| Research Article | ||
Open Vet. J.. 2025; 15(9): 4617-4634 Open Veterinary Journal, (2025), Vol. 15(9): 4617-4634 Research Article Phytogenic feed additive derived from Peronema canescens leaves: Bioactive profile, effects on broiler growth, carcass yield, blood biochemistry, and molecular interactionsWiwaha Anas Sumadja1*, Filawati Filawati1, Ilham Ifandi Ramadhan2, Nadia Rahmasari2 and Indra Lasmana Tarigan21Department of Animal Husbandry, Faculty of Animal Science, Universitas Jambi, Indonesia 2Department of Chemistry, Faculty of Science and Technology, Universitas Jambi, Jambi, Indonesia *Corresponding Author: Wiwaha Anas Sumadja. Department of Animal Sciences, Faculty of Animal Sciences, Universitas Jambi, Indonesia. Email: wiwahasumadja [at] unja.ac.id Submitted: 12/05/2025 Revised: 30/07/2025 Accepted: 17/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
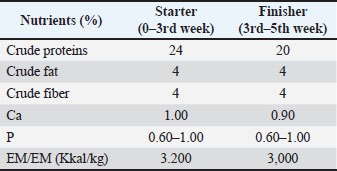
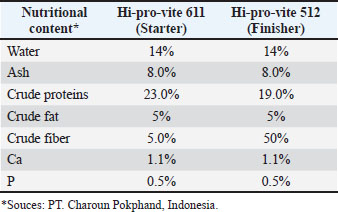
AbstractBackground: Several bioactive compounds of Peronema canescens Jack, including flavonoids, saponins, and phenolic constituents, exert anti-inflammatory, antimicrobial, antioxidant, and immunomodulatory effects. Understanding these active ingredients is essential because their combined metabolic and immune-modulating properties may influence broiler health, feed efficiency, and overall productivity. Aim: This study investigated the potential of P. canescens Jack (P. canescens) leaf infusion as a phytogenic feed additive for broiler chickens, evaluating its effects on growth performance, carcass characteristics, blood biochemical parameters, and its molecular interaction with inflammatory markers. Methods: Broilers were administered with varying concentrations of infusion over a 35-day period (a 3-day ON and 2-day OFF pattern). Performance metrics, such as feed intake, body weight gain, feed conversion ratio (FCR), carcass yield, and organ weights, were measured. Blood samples were analyzed for lipid profiles and hematological responses. Additionally, the phytochemical composition of the infusion was profiled, and selected bioactive compounds were subjected to molecular docking against inducible nitric oxide synthase (iNOS). Results: The result indicated P. canescens infusion did not significantly (p > 0.05) alter primary growth parameters; however, a biologically relevant improvement in FCR was observed at lower doses. We observed trends toward reduced abdominal fat, triglyceride, and low-density lipoprotein levels, accompanied by increased gizzard weight and lymphocyte counts, suggesting potential benefits for both digestive function and immune modulation. Molecular docking analysis showed that flavonoid and vanillyl derivatives from P. canescens exhibited moderate to strong binding affinities for the iNOS active site, indicating that enzyme inhibition could contribute to anti-inflammatory effects. Conclusion: Peronema canescens leaf infusion demonstrates promise as a natural feed additive with mild physiological effects and bioactive constituents capable of modulating inflammatory pathways. Future research involving standardized extracts and mechanistic studies is recommended to optimize its efficacy and application in poultry nutrition. Keywords: Bioactive compounds, Chicken broilers, Feed additive, Peronema canescens Jack. IntroductionThe poultry industry, particularly broiler chicken production, continues to expand in response to the growing demand for high-quality animal protein (Korver, 2023). However, improving production efficiency remains a key strategic challenge in modern poultry farming. Concerns surrounding the monitoring of growth performance, feed efficiency, carcass quality, and the physiological health of broiler chickens—without reliance on synthetic antibiotics—underscore the need for alternative approaches that are both environmentally friendly and sustainable (Teymouri et al., 2021; Wickramasuriya et al., 2024). These efforts extend beyond nutritional optimization and include the reduction or elimination of antibiotic growth promoters, which have been banned in many countries due to their contribution to AMR. The use of phytogenics—bioactive compounds derived from plants that serve as natural feed or water additives in animal husbandry—is a promising alternative (Zavyalov et al., 2022; Basiouni et al., 2023). Among the potential candidates, Peronema canescens Jack (commonly known as sungkai), a native Indonesian plant, has shown promise due to its rich content of active compounds, such as flavonoids, saponins, and tannins (Latief et al., 2021; Tarigan et al., 2022). Sungkai leaves (P. canescens Jack), a medicinal plant indigenous to Indonesia, are rich in secondary metabolites, including alkaloids, flavonoids, phenolics, polyphenols, terpenoid-steroids, saponins, and tannins (Latief, et al., 2021; Dillasamola, 2024). These leaves exhibit various bioactivities, such as antimalarial, antipyretic, antiplasmodial, antioxidant, antibacterial, antimicrobial, and anti-inflammatory effects (Shalihin et al., 2024). Key bioactive constituents—particularly flavonoids, saponins, and tannins—that hold promise for enhancing livestock health have been identified in several studies. Peronema canescens Jack (P. canescens) leaves are abundant in Indonesia and can be sourced sustainably from local cultivation. This availability supports their potential application in the poultry industry to help improve broiler productivity and contribute to safer, antibiotic-reduced production systems. However, the application of P. canescens leaf infusion in poultry production, especially as a phytogenic additive in drinking water, remains limited and warrants further scientific investigation. Herbal-derived flavonoids modulate energy metabolism by activating the adenosine monophosphate-activated protein kinase (AMPK) pathway, which plays a crucial role in suppressing lipogenesis, enhancing fatty acid oxidation, and improving blood lipid profiles (Al-ishaq et al., 2019). Saponins have hypocholesterolemic properties by inhibiting lipid absorption in the intestine and promoting sterol excretion (Jeepipalli et al., 2020; Cao et al., 2024). Meanwhile, low concentrations of tannins have been reported to enhance gizzard motility and suppress the proliferation of pathogenic microbes in the gastrointestinal tract (De la Mora et al., 2020; Gazwi et al., 2022). However, the effectiveness of these bioactive compounds is highly dependent on formulation, stability, and dosage. Although the infusion form is practical and easy to apply in the field, it often presents challenges in terms of the stability and bioavailability of active compounds compared with concentrated extracts or microencapsulated forms (Bhalani et al., 2022; Paul et al., 2025). Therefore, although the administration of P. canescens leaf infusion has not yet shown statistically significant effects on production performance and physiological parameters in broiler chickens, its therapeutic potential warrants further investigation because of several positive biological indicators, such as improved feed conversion efficiency, reduced fat accumulation, and enhanced blood lipid profiles. This study aimed to evaluate the potential of P. canescens leaf infusion in drinking water on broiler chicken production performance, digestive organ characteristics, meat chemical composition, and blood profile. Specifically, we assessed the bioadditive potential of P. canescens leaf infusion as a natural phytogenic in drinking water for broiler chicken. The parameters under investigation include growth performance, carcass quality, blood biochemical profile, and the molecular modeling of its bioactive compounds interacting with biological targets through molecular docking studies. Furthermore, this research seeks to characterize the chemical composition and distribution of bioactive compounds present in P. canescens leaf infusion, analyze changes in production performance and carcass components, and determine the effects of the infusion on the average weight of digestive organs. Additional objectives include evaluating meat composition and assessing the lipid profile—specifically, triglyceride, cholesterol, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) levels—in the blood of 35-day-old broiler chickens. Finally, molecular docking analysis of selected ligands against inducible nitric oxide synthase (iNOS) was conducted to explore potential mechanisms of action at the molecular level. This integrative approach is expected to offer a comprehensive understanding of the mechanisms and benefits of using P. canescens leaf infusion in modern, antibiotic-free poultry production systems. The findings of this study are anticipated to provide a scientific foundation for the use of P. canescens leaves as natural phytobiotics and to support the development of feed additives based on sustainable, locally sourced materials. Ultimately, this research aims to contribute to the advancement of safe, effective, and environmentally friendly bio-additive strategies that promote healthy and sustainable poultry production while supporting the achievement of several Sustainable Development Goals (SDGs), including SDG 2 (zero hunger) through enhanced food security, SDG 3 (good health and well-being) via improved animal and human health, SDG 12 (responsible consumption and production) by encouraging sustainable use of natural resources, and SDG 9 (industry, innovation, and infrastructure) through the development of innovative, science-based agricultural solutions. Materials and MethodsChemicals and equipmentThe materials used in this study included day-old chicks (DOC), P. canescens leaf extract (sourced from the Educational Forest of Universitas Jambi), distilled water, basal feed rations, Newcastle Disease vaccine, disinfectants, and reagents required for hematological analyses, such as erythrocyte count, leukocyte count, and differential leukocyte profiles. Blood samples were collected using blood collection tubes containing ethylenediaminetetraacetic acid (EDTA) (Sigma-Aldrich, USA) as an anticoagulant. The equipment used in this study