| Research Article | ||
Open Vet. J.. 2025; 15(9): 4558-4568 Open Veterinary Journal, (2025), Vol. 15(9): 4558-4568 Research Article Immunohistochemical and morphometric analysis of adult goose testes with special focus on sertoli cell localizationAli Ahmed Hasan1, Soadd Mohamd Al-Hamadany2, Thekra Fadel Saleh1 and Omar Younis Altaey1*1Department of Anatomy, College of Veterinary Medicine, University of Mosul, Mosul, Iraq 2Department of Pathology and Poultry Diseases, College of Veterinary Medicine, University of Mosul, Mosul, Iraq *Corresponding Author: Omar Younis Altaey. Department of Anatomy, College of Veterinary Medicine, University of Mosul, Mosul, Iraq. Email: Omar.younes [at] uomosul.edu.iq Submitted: 11/05/2025 Revised: 12/08/2025 Accepted: 27/08/2025 Published: 30/09/2025 © 2025 Open Veterinary Journal
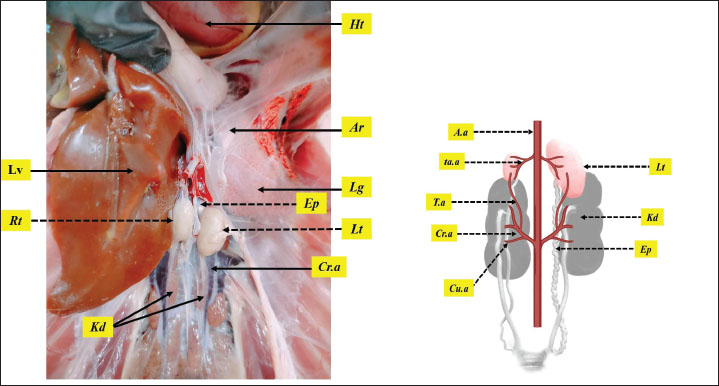
AbstractBackground: Geese breeding holds economic importance, but their reproductive efficiency lags behind other poultry species, due to male fertility challenges including variation in semen quality, genetics, environmental, and management factors. Aim: The current study presents a comprehensive histological and histomorphometric analysis of adult geese testes, employing two anti-vimentin immunostaining markers to localize Sertoli cells and quantify their spatial distribution. Methods: Seven adult male geese (Anser anser domesticus) were used in this study. The birds euthanized and testes were collected, weighed and measured for the total length and width, then testes were preserved with Davidson’s solution for 24 hours, followed by histological sectioning and staining with Harris hematoxylin and eosin, other sections were prepared for immunostaining with rabbit and mouse anti-vimentin antibody markers, histomorphometric measurements included numbers and diameters of seminiferous tubules, thickness of tunica albuginea, number and diameter of Sertoli cells, and Sertoli cell index, the data were statistically analyzed to interpret differences between left and right testes. Results: Testes were paired and bean-shaped, with the left testis being significantly larger, heavier, and situated more cranially than the right. Microscopic analysis revealed that the tunica albuginea was thicker in the left testis. The seminiferous tubules were densely packed, with a wider diameter and higher mitotic activity in the left testis compared to the right. Sertoli cells were more abundant in peripheral tubules compared with central tubules. Immunohistochemical analysis showed positive expression with rabbit polyclonal anti-vimentin antibody, with stronger staining in the left testis and higher cellular index, whereas the mouse monoclonal antibody exhibited no reactivity. Conclusion: Geese testes share some morphological and microscopic features with those of other avian species, and differ from mammal testes. These observations offer data for understanding reproductive physiology in geese, applications in the diagnosis of fertility-related diseases, and enhancing poultry farm management. Keywords: Geese, Immunohistochemical, Anti-vimentin, Sertoli cells, Testes. IntroductionGeese (Anser anser domesticus) hold significant economic value in global poultry production, providing meat, eggs, high-quality feathers, and special products such as fatty liver. Geese possess notable adaptability, breeding under various conditions, sometimes with modest nutritional and housing requirements compared to other poultry, making them suitable for specific agricultural systems, and their ability to adapt well to human-dominated landscapes, which contributes to their widespread presence (Djermanovic et al., 2021). However, their reproductive efficiency lags behind other poultry species, primarily due to pronounced seasonality in breeding behavior, broodiness tendency in females, and male fertility challenges, including variation in semen quality, such as volume, density, motility, and morphology (Shi et al., 2008). Morphological parameters such as testicular size and weight are often considered indicators of male reproductive health and can significantly impact fertilization rates (Hu et al., 2025). These limitations are influenced by genetic, environmental, and management factors (Shi et al., 2008). The testis serves as the cornerstone of flock fertility, and its development and function modulated by age, breed, and rearing systems (Pardyak et al., 2022). Avian testes exhibit some distinct structural features. Unlike in many mammals, the testes are located intra-abdominally, meaning spermatogenesis occurs at core body temperature (Lüpold et al., 2011). Histologically, testes of birds generally do not have the prominent connective tissue septa (septula testes) of the mammal’s testis (Estermann et al., 2021), and the outer capsule (tunica albuginea) is relatively thin. And the peritubular tissue contains multiple layers of smooth muscle-like cells (immunopositive for actin and desmin) which are absent in mammals (Aire and Ozegbe, 2007). Sertoli cells are pivotal for spermatogenesis, providing structural and metabolic support to germ cells while maintaining the blood-testis barrier (Costa et al., 2020 ). Their population density and functional capacity directly determine sperm production efficiency (Pintus et al., 2015). Despite the economic importance, detailed structural analyses of geese testes, especially Sertoli cells remain limited compared to other avian species such as chickens (Bozkurt et al., 2007), turkey (Parvez et al., 2023), ducks (Obeid et al., 2021), and guinea fowls (Dharani et al., 2017), making them a critical research focus for improving reproductive performance in this bird. Anti-vimentin is a well-established Sertoli cell immunohistochemical marker in mammals, for demonstration of the vimentin filamentous cytoskeleton protein (Saito et al., 2023). But this biomarker has never been validated in avian testes. Our work addresses this gap while interduce the quantitative morphometry to assess the seminiferous tubules architecture. The pattern and intensity of vimentin can change during different stages of spermatogenesis, reflecting dynamic interactions between Sertoli cell number and sexual activity and germ cell development (Niazi et al., 2022). This study presents the first histological and histomorphometric analysis of adult goose testes, employing rabbit polyclonal and mouse monoclonal anti-vimentin immunohistochemical markers to localize Sertoli cells and quantify