| Research Article | ||
Open Vet. J.. 2025; 15(8): 3693-3702 Open Veterinary Journal, (2025), Vol. 15(8): 3693-3702 Research Article The effect of Hepato Forte in reducing hypercholesterolemia and DNA fragmentation induced by high cholesterol in male albino ratsImtithal Ali Mohammed*Department of Optical Techniques, College of Health and Medical Techniques, Alnoor University, Nineveh, Iraq *Corresponding Author: Imtithal Ali Mohammed, Department of Optical Techniques, College of Health and Medical Techniques, Alnoor University, Nineveh, Iraq. Email: emtithal.ali [at] alnoor.edu.iq Submitted: 11/05/2025 Revised: 27/06/2025 Accepted: 01/07/2025 Published: 31/08/2025 © 2025 Open Veterinary Journal
ABSTRACTBackground: A “Hepato Forte” is essentially a built-in substance in plants that reconditions the functional aspects of the liver to act in manners like hepatoprotective drugs, aiding in liver repair and acting as an effect enhancer. Aim: The current investigation examines the protective benefits of Hepato Forte on (lipid profiles, DNA damage, and glycemic index) in healthy hypercholesterolemic and phosphatidylcholine-deficient male rats. Methods: Thirty-two adult male albino rats were included, and there were 8 rats in each group. The treatments were administered orally daily to the rats. The control group, labeled C, received distilled water, while the second group was given Hepato Forte alone at a dose of (500 mg/kg/day) via gavage needle. A third group received cholesterol at a dose of (10 g/day) via gavage needle, and the fourth group received both Hepato Forte at 500 mg/kg/day and high cholesterol at a dose of 10 g/day. Results: Routine administration of Hepato Forte led to a significant decline (p > 0.05) in total cholesterol and triglycerides among both healthy and hypercholesterolemic male rats. Additionally, shows the effect of cholesterol and Hepato Forte on the concentrations and the body’s resistance to insulin in the T1 group, which decreased blood glucose and insulin resistance compared to the increased insulin levels in the same T1 group. In the T3 group of Agarose Gel Electrophoresis, it was observed that in animals the combination of cholesterol and Hepato Forte enhanced DNA integrity by reducing fragmentation and enhancing condensation and concentration through agarose gel electrophoresis. Conclusion: The findings of this research investigation show that Hepato Forte supplements and reducing DNA fragmentation play a critical protective role for the cardiovascular system and liver in adult male albino rats with hypercholesterolemia, effectively mitigating the adverse effects of high cholesterol on both the heart and liver. Keywords: Hepato Forte, Cholesterol, Insulin resistance, DNA, Hypercholesterolemia. IntroductionA “Hepato Forte” is essentially a built-in substance in plants that reconditions the functional aspects of the liver to act in manners like hepatoprotective drugs, aiding in liver repair and acting as an effect enhancer. Hepato Forte has been shown to have several hepatoprotective effects in rats. Functionally, the liver acts as a filter and detoxifying organ by processing nutrients, cleaning the bloodstream, processing drugs and metabolites, as well as clearing free radicals. The liver is involved in a variety of digestive, detoxifying, exocrine, immune functions, and the control of metabolism. Toxic substances, drugs, vitamins, fatty acids, proteins, and carbohydrates are metabolized by liver detoxification processes (Saad et al., 2020). Therefore, genetic and molecular levels prove that the generation of proteins (TGF-1β, fibronectin, and collagen) mainly increases the degrading structures of the liver. Gene expression of TGF-1β, fibronectin, has been reported after treatment with CCl_4, producing enhanced liver injury. Moreover, oxidative stress (O.S.) initiated by free radicals can also cause liver damage and potentially trigger increased hepatic fibrosis. Therefore, the main active compound in Hepato Forte has shown anti-inflammatory, antioxidant, and stimulating effects on liver cell regeneration (Babatunde et al., 2017). The liver is the main organ affected by acute toxicity with the potential use of Hepato Forte and a complex of essential phospholipids in mitigating liver injuries (Todić et al., 2003; Olukiran et al., 2014). Cardiac surgery and cardiopulmonary bypass (CPB) are both highly associated with liver injury; the mechanism of this has a negative effect, resulting in ischemic or cholestatic type liver damage. Total bilirubin, aspartate aminotransferase (AST), lactate dehydrogenase, alanine aminotransferase (ALT), prothrombin time, haptoglobin, and ammonia levels are conventionally used to show both heart and liver function. This research aimed to investigate the beneficial influence of Hepato Forte on the serum levels of ALT, LDH, AST, total and conjugated bilirubin, direct bilirubin, and haptoglobin during cardiopulmonary bypass (CPB) (Zhonghua et al., 2008; El-Gendy et al., 2021). Moreover, the study on rats has found that oxidative stress as a result of using Ritalin leads to tissue damage as found in the heart, liver, and kidneys. This increased activity of the enzyme also changed the morphology of these organs as well as the histological appearance (Jarkovska et al., 2017). Thus, the riskiest contributor of CVD diseases for rats and humans is a high level of blood cholesterol, causing high amounts of total cholesterol, low density lipoprotein (LDL), and triglycerides (Luiza et al., 2021; Azemi et al., 2022; Maulana et al., 2023). As a result, rats have been reported to experience an increase in blood cholesterol when fed on a high-fat diet (Abdulaziz et al., 2020). Studies showed that rats with hypercholesterolemia, an appropriate diet, including linoleic acid, reduces serum lipid levels and promotes the normal course of liver function, comparable to the effect of fenofibrate used as a drug (Shalaby et al., 2020). The relationship between increase in saturated fatty acids and cholesterol ingestion with lipid homeostasis disturbance, predominantly with changes in blood cholesterol and atherosclerosis development, was