comprised a broiler chicken housing facility with a capacity of 20 litter cages (1.0 × 1.0 × 0.7 m), feeding and drinking containers, incandescent lamps, wood shavings (as litter material), weighing scales, buckets, tarpaulins, room thermometers, ropes, cleaning tools (brooms), and slaughter instruments. Blood samples were collected using 3-ml sterile syringes and EDTA-coated vacutainer tubes. For hematological analysis, flow cytometry instruments (BD FACSCanto II, Becton Dickinson, USA; equipped with three lasers and eight-color detection) and automated hematology analyzers (Sysmex XN-1000, Sysmex Corporation, Japan) were used. Additionally, chemical profiling of P. canescens leaf infusion was performed using liquid chromatography-mass spectrometry (LC-MS) with a Thermo Scientific™ Q Exactive™ Hybrid Quadrupole-Orbitrap™ Mass Spectrometer (Thermo Fisher Scientific Inc., Waltham, MA). Molecular docking simulations were performed on laptops equipped with Intel® Core™ i7 processors, 4 GB RAM, and Windows 10 operating systems. The software used for molecular docking and analysis included Discovery Studio Visualizer v20.1.0.19295 (BIOVIA), MGLTools 1.5.6 (Scripps Research Institute), comprising AutoDock Tools and AutoDock Vina, PyMOL (DeLano Scientific LLC), and publicly available databases such as the Protein Data Bank (https://www.rcsb.org/), UniProt, and PubChem (https://pubchem.ncbi.nlm.nih.gov/). Extraction and bioactive profiling (BPP) Fresh P. canescens leaves were obtained from the Educational Forest of Universitas Jambi. The leaves were collected, thoroughly washed, and air-dried at room temperature for 5 days. The dried material was then ground into a coarse powder. A 100-g portion of the powdered leaves was weighed and mixed with 1 l of water (ratio 1:10, w/v). Then, the mixture was heated at 60°C for 20 minutes. The decoction was then filtered through a clean cloth to obtain the P. canescens leaf infusion. The resulting filtered infusion was stored in a clean, airtight container to maintain stability. The prepared P. canescens leaf infusion was then added to the broiler drinking water according to the respective treatment groups (Williams et al., 2024). The infusion extract was analyzed using LC-MS. The chromatographic data, in the form of a peak height plot, were processed using MassLynx software to determine the molecular weight of the compounds present in the extract (Latief et al., 2024). Infusion preparation The drinking water was administered following the respective treatment protocols. The provision of treatment water followed a 3-day ON and 2-day OFF pattern, based on a previous study that showed that the continuous administration of herbal plants as feed additives may negatively affect nutrient absorption in the digestive tract (Hehanussa et al., 2022; Shittu et al., 2022). The intermittent administration of P. canescens leaf infusion was intended to allow the physiological functions of the broilers to use the extract more effectively. Moreover, this schedule was designed as a precautionary measure against the potentially high tannin content in the extract, which impairs protein digestibility and subsequently affects growth performance in poultry (Xu et al., 2025). Ration formulationThe commercial feed used in this study consisted of Hi-Pro-Vite 611 for chickens aged 1–3 weeks and Hi-Pro-Vite 512 for chickens aged 4–5 weeks. Table 1 outlines the nutrient requirements for the rations utilized in this study, while Table 2 presents the nutritional composition of the commercial feed. Experimental designThis study employed a Completely Randomized Design (CRD) with four treatments and five replications, each consisting of 10 chickens, resulting in 200 chickens. The treatments were as follows: P0 (0% P. canescens leaf infusion in drinking water/control), P1 (1.5% P. canescens leaf infusion in drinking water), P2 (2.0% P. canescens leaf infusion in drinking water), and P3 (2.5% P. canescens leaf infusion in drinking water). TreatmentsThe 200- DOC were randomly allocated into 20 litter cage units (1.0 × 1.0 × 0.7 m; length × width × height), with 10 chicks per unit serving as an experimental replicate. All cages were thoroughly cleaned and disinfected prior to chick placement. The cleaning procedure included disinfectant spraying inside and around each cage, followed by drying. Once dry, lime was applied to the floor surfaces and left in place for 1 week before the chicks arrived. The feeding and drinking containers were cleaned and installed before placement, after which lamps were set up and litter was provided in each cage. The litter material consisted of wood shavings (sawdust) to maintain dryness and ensure comfort throughout the rearing period. The chickens were provided with ad libitum feed and drinking water according to the treatment protocol. Specifically, water administration followed a schedule of 3 days on and 2 days off. During the on phase (three consecutive days), the chickens received drinking water mixed with P. canescens leaf infusion. During the off phase (two consecutive days), the patients were provided with regular drinking water without infusion. The chickens were provided with feed ad libitum and water according to the treatment protocol, with a 3-day ON and 2-day OFF schedule. The rearing period lasted for 35 days (Tahamtani et al., 2020). Biochemical profileThe observed variables included production performance, carcass components, abdominal fat, digestive organs, meat chemical composition, blood lipid profile, and blood profile. Blood samples were collected from 30-day-old broiler chickens. A random sample of one chicken was obtained from each replication unit. Blood was collected using a syringe from the brachial vein located beneath the wing. The blood samples were collected into 3 ml blood collection tubes, with approximately 2 ml of blood placed into tubes containing EDTA to prevent coagulation (Osadcha et al., 2023). The samples were then stored for subsequent analysis of erythrocyte and leukocyte numbers, and differential leukocyte numbers were analyzed using flow cytometry instruments (BD FACSCanto II, 3-laser, 8-color) and automated analyzers (Sysmex XN-1000) at the Provincial Health Laboratory of Jambi. Molecular dockingThe ligand structures were prepared and energy-minimized using PyRx software, while the macromolecule (receptor) was obtained from the RCSB Protein Data Bank (rscb.org) and subsequently optimized using AutoDock Tools. Molecular docking was performed using AutoDock Tools, where both the receptor and ligand structures—previously optimized independently—were placed in a single working directory for docking simulation. Following the docking process, the resulting ligand-receptor interactions were analyzed through a visualization step to observe binding poses and hydrogen bond formations. This interaction analysis was conducted using Discovery Studio Visualizer 2021, which provided detailed insight into the molecular interactions within the docking complexes (Latief et al., 2024). Data analysisThe obtained data were analyzed using a one-way analysis of variance (ANOVA) under a CRD. Duncan’s multiple range test was applied for post hoc comparisons to determine significant differences among treatment means. The results are shown in Table 1. Linear and quadratic trends of P. canescens leaf infusion treatments were assessed through polynomial contrasts within the ANOVA framework. All statistical analyses were conducted using IBM SPSS Statistics version 26.0 (IBM Corp., Armonk, NY). Differences were considered statistically significant at p < 0.05 (Xu et al., 2025). Ethical approvalAll experimental procedures involving animals were conducted in accordance with the ethical standards approved by the Ethical Clearance Committee for Animal Research, Faculty of Animal Sciences, University of Jambi (Ref. 03/U21.7/ECC/2024). Table 1. Nutritional needs of broiler chickens.
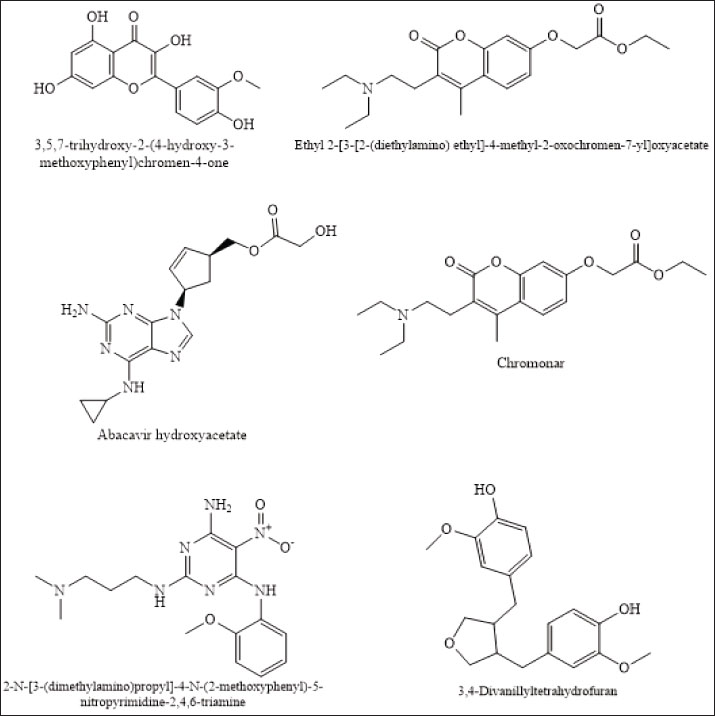
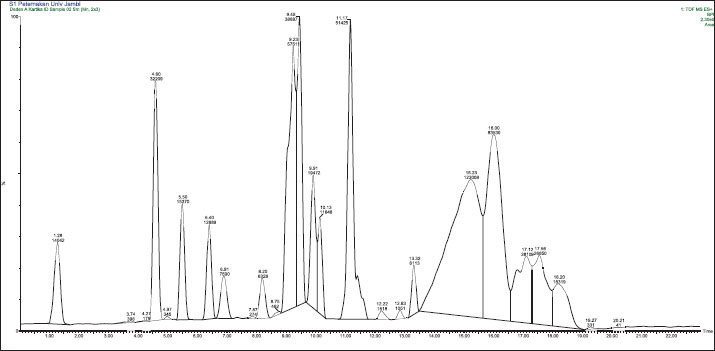
ResultsChemical composition and distribution in P. canescens leaf infusionBPP of P. canescens Jack leaf infusions across treatment levels (P1–P3) revealed a complex array of secondary metabolites, reflecting differences in extraction efficiency and compound stability, as shown by the LC-MS spectra in Figure S1–S3. Notably, P1 exhibited a narrower chemical diversity, with 13 identifiable compounds, including C20H33NO12 and C20H27NO5, whereas unique molecules, such as C57H105NO6 and C11H22N4O4, emerged, potentially indicating the formation of degradation products or novel oligomeric structures at higher concentrations. The recurrence of nitrogen- and oxygen-rich compounds across treatments suggests the presence of alkaloids, flavonoids, and peptide derivatives. For example, CH7N7—a low-molecular-weight nitrogenous compound consistently observed in P1 and P2—may contribute to antioxidant or antimicrobial activity. Conversely, P2 demonstrated the highest prevalence of oxygenated compounds, such as C20H33NO12 and C12H26N6O5, likely representing polyphenolic or glycosidic constituents. The shared presence of C23H26N6O12 and C20H33NO12 across treatments suggests continuity in the bioactive profile at moderate levels of infusion. Conversely, P3 displayed greater overall diversity (18 compounds), with CH7N7 disappearing, indicating potential thermal or chemical disappearing, indicating potential thermal or chemical instability under more intense extraction conditions. The distribution of chemical compounds among P1–P3 is presented in Table S1. Among the identified compounds, six major ones are likely to play a significant role in broiler feed efficiency (Fig. 1). The observed transition and loss of specific molecules at higher concentrations imply degradation or solubility limits, potentially diminishing bioavailability. This corresponds with performance outcomes, where higher infusion levels did not enhance broiler feed efficiency and, in some cases, negatively impacted growth—likely due to reduced beneficial bioactives or increased antinutritional factors. Table 2. Nutritional content of commercial rations.