their spatial distribution. The results could improve veterinary diagnostics by allowing for more accurate assessments of avian reproductive health, fertility, and testicular dysfunction. In avian farming systems, this understanding may help to improve breeding strategies by identifying biomarkers of optimal Sertoli cell organization, which is closely linked to sperm efficiency. Materials and MethodsStudy animalsSeven (N=7) healthy adult male geese (Anser anser domesticus), aged 4 years and weighing between 6.5 and 8.6 kg, were used in this study. The birds were obtained from certified breeders in Mosul, Iraq. During the experiment, the geese were housed in an outdoor pen cage of 3L*2W*2.5H m dimensions, with unrestricted access to food and water at ambient temperature ≈ 37Cº. During rearing time, birds were exposed to 14 hours light :10 hours dark (< 30 lux) to enhance the long photoperiod effect (Chang et al., 2015). The study was conducted in the Anatomy Department laboratory. College of Veterinary Medicine, University of Mosul, Iraq, between September 2024 and December 2024. Animal preparationThe animals were euthanized via cervical dislocation, the abdominal cavity was exposed, and visceral organs were evacuated. A gross examination of the testes was performed in situ before excision. The testes were then carefully harvested, left and right testes weighed using a sensitive scale (EK-IEWI, Japan), and measured for the total length and width with a digital Vernier caliper (LOUISWARE, China). Testes were preserved for histological processing in a container filled with Davidson’s solution for 24 hours (Latendresse et al., 2002). Histological processingFollowing fixation, the samples underwent sequential dehydration process using ethyl alcohol solutions at concentrations of 70%, 80%, and 95%, two changes (30 minutes), and subsequently immersed in alcohol 100% for (45 minutes). The samples were then cleared in xylene (50 minutes) before being embedded in paraffin wax, with two changes (1.5 hours). After embedding, the samples were cast into paraffin blocks of 30 × 24 × 5 mm size and sectioned into 5 µm thick slices using a rotary microtome (BIOBASE BK-2218, China). Later, 50 slides were stained with Harris hematoxylin and eosin, and mounted with DPX. Lastly, the stained slides were evaluated with a light microscope (Olympus CX21, Japan) (Carson and Cappellano., 2015; Altaey et al., 2025). Immunohistochemical analysisSlides were deparaffinized, washed in phosphate buffered saline (PBS), and incubated with 3% hydrogen peroxide and PBS for 15 minutes at 37°C, next samples were blocked with 5% serum albumin. The slides were separated into two groups, the first was incubated with rabbit anti-vimentin antibody 1:75 (SAB5700070, Sigma-Alderich, USA), and the second group was incubated with mouse monoclonal anti-vimentin antibody (MAB3400, Sigma-Aldrich, USA), in a humid chamber at 4°C for 24 hours. Following washing, slides were incubated with Biotin-labeled anti-rabbit IgG and anti-mouse IgG (Boster BioTechnology Co., Ltd, USA) for 1 hour. The sections were rehydrated in PBS (pH 7.2), then incubated with the avidin-biotinylated peroxidase complex for 45 minutes at 37°C after the PBS wash process, and then, the peroxidase reaction product was visualized using diaminobenzidine substrate and counterstained with Gill’s hematoxylin according to the manufacturer’s instructions (Ahmed et al., 2016). Histomorphometric analysisA group of images was taken from the chosen tissue sections using an OMAX 18.0 MP (A35180U3, China) microscopic camera. Afterward, processing and measurements were performed using Image J software (version 1.53, NIH, USA). For analysis, eight histological slides were randomly selected, and measurements were carried out across fifteen microscopic random fields (Al-Taey and Al-Haaik, 2022). The measurements involved: numbers of seminiferous (X100 field), diameters of seminiferous (µm), thickness of tunica albuginea(µm), number of Sertoli cells (sectional tubules), diameter of Sertoli cells (µm), and Sertoli cell index SCI (%). SCI was calculated by measuring the fractional area of Sertoli cells from the whole germ cells using image J color threshold properties. Statistical analysisData were collected from gross morphometric and microscopic measurements in the left and right testes were recorded, and normal distribution was confirmed using the Shapiro-Wilk test. And the data were presented as mean ± SEM (M ± SEM). Differences between groups were analyzed using an independent samples t-test. Statistical analysis was conducted using IBM SPSS Statistics v27 (UK), with a significance level of p ≤ 0.05. Ethical approvalThe study corresponding with American Veterinary Medical Association (AVMA) guidelines (Underwood and Anthony, 2020). and approved by the College of Veterinary Medicine - Animal Care and Use Committee, University of Mosul, with reference number of UM.VET.2024.026. ResultsMacroscopic analysisMacroscopic observations revealed that geese possess paired testes (left and right) that were bean-shaped and located bilaterally along the midline of the body. The testes were suspended on the dorsal aspect of the abdominal cavity and attached ventrally by a peritoneal fold. They were white to yellow in color, depending on sexual activity, with a smooth texture. The left testis was larger and heavier, located more cranially than the right testis, and covered partially by the liver. Both testes are situated cranially to the kidney and caudally to the lung, and surrounded by the abdominal air sacs (Fig. 1). The morphometric examination revealed that testes weight ranged from 0.63 to 0.95 g, and the left testis was significantly (p ≤ 0.01) larger, while the length and width measurements were 1.3–1.8 cm and 0.7–0.9 cm, respectively, and the left testis was significantly (p ≤ 0.01) longer and wider compared with the right testes. Each testis was supplied by the testicular artery that originates from the abdominal aorta and accessory testicular arteries that branching from the cranial renal artery. Unlike in certain mammals, geese lake a scrotum and pampiniform plexus venous flow (Table 1). The epididymis was short and positioned dorsomedially to the testes, emerged close to their cranial pole. It consisted of an expanded head, a tapered body, and a narrow tail. The efferent ductulues formed an expansion at their junction with the epididymis, represented by a convoluted seminal glomus (Fig. 1).