determined (Asashina et al., 2005). High cholesterol in the blood was raised in rats through commercial feeds fortified with cholesterol derived from fatty sources such as coconut, canola oil, lard, and palm oil (Raza et al., 2022). Hepato Forte, a supplement rich in essential phospholipids, has been reported to lower blood cholesterol levels as well as decrease DNA damage in adult albino rats (Shabbir et al., 2020). Other studies have reported that specific natural plant extracts may be effective in reducing elevated levels of blood cholesterol and DNA damage These are extracts of Maytenus royleanus leaves, Bacopa monniera, and casein hydrolysate, which have been tested in rodents (Venkatakrishnan et al., 2012; Sarah et al., 2018; Todić et al., 2003). As a natural dietary supplement, Hepato Forte has a potent effect on DNA damage, lipid, and glycemic reduction. Its antioxidant properties neutralize the damage done to the liver and heart by high cholesterol. It reduces the DNA damage of liver tissue, drops cholesterol, and protects your liver. Hepato Forte is a natural plant-based supplemental product that heals elevated cholesterol and its associated DNA damage in an experimental set up (Adil et al., 2018). It will be one of the roles of cholesterol in the future All the while affecting the lipid metabolism of adult albino rats; while the total and non-HDL cholesterol was high, the blood level decreased simultaneously with the HDL cholesterol (Yu-Ming et al., 2010). Hepatic lipid levels, including triglycerides and total cholesterol, were elevated in rats exposed to a cholesterol-rich diet. It resulted in a decline in the activities of crucial hepatic enzymes accountable for lipid metabolism (Mahfouz et al., 2000). Administering a supplement comprising elevated concentrations of unsaturated fatty acids, such as Lipostabil Forte, to individuals with coronary heart disease affects lipid metabolism (Balta et al., 2021). Several studies have demonstrated that dietary cholesterol can impact glucose metabolism and insulin regulation in rats. Consequently, meals that include cholesterol can decrease blood insulin levels by hastening insulin degradation in the liver (Basciano et al., 2009). Moreover, the merged administration of cholecalciferol and levocarnitine promoted results and significantly improved glycaemia regulation, elevated insulin secretion, and reduced insulin resistance in type 2 diabetic rats caused by high-fat diet (Anwar et al., 2013). Moreover, in the transition from healthy obesity to insulin resistance and diabetes, it is a critical step to decrease hepatic cholesterol25-hydroxylase (hepatic Ch25h) levels. Identifying low hepatic cholesterol 25-hydroxylase (hepatic Ch25h) levels indicates an increased risk for unhealthy obesity. This research analyzes the potential therapeutic effects of Hepato Forte in adult male albino rats with hypercholesterolemia caused by a high-cholesterol diet (Ling and Yang, 2004). Aim of the researchThe investigation studied the effects of Hepato Forte supplements which reduces DNA fragmentation and plays a critical protective role for the cardiovascular system and liver in adult male albino rats with hypercholesterolemia, effectively mitigating the damaging ePects of high cholesterol on not just the heart but also the liver. Materials and MethodsEthical considerations and best practices in experimental animal researchThe experiment was performed in the laboratories of Al Noor University from February 2023 to April 2023, and all the requirements were provided to complete the research. Thirty-two healthy male albino rats, aged 3 to 4 months and weighing 190 to 200 grams. They were raised in ideal conditions ranging from 20°C to 25°C in an air-conditioned environment with a daily photoperiod of 12 hours. The rats were confined for a minimum of 2 weeks to acclimatize before starting the experiment. Dose of Hepato ForteHepato Forte is a complex preparation containing herbal extracts and vitamins with proven efficacy against liver pathology. Hepato Forte activates phosphatidylcholine synthesis from methyl groups in the liver and helps to restore multinuclearity and DNA replication in hepatocytes following their partial damage. However, they possess functional superiority due to their unsaturated fatty acid content. Treatment of male albino rats at the dose of (500mg/kg/day/rat) via gastric intubation (Todić et al., 2003). Dose of cholesterolCholesterol is a fatlike, waxy material that is necessary for the body because it is a major component of cell membranes and is found in all animal tissues. Cholesterol is produced primarily in the liver and can also be obtained from the eat. Hypercholesterolemic diets contained (10 g) of powdered cholesterol per kg of the diets (Wu et al., 2004). Blood sample collectionThe steps of blood sample collection: 1-After 42 days of the investigation experiment, rats in experimental studies are subjected to blood collection for health monitoring or metabolic studies. 2- Blood collections are performed under anesthesia in all types of laboratory animals. Combining ketamine and xylazine is efficient when injected intramuscularly: xylazine (5 mg/kg/B.W.)–ketamine (100 mg/ kg/B.W.). 3-Samples were partitioned into two segments: the first one with anticoagulant tube for DNA damage analysis and the second one for serum (without anticoagulant) that was obtained after centri fuge from the first one at 3,000 rpm for 20 min. Serum collected was stored frozen (−18 °C) until it was used. (Colak et al., 2013; Mohammed and Al-Okaily, 2017; Mohammed et al., 2022). Experimental design in scientific researchAfter survival of rat’s groups was 2 weeks in gauges for adaptation. During this time, the rats were evenly subset into four groups:
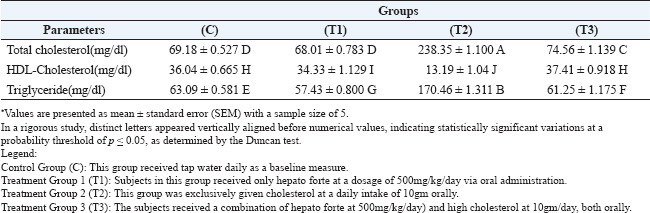
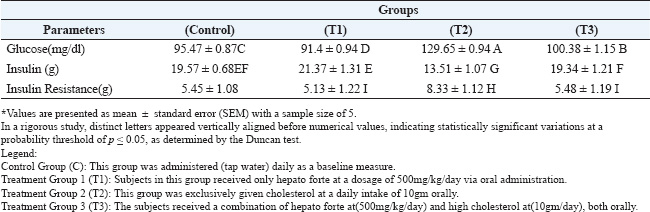
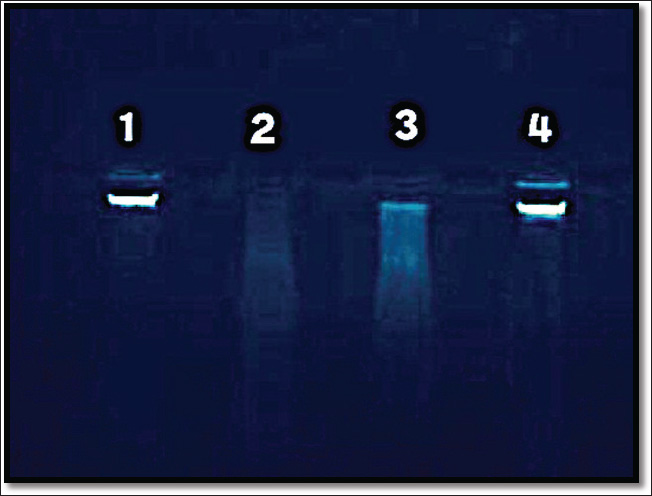