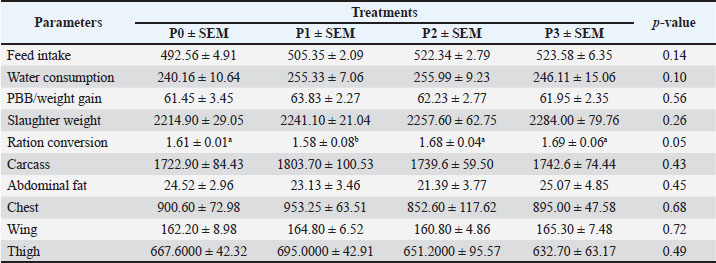
Table 3. Average performance, carcass percentage, abdominal fat percentage, and commercial cut percentage of chickens.
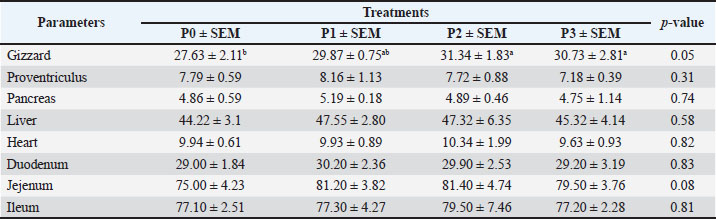
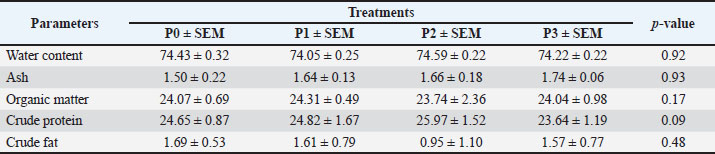
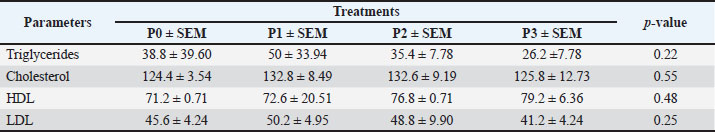
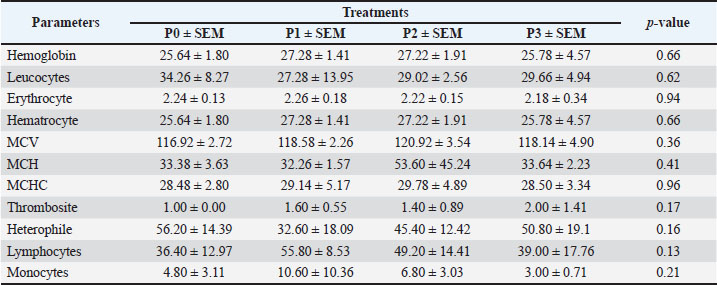
Changes in performance and carcass componentsTable 3 shows the average performance indicators, carcass percentage, abdominal fat content, and commercial carcass parts of 35-day-old broiler chickens given drinking water with P. canescens leaf infusion at different levels. The findings presented in Table 3 demonstrate that the administration of P. canescens leaf infusion in drinking water did not produce significant effects (p > 0.05) on feed intake, water intake, body weight gain (BWG), slaughter weight, carcass yield, abdominal fat, or the proportions of commercial carcass parts such as breast, wing, and thigh. The absence of significant results may be attributed to two primary factors: the potentially low concentration of active compounds entering the birds’ systems and the insufficient administration period to stimulate a pronounced physiological response. Effects of infusion on the average weight of the digestive organsThe data presented in Table 4 illustrate the effects of P. canescens leaf infusion on the average weight of digestive organs, indicating a range of physiological responses depending on the level of infusion provided through drinking water. The most notable effect was observed in the gizzard, which showed a significant increase (p > 0.05) in weight from the control group (P0) to P2, peaking at 31.34 ± 1.83 g before slightly decreasing in P3. This suggests that moderate levels of P. canescens leaf infusion may stimulate mechanical digestive activity, possibly due to the presence of bioactive compounds such as flavonoids and tannins. Meat compositionTable 5 shows the results of the average chemical composition of 35-day-old broiler chicken breast meat given drinking water with P. canescens leaf infusion at different levels. The results of this study indicate that the administration of P. canescens leaf infusion did not exert a statistically significant effect (p > 0.05) on the proximate composition of broiler chicken breast meat, including moisture, ash, organic matter, crude protein, and crude fat contents. The absence of significant variation suggests that the bioactive constituents of P. canescens leaves did not substantially affect the biochemical profile of muscle tissue at the administered dosage and in the infusion form used. This stability may indicate the inherent resilience of muscle metabolism to dietary modulation under non-stress conditions. Lipid profileTable 6 presents the average results of the triglyceride, cholesterol, HDL, and LDL levels in the blood of 35-day-old broiler chickens, which were provided with drinking water containing P. canescens leaf infusion at different concentrations. These values represent the biochemical parameters that were monitored to assess the impact of the P. canescens leaf infusion on lipid metabolism and cardiovascular health in broiler chickens. The analysis of these blood parameters provides valuable insights into the potential therapeutic effects of P. canescens leaf infusion in regulating lipid profiles, which could contribute to improving poultry health and performance. The findings describe the lipid profiles of 35-day-old broiler chickens administered with P. canescens leaf infusion at varying concentrations through drinking water. Blood profile of broiler chickens infused with P. canescens leafTable 7 presents the average blood profile results of 35-day-old broiler chickens, which were provided with drinking water containing P. canescens leaf infusion at varying concentrations. These blood profiles include key parameters such as red and white blood cell counts, hemoglobin levels, and hematocrit values, which are critical indicators of the chicks’ overall health and physiological status. This study aimed to evaluate the potential effects of P. canescens leaf infusion on the hematological health of broiler chickens, offering insights into its possible benefits as a natural supplement in poultry farming. The hematological profile of broiler chickens at 35 days of age reflects the physiological response to the administration of P. canescens leaf infusion at varying concentrations in their drinking water (Table 6). Table 4. Average digestive organs of commercial broiler chickens.
Table 5. Average chemical composition of chicken breast meat.
Table 6. Average levels of TG, cholesterol, HDL, and LDL in the blood of 35-day-old broiler chickens.