Fig. 1. illustrating geese’s testes within the abdominal viscera. Both testes left (Lt) and right (Rt) are positioned between the cranial lobe of the kidney (Kd) and the lungs (Lg). enveloped by the abdominal air sacs (Ar), and the right testis further covered by the caudal lobe of the liver (Lv). The figure also highlights the epididymis (Ep), extend from the dorsomedial aspect of the testes, figure also shows the cranial renal artery (Cr.a) and the heart (Ht). diagram in the left show the testicular arterial supply by the testicular artery (T.a) which branches from the cranial renal artery (Cr.a) and by the accessory testicular arteries (ta.a) which branch from cabdominal aorta (A.a). Table 1. The dimensions and weight of left and right geese testes.
Table 2. The microscopic measurements of left and right geese testes.
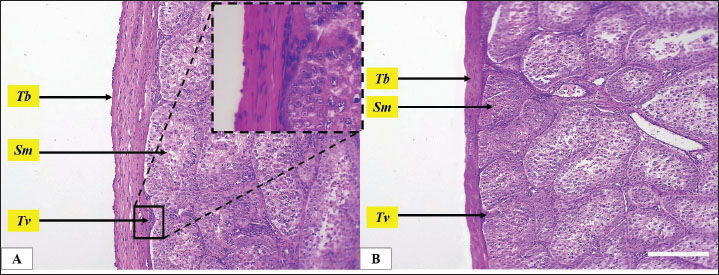
Fig. 2. Microphotograph showing the tunica albuginea (Tb) and the testicular seminiferous tubules (Sm), in the left testis (A) and right testis (B). Beneath the tunica albuginea, a thin layer of small blood vessels and connective tissue forms the tunica vasculosa (Tv) (magnified figure), H&E 100×. Scale bar=100 µm. Histomorphometric analysisMicroscopically, geese’s testes were covered by the tunica albuginea, a fibrous connective tissue capsule that consisted mainly of thick collagen and smooth muscle fibers, extending along the testicular surface. The tunica albuginea was significantly thicker in the left testis compared with the right testis, Beneath the tunica albuginea there was a relatively thin layer of connective tissue that contains interspersed arterioles and venules network represent the tunica vasculosa (Table 2) (Fig. 2). The testicular parenchyma consisted of numerous oval seminiferous tubules, densely packed with spermatogenic cells. Tubules in the periphery were larger than those in the central area. The geese testes lack testicular septa and, unlike mammals, the testis was not divided into lobules. The diameter and number of seminiferous tubules varied between the left and right testes. The diameter was significantly (p ≤ 0.01) wider in the left testis compared to the right, and the number of seminiferous tubules was also higher in the left testis (Table 2) (Fig. 3). The seminiferous tubules filled with germ cells and cellular density was high in both testes. Seminiferous tubules comprised spermatogonia, primary spermatocyte, secondary spermatocytes, spermatids, and Sertoli cells. Spermatogonia, characterized by large euchromatic nuclei undergoing active mitosis, rested on the basement membrane. While primary spermatocytes, the largest germ cells, occupied the central region of the tubules and displayed clear cytoplasm with large, spherical, nuclei, and prominent nucleolus. In contrast, secondary spermatocytes were fewer, smaller, and contained a central heterochromatic nucleus. And the smallest and least abundant cells, spermatids, were clustered near the tubular lumen, and typically arranged in groups (Figs. 4 and 5).
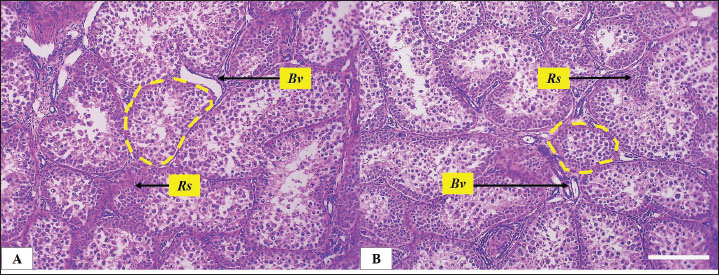
Fig. 3. Microphotograph showing the testicular parenchyma, focusing in the seminiferous tubules (dashed circles) at the center and peripheral region in both left (A) and right testis (B), the figure also shows interstitial testicular tissue (Rs) and the interstitial blood vessels, H&E 100×. Scale bar=100 µm.
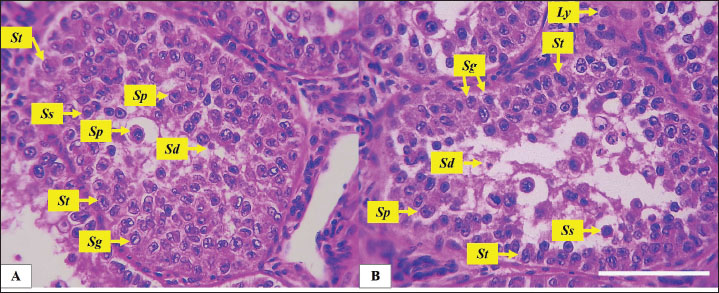
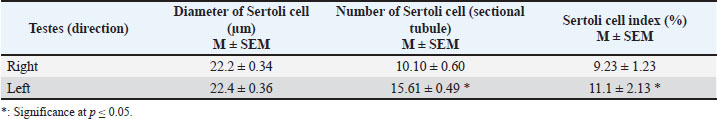
Fig. 4. Microphotograph showing the spermatogenic and Sertoli cells, in the left (A) and right testis (B) within testicular parenchyma including: the spermatogonia (Sg), primary spermatocytes (Sp), secondary spermatocytes (Ss), spermatids (St) and Sertoli cells (St). Leydig cells (Ly) prominent in the interstitial tissue around the seminiferous tubules, H&E 400×, Scale bar=100 µm. The mitotic activity was remarkably distinct observable in the spermatogonia, primary spermatocytes, and Sertoli cells. It exhibited a higher division activity in the peripheral tubules compared to the central ones and was more pronounced in the left testis than in the right. Histological examination showed that the Sertoli cell exhibited an irregular cylindrical shape, eccentrical nucleus, with clear, pale cytoplasm, the nucleus was oval or triangular in shape, with prominent nucleolus (Fig. 4). The density of distribution of Sertoli cell has regional difference, the ratios of Sertoli cell were much higher in peripheral seminiferous tubules than those in the center of testis. The diameter of the Sertoli cells ranged from 22.2 to 23 µm in both testes, but their density and number varied. Sertoli cell count in the left testis was higher (p ≤ 0.01) than in the right testis, and Sertoli cell index was also higher in left testis compared with the right one (Table 3). The interstitial spaces between seminiferous tubules were filled with connective tissue and clusters of small cells characterized by a central nucleus and acidophilic cytoplasm. These cells are identified as Leydig cells. The interstitial tissue also contains numerous small blood vessels.