After 42 days of the experiment, the samples were collected and partitioned into two segments: the first segment was collected using an anticoagulant tube for DNA damage analysis, while the second segment was designated for serum collection (nor anticoagulant), which was collected after centrifugation at 3000 rpm for 20 min. Serum samples were kept in a deep-freezer at −18 °C until analyzed. Statistical analysisThe data were analyzed using a simple experimental system (Microsoft Excel 365) and a completely randomized design. The coefficients were analyzed using Duncan’s multiple range test under the probability level of p < 0.05. Different coefficients are distinguished significantly by different alphabetical letters (Tallarida et al., 1987; Abd El-Mohsen et al., 2015). ResultsThe results in Table 1 indicate that the mean levels of serum total cholesterol and of serum triglycerides significantly (p ≤ 0.05) decreased after 42 days during the oral intubation of the hepatic forte (T1) alone plus high cholesterol (T3) groups compared to the cholesterol-only (T2) groups, indicating the hypercholesterolemia effect of hepatic forte. Therefore, the result showed that serum HDL-cholesterol increased for the T3 group was (37.41 ± 0.918H), compared to the T2 group (13.19 ± 1.04J) and the control group (36.04 ± 0.665H) at significant (p ≤ 0.05). The effects of oral intubation of Hepato Forte (T3) on the average values of specific glycemic indices are detailed in Table 2. Statistical analysis demonstrated significant reductions (p > 0.05) in both glucose levels and insulin resistance observed in the T3 group, with average values recorded at 100.38 ± 1.15B and 5.48 ± 1.19I, respectively. In contrast, the (T2) group exhibited mean values of (129.65 ± 0.94A) and (8.33 ± 1.12H), alongside the control group. Moreover, the concentration of insulin was notably increased (p > 0.05) in the T3, 19.34 ± 1.21F, compared to the T2 group, which showed a lower average of 13.51 ± 1.07G. The present study showed that orally administrated pure cholesterol to male rats significantly increased serum total cholesterol, LDL cholesterol and triglycerides, but decreased HDL cholesterol within the hypercholesterolemic treatment group. Furthermore, the current research in Figure 1 indicates that hypercholesterolemia may induce DNA damage. Thus, the T3group of Agarose Gel Electrophoresis observed that in animals, the combination of cholesterol and Hepato Forte enhanced DNA integrity by reducing fragmentation and enhancing condensation and concentration through agarose gel electrophoresis. Table 1. The impact of cholesterol and Hepato Forte on the concentrations of total cholesterol, HDL, and triglycerides in the serum following 42 days of experimentation (Mean ± Standard error).
Table 2. The impact of cholesterol and Hepato Forte on the concentrations of glucose, insulin, and the body’s resistance to insulin (Mean ± Standard error).
DiscussionEffects of cholesterol on different lipid profiles, blood sugar, insulin, insulin resistanceThese findings noted in Table 1 align with earlier studies gain (Adams et al., 2006), which has demonstrated that a high cholesterol diet in rats leads to an increased risk of atherosclerosis and cardiovascular diseases. This includes dyslipidemia, distinguished by heightened triglyceride concentrations, total cholesterol, and LDL cholesterol, along with reduced HDL cholesterol. Additionally, the diet prompted increased food consumption, contributing to a corresponding weight gain (Gallou-Kabani et al., 2007). In support of this, an additional study revealed that a diet high in fat and cholesterol raised total blood cholesterol levels. However, a separate study indicated that such a diet did not impact body weight because of a decrease in testosterone, which is essential for muscle development and fat storage (Calvo-Ochoa et al., 2014). Insulin levels decreased a marked decline by the 42 days of the experiment in the group of hypercholesterolemic rats. Insulin plays an essential function in regulating food metabolism, promoting the activity of lipoprotein lipase and the synthesis of fatty acids, while also suppressing lipolysis during lipid metabolic processes (Avramoglu et al., 2006). Consequently, a diet high in cholesterol promotes the elimination of cholesterol esters and phospholipids from the bloodstream. Our current investigation shows elevated glucose levels due to excessive cholesterol activity. Hyperglycemia in the cholesterol group may be attributed to the increased metabolic energy obtained from fat, resulting in a modest elevation of glucose levels after the trial. A meal rich in fats and energy modifies intestinal paracellular permeability, resulting in elevated plasma levels of lipopolysaccharide (LPS), which correlates with alterations in gut microbiota. The research results indicate that LPS significantly influences glucose intolerance and atherosclerosis (Ghosh et al., 2015). Rats receiving a daily dose of 10g pure cholesterol, insulin resistance was significantly accelerated. Insulin is crucial in the controlling of multiple aspects of lipoprotein metabolism. When insulin resistance occurs, the liver’s production of triglycerides and apolipoprotein B is increased, leading to an enhanced release of very low-density lipoproteins from the liver (Lewis et al., 1995). Insulin resistance impairs the function of lipoprotein lipase, leading to an extended duration in the clearance of triglyceride-rich lipoproteins. This delayed process is frequently linked to the production of small dense LDL particles and reduced HDL-C levels (Zambon et al., 1998). A significant relationship exists between insulin resistance and various lipid markers, including triglycerides, apolipoprotein B, HDL-C, and LDL particle size. Together, the data suggest that insulin resistance profoundly affects lipid metabolism. Increased visceral fat contributes to higher levels and flow of free fatty acids in the portal circulation, impairing insulin breakdown and exacerbating insulin resistance (Kahn and Flier 2000). Additionally, altered adipocytokine secretion, including factors such as leptin, TNF-α, and adiponectin, is thought to promote insulin resistance (Kadowaki et al., 2003). Insulin resistance shows a direct association with elevated triglyceride and LDL-c levels, while inversely associated with HDL-c levels (Lin et al., 2006).
