of the total androgen in women, underscoring the important role of the adrenal glands in endocrine regulation in women (Turcu et al., 2015; Vinson, 2016). The adrenal medulla is made up of chromaffin cells that come from the neural crest and produce catecholamines, such as adrenaline and noradrenaline. The sympathetic nervous system directly innervates these cells, which mediates a rapid response to stress (Vinson, 2016). Although AR expression in the medulla is not yet fully understood, androgens may affect medulla function through non-genomic signaling pathways. AR is thought to contribute to the modification of the sensitivity of chromaffin cells to sympathetic stimuli and possibly affect the capacity of catecholamine synthesis during stressful conditions (Lagunas et al., 2022). More research is needed to clarify this role in the context of mammalian physiology as a whole. In a study of the Sunda porcupines (H. javanica), there were interesting differences compared to other mammalian general patterns, especially related to the expression of ARs. Specifically, in most mammals, AR is typically distributed in all three zones of the adrenal cortex and in the medulla. However, in the Sunda porcupines (H. javanica), IHC staining results showed that AR expression was found in ZG, ZF, and medulla but not in ZR. Among the three zones that exhibit AR expression, ZG has the most potent immunoreactivity, followed by ZF, which has lower immunoreactivity. In addition, AR was clearly detected in the adrenal medulla. This pattern suggests that the cells in these zones can respond to androgens. The absence of AR in ZR may also indicate that adrenal androgen production in Sunda porcupines (H. javanica) is not dependent on local androgen signaling via AR or may be replaced by other regulatory mechanisms that are not yet fully understood. Some studies have stated that AR expression is generally more dominant in ZG and ZF, as well as in transient zones such as the X-zone in rodents. AR is clearly detected in ZG and ZF, but little or no detection at all in ZR (Gannon et al., 2019; Gannon et al., 2021). The finding demonstrates the specificity of the zonal distribution of AR and its role in the synthesis of mineralocorticoids and glucocorticoids, rather than in the production of adrenal androgens. Several reasons can explain the absence of AR in the reticular zone, both in Sunda porcupines (H. javanica) and in other species. First, ZR can have very low levels of AR gene expression or AR protein production to the point that it is below the threshold of IHC detection, so it technically appears undetectable (Trejter et al., 2015). Second, differences in ZR development between species complicate interpretation (Dumontet and Martinez, 2021). ZR develops after birth, and its shape varies greatly between mammals. In some species, including rodents, ZR is often not clearly defined and even appears to merge with ZF, which can lead to inconsistent AR expression patterns (Mitani et al., 1999; Gannon et al., 2019). The distribution pattern and AR expression levels tend to show sex-dependent variations. In general, AR expression in the adrenal glands of male animals is higher than that in females, as observed in studies conducted in mice. This difference in expression is also related to the expression of other steroid hormone receptors, such as estrogen receptors (ERα and ERβ), which exhibit distinct expression profiles between males and females (Bentvelsenj et al., 2015; Lagunas et al., 2022). The unclear sex of the specimens in this study is one of the limitations that need to be considered based on the literature. Sex uncertainty can affect the interpretation of results related to the distribution of AR-IR cells in the adrenal glands, as sex differences can cause significant biological variations in the expression and distribution of ARs. Therefore, in future studies, ensuring the accurate identification of the sex of the specimens and considering comparative analysis between males and females to gain a more comprehensive understanding of the role of androgens and their distribution in the adrenal glands are essential. Differences in network regulatory mechanisms and network-specific expressions also play a role. Various local