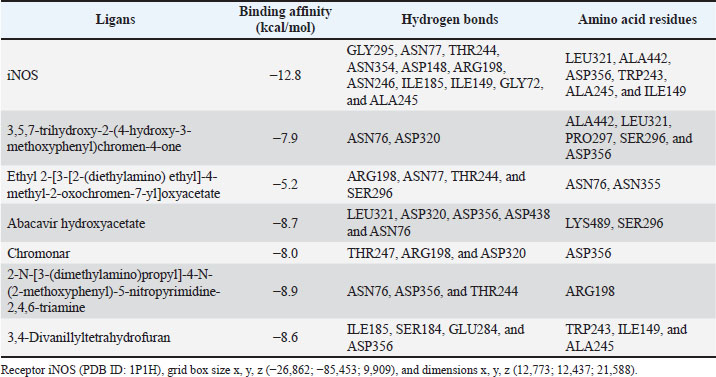
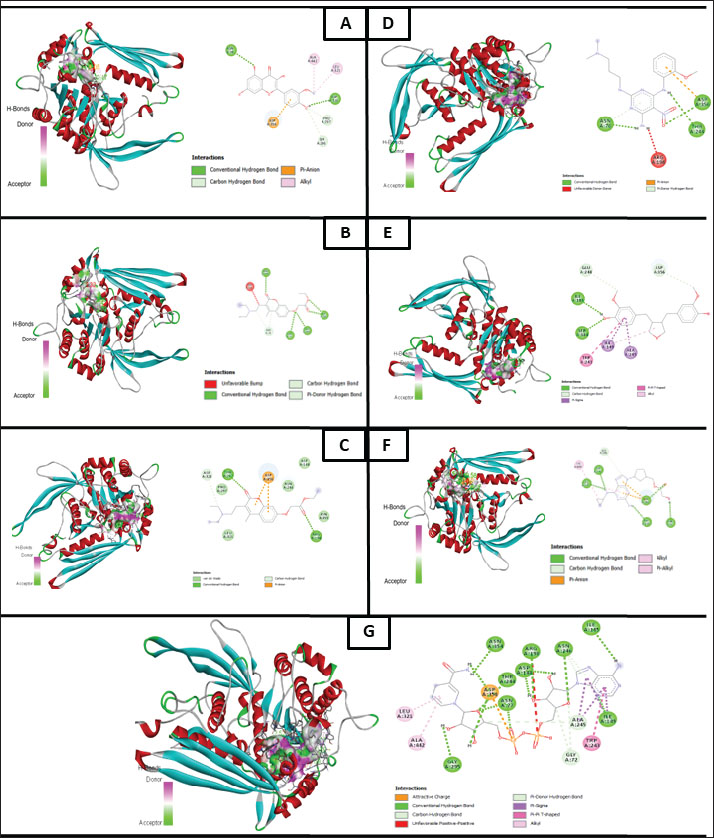
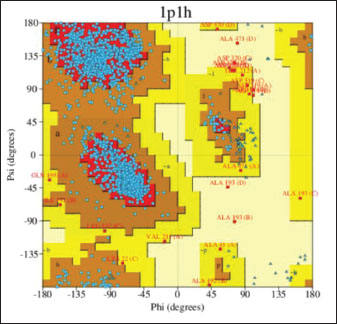
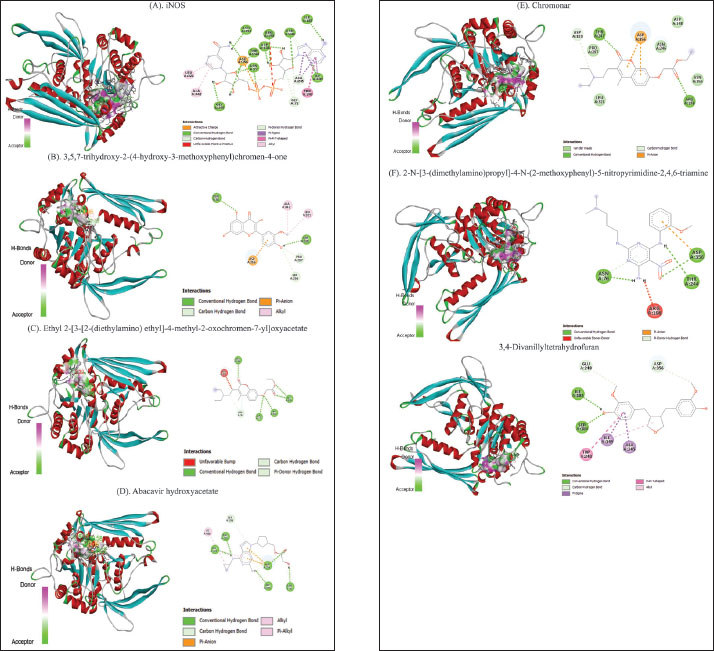
Molecular docking analysis of selected iNOS ligandsMolecular docking analysis was performed on six potential compounds identified in P1—3, namely:3,5,7-trihydroxy-2-(4-hydroxy-3-methoxyphenyl)chromen-4-one, Ethyl 2-[3-[2-(diethylamino)ethyl]-4-methyl-2-oxochromen-7-yl]oxyacetate, abacavir hydroxyacetate, and abacavir hydroxyacetate. Chromonar, 3,4-Divanillyltetrahydrofuran, and 2-N-[3-(dimethylamino)propyl]-4-N-(2-methoxyphenyl)-5-nitropyrimidine-2,4,6-triamine. These compounds were screened using Lipinski’s Rule of Five and were found to meet the ADMET profiles (Tables S2 and S3). Based on the Ramachandran Plot for the 1P1H receptor (Fig. S4), approximately 85% of residues fall within the most favored regions, indicating good conformational stability. Only 0.3% of residues are in the disallowed regions, indicating that the protein structure has good stereochemical quality and is suitable for molecular docking studies (Fig. S5). Simulation results of test ligand tethering and comparative ligand obtained bond energy values (ΔG) from the most stable iNOS ligand-receptor interactions (Table 8). Visualization of iNOS ligand-receptor interactions (Figs. 2 and S6) shows residues from iNOS receptors that play an essential role in the binding site area. DiscussionChanges in performance and carcass components treated by infusionP. canescens leaves are known to contain a range of bioactive compounds, including flavonoids, saponins, and tannins, which demonstrate antioxidant, antimicrobial, and metabolic-regulating activities (Zhao et al., 2020; Shalihin et al., 2024). However, these phytochemicals are only effective within specific dosage thresholds. For instance, dietary flavonoids in the range of 100–300 mg/kg feed have been shown to improve feed efficiency and BWG in broiler chickens by activating the AMPK signaling pathway and modulating gut microbiota (Wang et al., 2021). Flavonoid-mediated AMPK activation promotes energy metabolism and reduces fat accumulation by downregulating lipogenic genes such as ACC and FAS while upregulating CPT1, a critical enzyme in fatty acid oxidation (Ye et al., 2023; Moon, 2024). Although abdominal fat reductions were not statistically significant in this study, the observed downward trend points to a potential biological impact consistent with these mechanisms. Table 7. Average blood profile of 35-day-old broiler chickens.
Table 8. Binding affinity of the six potential compounds.
Tannins also exert dose-dependent effects. At low dietary concentrations (<0.5%), tannins can enhance gut health by suppressing pathogenic bacteria, but excessive intake (>0.5%–1%) may cause antinutritional effects, such as protein and mineral binding and digestive enzyme inhibition (Nath et al., 2022). This is aligned with the present finding that higher infusion levels (P2 and P3) worsened the feed conversion ratio (FCR), likely reflecting tannin-induced digestive inefficiencies.
Fig. 1. The six major compounds in P1–P3. Similarly, saponins in the range of 50–150 mg/kg feed can reduce serum cholesterol and improve digestive processes, whereas higher concentrations (>300 mg/kg) have been associated with intestinal irritation and decreased feed intake (Francis et al., 2002; Cheok et al., 2014). The saponin content may have been suboptimal or excessive because the P. canescens infusion used here was neither standardized nor purified, contributing to the inconsistent effects observed. The limited performance improvements noted in this study are likely attributable to the low concentrations of active compounds or to the instability of phytochemicals during infusion preparation (Abd El-Hack et al., 2018, 2022; Cisse et al., 2025). Nevertheless, the significantly improved FCR observed in the P1 group (1.58) suggests that lower infusion doses may have beneficial effects on digestive efficiency. In contrast, the decline in FCR at higher doses underscores the risk of antinutritional impacts, as also reported by (Zhao et al., 2020; Abd El Latif et al., 2023). Digestive organ response to P. canescens leaf infusionSeveral bioactive constituents of P. canescens—notably flavonoids, phenolics, and saponins—are recognized for their astringent and gastrointestinal-modulatory properties, which can influence digestive organ development and function. An increase in gizzard weight was observed, aligning with reports in broilers supplemented with phytochemical-rich papaya leaf extract (Ezenwosu et al., 2022), suggesting enhanced mechanical digestion from dietary fiber or secondary metabolite stimulation.