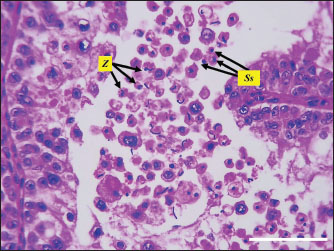
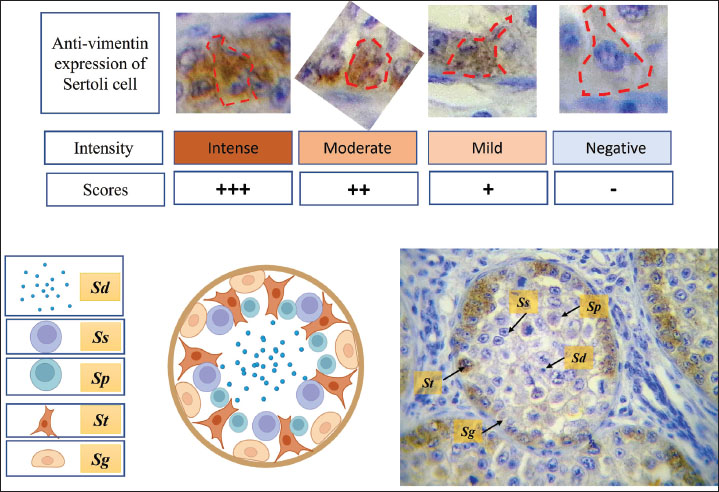
Fig. 5. Microphotograph showing the spermatids (St) and sperms cells (Z).in both testes H&E 400×, Scale bar=100 µm. The seminiferous tubules were connected to a network of smaller anastomosing tubules with a wider lumen, collectively forming the rete testis. These tubules were lined with short columnar epithelium. As the rete testis tubules approached the efferent ductulues, they became wider and lined with pseudostratified columnar epithelium, composed different lengths cells. Surrounding these ductulus were layers of connective tissue, smooth muscle fibers, blood vessels, and aggregations of lymphoid tissue and lymphoid follicles. The efferent ductulues connected to the head of the epididymis, which was characterized by a wider lumen and a regular diameter lined with pseudostratified columnar epithelium (Fig. 6). Immunohistochemical analysisThe immunohistochemical analysis of the Sertoli cells exhibited positive expression with rabbit polyclonal anti-vimentin marker, Sertoli cells were distributed along the basement membrane of the seminiferous tubules, among various neighboring germ cells, that lodged through their cytoplasmic extensions. As Sertoli cells approached the lumen, their shape became narrower and cytoplasmic extensions became longer (Fig. 7). The intensity of vimentin expression varied, ranging from intense to moderate and mild. Notably, the spermatogenic and interstitial Leydig cells showed no immunoreactivity (negative) to anti-vimentin marker, make this technique the best to the identification of Sertoli cells within testicular tissue, The left testis demonstrated a higher cellular density compared to the right testis, with correspondingly increased staining intensity (Fig. 8). The sections stained with the mouse monoclonal anti-vimentin antibody (MAB3400, Sigma-Aldrich, USA) exhibited negative immunoreactivity in Sertoli cells and all other spermatogenic cell types (Fig. 9, Table 4). DiscussionThe current study intended to investigate the histological and histomorphometric characteristics of adult goose testes, employing anti-vimentin immunohistochemical techniques to localize Sertoli cells and quantify their spatial distribution. The anatomical observations in the present study revealed that geese have paired bean-shaped, asymmetrical testes (left and right) positioned within the abdominal cavity, along body midline, and the left testis was larger and heavier and extend more cranially compared to the right testis. Similar observations regarding the shape, symmetry, position recoded by (Elbajory et al., 2013) in duck’s testes, (Elbajory et al., 2013) also mentioned that the left testis was larger and present in higher position than the right testis. Chicken rooster’s testes were similar in shape and position, and the left testis was larger, thicker, and heavier than the right testis (Althnaian, 2022). Furthermore (Parvez et al., 2023), mentioned that the turkey’s testicles were bean-shaped, located within abdominal cavity, ventrally to the kidneys, and caudally to the lungs, and the left testis was longer, wider, and heavier, and gonadosomatic index was greater than the right testis. These findings explain the unique intra-abdominal position of the testis in geese and the adaptive thermal regulation mechanisms effected by the lung, air sacs, and neighboring organs. Microscopically, the current study revealed that geese’s testes were covered by a thick fibrous connective tissue tunica albuginea, Beneath the tunica albuginea there was tunica vasculosa, a thin layer of connective tissue, and blood vessels network. Similar observations noted by (Dharani et al., 2017; Obeid et al., 2021; Mfoundou et al., 2022) in chicken, ducks, and guinea fowls. The thickness of tunica albuginea in duck’s testes was 52.12 ± 0.33 µm (Obeid et al., 2021), which is very close to our measurements in geese. The author also mentioned that tunica albuginea in the left testis was thicker than that of the right testis. The testicular parenchyma contained many oval seminiferous tubules densely packed with spermatogenic cells, and the peripheral tubules were larger than central ones. The geese testes were lacked septa and lobules. Additionally, the left testis had significantly more numerous and wider tubules than the right. These findings were also mentioned by (Kareem et al., 2020) in ducks, authors found that spermatogenic activity was higher in the left testis. Mfoundou et al. (2022) also mentioned that seminiferous tubules were more developed and spermatogenic density was higher in the left testis of yellow-feathered chickens. The absence of a mediastinum testis in geese aligns with the avian strategy of direct sperm transport via the rete testis and efferent ductules. The lymphoid aggregates surrounding goose efferent ductules may underscore an immunological adaptation to protect sperm antigens, which is less prominent in mammals. Table 3. Sertoli cell’s dimeter and number in left and right geese testes.