factors control AR expression, including the presence of specific transcription factors and co-regulators. If ZR does not have a transcription device or a molecular environment that supports AR expression, it is not surprising that AR is undetectable. In addition, the main function of ZR is not to respond to androgens but, rather, to synthesize androgen precursors such as DHEA and androstenedione. Therefore, AR may not be functionally necessary in this zone (Bird, 2012; Nicolaides et al., 2023). This study highlights the importance of comparative approaches in the study of adrenal anatomy and physiology and emphasizes the need for further exploration of molecular expression and hormonal regulatory pathways across species. Differences such as those found in Sunda porcupines (H. javanica) not only reflect evolutionary adaptations but also provide insights into the biological diversity of the endocrine system among mammals. This diversity is essential to continue exploring, as it can provide new insights into how hormonal regulatory mechanisms, including AR expression, vary across species. Cross-species studies that combine aspects of anatomy, histology, and molecular expression are expected to elucidate the relationship between each animal’s structure, function, and biological adaptation. Future research development in molecular approaches, such as RNA sequencing, is needed to map AR gene expression in each zone of the adrenal cortex. This approach is essential for identifying actantial variations in genetic expression that cannot be observed using IHC alone. In addition, the use of an in vitro culture system can serve as an alternative to test the functional response of adrenal cells to androgen stimulation, especially in the reticular zone, which, in this study, showed AR expression that warrants further functional investigation. Future studies involving transcriptome analysis, cell culture-based functional assays, and comparative analysis with other mammalian species that exhibit different ecological characteristics will enrich our understanding of endocrine adaptation mechanisms and provide a more comprehensive contribution to animal physiology and wildlife conservation. ConclusionThis study showed that AR-IR cells were detected in the adrenal glands of Sunda porcupines (H. javanica), especially in the layers of the cortex, namely the ZG and ZF, including the medulla. The distribution patterns of these receptors show variations between layers, indicating the possible role of different ARs in each adrenal zone’s physiological function. This study also highlighted the species-specific distribution patterns of AR-IR cells, which may clarify the role of ARs in the physiological adaptation of Sunda porcupines (H. javanica). AcknowledgmentsThe authors would like to thank Universitas Gadjah Mada for their support through the research grant “The Program Asistensi Riset Tahun Anggaran 2024” Nomor: 10541/UN1.P4/PT.01.03/2024. Conflict of interestThe authors declare that there were no financial or commercial relationships that could be construed as a potential conflict of interest during the research. FundingThe author acknowledges the financial support received for authorship and publication from the research grant “The Program Asistensi Riset Tahun Anggaran 2024” of Universitas Gadjah Mada (number: 10541/UN1. P4/PT.01.03/2024). Authors’ contributionsTB contributed to the research design and manuscript development and supervised the research process. ADL, SAKP, WDW, and YHF were responsible for sample collection and data analysis. ADL, SAKP, and WDW prepared the manuscript following a critical analysis of the histology data. All authors have read and approved the final version of the manuscript. Data availabilityThe original data and contributions from this study are available in the article and/or supplementary materials. Further inquiries should be directed to the corresponding author. ReferencesAbdalla, M.A. and Ali, A.M. 1988. Morphometric and histological studies on the adrenal glands of the camel Camelus dromedarius. Acta Morphol. Neerlando-Scand. 