Fig. 2. Molecular docking results: (A) 3,5,7-trihydroxy-2-(4-hydroxy-3-methoxyphenyl)chromen-4-one; (B) ethyl 2-[3-[2-(diethylamino) ethyl]-4-methyl-2-oxochromen-7-yl]oxyacetate; (C) abacavir hydroxyacetate; (D) chromonar; (E) 2-N-[3-(dimethylamino)propyl]-4-N-(2-methoxyphenyl)-5-nitropyrimidine-2,4,6-triamine; (F) 3,4-divanillyltetrahydrofuran; (G) native ligand structure. In contrast, the weight of the proventriculus declined with higher infusion levels, reaching its lowest value in P3 (7.18 ± 0.39 g). This may reflect compensatory physiological responses to elevated antinutritional factors, such as saponins and alkaloids, which impair mucosal integrity and reduce enzyme secretion (Obianwuna et al., 2024; Paul et al., 2025). Pancreatic weight also progressively decreased, with the lowest in P3 (4.75 ± 1.14 g), possibly indicating reduced enzymatic demand due to antimicrobial actions lowering gut microbial load, consistent with reports of pancreatic atrophy in broilers receiving high-flavonoid phytobiotics (Buryakov et al., 2023). The liver weight increased in P1 and P2 but declined in P3, suggesting a biphasic response: initial hepatostimulation from detoxification demands, followed by early signs of overload or toxicity. Although moderate flavonoid intake supports liver metabolism, excessive levels may provoke oxidative stress (Jomova et al., 2025). Cardiac weights remained relatively stable, with only minor non-significant fluctuations, consistent with the findings of Sultana and Islam (2023), who found that phytobiotics do not directly impact cardiac tissues. Jejunal weight peaked in P2 (81.40 ± 4.74 g), possibly reflecting improved mucosal development from polyphenol-stimulated villi elongation, whereas lower jejunal weight in P3 may indicate epithelial stress (Camilleri and Vella, 2022). Importantly, no significant differences (p > 0.05) were observed in duodenal, jejunal, or ileal lengths, suggesting that although microscopic features may be enhanced, infusions do not alter gross intestinal structure (Hidayati et al., 2022). Collectively, these findings support the beneficial effects of moderate infusion on digestive organ development, while highlighting potential adverse outcomes at higher concentrations. Chemical composition of meatProximate composition analysis of the pectoralis major muscle in 35-day-old broiler chickens administered P. canescens leaf infusion at graded inclusion levels (P1–P3) showed no significant differences (p > 0.05) in moisture, ash, organic matter, crude protein, or crude fat content. Moisture remained stable (74.05%–74.59%), supporting reports that water retention in the muscle is mainly governed by environmental factors and water regulation rather than moderate phytogenic supplementation (Oleforuh-Okoleh et al., 2015; Ayalew et al., 2022). Slight nonsignificant increases in ash in P3 (1.74%) may reflect minor improvements in mineral uptake due to flavonoids and saponins, though the small magnitude suggests limited systemic bioavailability in aqueous infusions (Prihambodo et al., 2021). However, the modest magnitude of these differences suggests that the aqueous infusion form employed has limited systemic bioavailability. Rude protein peaked in P2 (25.97%), possibly indicating enhanced amino acid utilization or mild stimulation of protein synthesis, while P3 declined (23.64%), consistent with the antinutritional effects of higher tannin and saponin doses that can impair proteolysis and nutrient absorption (Hidayati et al., 2022). Crude fat tended to decrease in P2 (0.95%) versus control (1.69%), aligning with known hypolipidemic actions of saponins, which reduce lipid uptake and stimulate β-oxidation. Previous studies have shown that saponins downregulate lipogenesis genes (SREBP-1c, FAS) and activate the CPT-1 and ACOX-1 pathways (Di et al., 2018; Yu et al., 2024). Overall, while the absence of significant compositional changes highlights limited efficacy under the tested conditions, trends such as higher protein and lower fat at moderate doses suggest bioactivity worth further study. Concentrated extracts, histological assessments, and gene expression analyses should be employed in future research to elucidate the functional relevance of P. canescens in broiler nutrition (Abd El-Hack et al., 2018, 2022). Changes in the lipid profiles of broiler chickens treated with leaf infusionSerum lipid parameters—including triglycerides, total cholesterol, HDL, and LDL—are essential biomarkers of lipid metabolism and cardiovascular health in poultry (Hassan et al., 2022). Although intergroup differences were not statistically significant (p > 0.05), notable trends were observed that may suggest biological relevance. Triglyceride levels exhibited a dose-dependent decline, with the lowest concentration observed in the P3 group (26.24 ± 7.88 mg/dl) compared with the control (38.83 ± 9.60 mg/dl). Total cholesterol also decreased slightly across treatments, with P3 showing the lowest mean value (125.82 ± 12.73 mg/dl). LDL levels declined from 45.64 ± 7.24 mg/dl (P0) to 41.24 ± 4.24 mg/dl (P3), while HDL concentrations increased favorably, reaching 79.24 ± 6.36 mg/dl in P3 compared to 71.20 ± 7.11 mg/dl in the control group. These trends may reflect the hypolipidemic potential of bioactive phytochemicals in P. canescens, particularly saponins and flavonoids. Saponins have been reported to reduce intestinal lipid absorption by forming insoluble complexes with cholesterol, thereby facilitating excretion (Cao et al., 2024; Yu et al., 2024). Meanwhile, flavonoids may inhibit hepatic 3-hydroxy-3-methylglutaryl coenzyme Areductase and stimulate lipoprotein lipase activity, promoting triglyceride breakdown and cholesterol homeostasis (Tan et al., 2022). The increase in HDL levels is especially relevant because HDL mediates reverse cholesterol transport and is associated with reduced cardiovascular risk. Flavonoids may enhance HDL biosynthesis via apolipoprotein A1 upregulation (Xu et al., 2022). Despite the promising shifts, the absence of statistical significance could stem from small sample sizes, biological variability, or limited absorption of active compounds in infusion form. Prior research indicates that concentrated extracts, such as ethanolic or pressurized aqueous forms, may yield stronger effects than crude infusions (Abd El-Hack et al., 2022). Comparable phytogenic interventions—such as Moringa oleifera, Curcuma longa, and Allium sativum—have demonstrated lipid-lowering effects in broilers (Abd El-Hack et al., 2018; Parvin et al., 2021). Furthermore, saponin-rich additives and flavonoids, such as quercetin, are known to reduce serum lipids and elevate HDL levels (Tan et al., 2022; Wang et al., 2024). In summary, while the administration of P. canescens infusion did not result in statistically significant changes, the observed trends indicate a potential hypolipidemic effect. Further studies using refined extract forms, higher dosages, and extended durations are recommended to confirm and expand upon these findings. Hematological and immunological assessmentIn poultry, hematological parameters are key indicators of physiological status, immune competence, and stress (Oluwagbenga and Fraley, 2023). In this study, no statistically significant differences (p > 0.05) were observed in Hb, HCT, or erythrocyte counts among the treatment groups. Nonetheless, a modest increase in Hb (27.28 ± 1.41 g/dl) and HCT in the P1 group (1.5% infusion) may suggest enhanced oxygen-carrying capacity, potentially attributable to flavonoids and saponins in P. canescens known to support erythropoiesis and protect RBCs from oxidative stress (Piao et al., 2023; Zahra et al., 2024). Erythrocyte indices—mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC)—remained within physiological norms, indicating no adverse effects on red cell morphology or hemoglobin synthesis. Similarly, leukocyte counts showed no significant variation, although the P3 group (2.5% infusion) exhibited increased lymphocyte percentages and decreased monocytes, suggesting possible immunomodulatory activity. Phytochemicals, such as polyphenols and tannins, are known to stimulate lymphocyte proliferation and modulate inflammatory pathways (Hassan et al., 2022; Mamun et al., 2024). Platelet counts showed a non-significant rise in P1 and P2, potentially reflecting mild thrombopoietic stimulation by bioactive compounds. The heterophil-to-lymphocyte (H/L) ratio decreased