Fig. 1. Agarose Gel Electrophoretic result for DNA damage assessment using in % agarose gel. From agarose gel electrophoresis illustrating: Line 1: DNA extracted from control leukocytes. Line 2: DNA from white blood cells subjected to 10 g/kg body weight of pure cholesterol. Line 3: DNA from white blood cells exposed to a combination of 500 mg/kg body weight plus 10 g/kg body weight of pure cholesterol. Line 4: DNA from white blood cells treated with 500 mg/kg body weight of Hepato Forte. The safeguarding influence of Hepato Forte on specific lipid levels, glucose balance, insulin hormone secretion, and insulin resistanceHepato Forte has been extensively studied for its potential in reducing plasma cholesterol levels and contributing to cardiovascular protection. Research has shown that dietary phosphatidylcholines can indeed impact lipid metabolism, with studies indicating that phosphatidylcholine treatment can lead to a significant decrease in transaminase levels and an increase in antioxidant enzymes, ultimately reducing lipid peroxidation markers (Cheen et al., 2023). Additionally, phosphatidylcholine supplementation has been linked to improvements in lipid profiles, protein profiles, and atherogenic index in animal models with metabolic syndrome induced by fructose consumption. Additionally, phosphatidylcholine supplementation has been linked to improvements in lipid profiles, protein profiles, and atherogenic index in animal models with metabolic syndrome induced by fructose consumption (Sugimoto et al., 2022). Furthermore, phosphatidylcholine has been found to increase hepatocyte membrane fluidity, decrease apoptosis, and enhance hepatocellular export, potentially improving liver function. These findings collectively suggest that dietary phosphatidylcholines, such as Hepato Forte, may play a crucial role in modifying cholesterol homeostasis and lipoprotein metabolism, highlighting their potential in managing hypercholesterolemia and promoting cardiovascular health (Surour et al., 2022). Hepato Forte demonstrates remarkable effects on liver health and cholesterol levels. Studies have shown that Hepato Forte, a supplement containing essential phospholipids, vitamins, and other nutrients, plays a significant role in improving liver function and reducing the risk of liver damage (Babatunde et al., 2017; Lai et al., 2023). Additionally, Hepato Forte has been found to ameliorate hepatic injuries induced by substances such as carbon tetrachloride, restoring liver histomorphology and protecting against degenerative changes Furthermore, research indicates that Hepato Forte can effectively reduce liver cancer risk in a dose-dependent and time-dependent manner, showcasing its potential as a protective agent against liver malignancies (Shankaraiah and Ram Charan, 2019). Overall, Hepato Forte’s multifaceted benefits on liver health, including its impact on cholesterol synthesis and liver function, highlight its importance in promoting overall liver well-being and potentially reducing the excess of LDL cholesterol while increasing the synthesis of HDL cholesterol (Ilic et al., 1992). In mice studies, sodium cholate (SC) demonstrated anti-NASH effects by activating hepatic and intestinal FXR signaling, leading to reduced bile acid levels and improved liver health (Pan et al., 2023). Additionally, a study on broilers revealed that bile acid and lipase supplementation did not significantly affect serum lipid profiles or growth performance, indicating no improvement in broiler performance or cholesterol levels. Therefore, while hepato forte-rich diets may encourage bile acid secretion, the direct hypercholesterolemic effects of Hepato Forte on patients with hypercholesterolemia require further investigation to determine its cholesterol-lowering capacity and overall impact on health (Chun et al., 2022). The research was conducted on rats fed with diets enriched in cholesterol, such as a low-carbohydrate–high-protein diet with added cholesterol (LCHPch) (Kostogrys et al., 2022; Saigo et al., 2023). A western diet with high fat and sucrose, and a diet-induced hypercholesterolemia diet, collectively demonstrates significant impacts on hepatic cholesterol metabolism. These studies reveal that such diets lead to alterations in cholesterol metabolism, including increased total cholesterol levels, changes in fatty acid profiles, and the development of non-alcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) (Roxane et al., 2020; Simoni-Nieves et al., 2021; Mikashinovich et al., 2021). Dietary strategies are essential in regulating bile acid metabolism and maintaining liver health. Studies indicate that the composition of fatty acids in one’s diet has the potential to affect bile acid metabolism, playing a role in the onset of NAFLD (Manjarín et al., 2022). Moreover, introducing cholic acid in diets high in fat and cholesterol can intensify hepatic steatosis, inflammation, and fibrosis, thereby disrupting bile acid metabolism and liver inflammation (Ichimura-Shimizu et al., 2022). These observations underline the complex interaction between dietary elements, bile acid metabolism, and liver health, highlighting the importance of dietary interventions in managing liver conditions (Agostino et al., 2022; Linyu et al., 2023). However, the daily administration of Hepato Forte in male rats from the T3 group led to a marked decrease in the glucose levels in comparison to the T2 group, alongside a rise in insulin levels. This suggests that Hepato Forte, along with its isoflavones, enhances insulin secretion in response to glucose. Even with a notable increase in insulin secretion, glucose uptake did not show any significant change. Moreover, the insulin-to-glucose AUC ratio from IVGTTs exhibited a dose-dependent elevation, pointing to heightened peripheral insulin resistance because of isoflavone exposure (Zhang et al., 2022). These