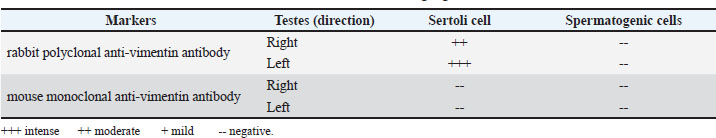
Table 4. The immunohistochemical demonstration of vimentin in left and right geese testes.
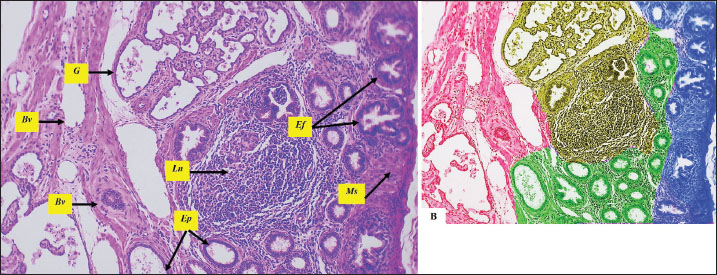
Fig. 6. Microphotograph showing the rete testis (t), the head of epididymis (Ep) and the efferent ductulues (Ef), surrounded by thick connective tissue mass studded with lymphoid follicles (Ln), blood vessels (Bv), and segments of muscular tissue (Ms), the figure in the left shows the zones of efferent ductulues (blue) , epididymis (green), and seminal glomus (yellow) in between surrounded from one side with the rete testis (red) , H&E 100×, Scale bar=100 µm. The mitotic activity was remarkably distinct and observable in the spermatogonia, primary spermatocytes, and Sertoli cells. It exhibited a higher division activity in the peripheral tubules compared to the central ones and was more pronounced in the left testis than in the right (Estermann et al., 2021) notes in avian, revealed that seminiferous tubules in peripheral region are less mature with higher mitotic activity in addition to the spermatogenic cells like spermatogonia, primary spermatocytes and Sertoli cells (Aire, 1997) also referred to the dominance of left testis mitotic activity and the germ cell development during sexual maturation in guinea fowls, ducks and Japanese quails, and mentioned that right testis was smaller with slightly reduced germ cell proliferation. The density and number of Sertoli cells varied. Sertoli cell in the left testis were higher than the right testis, and Sertoli cell index was also higher in the left testis compared with the right one. Similar studies documented that the density of Sertoli cells effected by the size of testes and seminiferous tubules (Okpe et al., 2010) linked the Sertoli cells density with seminiferous tubules numbers and size in Nigerian chicken. Obeid et al. (2021) found that Sertoli cell diameter was 10.4 ± 0.23 µm in duck, which is close to our measurements in the testis of geese, and found that Sertoli cells in the left testis were greater than the right testis
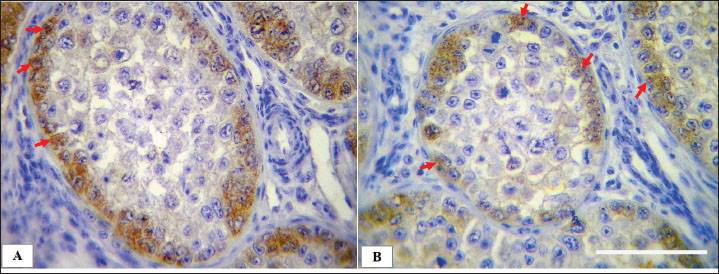
Fig. 7. Microphotograph showing the positive immunohistochemical expression of Sertoli cells with rabbit polyclonal anti-vimentin in the left (A) and right testis (B), Sertoli cells (arrows) distributed along the basement membrane of the seminiferous tubules and their cytoplasmic processes became narrower and extend toward tubular lumen, IHC 400×, Scale bar=100 µm.
Fig. 8. Microphotograph showing the scoring scale of immunohistochemical expressed Sertoli cells, explaining the degrees of intensity (upper set), figure below shows the location of Sertoli cells within the spermatogenic epithelium. Rabbit polyclonal anti-vimentin shows specificity to demonstrate Sertoli cells (St) compared with spermatogonia (Sg), primary spermatocyte (Sp), secondary spermatocyte (Ss) and spermatid (Sd) (lower set), IHC upper set 1,000×, lower set 400×. Testes in geese was lack of mediastinum testis and testicular septa, the rete testis was lined by columnar-to-cuboidal cells with microvilli. These observations correspond with other birds, such as the domestic fowl, Japanese quail, guinea-fowl, and ducks, according to (Aire, 1997; Dharani et al., 2017; Althnaian, 2022). Rete testis structure, facilitates its functional role, bird’s external rete testis connection with the seminiferous tubule enhances sperm passage directly into efferent ductulues, while mammals use an internal rete system for initial sperm collection. The left testis showed markedly higher mitotic activity, might as result of asymmetric hormonal regulation, developmental differences, and evolutionary adaptation to maximize sperm output and energy conserving.