slightly in the treated groups, indicating reduced physiological stress—likely linked to the antioxidant and anti-inflammatory properties of P. canescens (Sumadja and Hani, 2023; Cao et al., 2024; Yu et al., 2024). Although most findings lacked statistical significance, the observed consistent trends support the safety and potential hematological benefits of P. canescens infusion in broilers. However, depending on their concentration and bioavailability, tannins have also been associated with pro-inflammatory effects and impaired nutrient absorption in poultry (Choi et al., 2022). Therefore, further research is recommended to specifically assess whether the tannin content in P. canescens extracts contributes to inflammatory responses or other subclinical alterations in hematological parameters. To better elucidate the mechanisms underlying these observations, future studies should incorporate extended trial durations, larger sample sizes, and the isolation or standardization of individual phytochemical fractions. Molecular interaction revealed bioactive mechanismsMolecular docking analysis elucidated the binding affinities and interaction profiles of diverse phytochemicals and synthetic compounds with iNOS, a critical enzyme implicated in inflammatory and oxidative processes. iNOS mediates the production of nitric oxide, a reactive nitrogen species that contributes to cellular injury, chronic inflammation, and immune dysregulation when overproduced (Herawati et al., 2021). The native ligand exhibited the highest binding affinity (−12.8 kcal/mol), forming extensive hydrogen bonds with key residues such as GLY295, ASN77, THR244, and ARG198, underscoring its relevance as a benchmark for assessing test ligands. Among the evaluated compounds, the flavonoid derivative 3,5,7-trihydroxy-2-(4-hydroxy-3-methoxyphenyl)chromen-4-one exhibited a binding affinity of −7.9 kcal/mol, exhibiting hydrogen bonding with ASN76 and ASP320 and hydrophobic interactions with LEU321 and ALA442. This indicates a binding orientation analogous to the native ligand, supporting its candidacy as a potential iNOS inhibitor (Hasibuan et al., 2024). Conversely, ethyl 2-[3-[2-(diethylamino)ethyl]-4-methyl-2-oxochromen-7-yloxy]acetate displayed the weakest binding (−5.2 kcal/mol), reflecting limited stability and possibly suboptimal alignment within the active site. The antiviral agent abacavir hydroxyacetate achieved a notable binding affinity (−8.7 kcal/mol), forming hydrogen bonds with LEU321, ASP320, and ASP438, suggesting potential repurposing for inflammatory indications (Zhan et al., 2022). Chromonar, known primarily as a vasodilator, exhibited a binding affinity of −8.0 kcal/mol, interacting with THR247 and ASP356—an interaction profile potentially underlying its reported antioxidant and anti-inflammatory properties. The NP compound 2-N-[3-(dimethylamino)propyl]-4-N-(2-methoxyethyl)-5-nitropyrimidine-2,4,6-triamine demonstrated a binding energy of −8.9 kcal/mol, engaging residues overlapping with the native ligand, highlighting its promise as a lead scaffold. Finally, 3,4-divanillyltetrahydrofuran, a lignan derivative, presented a binding affinity of −8.6 kcal/mol and formed stabilizing interactions via hydrogen bonding and π–π stacking. Collectively, these findings identify several promising candidates—especially the flavonoid, abacavir hydroxyacetate, and the nitropyridine derivative—for further in vitro and in vivo validation as prospective iNOS inhibitors in anti-inflammatory therapy. ConclusionSupplementing broiler drinking water with P. canescens leaf infusion for up to 35 days did not produce significant changes in growth performance, meat composition, digestive organ morphology, or hematological profiles. However, trends such as improved feed conversion at lower doses, reduced meat fat, triglycerides, and LDL, along with increased gizzard weight and lymphocyte counts, indicate mild effects on digestion and immunity. The limited impact may stem from non-standardized preparation and low levels of bioactive compounds. Future studies should use standardized extracts with quantified flavonoid and saponin contents, combined with histopathological and gene expression analyses to clarify the underlying mechanisms. Additionally, molecular docking showed that the native iNOS ligand had the strongest binding affinity, followed by 2-N-[3-(dimethylamino)propyl]-4-N-(2-methoxyethyl)-5-nitropyrimidine-2,4,6-triamine, abacavir hydroxyacetate, and 3,4-divanillyltetrahydrofuran. These compounds interacted favorably with key iNOS residues, highlighting flavonoid and vanillyl-derived structures as promising scaffolds for selective iNOS inhibitors. Further in vitro and in vivo studies are needed to confirm their efficacy and safety. AcknowledgmentsWe gratefully acknowledge the Faculty of Animal Science, University of Jambi, for providing research facilities and the Natural Products and Bioactive Compounds Laboratory for their support with the metabolite analysis. Conflict of interestThe author declares that there is no conflict of interest. FundingThe authors received no financial support for the research, authorship, and publication of this article. Authors’ contributionsResearch concept, W.A.S; F.F; I.L.T; Preparation, W.A.S, F.F; Methodology, W.A.S, I.L.T, I.I.R, N.R; Analysis, W.A.S, I.L.T, I.I.R, N.R; Data processing, W.A.S, I.L.T, I.I.R; Software management, I.I.R, N.R; I.L.T; Literature study, W.A.S, I.L.T; Manuscript, W.A.S, I.L.T; Review and Editing, W.A.S, I.L.T.; Visualization, I.L.T, I.I.R., N.R. Data availabilityAll data supporting this study’s findings are available within the manuscript. ReferencesAbd El Latif, M.A., El-Saadony, M.T., Elkomy, A.A., Mohamed, M.M.Y., Desoky, E.S.M., Saad, A.M., Elnesr, S.S., Khafaga, A.F., Taha, A.E. and Swelum, A.A. 2023. Effect of dietary OPM and multi-enzymes on productive, physiological, and nutritional responses of broiler chickens. Animals 13(15), doi:10.3390/ani1315247 Abd El-hack, M.E., Alagawany, M., El-Saadony, M.T., Arif, M., Batiha, G.E., Khafaga, A.F., Elnesr, S.S. and Swelum, A.A. 2018. Effect of Moringa oleifera L. (moringa) forage on animal health and nutrition and its beneficial applications in soil, plants, and water purification. Agriculture 8(9), 1–22; doi:10.3390/agriculture8090145 Abd El-hack, M.E., Swelum, A.A., Arif, M., Alagawany, M., Hussein, E.O.S., Saadeldin, I.M., Taha, A.E., Khafaga, A.F., El-Saadony, M.T. and Elwan, H.A. 2022. Alternatives to antibiotics for organic poultry production: types, modes of action, and impacts on bird health and production. Poultry Sci. 101(4), 696; doi:10.1016/j.psj.2022.101696 Al-Ishaq, R.K., Abotaleb, M., Kubatka, P., Kajo, K. and Büsselberg, D. 2019. Flavonoids and their anti-diabetic effects: cellular mechanisms and effects to improve blood sugar levels. Biomolecules 9(430), 430; doi:10.3390/biom9090430 Ayalew, H., Zhang, H., Wang, J., Wu, S., Qiu, K., Qi, G., Tekeste, A., Wassie, T. and Chanie, D. 2022. Potential feed additives as antibiotic alternatives in broiler production. Front. Vet. Sci. 9(916473), 1–15; doi:10.3389/fvets.2022.916473 Basiouni, S., Tellez-Isaias, G., Latorre, J.D., Graham, B.D., Petrone-Garcia, V.M., El-Seedi, H.R., Yalçın, S., El-Wahab, A.A., Visscher, C., May-Simera, H.L., Huber, C., Eisenreich, W. and Shehata, A.A. 2023. Anti-inflammatory and antioxidative phytogenic substances against secret killers in poultry: current status and prospects. Vet. Sci. 10(1), 55; doi:10.3390/vetsci10010055 Bhalani, D.V., Nutan, B., Kumar, A. and Singh Chandel, A.K. 2022. Bioavailability enhancement techniques for poorly aqueous soluble drugs and therapeutics. Biomedicines 10(9), 2055; doi:10.3390/biomedicines10092055 Buryakov, N.P., Gorlov, I.F., Mosolov, A.A., Slozhenkina, M.I., Omarov, R.S., Randelin, D.A., Gizatullin, R.S., Pozdnyakova, M.V., Makarov, A.Y. and Brazhnik, E.A. 2023. Role of supplementing a complex phytobiotic feed additive containing Castanea sativa Mill extract along with calcium butyrate, zinc–methionine, and essential oils on broiler chicken growth indicators, blood profile, and carcass quality. Vet. Sci. 10(3), 1–18; doi:10.3390/vetsci10030212 Camilleri, M. and Vella, A. 2022. What to do about the leaky gut. Gut 71(2), 424–435; doi:10.1136/gutjnl-2021-325428 Cao, S., Liu, M., Han, Y., Li, S., Zhu, X., Li, D., Shi, Y. and Liu, B. 2024. Effects of saponins on lipid metabolism: the gut–liver axis plays a key role. Nutrients 16, 1514; doi:10.3390/nu16101514 Cheok, C.Y., Salman, H.A.K. and Sulaiman, R. 2014. Extraction and quantification of saponins: a review. Food Res. Int. 59, 16–40. Choi, J., Lee, S.R., Kim, H.W., Lee, D.S., Oh, S.T., Mun, J.Y., Park, B.C., Kim, Y.H., Kim, K.H. and Park, H.C. 2022. Effects of supplemental TA on growth performance, gut health, microbiota, and fat accumulation and optimal dosages of TA in broilers. Front. Physiol. 