findings highlight the complex interplay between isoflavones, insulin secretion, glucose metabolism, and insulin resistance in the context of Hepato Forte supplementation in rats (Shali et al., 2022). Research findings suggest that proteins can positively impact metabolic responses related to glucose tolerance and insulin sensitivity, while protein supplementation has been linked to reduced postprandial glucose concentrations (Hovland et al., 2020), decreased levels of potential early markers of impaired glucose metabolism, and improved fasting insulin sensitivity in healthy individuals (Iselin et al., 2020; Dale et al., 2018). Furthermore, Alaska pollock protein intake has been associated with improved insulin sensitivity and altered gut microbiota composition, leading to better glucose metabolism and potentially preventing hyperglycemia and type 2 diabetes. The collective results underscore the potential of cod and Alaska pollock proteins in boosting metabolic activity, as well as enhancing glucose tolerance and insulin sensitivity in contrast to casein. Significantly, the levels of IGF1 and insulin resistance exhibited no considerable variations between the group and the Hepato Forte plus high cholesterol group in comparison to the control group (Ryota et al., 2020). Impact of cholesterol and Hepato Forte on DNA damageFigure 1 shows that hypercholesterolemia may lead to DNA damage. Thus, the combination of cholesterol and Hepato Forte (T3 group) was observed to enhance DNA integrity in animals by reducing fragmentation and enhancing condensation and concentration through agarose gel electrophoresis. Recent observations in rats indicate that hypercholesterolemia affects DNA damage. (Behling-Kelly and Wong, 2022). Furthermore, we may also wish to evaluate the significance (R.O.). The recent research findings indicate that the oxidation of cholesterol by of reactive oxygen species leads to the formation of hydroperoxides, which have the potential to induce DNA mutations and modifications (Ying et al., 2005). Hypercholesterolemia triggers increased generation of (ROS), which fosters oxidative stress, ultimately resulting in cellular damage and impaired function (Ronsein et al., 2011). Elevated ROS levels are capable of damaging various molecular structures, including nucleic acids (Vasquez et al., 2012). Recognizing the role of Hepato Forte as an antioxidant, ROS seem to play a substantial part in causing DNA damage. While the research conducted in vitro indicates that antioxidants can defend sperm DNA against external oxidative stress, their ability to protect WBC from internally generated ROS remains less defined. Evidence suggests that dietary antioxidants may have the potential to lower WBC DNA damage, especially in men experiencing heightened DNA fragmentation. Nonetheless, the specific mechanisms by which dietary antioxidants act are not fully understood, and the clinical trials investigating these effects are relatively small (Taylor et al., 2009). ConclusionThe present study showed that Hepato Forte is a dietary supplement that effectively lowers high blood cholesterol levels. It was found that diets abundant in Hepato Forte affect the release of fatty acids and the levels of increased cholesterol. A significant decrease in particularly in contrast to diets that lack Hepato Forte. A significant decrease in total cholesterol levels was observed in this study, suggesting that frequent consumption of Hepato Forte may be crucial in the management of high blood cholesterol. As a result, it significantly reduces the absorption of nutrients in the intestine or increases the release of bile acids containing cholesterol. Thus, the Hepato Forte dietary supplement has a protective effect against cardiovascular and hepatic disorders. AcknowledgmentsThe author expresses their appreciation to Alnoor-University for their invaluable help and provision of information, which significantly contributed to the completion of the research paper. FundingThis study did not obtain external support. All authors contributed to this work in a self-sustaining manner. Conflict of interestThe authors of this study have no conflicts of interest to report regarding its publication. Ethics approval and consent to participateThe current research has followed the accepted principles of ethical conduct by the committee of the cCollege of vVeterinary mMedicine, University of Mosul, Iraq number 24/ 2024. ReferencesAbd El-Mohsen, A.A., Abd El-Shafi, M.A., Gheith, E.M.S. and Suleiman, H.S. 2015. Using different statistical procedures for evaluating drought tolerance indices of bread wheat genotypes. Adv. Agric. Biol. 4(1), 19–30; doi:10.15192/pscp.aab.2015.4.1.1930. Abdulaziz, M., AlSaad., M., Mohamed, M., Mohammed, S., Almalk, I., Ibrahim, Almutham., Abdulwahab, A, Alahmari, M., AlSulaiman, M., Salim, S. and Al-Rejaie, S. 2020. Baicalein neutralizes hypercholesterolemia-induced aggravation of oxidative injury in rats. Int. J. Med. Sci. 17(9), 1156–1166; doi:10.7150/IJMS.46108. Adams, K.F., Schatzkin, A., Harris, T.B., Kipnis, V., Mouw, T., Ballard-Barbash, R. and Leitzmann, M.F. 2006. Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old. N. Engl. J. Med. 355(8), 763–777. Adil, A., Alsultani., A., Luma, W.K., Sabah, M. and Hadi, H. 2018. Role and importance of casein hydrolysate in reducing fructose-induced hyperlipidemia and DNA fragmentation in adult male rats. J. Entomol. Zool. Stud. 6(2), 2062–2067. Anwar, M.K., Hussain, M.M., Khan, M.A. and Ahmad, T. 2013. Effect of cholecalciferol and levo carnitine on plasma glucose, plasma insulin and insulin resistance in type 2 diabetic rats. J. Pak. Med. Assoc. 