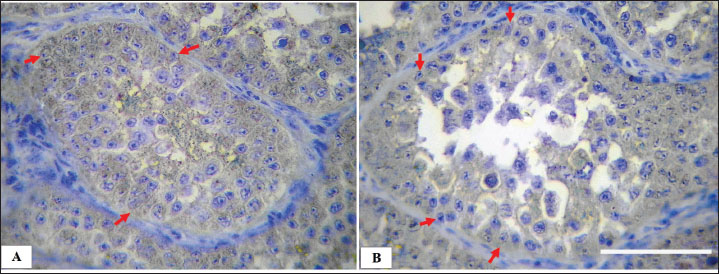
Fig. 9. Microphotograph showing the negative immunohistochemical expression of Sertoli cells with mouse monoclonal anti-vimentin in the left (A) and right testis (B), Sertoli cells (arrows) distributed along the basement membrane of the seminiferous tubules, IHC 400×, Scale bar=100 µm. Geese efferent ductulues was wide and lined with pseudostratified columnar epithelium Surrounded with layers of connective tissue, smooth muscle fibers, blood vessels, and aggregations of lymphoid tissue and lymphoid follicles, similar observations noted by (Aire, 1997) in guinea fowl and quails and by (Parvez et al., 2023) in turkey. The author believes that this unique structure differs from mammals efferent ductulues, which is more coiled and surrounded by less fibrous mass and with no muscular and lymphoid impression, due to the functional and immunological role, mammalian efferent ductules actively regulate fluid balance, while birds prioritize passive transport via muscular contractions. The immunohistochemical analysis revealed that Sertoli cells showed positive expression for the rabbit polyclonal anti-vimentin antibody, with distinct cytoplasmic fibrous protein demonstration. The anti-vimentin antibody is regarded as the most reliable biomarker for visualizing the vimentin cytoskeletal filamentous network in Sertoli cells of both mammalian and human tissues, as reported by Saito et al. (2023) and Camacho-Moll et al. (2022). However, this technique has not been employed in avian species. Previous studies have experimentally utilized SOX9, AMH (Anti-Müllerian Hormone), and DMRT1 as alternative markers for Sertoli cell identification in birds such as doves, chickens, and quails (Guibert et al., 2011; Mustafa and Elhanbaly, 2021; Ibrahim et al., 2022). The described immunohistochemical method is comparatively more expensive, less widely accessible, and exhibits limited species specificity in non-mammalian models. The mouse monoclonal anti-vimentin antibody showed no immunoreaction in Sertoli or other spermatogenic cells, despite manufacturer specifications indicating its applicability for detecting vimentin in avian tissues, including chickens and other birds. The absence of reactivity shows that the antibody's suitability for these species may require further experimental validation to confirm its effectiveness in avian. Danahey et al. (1995) mentioned that monoclonal antibodies have a sensitive distinguish between different forms of vimentin, allowing for more precise analysis of functional and diseased Sertoli cell. Vimentin expression varied in intensity; the left testis exhibited higher staining intensity compared to the right testis. The authors propose that variations in Sertoli cell density between the left and right testes contribute to an expansion of the vimentin-positive expressed area, the higher cellular density correlating with increased immunostaining intensity. This relationship of testicular architecture and cellular distribution may influence the extent and visibility of vimentin expression within Sertoli cells (Guibert et al., 2011; Ibrahim et al., 2022). Several limitations need to be mentioned. First, the small sample might restrict the statistical generalization of the findings. Moreover, the exclusive recognition of a single avian species (geese) limits comparative applicability throughout different birds, despite similarities mentioned in geese, chickens, and turkeys. The ability of antibody reactivity, especially the inconsistent performance of the mouse monoclonal anti-vimentin antibody, highlights the need for similar validation of immunohistochemical markers in avian tissues. The reliance on polyclonal anti-vimentin, although powerful, warrants cross-validation with opportunity Sertoli cell markers (e.g., SOX9 and AMH) to confirm specificity in birds. Future research must incorporate a broader variety of avian species (e.g., geese, quails, and turkeys) to assess the Sertoli cell distribution patterns. Standardizing antibody protocols, which include validation of rabbit-polyclonal anti-vimentin antibodies, to enhance reproducibility and solve the inconsistencies in immunoreactivity. Searching for alternative Sertoli cell markers should provide supplementary data, particularly in species in which vimentin expression is variable or weak. Incorporating functional reproductive assays, such as hormonal profiling (e.g., testosterone and FSH), and gene expression research (e.g., DMRT1 and AR), would help to link histological observations to physiological results. ConclusionThe current study describes the histological structure of adult goose testes, and the localization of Sertoli cells using anti-vimentin immunohistochemistry. The findings revealed marked asymmetry was observed, the left testis was larger, heavier, and exhibited greater cellular density. Indicating enhanced spermatogenic activity compared to the right testis. Immunohistochemical localization of Sertoli cells using the rabbit polyclonal anti-vimentin antibody proved an effective differential expression pattern correlating with testicular activity, whereas the monoclonal antibody failed to yield reactivity. These observations contribute to offering valuable data for understanding reproductive physiology in geese, presenting potential applications in the diagnosis of fertility-related diseases and