13, 1–23; doi:10.3389/fphys.2022.912797 Cisse, S., Ranjitkar, S., Tonda, R., Dumorné, K., Colin, T., Herve, B. and Rudeaux, F. 2025. Effects of standardized natural citrus extract on broiler chicken growth, gut health, carcass quality, and welfare. Animals 15(2), 1–16; doi:10.3390/ani15020127 De La Mora, Z.V., Aguilar, Y.M., López-Arellano, R., Angeles, M.L., Torres, I. and Rivas-Caceres, R.R. 2020. Clostridium perfringens as a foodborne pathogen in broiler production: pathophysiology and potential strategies for controlling necrotic enteritis. Animals 10(9), 1–28; doi:10.3390/ani10091718 Di, T.M., Hu, J.N., Yu, J., Wang, J.S., Xiao, X., Wang, M.M., Liu, X., Zhang, L. and Liang, Y. 2018. Oleiferasaponin A2, a novel saponin from Camellia oleifera Abel. seeds, inhibits HepG2 cell lipid accumulation by regulating fatty acid metabolism. Molecules 23(12), 1232–1296; doi:10.3390/molecules23123296 Dillasamola, D. 2024. Efficacy of the Sungkai plant (Peronema canescens Jack.) As COVID-19 Protection. Quest J. J. Res. Pharm. Sci. 10(2), 21–25. Ezenwosu, C., Okeke, C.O. and Machebe, N.S. 2022. Impact of pawpaw (Carica papaya) leaf extract on biochemical markers, organs, and carcass traits of broiler chickens in Nsukka, Nigeria. Asian J. Res. Biosci. 4(2), 7–14. Francis, G., Kerem, Z., Makkar, H.P. and Becker, K. 2002. The biological action of saponins in animal systems: a review. Br. J. Nutr. 88(6), 587–605; doi:10.1079/bjn2002725 Gazwi, H.S.S., Mahmoud, M.E. and Toson, E.M.A. 2022. The phytochemicals of Coriandrum sativum and Cichorium intybus aqueous extracts and their biological effects on broiler chickens were analyzed. Sci. Rep. 12(1), 1–11; doi:10.1038/s41598-022-14645-5 Hasibuan, P.A.Z., Napitupulu, R.J., Ginting, C.N.A., Satria, D., Batubara, T.S. and Ramadhan, R. 2024. Unlocking the potential of flavonoids: natural solutions in the fight against colon cancer. Biomed. Pharmacother. 176, 116827; doi:10.1016/j.biopha.2024.116827 Hassan, F.U., Alagawany, M. and Jha, R. 2022. Editorial: interplay of nutrition and genomics: Potential for improving poultry performance and health. Front. Physiol. (Switzerland). 13, 1030995; doi:10.3389/fphys.2022.1030995 Hehanussa, S.C.H., Bata, M. and Rumokoy, J.F. 2022. Performance and hematological profile of broiler chickens fed a diet containing Atung (Parinarium glaberrimum Hassk.) seed powder. Buletin. Peternakan. 46(2), 104; doi:10.21059/buletinpeternak.v46i2.73251 Herawati, H., Oktanella, Y. and Kaltaria Anisa, A. 2021. Molecular docking analysis of curcuminoids from Curcuma longa extract on iNOS as an immunomodulator candidate in broilers. Adv. Anim. Vet. Sci. 9(4), 519–524; doi:10.17582/journal.aavs/2021/9.4.519.524 Hidayati, N.A., Isnawati, R., Chusniati, S., Syahruddin, E., Widodo, A. and Setiawan, E. 2022. Intestinal health in broiler chickens treated with T. catappa leaf extract nanoencapsulation as an antibacterial agent. Trop. Anim. Sci. J. 45(4), 443–450; doi:10.5398/tasj.2022.45.4.443 Jeepipalli, S.P.K., Du, B., Sabitaliyevich, U.Y. and Xu, B. 2020. New insights into potential nutritional effects of dietary saponins in protecting against the development of obesity. Food Chem. 318, 126474; doi:10.1016/j.foodchem.2020.126474 Jomova, K., Alomar, S.Y., Valko, R., Liska, J., Nepovimova, E., Kuca, K. and Valko, M. 2025. Flavonoids and their role in oxidative stress, inflammation, and human diseases. Chem. Biol. Interact. 413, 111489; doi:10.1016/j.cbi.2025.111489 Korver, D.R. 2023. Review: current challenges in poultry nutrition, health, and welfare. Animal 17 Suppl 2, 100755; doi:10.1016/j.animal.2023.100755 Latief, M., Arum, D., Nurjannah, S., Tarigan, I.L., Khatib, A. and Yusnaidar, Y. 2024. LC-MS based metabolite profiling of ethanol extract from the Sungkai (Peronema canescens Jack) and In Silico prediction of antidiabetic activity with α-glucosidase. Molekul 19(3), 455–462; doi:10.20884/1.jm.2024.19.3.8806 Latief, M., Sari, P.M., Fatwa, L.T. and Tarigan, I. L. Rupasinghe, H. P. V. 2021. Antidiabetic activity of Sungkai (Peronema canescens Jack.) ethanol extract from leaves of male mice induced alloxan monohydrate pharmacology and clinical pharmacy research. Pharmacol. Clin. Pharm. Res. 6(2), doi:10.15416/pcpr.v4i3.31666 M., Ye, S., Fan, X., Li, S., Yang, C., Ji, F., Ji, B. and Zhou. 2023. Four flavonoids from propolis ameliorate free fatty acids-induced non-alcoholic steatohepatitis in HepG2 cells: involvement of enhanced AMPK activation, mTOR-NF-κBp65 interaction, and PTEN expression. J. Funct. Foods 102, 105460; doi:10.1016/j.jff.2023.105460 Mamun, M.A.A., Rakib, A., Mandal, M., Kumar, S., Singla, B. and Singh, U.P. 2024. Polyphenols: role in modulating immune function and obesity. Biomolecules 14(2), 221; doi:10.3390/biom14020221 Moon, D.O. 2024. Plant-derived flavonoids as AMPK activators: unveiling their potential in type 2 diabetes management through mechanistic insights, docking studies, and pharmacokinetics. Appl. Sci. 14, 8607; doi:10.3390/app14198607 Nath, H., Samtiya, M. and Dhewa, T. 2022. Beneficial attributes and adverse effects of major plant-based food anti-nutrients on health: a review. Hum. Nutr. Metab. 28, 200147; doi:10.1016/j.hnm.2022.200147 Obianwuna, U.E., Chang, X., Oleforuh-Okoleh, V.U., Onu, P.N., Zhang, H., Qiu, K. and Wu, S. 2024. Phytobiotics in poultry: revolutionizing broiler chicken nutrition with plant-derived gut health enhancers. J. Anim. Sci. Biotechnol. 15, 169; doi:10.1186/s40104-024-01101-9 Oleforuh-Okoleh, V.U., Onu, P.N., Udeh, F.U. and Ijeoma, J.A. 2015. Evaluation of growth performance and hematological and serum biochemical responses of broiler chickens to aqueous extracts of ginger and garlic. J. Agricult. Sci. 7(4), 167–173; doi:10.5539/jas.v7n4p167 Oluwagbenga, E.M. and Fraley, G.S. 2023. Heat stress and poultry production: a comprehensive review. Poultry Sci. 102(12), 103141; doi:10.1016/j.psj.2023.103141 Osadcha, Y., Muzyka, I., Shevtsov, O., Reheda, M., Nazaryuk, I., Zakrutko, O. and Hlushko, O. 2023. Biochemical parameters of chicken blood under the influence of various technological stimuli. Scientific Horizons 26(9), 70–80; doi:10.48077/scihor9.2023.70 Parvin, M.M., Howlider, M.A.R., Akter, S. and Islam, M.N. 2021. Effects of using garlic (Allium sativum) and turmeric (Curcuma longa) powder on broiler chicken production performance and biochemical parameters. Int. J. Vet. Sci. Anim. Husbandry 6(4), 53–57; doi:10.22271/veterinary.2021.v6.i4a.369 Paul, B., Xie, L., Yahia, Z.O. and Chen, W. 2025. Recent review on the stability of bioactive substances through encapsulation and their application in dairy products. Food Rev. Int. 41(6), 1–27; doi:10.1080/87559129.2025.2492338 Piao, M., Tu, Y., Zhang, N., Diao, Q. and Bi, Y. 2023. Advances in the application of phytogenic extracts as antioxidants and their potential mechanisms in ruminants. Antioxidants 12(4), 879; doi:10.3390/antiox12040879 Prihambodo, T.R., Indarsih, B., Nahrowi, N., Suprayogi, A. and Suthama, N. 2021. Effects of dietary flavonoids on broiler performance, blood constituents, carcass composition, and small intestinal morphology: a meta-analysis. Anim. Biosci. 34(3), 434–442; doi:10.5713/ajas.20.0379. Shalihin, M.I., Khatib, A., Yusnaidar, Y., Tarigan, I.L. and Latief, M. 2024. An in-vogue plant, Peronema canescens Jack: traditional uses and scientific evidence of its bioactivities. Discover Plants 1, 58; doi:10.1007/s44372-024-00048-5 Shittu, M.D., Oke, O.E., Abioja, M.O., Oso, A.O. and Akinlade, J.A. 2022. Growth performance and hematological and serum biochemical parameters of broiler chickens treated with varying concentrations of P. longifolia leaf extract instead of conventional antibiotics. Anim. Sci. Genet. 