63(3), 374–379. Asashina, M., Sato, M. and Imaizumi, K. 2005. Genetic analysis of diet- induced hypercholesterolemia in exogenously hypercholesterolemic (ExHC) rats. J. Lipid. Res. 46, 2289–2294; doi:10.1194/jlr.M500257-JLR200. Avramoglu, R.K., Basciano, H. and Adeli, K. 2006. Lipid and lipoprotein dysregulation in insulin resistant states. Clin. Chim. Acta. 368(1–2), 1–19; doi:10.1016/j.cca.2005.12.026. Azemi, N.A., Azemi, A.K., Abu-Bakar, L., Sevakumaran, V., Sifzizul, T., Muhammad, T. and Ismail, N. 2022. Effect of linoleic acid on cholesterol levels in a high-fat diet-induced hypercholesterolemia rat model. Metabolites, 13(1), 53; doi:10.3390/metabo13010053. Babatunde, E., Arayombo, O., Adewole, S., Ofusori, D.A., Adelodun, T.S., Olusola, S.S. and Bejide, R.A. 2017. Effect of zinc sulphate and Essentiale® Forte on carbon tetrachloride-induced hepatotoxicity in adult Wistar rats, 11(2), 93–98; doi:10.2399/ANA.17.016. Balta, I., Stef, L., Pet, I., Iancu, T., Stef, D. and Corcionivoschi, N. 2021. Essential fatty acids as biomedicines in cardiac health. Biomedicine. (Taipei), 9(10), 1466; doi:10.3390/biomedicines9101466. Basciano, H., Miller, A.E., Naples, M., Baker, C., Kohen, R., Xu, E., Su, Q., Allister, E.M., Wheeler, M.B., and Adeli, K.. 2009. Metabolic effects of dietary cholesterol in an animal model of insulin resistance and hepatic steatosis. Am. J. Physiol. Endocrinol. Metab. 297(2), 764; doi:10.1152/AJPENDO.90764.2008. Behling-Kelly, E. and Wong, C. 2022. Agarose gel electrophoresis determination of bovine lipoproteins compared with a wet chemistry method. JDS Commun. 3(5), 373–376; doi:10.3168/jdsc.2022-0223. Calvo-Ochoa, E., Hernández-Ortega, K., Ferrera, P., Morimoto, S. and Arias, C. 2014. Short-term high-fat-and-fructose feeding produces insulin signaling alterations accompanied by neurite and synaptic reduction and astroglial activation in the rat hippocampus. J. Cereb. Blood Flow Metab. 34(6), 1001–1008; doi:10.1038/jcbfm.2014.48 Chin, C.F., Galam, D., Gao, L., Tan, B.C., Wong, B.H., Chua, G.L., Loke, R.Y., Lim, Y.C., Wenk, M.R., Lim, M.S., Leow, W.Q., Goh, G.B., Torta, F. and Silver, D.L.. 2023. Blood-derived lysophospholipid sustains hepatic phospholipids and fat storage necessary for hepatoprotection in overnutrition. J. Clin. Invest. 33(17), e17126. doi:10.1172/JCI171267 Chun, H.J., Shim, Y.J. and Kwon, Y.H. 2022. Cholic acid supplementation accelerates the progression of nonalcoholic fatty liver disease to the procarcinogenic state in mice fed a high-fat and high-cholesterol diet. J. Nutr. Biochem. 100, 108869; doi:10.1016/j.jnutbio.2021.108869 Colak, A., Toprak, B., Dogan, N. and Ustuner, F. 2013. Effect of sample type, centrifugation and storage conditions on vitamin D concentration. Biochem. Med. 23(3), 321–325; doi:10.11613/BM.2013.039 Cunha, L.F., Ongaratto, M.A., Endres, M. and Barschak, A.G. 2021. Modelling hypercholesterolaemia in rats using high cholesterol diet. Int. J. Exp. Pathol. 102(2), 74–79; doi:10.1111/IEP.12387 Dale, H.F., Jensen, C., Hausken, T., Lied, E., Hatlebakk, J.G., Brønstad, I., Lihaug Hoff, D.A. and Lied, G.A.. 2018. Effect of a cod protein hydrolysate on postprandial glucose metabolism in healthy subjects: a double-blind cross-over trial. J. Nutr. Sci. 8, 23; doi: 10.1017/JNS.2018.23 Di Ciaula, A., Bonfrate, L., Baj, J., Khalil, M., Garruti, G., Stellaard, F., Wang, H.H., Wang, D.Q. and Portincasa, P.. 2022. Recent advances in the digestive, metabolic, and therapeutic effects of farnesoid X receptor and fibroblast growth factor 19: from cholesterol to bile acid signaling. Nutrients 14, 4950; doi:10.3390/nu14234950 El-Gendy, M.A.E., El-Dabbah, F.H., Hassan, A.I. and Awad, N.A.E. 2021. Hepatotoxic and cardiotoxic effects of testosterone enanthate abuse in adult male albino rats. Al-azhar Med. J. 50(2), 1335–1348; doi:10.21608/AMJ.2021.158480 Gallou-Kabani, C., Vigé, A., Gross, M.S. and Junien, C. 2007. Nutri-epigenomics: lifelong remodelling of our epigenomes by nutritional and metabolic factors and beyond. Clin. Chem. Lab. Med. 45(3), 321–7; doi:10.1515/cclm.2007.081 Ghosh, S., Parihar, V.S., More, P., Dhavale, D.D. and Chopade, B.A. 2015. Phytochemistry and therapeutic potential of medicinal plant: Dioscorea bulbifera. Med. Chem. 5(4), 160–172; doi:10.4172/2161-0444.1000259 Hovland, I.H., Leikanger, I.S., Stokkeland, O., Waage, K.H., Mjøs, S.A., Brokstad, K.A., McCann, A., Ueland, P.M., Slizyte, R., Carvajal, A., Mellgren, G., Remman, T., Høgøy, I. and Gudbrandsen, O.A.. 2020. Effects of low-dose of fish and milk proteins on glucose regulation and markers of insulin sensitivity in overweight adults: a randomised, double blind study. Eur. J. Nutr. 59(3), 1013–1029; doi:10.1007/S00394-019-01963-0 Ichimura-Shimizu, M., Watanabe, S., Kashirajima, Y., Nagatomo, A., Wada, H., Tsuneyama, K., and Omagari, K. 2022. Dietary cholic acid intensifies liver fibrosis in NASH model of sprague–Dawley rats fed a high-fat and high-cholesterol diet. Int. J. Mol. Sci. 23(16), 9268–9268; doi:10.3390/ijms23169268 Ilic., K., Kordac., A. and Alvarez, S. 1992. Clinical experience with long-term administration of “essential” phospholipids in chronic active hepatitis. Review of 3 double-blind studies. Cas. Lek. Cesk. 131(26), 801–804. Jarkovska, D., Bludovska, M., Mistrova, E., Krizkova, V., Kotyzova, D., Kubikova, T., Slavikova, J., Erek, S.N., Djordjevic, A. and Chottova Dvorakova, M.. 2017. Expression of classical mediators in hearts of rats with hepatic dysfunction. Can. J. Physiol. Pharmacol. 95(11), 1351–1359; doi:10.1139/CJPP-2017-0060 Kadowaki, T., Hara, K., Yamauchi, T., Terauchi, Y., Tobe, K. and Nagai, R. 2003. Molecular mechanism of insulin resistance and obesity. Exp. Biol. Med. 228(10), 1111–1117. Kahn, B.B. and Flier, J.S. 2000. Obesity and insulin resistance. J. Clin. Invest. 106(4), 473–481; doi:10.1172/jci10842 Kostogrys, R.B., Franczyk-Zarow, M., Kus, E. and Topolska, K. 2022. The LCHP diet enriched with cholesterol promotes non-alcoholic fatty liver disease in Wistar rats. Appl. Sci. 12(16), 8266–8266; doi:10.3390/app12168266 Lai, H.C., Lin, H.J., Shih, Y.H., Chou, J.W., Lin, K.W., Jeng, L.B., and Huang, S.T.. 2023. LipoCol Forte capsules reduce the risk of liver cancer: a propensity score-matched, nationwide, population-based cohort study. World J. Gastrointest. Oncol. 