enhancing productivity in avian breeding and food production systems. AcknowledgmentsThe authors thank the College of Veterinary Medicine, University of Mosul, for their encouragement. Conflict of interestThere is no conflict of interest. FundingSelf-funded study Authors’ contributionsAli A. conceptualized the research idea, wrote the draft, Soadd M. conducted data analysis Thekra F. performed the histological processing. Omar Y. conducted the microscopic examination, collected and analyzed data, and all authors contributed equally in reviewing, analysis, and presentation of the final work. Data availabilityThe data of this study are available within the manuscript. ReferencesAhmed, N., Yufei, H., Yang, P., Muhammad Yasir, W., Zhang, Q., Liu, T., Hong, C., Lisi, H., Xiaoya, C. and Chen, Q. 2016. Cytological study on Sertoli cells and their interactions with germ cells during annual reproductive cycle in turtle. Ecol. Evol. 6(12), 4050–4064; doi:10.1002/ece3.2193 Aire, T.A. 1997. The structure of the interstitial tissue of the active and resting avian testis. Onderstepoort J. Vet. Res. 64(4), 291–299; Aire, T.A. and Ozegbe, P.C. 2007. The testicular capsule and peritubular tissue of birds: morphometry, histology, ultrastructure and immunohistochemistry. J. Anatomy 210(6), 731–740; doi:10.1111/j.1469-7580.2007.00726.x Altaay, O.Y. and Al-Haaik, A.G. 2022. Histomorphometrical and histochemical postnatal development of cornea in indigenous rabbits. Iraqi J. Vet. Sci. 36(2), 291–296; doi: 10.33899/ijvs.2021.130031.1722 Altaey, O.Y., Hasan, A.A. and Alhaaik, A.G. 2025. Early-life development of spleen in white rabbit (Oryctolagus cuniculus): a morphometric and histochemical analysis. Vet. Integr. Sci. 23(1), 1–13. Althnaian, T. 2022. Morphological studies on the testis, epididymis and vas deferens of Al-Ahsa native rooster. Braz. J. Poultry Sci. 24(4), eRBCA-2021; doi:10.1590/1806-9061-2021-1596 Bozkurt, H.H., Aktaş, A., Ulkay, M.B. and Firat, U.B. 2007. Sertoli cell proliferation during the post hatching period in domestic fowl. J. Vet. Sci. 8(3), 219–222; doi:10.4142/jvs.2007.8.3.219 Camacho-Moll, M.E., Looijenga, L.H.J., Donat, R., Shukla, C.J., Jørgensen, A. and Mitchell, R.T. 2022. Expression of intermediate filaments in the developing testis and testicular germ cell cancer. Cancers 14(22), 5479; doi:10.3390/cancers14225479 Carson, F.L. and Cappellano, C.H. 2015. Fixation. In histotechnology. A self-instructional text. Eds., Carson, F.L. and Cappellano C.H. Chicago, IL: American Society, pp: 12–15. Chang, S.C., Lin, M.J., Zhuang, Z.X., Huang, S.Y., Lin, T.Y., Jea, Y.S., Fan, Y.K. and Lee, T.T. 2015. Effect of monochromic light-emitting diode light with different color on the growth and reproductive performances of breeder geese. Asian-Australasian J. Anim. Sci. 29(6), 830; doi:10.5713/ajas.15.0613 Costa, G.M.J., Figueiredo, A.F.A. and de França, L.R. 2020. The Sertoli cell: what can we learn from different vertebrate models?. Anim. Reprod. 16(1); doi:10.21451/1984-3143-AR2018-125 Danahey, D.G., Wu, J.C., Lin, L.H. and Dephilip, R.M. 1995. A monoclonal antibody identifies vimentin filaments in Sertoli cells and in a subset of epithelial cells in the rat epididymis, urinary bladder, and prostate. J. Urol. 154(6), 2190–2196. Dharani, P., Ushakumary, S., Sundaram, V., Joseph, C. and Ramesh, G. 2017. Morphometry and histology of the testicular capsule and peritubular tissue of testis of guinea fowl (Numida meleagris). Indian J. Vet. Anat. 29, 67–69; doi:10.4067/S0717-95022018000300909 Djermanovic, V., Milojevic, M., Mitrovic, S. and Bozickovic, I. 2021. Possibilities of productive and reproductive performance improvement in geese: part I-genetic factors. World's Poultry Sci. J. 77(4), 1027–1036; doi:10.1080/00439339.2021.1960233 Elbajory, S.I.A., El Tingari, M.D. and Abdalla, M.A. 2013. Morphological study of the testis of adult Sudanese duck (Anas platyrhynchos). Int. J. Anim. Vet. Adv. 5(3), 103–107; doi:10.19026/ijava.5.5584 Estermann, M.A., Major, A.T. and Smith, C.A. 2021. Genetic regulation of avian testis development. Genes 12(9), 1459; doi:10.3390/genes12091459 Guibert, E., Brière, S., Pelletier, R., Brillard, J.P. and Froment, P. 2011. Characterization of chicken Sertoli cells in vitro. Poultry Sci. 90(6), 1276–1286; doi:10.3382/ps.2010-01081 Hu, S., Li, X., Qing, E., Wang, J., Chen, Q., Song, Y., Chen, J., Hu, J., Li, L. and Wang, J. 2025. Molecular mechanisms underlying age-dependent effects of rearing system on the goose testicular development and semen quality. Poultry Sci. 104(1), 104589; doi:10.1016/j.psj.2024.104589 Ibrahim, M.I.A., Williams, J.H. and Botha, C.J. 2022. Immunohistochemical changes in the testicular excurrent duct system of healthy, male Japanese quail (Coturnix coturnix japonica) observed at 4, 6–7, 12, and 52 weeks of age. Int. J. Mol. Sci. 23(22), 14028; doi:10.3390/ijms232214028 Kareem, D.A., Jassem, E.S., Daaj, S.A. and Al-Khalad, W.J. 2020. Morphological and histological study of the testes in adult duck. Plant Arch. 20(S2), 751–755. Lara, N.L.E.M., Costa, G.M.J., Figueiredo, A.F.A. and De França, L.R. 2020. The Sertoli cell: what can we learn from different vertebrate models?. Anim. Reprod. 16(1), 81; doi:10.21451/1984-3143-AR2018-125 Latendresse, J.R., Warbrittion, A.R., Jonassen, H. and Creasy, D.M. 2002. Fixation of testes and eyes using a modified Davidson's fluid: comparison with Bouin's fluid and conventional Davidson's fluid. Toxicologic Pathol. 