18(2), 57–71; doi:10.5604/01.3001.0015.9185 Sultana, N. and Islam, R. 2023. Impact of phytobiotic growth promoter supplementation on the morphology and biometry of vital organs in broilers. Braz. J. Poultry Sci. 25(4), 1–12; doi:10.1590/1806-9061-2023-1805. Sumadja, W.A. and Hani, S.F. 2023. The effect of the addition of Glodokan Tiang leaf flour (Polyalthia longifolia) to the diet on the growth of broiler chickens. J. Ilmiah Ilmu-Ilmu Peternakan 26(2), 98–108; doi:10.22437/jiiip.v26i2.26767 Tahamtani, F.M., Moradi, H. and Riber, A.B. 2020. Effect of qualitative feed restriction on stress and clinical welfare indicators in broiler breeders. Front. Vet. Sci. 7, 316; doi:10.3389/fvets.2020.00316 Tan, Z., Halter, B., Liu, D., Gilbert, E.R. and Cline, M.A. 2022. Dietary flavonoids as lipid metabolism modulators in poultry. Front. Physiol. 13, 863860; doi:10.3389/fphys.2022.863860 Tarigan, I.L., Sutrisno., Rumaida., Aini, I.P.S. and Latief. 2022. Isolation of the flavone apigenin and the steroid squalene from Peronema canescens jack leaves with anti-inflammatory activities. Pharmacogn. J. 14(6), 744–752; doi:10.5530/pj.2022.14.162 Teymouri, P., Irani, M., Pournazari, M. and Pirsaraei, Z.A. 2021. Efficacy of natural antibiotic alternatives on broiler chicken growth performance, gut microbial population, intestinal morphology, and serum biochemical metabolites. Italian J. Anim. Sci. 20(1), 1801–1809; doi:10.1080/1828051X.2021.1954558 Wang, J., Deng, L., Chen, M., Che, Y., Li, L., Zhu, L., Chen, G. and Feng, T. 2024. Phytogenic feed additives as natural antibiotic alternatives in animal health and production: a review of the literature of the last decade. Anim. Nutr. 17, 244–264; doi:10.1016/j.aninu.2024.01.012 Wang, M., Wang, B., Wang, S., Lu, H., Wu, H., Ding, M., Ying, L., Mao, Y. and Li, Y. 2021. Effect of quercetin on lipid metabolism through modulation of the gut microbial and AMPK/PPAR signaling pathways in broilers. Front. Cell Develop. Biol. 9, 1–11; doi:10.3389/fcell.2021.616219 Wickramasuriya, S.S., Ault, J., Ritchie, S., Gay, C.G. and Lillehoj, H.S. 2024. Alternatives to antibiotic growth promoters for poultry: a bibliometric analysis of the research journals. Poultry Sci. 103(9), 103987; doi:10.1016/j.psj.2024.103987 Williams, G.A., Mafimidiwo, A.N., Oyekan, I.A., Lawal, A.H., Muibi, S.A., Arabambi, J., Ganiyu, Z.T., Nudewhenu, G.M., Sole, F.D. and Saula, A.A. 2024. Performance of broilers administered aqueous bamboo (Bambusa vulgaris) leaf extract in drinking water. In Proceedings of the 49th Conference of the Nigerian Society for Animal Production. Oyo, Nigeria: Univ. of Ibadan, pp: 1271–1275; doi:10.51791/njap.vi.6983 Xu, H., Gong, L., Zhang, X., Li, Z., Fu, J., Lv, Z. and Guo, Y. 2025. Effects of tannic acid on growth performance, intestinal health, and tolerance in broiler chickens. Poultry Sci. 104(2), 104676; doi:10.1016/j.pss.2025 Xu, X., Song, Z., Mao, B. and Xu, G. 2022. Apolipoprotein A1-related proteins and reverse cholesterol transport in antiatherosclerosis therapy: recent progress and future perspectives. Cardiovasc. Therapeutics 2022, 1–9; doi:10.1155/2022/4610834 Ye, M., Fan, S., Li, X., Yang, S., Ji, C., Ji, F. and Zhou, B. 2023. Four flavonoids from propolis ameliorate free fatty acids-induced non-alcoholic steatohepatitis in HepG2 cells: involvement of enhanced AMPK activation, mTOR-NF-κBp65 interaction, and PTEN expression. J. Funct. Foods 102, 105460; doi:10.1016/j.jff.2023.105460 Yu, Y., Ji, X., Song, L., Cao, Y., Feng, J., Zhang, R., Tao, F., Zhang, F. and Xue, P. 2024. Saponins from Chenopodium quinoa Willd. husks alleviated high-fat-diet-induced hyperlipidemia via modulating the gut microbiota and multiple metabolic pathways. J. Sci. Food Agric. 104(4), 2417–2428; doi:10.1002/jsfa.13127 Zahra, M., Abrahamse, H. and George, B.P. 2024. Flavonoids: antioxidant powerhouses and their role in nanomedicine. Antioxidants 13(8), 922; doi:10.3390/antiox13080922 Zavyalov, O., Galimzhan, D. and Marina, K. 2022. Effect of feeding bioactive compounds identified from plant extracts (4-hexylresorcinol, 7-hydroxycoumarin, and gamma-octalactone) on the productivity and quality of broiler meat. Vet. World 15(12), 2986–2996; doi:10.14202/vetworld.2022.2986-2996 Zhan, P., Yu, B. and Ouyang, L. 2022. Drug repurposing: an effective strategy for accelerating contemporary drug discovery. Drug Discov. Today 27(7), 1785–1788; doi:10.1016/j.drudis.2022.05.026 Zhao, X., Hu, M., Zhang, Q., Zhao, C., Zhang, Y., Li, L., Qi, J., Luo, Y., Zhou, D. and Liu, Y. 2020. Characterization of integrons and antimicrobial resistance in Salmonella from broilers in Shandong, China. Poultry Sci. 99(12), 7046–7054; doi:10.1016/j.psj.2020.09.071 Supplementary Material
Figure S1. LC-MS/MS chromatogram of P1.
Figure S2. LC-MS/MS chromatogram of P2.
Figure S3. Chromatogram of P3.
Figure S4. Receptor 1P1H. Ligan uji yang digunakan meliputi 3,5,7-trihydroxy-2-(4-hydroxy-3-methoxyphenyl)chromen-4- one, Ethyl 2-[3-[2-(diethylamino) ethyl]-4-methyl-2-oxochromen-7-yl]oxyacetate, Abacavir hydroxyacetate, Chromonar, 2-N-[3-(dimethylamino)propyl]-4-N-(2-methoxyphenyl)-5- nitropyrimidine-2,4,6-triamine dan 3,4-Divanillyltetrahydrofuran.
Figure S5. Ramachandran plot 1P1H Dari Ramachandran plot diatas, diperoleh bahwa reseptor 1P1H memiliki most favoured regions sebesar 85%, additional allowed regions 13,7%, generously allowed regions 0,9% dan dissalowed regions 0,3% yang menandakan bahwa reseptor ini dapat digunakan untuk molecular docking.
Figure S6. Visualisasi molecular docking. | ||
| How to Cite this Article |
| Pubmed Style Sumadja WA, Filawati F, Ramadhan II, Rahmasari N, Tarigan IL. Phytogenic feed additive derived from Peronema canescens leaves: Bioactive profile, effects on broiler growth, carcass yield, blood biochemistry, and molecular interactions. Open Vet. J.. 2025; 15(9): 4617-4634. doi:10.5455/OVJ.2025.v15.i9.67 Web Style Sumadja WA, Filawati F, Ramadhan II, Rahmasari N, Tarigan IL. Phytogenic feed additive derived from Peronema canescens leaves: Bioactive profile, effects on broiler growth, carcass yield, blood biochemistry, and molecular interactions. https://www.openveterinaryjournal.com/?mno=257787 [Access: January 26, 2026]. doi:10.5455/OVJ.2025.v15.i9.67 AMA (American Medical Association) Style Sumadja WA, Filawati F, Ramadhan II, Rahmasari N, Tarigan IL. Phytogenic feed additive derived from Peronema canescens leaves: Bioactive profile, effects on broiler growth, carcass yield, blood biochemistry, and molecular interactions. Open Vet. J.. 2025; 15(9): 4617-4634. doi:10.5455/OVJ.2025.v15.i9.67 Vancouver/ICMJE Style Sumadja WA, Filawati F, Ramadhan II, Rahmasari N, Tarigan IL. Phytogenic feed additive derived from Peronema canescens leaves: Bioactive profile, effects on broiler growth, carcass yield, blood biochemistry, and molecular interactions. Open Vet. J.. (2025), [cited January 26, 2026]; 15(9): 4617-4634. doi:10.5455/OVJ.2025.v15.i9.67 Harvard Style Sumadja, W. A., Filawati, . F., Ramadhan, . I. I., Rahmasari, . N. & Tarigan, . I. L. (2025) Phytogenic feed additive derived from Peronema canescens leaves: Bioactive profile, effects on broiler growth, carcass yield, blood biochemistry, and molecular interactions. Open Vet. J., 15 (9), 4617-4634. doi:10.5455/OVJ.2025.v15.i9.67 Turabian Style Sumadja, Wiwaha Anas, Filawati Filawati, Ilham Ifandi Ramadhan, Nadia Rahmasari, and Indra Lasmana Tarigan. 2025. Phytogenic feed additive derived from Peronema canescens leaves: Bioactive profile, effects on broiler growth, carcass yield, blood biochemistry, and molecular interactions. Open Veterinary Journal, 15 (9), 4617-4634. doi:10.5455/OVJ.2025.v15.i9.67 Chicago Style Sumadja, Wiwaha Anas, Filawati Filawati, Ilham Ifandi Ramadhan, Nadia Rahmasari, and Indra Lasmana Tarigan. "Phytogenic feed additive derived from Peronema canescens leaves: Bioactive profile, effects on broiler growth, carcass yield, blood biochemistry, and molecular interactions." Open Veterinary Journal 15 (2025), 4617-4634. doi:10.5455/OVJ.2025.v15.i9.67 MLA (The Modern Language Association) Style Sumadja, Wiwaha Anas, Filawati Filawati, Ilham Ifandi Ramadhan, Nadia Rahmasari, and Indra Lasmana Tarigan. "Phytogenic feed additive derived from Peronema canescens leaves: Bioactive profile, effects on broiler growth, carcass yield, blood biochemistry, and molecular interactions." Open Veterinary Journal 15.9 (2025), 4617-4634. Print. doi:10.5455/OVJ.2025.v15.i9.67 APA (American Psychological Association) Style Sumadja, W. A., Filawati, . F., Ramadhan, . I. I., Rahmasari, . N. & Tarigan, . I. L. (2025) Phytogenic feed additive derived from Peronema canescens leaves: Bioactive profile, effects on broiler growth, carcass yield, blood biochemistry, and molecular interactions. Open Veterinary Journal, 15 (9), 4617-4634. doi:10.5455/OVJ.2025.v15.i9.67 |