26(4), 269–281. Alturkistani, H.A., Tashkandi, F.M. and Mohammedsaleh, Z.M. 2015. Histological stains: a literature review and case study. Global J. Health Sci. 8(3), 72–79; doi:10.5539/gjhs.v8n3p72 Barszcz, K., Przespolewska, H., Olbrych, K., Czopowicz, M., Klećkowska-Nawrot, J., Goździewska-Harłajczuk, K. and Kupczyńska, M. 2016. The morphology of the adrenal gland in the European bison (Bison bonasus). BMC Vet. Res. 12(1), 1–11; doi:10.1186/s12917-016-0783-8 Beéry, E., Middel, P., Bahn, A., Willenberg, H.S., Hagos, Y., Koepsell, H., Bornstein, S.R., Müller, G.A., Burckhardt, G. and Steffgen, J. 2003. Molecular evidence of organic ion transporters in the rat adrenal cortex with adrenocorticotropin-regulated zonal expression. Endocrinology 144(10), 4519–4526; doi:10.1210/en.2002-221001 Benmouloud, A. and Z. Amirat, F. Khammar, A. V. Patchev, J. M. Exbrayat,, O. F. X. Almeida. 2014. Androgen receptor-mediated regulation of adrenocortical activity in Psammomys obesus, a sand rat. J. Comp. Physiol. B. Biochem. Syst. Environ. Physiol. 184(8), 1055–1063; doi:10.1007/s00360-014-0859-3 Bentvelsen, F.M., McPhaul, M.J., Wilson, C.M., Wilson, J.D. and George, F.W. 1996. Regulation of immunoreactive androgen receptor in the adrenal gland of the adult rat. Endocrinology 137(7), 2659–2663; doi: 10.1210/endo.137.7.8770883. Bird, I.M. 2012. In the zone: understanding zona reticularis function and its transformation by adrenarche. J. Endocrinol. 214(2), 109–111; doi:10.1530/JOE-12-0246 Chang, C., Lee, S.O., Wang, R.S., Yeh, S. and Chang, T.M. 2013. Androgen receptor (AR) physiological roles in male and female reproductive systems: lessons learned from AR-Knockout mice lacking AR in selective cells. Biol. Reprod. 89(1), 1–16; doi:10.1095/biolreprod.113.109132 Dart, D.A., Waxman, J., Aboagye, E.O. and Bevan, C.L. 2013. Visualising androgen receptor activity in male and female mice. PLoS. One 8(8), e71694; doi:10.1371/journal.pone.0071694 Dumontet, T. and Martinez, A. 2021. Adrenal androgens, adrenarche, and zona reticularis: a human affair? Mol. Cell Endocrinol. 528, 111239; doi: 10.1016/j.mce.2021.111239 Dunk, L. 2007. Managing the laboratory. In Bancroft’s theory and practice of histological techniques, 7th ed. Eds., Suvarna, S.K. Layton, C. and Bancroft, J.D. Nottingham, UK: Churchill Livingstone (Elsevier); doi: 10.1016/B978-0-7020-4226-3.00001-9 Dutt, M., Wehrle, C.J. and Jialal, I. 2025. Physiology, adrenal gland. Adrenal Gland 1(1), 1–8. Farida, W.R., Sari, A.P., Sofyani, U. and Amalia, R.L.R. 2022. Growth performance and prediction of carcass production of captive Javan porcupine (Hystrix javanica). E3S Web of Conferences 335, 00023; doi: 10.1051/e3sconf/202233500023 Fischer, A.H., Jacobson, K.A., Rose, J. and Zeller, R. 2008. Hematoxylin and eosin staining of tissue and cell sections. Cold Spring Harbor Protocols 2008(5, 4986–4988), pdb.prot4986–pdb.prot4988; doi:10.1101/pdb.prot4986 Gannon, A.L., O’Hara, L., Mason, J.I., Jørgensen, A., Frederiksen, H., Milne, L., Smith, S., Mitchell, R.T. and Smith, L.B. 2019. Androgen receptor in the male adrenal X-zone regression, cell turnover and protects against. Sci. Rep. 9(1), 1–16; doi:10.1038/s41598-019-46049-3 Gannon, A.-L., O’Hara, L., Mason, I.J., Jørgensen, A., Frederiksen, H., Curley, M., Milne, L., Smith, S., Mitchell, R.T. and Smith, L.B. 2021. Androgen receptor is dispensable for x-zone regression in the female adrenal but regulates post-partum corticosterone levels and protects cortex integrity. Front. Endocrinol. 11, 599869; doi: 10.3389/fendo.2020.599869 Grossman, A.B. 2024. Overview of the Merck manual, pp. 1–3. Available via http://www.merckmanuals.com/home/hormonal-and-metabolic-disorders/adrenal-gland-disorders/overview-of-the-adrenal glands Jelinek, F. and Konecny, R. 2011. Adrenal glands of slaughtered bulls, heifers, and cows: a histological study. J. Vet. Med. Ser. C. Anatomia. Histol. Embryol. 40(1), 28–34; doi:10.1111/j.1439-0264.2010.01034.x Jennings, R. and Premanandan, C. 2025. Veterinary histology. Ohio State University. Available via https://ohiostate.pressbooks.pub/vethisto/chapter/adrenal-gland/ Lagunas, N., Fernández-García, J.M., Blanco, N., Ballesta, A., Carrillo, B., Arevalo, M.A., Collado, P., Pinos, H. and Grassi, D. 2022. Organizational effects of estrogens and androgens on estrogen and androgen receptor expression in the pituitary and adrenal glands of adult male and female rats. Front. Neuroanatomy 16, 902218; doi:10.3389/fnana.2022.902218 Louw, M.A., Haussmann, N.S. and Le Roux, P.C. 2019. Testing for consistency in the impact of a burrowing ecosystem engineer on soil and vegetation characteristics across biomes. Sci. Rep. 9(1), 1–12; doi:10.1038/s41598-019-55917-x Mitani, F., Mukai, K., Miyamoto, H., Suematsu, M. and Ishimura, Y. . 