30(4), 524–533; doi:10.1080/01926230290105721 Lüpold, S., Wistuba, J., Damm, O.S., Rivers, J.W. and Birkhead, T.R. 2011. Sperm competition leads to functional adaptations in avian testes to maximize sperm quantity and quality. Reproduction 141(5), 595–605; doi:10.1530/rep-10-0501 Mfoundou, J.D.L., Guo, Y., Yan, Z. and Wang, X. 2022. Morpho-histology and morphometry of chicken testes and seminiferous tubules among yellow-feathered broilers of different ages. Vet. Sci. 9(9), 485; doi:10.3390/vetsci9090485 Mustafa, F.E.Z.A. and Elhanbaly, R. 2021. Histological, histochemical, immunohistochemical and ultrastructural characterization of the testes of the dove. Zygote 29(1), 33–41; doi:10.1017/s0967199420000477 Niazi Tabar, A., Azizi, H., Hashemi Karoii, D. and Skutella, T. 2022. Testicular localization and potential function of vimentin positive cells during spermatogonial differentiation stages. Animals 12(3), 268; doi:10.3390/ani12030268 Obeid, A.K., Al-Bazii, S.J. and Alsafy, A.H.M. 2021. Histological and morphomterical features of domesticus duck testes (Anas platyrhynchos). Ann. Romanian Soc. For Cell Biol. 25(3), 336–341; Available via Okpe, G.C., Nwatu, U. and Anya, K. 2010. Morphometric study of the testes of the Nigerian local breed of chicken. Anim. Res. Int. 7(2), 1163–1168. Pardyak, L., Chmurska-Gasowska, M., Jaszcza, K., Lonc, G., Pawlus, A., Trela, M., Lis, M.W., Bojarski, B., Krakowska, I. and Kotula-Balak, M. 2022. Diverse effect of in ovo treatment with metaloestrogen: selenium on chick testis histology and estrogen signaling. Medycyna Weterynaryjna 78(10), 526–531; doi:10.21521/mw.6701 Parvez, M.N.H., Sumon, K. and Rashid, S.M.H. 2023. Biometric and morphometric characteristics of turkey testes in Bangladesh. Bangladesh J. Vet. Med. 21(1), 1–5; doi:10.33109/bjvmjj2023am1 Pintus, E., Ros-Santaella, J.L. and Garde, J.J. 2015. Beyond testis size: links between spermatogenesis and sperm traits in a seasonal breeding mammal. PLos One 10(10), 139240; doi:10.1371/journal.pone.0139240 Saito, H., Yokota, S. and Kitajima, S. 2023. Immunohistochemical analysis of the vimentin filaments in Sertoli cells is a powerful tool for the prediction of spermatogenic dysfunction. Acta Histochemica 125(5), 152046; doi:10.1016/j.acthis.2023.152046 Shi, Z.D., Tian, Y.B., Wu, W. and Wang, Z.Y. 2008. Controlling reproductive seasonality in the geese: a review. World's Poultry Sci. J. 64(3), 343–355; doi:10.1017/S0043933908000081 Underwood, W. and Anthony, R. 2020. AVMA guidelines for the euthanasia of animals: 2020 edition. Retrieved on March, 2013(30), 2020. Available via https://www.avma.org/sites/default/files/2020-02/Guidelines-on-Euthanasia-2020.pdf | ||
| How to Cite this Article |
| Pubmed Style Hasan AA, Al-hamadany SM, Saleh TF, Altaey OY. Immunohistochemical and morphometric analysis of adult goose testes with special focus on sertoli cell localization. Open Vet. J.. 2025; 15(9): 4558-4568. doi:10.5455/OVJ.2025.v15.i9.62 Web Style Hasan AA, Al-hamadany SM, Saleh TF, Altaey OY. Immunohistochemical and morphometric analysis of adult goose testes with special focus on sertoli cell localization. https://www.openveterinaryjournal.com/?mno=257575 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i9.62 AMA (American Medical Association) Style Hasan AA, Al-hamadany SM, Saleh TF, Altaey OY. Immunohistochemical and morphometric analysis of adult goose testes with special focus on sertoli cell localization. Open Vet. J.. 2025; 15(9): 4558-4568. doi:10.5455/OVJ.2025.v15.i9.62 Vancouver/ICMJE Style Hasan AA, Al-hamadany SM, Saleh TF, Altaey OY. Immunohistochemical and morphometric analysis of adult goose testes with special focus on sertoli cell localization. Open Vet. J.. (2025), [cited January 25, 2026]; 15(9): 4558-4568. doi:10.5455/OVJ.2025.v15.i9.62 Harvard Style Hasan, A. A., Al-hamadany, . S. M., Saleh, . T. F. & Altaey, . O. Y. (2025) Immunohistochemical and morphometric analysis of adult goose testes with special focus on sertoli cell localization. Open Vet. J., 15 (9), 4558-4568. doi:10.5455/OVJ.2025.v15.i9.62 Turabian Style Hasan, Ali Ahmed, Soadd Mohamd Al-hamadany, Thekra Fadel Saleh, and Omar Younis Altaey. 2025. Immunohistochemical and morphometric analysis of adult goose testes with special focus on sertoli cell localization. Open Veterinary Journal, 15 (9), 4558-4568. doi:10.5455/OVJ.2025.v15.i9.62 Chicago Style Hasan, Ali Ahmed, Soadd Mohamd Al-hamadany, Thekra Fadel Saleh, and Omar Younis Altaey. "Immunohistochemical and morphometric analysis of adult goose testes with special focus on sertoli cell localization." Open Veterinary Journal 15 (2025), 4558-4568. doi:10.5455/OVJ.2025.v15.i9.62 MLA (The Modern Language Association) Style Hasan, Ali Ahmed, Soadd Mohamd Al-hamadany, Thekra Fadel Saleh, and Omar Younis Altaey. "Immunohistochemical and morphometric analysis of adult goose testes with special focus on sertoli cell localization." Open Veterinary Journal 15.9 (2025), 4558-4568. Print. doi:10.5455/OVJ.2025.v15.i9.62 APA (American Psychological Association) Style Hasan, A. A., Al-hamadany, . S. M., Saleh, . T. F. & Altaey, . O. Y. (2025) Immunohistochemical and morphometric analysis of adult goose testes with special focus on sertoli cell localization. Open Veterinary Journal, 15 (9), 4558-4568. doi:10.5455/OVJ.2025.v15.i9.62 |