15(5), 828–842; doi:10.4251/wjgo.v15.i5.828 Lavigne, C., Marette, A. and Jacques, H. 2000. Cod and soy proteins compared with casein improve glucose tolerance and insulin sensitivity in rats. Am. J. Physiol. Endocrinol. Metab. 278(3), E491–E500; doi:10.1152/ajpendo.2000.278.3.e491 Lewis, G.F., Uffelman, K.D, Szeto, L.W., Weller, B. and Steiner, G. 1995. Interaction between free fatty acids and insulin in the acute control of very low- density lipoprotein production in humans. J. Clin. Invest. 95(1), 158–166; doi:10.1172/jci117633 Lin, M.W., Hwu, C.M., Huang, Y.H., Sheu, W.H.H., Shih, K.C., Chiang, F.T. and Ho, L.T. 2006. Directly measured insulin resistance and the assessment of clustered cardiovascular risks in hypertension. Am. J. Hypertens. 19(11), 1118–1124; doi:10.1016/j.amjhyper.2006.04.003 Ling, L. and Yang, G.-Y. 2004. Effect of hepatic glucose production on acute insulin resistance induced by lipid-infusion in awake rats. World J. Gastroenterol. 10(21), 3208–3211; doi:10.3748/WJG.V10.I21.3208 Mahfouz, M.M. and Kummerow, F.A. 2000. Cholesterol-rich diets have different effects on lipid peroxidation, cholesterol oxides, and antioxidant enzymes in rats and rabbits. J. Nutr. Biochem. 11(5), 293–302; doi:10.1016/S0955-2863(00)00083-8 Manjarín, R., Dillard, K., Coffin, M., Hernandez, G.V., Smith, V.A., Noland-Lidell, T., Gehani, T.R., Smart, H.J., Wheeler, K., Sprayberry, K.A., Edwards, M.S., Fanter, R.K., Glanz, H., Immoos, C., Santiago-Rodriguez, T.M., Blank, J.M., Burrin, D.G., Piccolo, B.D., Abo-Ismail, M., La Frano, M.R. and Maj, M. 2022. Dietary fat composition shapes bile acid metabolism and severity of liver injury in a pig model of pediatric NAFLD. Am. J. Physiol. Endocrinol. Metab. 323(3), E187–E206; doi:10.1152/ajpendo.00052.2022 Maulana, H., Ridwan, A., Suprijanto, Kusumawardani, S.R. and Fitri, L.L. 2023. Electrocardiogram analysis of Wistar rats with hyperlipidemia using wireless mouse electrocardiogram (Wim ECG) in a coronary heart disease study. Sains Malays. 52(2), 589–597; doi:10.17576/jsm-2023-5202-20 Mikashinovich, Z.I., Romashenko, A.V. and Semenets, I.A. 2021. Effects of diet-induced hypercholesterolemia on metabolic processes in the heart, liver, and pancreas in rats. Kazanskiy Meditsinskiy Zhurnal, 102(5), 663–668; doi:10.17816/KMJ2021-663 Mohammed, I.A. and Al-Okaily, B.N. 2017. Effect of sodium fluoride on the liver function of rats and reduction by CoQ10. J. Entomol. Zool. Stud. 5(5), 887–893. Mohammed, I.A., Shaban, K.A. and Albadrany, Y.M. 2022. Hepato-renal and hematological effects of flunixin and silymarin coadministration in rats. I.J.V.S. 36(2), 367–373; doi:vetmedmosul.com/article_170673.html Olukiran, O.S., Akomolafe, R.O., Bamitale, K.O., Ajayi, A.O., Okonji, R.E. and Bejide, R.A. 2014. Prophylactic and curative assessment of essentiale forte® On carbon tetrachloride-induced liver damage in wistar rats. Maced. J. Med. Sci. 7(3), 408–414; doi:10.3889/MJMS.1857-5773.2014.0364 Pan, L., Yu, Z., Liang, X., Yao, J., Fu, Y., He, X., Ren, X., Chen, J., Li, X., Lu, M. and Lan, T. 2023. Sodium cholate ameliorates nonalcoholic steatohepatitis by activation of FXR signaling. Hepatol. Commun. 7(2), e0039; doi:10.1097/hc9.0000000000000039 Raza, Q.S., Saleemi, M.K., Gul, S., Irshad, H., Fayyaz, A., Zaheer, I. and Khan, A. 2022. Role of essential oils and volatile oils in poultry production—A review of present, past and future contemplation. Agrobiol. Rec. 7, 40–56. Ronsein, G.E., de Oliveira, M.C.B., Medeiros, M.H., Miyamoto, S. and Di Mascio, P. 2011. DNA strand breaks and base modifications induced by cholesterol hydroperoxides. Free Radic. Res. 45(3), 266–275; doi:10.3109/10715762.2010.524215 Roxane, A., Emilienne, T., Ngo, S., Samantha, Q., Jean-Marc, L. and David, H.P. 2020. Two weeks of western diet disrupts liver molecular markers of cholesterol metabolism in rats. Lipids Health Dis. 19(1), 1–11; doi:10.1186/S12944-020-01351-2 Ryota, H., Ayano, N., Toshihiro, K., Yuki, I., Megumi, M., Takaki, S., Seiji, K., Toshimasa, N., Munehiro, Y. and Kenji, F. 2020. Dietary Alaska pollock protein alters insulin sensitivity and gut microbiota composition in rats. J. Food Sci. 85(10), 3628–3637; doi:10.1111/1750-3841.15413 Saad, H.S., Samah, S., Oda, S. and Sedeek, E.K. 2020. Protective effect of lactéol® forte against thioacetamide-induced hepatic injury in male albino rats. Lex. J. Vet. Sci. 67(1), 92–98; doi:10.5455/AJVS.17323 Saigo, Y., Sasase, T., Tohma, M., Uno, K., Shinozaki, Y., Maekawa, T., Sano, R., Miyajima, K. and Ohta, T.. 2023. High-cholesterol diet along with in combination with hydroxypropyl-beta-cyclodextrin induces NASH-like disorders in the liver of rats. Physiol. Res. 72, 371–382; doi:10.33549/physiolres.934981 Sarah, M., Alshammary, L. and Waleed, K. 2018. Protective effects of soybean lecithin against hypercholesterolemia and DNA fragmentation induced by high levels of cholesterol in adult male rats. K.J.V.S. 9, 1. Shabbir, M., Afsar, T., Razak, S., Almajwal, A., and Khan, M.R. 2020. Phytochemical analysis and evaluation of hepatoprotective effect of Maytenus royleanus leaves extract against antituberculosis drug induced liver injury in mice. Lipids Health Dis. 19(1), 46–46; doi:10.1186/S12944-020-01231-9 Shalaby, H.S., Amira, S. and Hassenin, H. 2020. Effects of fortification stirred yoghurt with red beet powder (RBP) on hypercholesterolemia rats. Eur. J. Agric. Food Sci. 2(5), 61; doi:10.24018/EJFOOD.2020.2.5.61 Shali, K.S., Soumya, N.P., Sukanta, S. and Mini, M.S. 2022. Hepatoprotective effects of morin by regulating the oxidative stress and carbohydrate metabolism in STZ induced diabetic rats. Bioact. Compd. Health Dis. 5(3), 53–53; doi:10.31989/bchd.v5i3.893 Shankaraiah, R.C. 2019. Early metformin intervention prevents hepatocellular carcinoma in a carbon tetrachloride induced mouse model of cirrhosis. SFERA. Simoni-Nieves, A., Salas-Silva, S., Chávez-Rodríguez, L., Escobedo-Calvario, A., Desoteux, M., Bucio, L., Souza, V., Miranda-Labra, R.U., Muñoz-Espinosa, L.E., Coulouarn, C., Gutiérrez-Ruiz, M.C., Marquardt, J.U. and Gomez-Quiroz, L.E.. 2021. The consumption of cholesterol-enriched diets conditions the development of a subtype of HCC with high aggressiveness and poor prognosis. Cancers 13(7), 1721; doi:10.3390/CANCERS13071721 Sugimoto, N.D. 2022. Treatment with phosphatidylcholine of patients with nonalcoholic fatty liver disease: a prospective pilot study. Miner. Gastroenterol. 