1999. Development of functional zonation in the rat adrenal cortex. Endocrinology 140(7), 3342–3353; doi:10.1210/endo.140.7.6859 Nicolaides, N.C., Willenberg, H.S., Bornstein, S.R. and Chrousos, G.P. 2023. Adrenal cortex: embryonic development, anatomy, histology and physiology. Endotext 450, 1–8. Santos., dos, A.C., Viana, D.C., Leandro, R.M., Rodrigues, R.F., Assis-Neto, A.C. and Melo, A.P.F. 2016. Morphology of the adrenal glands of the giant anteater (Myrmecophaga tridactyla, Linnaeus, 1758) of wild life. Bioscience J. 1559, 1559–1566; doi:10.14393/bj-v32n6a2016-32810 Tomich, . andP. Q.. 1965. Weight variation in adrenal glands of the mongoose in Hawaii. Pacific Sci. 19(2), 238–243; . Trejter, M., Jopek, K., Celichowski, P., Tyczewska, M., Malendowicz, L.K. and Rucinski, M. 2015. Expression of estrogen, estrogen related and androgen receptors in adrenal cortex of intact adult male and female rats. Folia Histochem. Cytobiol. 53(2), 133–144; doi:10.5603/FHC.a2015.0012 Turcu, A., Smith, J.M., Auchus, R., Rainey, W.E., Arbor, A., Physiology, I. and Arbor, A. 2015. HHS public access. Cphy 4(4), 1369–1381; doi:10.1002/cphy.c140006 Vinson, G.P. 2016. Functional zonation of the adult mammalian adrenal cortex. Front. Neurosci. 10, 238; doi:10.3389/fnins.2016.00238 | ||
| How to Cite this Article |
| Pubmed Style Budipitojo T, Larasati AD, Putri SAK, Wendo WD, Fibrianto YH. Distribution of androgen receptor immunoreactive cells in the adrenal glands of Hystrix javanica. Open Vet. J.. 2025; 15(9): 4090-4096. doi:10.5455/OVJ.2025.v15.i9.13 Web Style Budipitojo T, Larasati AD, Putri SAK, Wendo WD, Fibrianto YH. Distribution of androgen receptor immunoreactive cells in the adrenal glands of Hystrix javanica. https://www.openveterinaryjournal.com/?mno=258727 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i9.13 AMA (American Medical Association) Style Budipitojo T, Larasati AD, Putri SAK, Wendo WD, Fibrianto YH. Distribution of androgen receptor immunoreactive cells in the adrenal glands of Hystrix javanica. Open Vet. J.. 2025; 15(9): 4090-4096. doi:10.5455/OVJ.2025.v15.i9.13 Vancouver/ICMJE Style Budipitojo T, Larasati AD, Putri SAK, Wendo WD, Fibrianto YH. Distribution of androgen receptor immunoreactive cells in the adrenal glands of Hystrix javanica. Open Vet. J.. (2025), [cited January 25, 2026]; 15(9): 4090-4096. doi:10.5455/OVJ.2025.v15.i9.13 Harvard Style Budipitojo, T., Larasati, . A. D., Putri, . S. A. K., Wendo, . W. D. & Fibrianto, . Y. H. (2025) Distribution of androgen receptor immunoreactive cells in the adrenal glands of Hystrix javanica. Open Vet. J., 15 (9), 4090-4096. doi:10.5455/OVJ.2025.v15.i9.13 Turabian Style Budipitojo, Teguh, Arinda Devi Larasati, Sekar Arum Krisna Putri, Woro Danur Wendo, and Yuda Heru Fibrianto. 2025. Distribution of androgen receptor immunoreactive cells in the adrenal glands of Hystrix javanica. Open Veterinary Journal, 15 (9), 4090-4096. doi:10.5455/OVJ.2025.v15.i9.13 Chicago Style Budipitojo, Teguh, Arinda Devi Larasati, Sekar Arum Krisna Putri, Woro Danur Wendo, and Yuda Heru Fibrianto. "Distribution of androgen receptor immunoreactive cells in the adrenal glands of Hystrix javanica." Open Veterinary Journal 15 (2025), 4090-4096. doi:10.5455/OVJ.2025.v15.i9.13 MLA (The Modern Language Association) Style Budipitojo, Teguh, Arinda Devi Larasati, Sekar Arum Krisna Putri, Woro Danur Wendo, and Yuda Heru Fibrianto. "Distribution of androgen receptor immunoreactive cells in the adrenal glands of Hystrix javanica." Open Veterinary Journal 15.9 (2025), 4090-4096. Print. doi:10.5455/OVJ.2025.v15.i9.13 APA (American Psychological Association) Style Budipitojo, T., Larasati, . A. D., Putri, . S. A. K., Wendo, . W. D. & Fibrianto, . Y. H. (2025) Distribution of androgen receptor immunoreactive cells in the adrenal glands of Hystrix javanica. Open Veterinary Journal, 15 (9), 4090-4096. doi:10.5455/OVJ.2025.v15.i9.13 |