68(4), 7; doi:10.23736/s2724-5985.21.03066-7 Surour, M.A., Ramadhan, S.J. and Khudair, K.K. 2022. Effect of phosphatidylcholine on dyslipidemia and the atherogenic index in high fructose exposed rats. Iraqi J. Vet. Med. 46(2), 20–28; doi:10.30539/ijvm.v46i2.1404 Sutharinee, L., Theerayuth, K., Arunrat, Y., Chanathip, D., Supachoke, M., Darawan, P. and Masahiko, I. 2023. Pyridylnidulin exerts antidiabetic properties and improves nonalcoholic fatty liver disease in diet-induced obesity mice. Front. Mol. Biosci. 10, 1208215; doi:10.3389/fmolb.2023.1208215 Tallarida, R.J., Murray, R.B., Tallarida, R.J. and Murray, R.B. 1987. Duncan multiple range test. Manual Pharmacol. Calcul. 124, 125–127; doi:10.4135/9781412961288.n124 Taylor, K., Roberts, P., Sanders, K. and Burton, P. 2009. Effect of antioxidant supplementation of cryopreservation medium on post-thaw integrity of human spermatozoa. Reprod. Biomed. Online. 18(2), 184–189; doi:10.1016/s1472-6483(10)60254-4 Todić, M., Bakić, S., Zulić, I., Krosnjar, S., Begovic, B., Dorić, M. and Babić, M. 2003. Oral acute toxicity of HEPALIP FORTE in rats. Bosn. J. Basic Med. Sci. 3(4), 30–36; doi:10.17305/BJBMS.2003.3489 Vasquez, E.C., Peotta, V.A., Gava, A.L, Pereira, T.M. and Meyrelles, S. 2012. Cardiac and vascular phenotypes in the apolipoprotein E-deficient mouse. J. Biomed. Sci. 19, 1–9; doi:10.1186/1423-0127-19-22 Venkatakrishnan, K. and Thangarajan, S. 2012. Antihypercholesterolemic effect of Bacopa monniera linn. on high-cholesterol diet-induced hypercholesterolemia in rats. Asian Pac. J. Trop. Med. 5(12), 949–955. Wang, Y-M., Zhang, B., Xue, Y., Li, Z., Wang, J., Xue, C. and Yanagita, T. 2010. The mechanism of dietary cholesterol effects on lipids metabolism in rats. Lipids Health Dis. 9(1), 4; doi:10.1186/1476-511X-9-4 Wu, X., Beecher, G.R., Holden, J.M., Haytowitz, D.B., Gebhardt, S.E. and Prior, R.L. 2004. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J. Agric. Food Chem. 52(12), 4026–4037; doi:10.1021/jf049696w Ying, Y., Xingkui, T., Xiaocheng, L., Jufang, S. and Yongman, L.V. 2005. The p53-mediated apoptosis in hypercholesterolemia-induced renal injury of rats. J. Huazhong Univ. Sci. Technolog. Med. Sci. 25, 408–411; doi:10.1007/bf02828209 Zambon, A., Deeb, S., Hokanson, J.E., Brown, B.G. and Brunzell, J.D. 1998. Common variants in the promoter of the hepatic lipase gene are associated with lower levels of hepatic lipase activity, buoyant LDL, and higher HDL2 cholesterol. Arterioscl. Thromb. Vasc. Biol. 18(11), 1723–1729; doi:10.1161/01.atv.18.11.1723 Zhang, N., Zhang, W., Liu, J., Li, S., Zhang, H. and Fan, B. 2022. Genistein protects mice with diet-induced prediabetes against hyperglycemia and fatty liver disease by activating the hepatic insulin signaling pathway. Front. Nutr. 9, 1072044; doi:10.3389/fnut.2022.1072044 | ||
| How to Cite this Article |
| Pubmed Style Imtithal Ali Mohammed. The effect of Hepato Forte in reducing hypercholesterolemia and DNA fragmentation induced by high cholesterol in male albino rats. Open Vet. J.. 2025; 15(8): 3693-3702. doi:10.5455/OVJ.2025.v15.i8.33 Web Style Imtithal Ali Mohammed. The effect of Hepato Forte in reducing hypercholesterolemia and DNA fragmentation induced by high cholesterol in male albino rats. https://www.openveterinaryjournal.com/?mno=257567 [Access: January 25, 2026]. doi:10.5455/OVJ.2025.v15.i8.33 AMA (American Medical Association) Style Imtithal Ali Mohammed. The effect of Hepato Forte in reducing hypercholesterolemia and DNA fragmentation induced by high cholesterol in male albino rats. Open Vet. J.. 2025; 15(8): 3693-3702. doi:10.5455/OVJ.2025.v15.i8.33 Vancouver/ICMJE Style Imtithal Ali Mohammed. The effect of Hepato Forte in reducing hypercholesterolemia and DNA fragmentation induced by high cholesterol in male albino rats. Open Vet. J.. (2025), [cited January 25, 2026]; 15(8): 3693-3702. doi:10.5455/OVJ.2025.v15.i8.33 Harvard Style Imtithal Ali Mohammed (2025) The effect of Hepato Forte in reducing hypercholesterolemia and DNA fragmentation induced by high cholesterol in male albino rats. Open Vet. J., 15 (8), 3693-3702. doi:10.5455/OVJ.2025.v15.i8.33 Turabian Style Imtithal Ali Mohammed. 2025. The effect of Hepato Forte in reducing hypercholesterolemia and DNA fragmentation induced by high cholesterol in male albino rats. Open Veterinary Journal, 15 (8), 3693-3702. doi:10.5455/OVJ.2025.v15.i8.33 Chicago Style Imtithal Ali Mohammed. "The effect of Hepato Forte in reducing hypercholesterolemia and DNA fragmentation induced by high cholesterol in male albino rats." Open Veterinary Journal 15 (2025), 3693-3702. doi:10.5455/OVJ.2025.v15.i8.33 MLA (The Modern Language Association) Style Imtithal Ali Mohammed. "The effect of Hepato Forte in reducing hypercholesterolemia and DNA fragmentation induced by high cholesterol in male albino rats." Open Veterinary Journal 15.8 (2025), 3693-3702. Print. doi:10.5455/OVJ.2025.v15.i8.33 APA (American Psychological Association) Style Imtithal Ali Mohammed (2025) The effect of Hepato Forte in reducing hypercholesterolemia and DNA fragmentation induced by high cholesterol in male albino rats. Open Veterinary Journal, 15 (8), 3693-3702. doi:10.5455/OVJ.2025.v15.i8.33 |